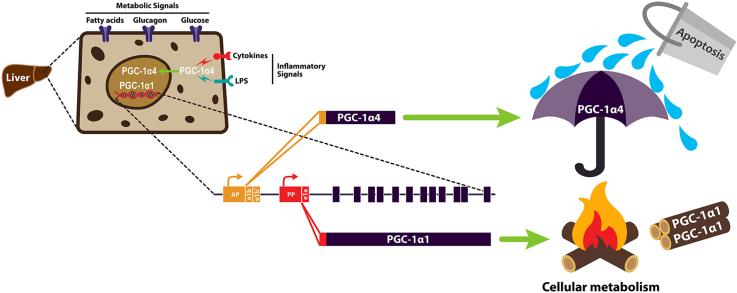
Abstract
Objective
The liver is regularly exposed to changing metabolic and inflammatory environments. It must sense and adapt to metabolic need while balancing resources required to protect itself from insult. Peroxisome proliferator activated receptor gamma coactivator-1 alpha (PGC-1α) is a transcriptional coactivator expressed as multiple, alternatively spliced variants transcribed from different promoters that coordinate metabolic adaptation and protect against inflammation. It is not known how PGC-1α integrates extracellular signals to balance metabolic and anti-inflammatory outcomes.
Methods
Primary mouse hepatocytes were used to evaluate the role(s) of different PGC-1α proteins in regulating hepatic metabolism and inflammatory signaling downstream of tumor necrosis factor alpha (TNFα). Gene expression and signaling analysis were combined with biochemical measurement of apoptosis using gain- and loss-of-function in vitro and in vivo.
Results
Hepatocytes expressed multiple isoforms of PGC-1α, including PGC-1α4, which microarray analysis showed had common and isoform-specific functions linked to metabolism and inflammation compared with canonical PGC-1α1. Whereas PGC-1α1 primarily impacted gene programs of nutrient metabolism and mitochondrial biology, TNFα signaling showed several pathways related to innate immunity and cell death downstream of PGC-1α4. Gain- and loss-of-function models illustrated that PGC-1α4 uniquely enhanced expression of anti-apoptotic gene programs and attenuated hepatocyte apoptosis in response to TNFα or lipopolysaccharide (LPS). This was in contrast to PGC-1α1, which decreased the expression of a wide inflammatory gene network but did not prevent hepatocyte death in response to cytokines.
Conclusions
PGC-1α variants have distinct, yet complementary roles in hepatic responses to metabolism and inflammation, and we identify PGC-1α4 as an important mitigator of apoptosis.
Keywords: Liver, PGC-1 isoforms, Inflammation, Apoptosis, Metabolism
Abbreviations: Peroxisome proliferator activated receptor gamma coactivator-1 alpha, PGC-1α, PPARGC1A; nuclear factor of kappa-light-chain-enhancer of activated B cells, NF-κB; tumor necrosis factor alpha, TNFα; lipopolysaccharide, LPS.
Graphical abstract
Highlights
-
•
Multiple isoforms of PGC-1α are expressed in hepatocytes, including PGC-1α4.
-
•
PGC-1α1 and PGC-1α4 share many metabolic targets, but PGC-1α4 has unique functions linked to hepatic inflammatory signalling.
-
•
PGC-1α4 attenuates hepatocyte apoptosis in response to TNFα and LPS in vitro and in vivo.
-
•
Inflammatory signaling influences PGC-1α4 localization in hepatocytes.
1. Introduction
The unique anatomical architecture of the liver allows it to perform a broad range of metabolic functions, but also exert powerful immunocompetence by surveilling portal blood and acting as a protective barrier [1]. The liver must adapt quickly to various metabolic and inflammatory signals from the digestive tract or systemic circulation, while at the same time responding to changing glucose, metabolite, and lipid homeostasis. Importantly, hepatic metabolism can be reprogramed by an inflammatory response [2], allowing a trade-off between energy destined for nutrient metabolism versus tolerance to infection. However, it is unclear which mechanisms help to balance responses to metabolic demand with inflammation.
An important regulator of metabolic adaptation is the peroxisome proliferator activated receptor gamma coactivator-1 alpha (PGC-1α), a transcriptional coactivator that regulates many gene programs related to nutrient metabolism, energy homeostasis, and mitochondrial respiration [3] by binding to transcription factors to enhance their activity [4]. In addition to mitochondrial metabolism, PGC-1α also activates expression of gene programs within a broader set of biological functions, including inflammation, in muscle [[5], [6], [7], [8]] and liver [[9], [10], [11], [12], [13], [14]]. Most biological functions for PGC-1α were discovered through study of PGC-1α1, which is only one of many isoforms for this protein [15,16]. PGC-1α is expressed as multiple splice variants initiating transcription from two distinct promoters, one located just before canonical exon 1 (proximal promoter, PP) and one located approximately 13 kilobases upstream (alternative promoter, AP) [16]. The proximal promtoer appears to have higher basal expression, whereas the alternative promoter is more responsive to stimulation such as physical exercise or cold exposure [[17], [18], [19]].
Transcription initiated from the PGC-1α proximal promoter generates canonical PGC-1α1 (also known as PGC-1α-a) and NT-PGC-1α-a, a truncated version containing a 31 nucleotide insertion between exons 6 and 7 that generates a premature stop codon [20] (Figure 1A). Over-expression of NT-PGC-1α-a and PGC-1α1 drive similar gene programs in brown adipocytes and liver, including mitochondrial biogenesis, fatty acid oxidation, thermogenesis, and gluconeogenesis [15,[20], [21], [22]]. In human liver, a third promoter and new exon 1L within intron 2 (liver promoter, LP) drives expression of a liver-specific variant L-PGC-1α with similar properties to PGC-1α1 [23]. Transcripts from the alternative promoter contain one of two possible novel exon 1 sequences: a long exon 1b (12 amino acids) or a short exon 1b’ (3 amino acids). The alternative promoter gives rise to: two full-length isoforms named PGC-1α-b and PGC-1α-c [18,24]; PGC-1α2 and PGC-1α3 that lack exons 4–6 and 9–13 [16]; and, NT-PGC-1α-c [15,19] and PGC-1α4 (also known as NT-PGC-1α-b) [16], versions truncated between exons 6 and 7. PGC-1α-b and PGC-1α-c appear to have a similar function to canonical PGC-1α1 [18]. PGC-1α2 and PGC-1α3 regulate differential splicing of mRNA targets [25], and PGC-1α4 induces skeletal muscle hypertrophy [16].
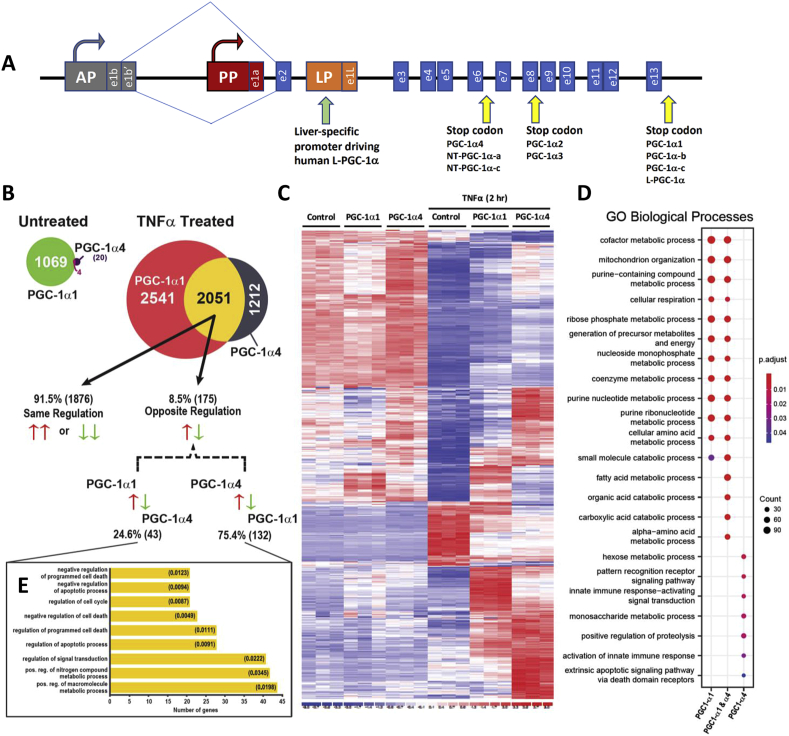
Figure 1.
PGC-1α isoforms differentially regulate inflammatory and metabolic signaling pathways downstream of TNFα. A) Schematic representation of the PPARGC1A gene with known promoters and transcript changes that give rise to different PGC-1α variants. B-E) Gene expression microarrays of mRNA isolated from wild type primary mouse hepatocytes over-expressing either PGC-1α1, PGC-1α4, or vector control by adenoviral infection. B) Number of genes changed greater than two-fold 48 h following transduction in the absence or presence of 2 ng/mL TNFα (2 h) (n = 3 biological replicates, adj. p-value <0.01). C) Clustering of genes significantly changed by over-expression of PGC-1α4 in primary hepatocytes in the presence of TNFα. D) Top 10 GO biological processes (adj. p-value <0.05) were identified from each list generated from TNFα-treated samples in B and listed on x-axis. Size of dot represents number of genes identified in each pathway, in comparison to other genotypes. E) GO biological processes (adj. p-value <0.05) associated with 175 genes regulated in the opposite direction. Data sets were generated using biological replicates (n = 3) of each condition from one experiment.
Despite characterization of multiple transcription factors and gene networks downstream of these various PGC-1α proteins, a mechanistic understanding of links between inflammatory signaling and PGC-1α activity remains limited. PGC-1α appears to be an essential component of the inflammatory response. Over-expression in muscle protects mice from disease, exercise, and age-related inflammatory damage [[26], [27], [28], [29]], and preservation of PGC-1α activity blunts lipopolysaccharide (LPS)-induced inflammatory damage to heart and kidney [30,31]. Consistently, low levels of PGC-1α increase pro-inflammatory cytokine expression and inflammatory damage to muscle and liver tissue in response to cellular stress [28,32,33]. Over-expression of PGC-1α decreases expression of pro-inflammatory cytokines, while simultaneously inducing expression of secreted anti-inflammatory factors [28,34].
Although PGC-1α is generally considered a coactivator, data suggest that PGC-1α indirectly represses nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) target gene transcription though coactivation of anti-inflammatory transcriptional networks linked to PPARs [29]. It may also bind directly to the p65 subunit of NF-κB [35]. The primary function of PGC-1α is to increase the number and efficiency of mitochondria, an organelle important for energy production, but also for responding to extra- and intra-cellular signals to coordinate metabolic, inflammatory, and cytotoxic responses [2,36]. In this study, we show that differentially spliced variants of the PGC-1α protein have unique functions in regulating hepatocyte responses to concurrently integrate metabolic and inflammatory signals.
2. Materials and methods
2.1. Mice
Mice were maintained on ad libitum chow (Tekland #2918) at 22 °C (12-h light/dark cycle). Hepatocyte-specific PGC-1α knockout mice (LKO: Ppargc1afl/fl, Alb-cre) were generated as previously described [10,11,33]. Age- and sex-matched mice on a C57BL/6J background were used (male or female, as indicated). The tissue-specific PGC-1α4 over-expressing mouse line (LSLPGC-1α4) was generated by inserting PGC-1α4 cDNA downstream of a Lox-stop-Lox cassette at the ROSA26 locus (Supplementary Fig 1A). Ppargc1a Alternative Promoter Knock-Out (AltPromKO) mice were generated by inserting LoxP sites flanking the alternative Ppargc1a promoter including exons 1b and 1b’ (Supplementary Fig 1B). For LPS treatment, livers of 10-week-old mice were harvested 6 h after tail-vein injection of LPS (2 mg/kg, Invivogen) or vehicle [phosphate-buffered saline (PBS)]. Experiments were performed in accordance with IRCM institutional animal care and use committee regulations.
2.2. Primary hepatocyte isolation, cytokine treatment, and luciferase
Primary mouse hepatocytes from 12-week-old mice were isolated and cultured as previously described [33]. One day after isolation, hepatocytes were infected with adenovirus expressing vector control (AdTrack-CMV-GFP), PGC-1α1, or PGC-1α4 (5 MOI) overnight in maintenance media. Cells were starved of insulin and dexamethasone for 24 h prior to treatment with TNFα (Fitzgerald) at 2 ng/mL for 2 h for signaling/gene expression, or 20 ng/mL for 8 h for apoptosis. Rat INS-1 β-cells were infected with indicated adenoviruses at a titer of 0.625 × 107 ifu/mL for 8 h. Thirty hours post-infection, starved cells were treated with vehicle (PBS) or cytokines (TNFα: 50 ng/mL, IFNγ: 50 ng/mL, IL-1β: 10 ng/mL) for an additional 18 h. Apoptosis was measured by enzyme-linked immunosorbent assay (ELISA) using the Cell Death Detection ELISA kit (Roche). For reporter assays, cells were transfected (Lipofectamine) with a construct expressing firefly luciferase downstream of 3x NF-κB response elements. Activity was normalized to total protein following quantification using the Dual Luciferase Reporter Assay System (Promega).
2.3. Protein isolation, immunoprecipitation, and immunoblotting
Proteins were solubilized in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors. Elutes and total proteins were resolved by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted, and probed with antibodies (Supplementary Table 1).
2.4. Immunofluorescence
The mouse hepatocyte cell line (H2.35) was cultured in Dulbecco modified Eagle medium (DMEM; Wisent), supplemented with 5% fetal bovine serum (FBS, Wisent), 1% penicillin/streptomycin (Wisent), and 0.2 μM dexamethasone (Sigma). The immortalized human hepatocyte (IHH) cell line was cultured in DMEM, supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were incubated on poly-L-lysine (Sigma) coated coverslips and transfected with V5-tagged PGC-1α variants for 24 h (Lipofectamine). Cells were starved overnight prior to TNFα treatment (20 ng/mL) for 3 h. Cells were fixed with pre-warmed 5% p-fluorophenylalamine (PFA) added directly to cells after removal of the culture medium. After incubation at 37 °C for 15 min, the PFA was quenched with 50 mM NH4Cl/PBS for 10 min at room temperature. Cells were permeabilized with 0.1% Triton X-100/PBS (v/v) for 10 min and blocked with 5% FBS/PBS for 30 min at room temperature. Cells were incubated with indicated primary antibodies (Supplementary Table 1) overnight at 4 °C, washed three times with PBS, incubated with secondary antibodies (90 min), and washed and stained with 4,6-diamino-2-phenylindole (DAPI). Cells were visualized using a spinning disk confocal microscope (Olympus IX81) with the Andor/Yokogawa spinning disk system (CSU-X), sCMOS camera, and 100× objective lenses (NA1.4). Images were taken with the same exposure time and laser power. Images were processed with Fiji [37] using a batch workflow (one batch for each cell line) to allow for comparable adjustments.
2.5. Cell fractionation
H2.35 cells transduced with indicated adenoviral vector were starved overnight of dexamethasone and FBS prior to TNFα treatment (50 ng/mL) for 3 h. Cell pellets were washed in PBS and resuspended in lysis buffer [10 mM HEPES (pH 7.5), 10 mM KCl, 3 mM MgCl2, 0.35 M sucrose, 0.1% NP40, 3 mM 2-mercaptoethanol, 0.4 mM phenylmethylsulfonyl fluoride (PMSF), 1 μM pepstatin A, 1 μM leupeptin, and 5 μg/mL aprotinin]. After centrifugation, supernatants were kept as cytoplasmic fraction. The pellet (nuclear fraction) was washed twice with lysis buffer, resuspended in buffer A [3 mM ethylenediamine tetraacetic acid (EDTA), 0.2 mM ethyleneglycol-bis-(β-aminoethylether)-N,N,N',N′-tetraacetic acid (EGTA), 1 mM dithiothreitol, 100 mM NaCl, and 0.8% NP40], and sonicated for 10 min (cycles of 30 s ON, 30 s OFF). Equal amounts of proteins were resolved by SDS-PAGE.
2.6. Microarray and gene set enrichment analysis
mRNA was isolated from primary mouse hepatocytes infected with adenovirus expressing PGC-1α1, PGC-1α4, or vector control treated with 2 ng/mL TNFα or vehicle (PBS) for 2 h (n = 3), and gene expression profiles were generated using Affymetrix Mouse Genome 430 2.0 Arrays. Raw CEL files were normalized using RMA [PMID: 12925520] and annotated using biomaRt [PMID: 16082012]. Raw data and sample annotation are available on GEO (GSE132458).
Gene set enrichment analysis was performed using javaGSEA software (version 3.0 – build: 01600) on chip data using the Gene Ontology processes (number of permutations = 1000, Permutation type = gene_set, Chip platform = Affy_430_2.0_mgi (version 2011) from the Mouse Genome Database. The Ppargc1a probe (1434099_at) was removed prior to analysis to eliminate over-expression bias. Full GSEA results are provided in Supplementary File 1. A MySQL database generated lists of genes significantly regulated (adj. p-value < 0.01, Log10 FC ≥ 0.3 or ≤ −0.3). Full lists are provided in Supplementary File 2 (untreated samples) and 3 (TNFα–treated samples).
Clustering based on PGC-1α4-regulated genes was performed using dChip software. Over-representation analysis (ORA) of Gene Ontology processes was performed using ClusterProfiler and the mouse genome-wide annotation in R www.r-project.org. The top 10 statistically over-represented processes were determined for each condition, merged into one list, and represented as a dot plot (adj. p-value < 0.05, correction method = Bonferroni). For 175 genes regulated oppositely by the variants, ORA was performed using g:Profiler (adj. p-value < 0.05, correction method = g:SCS threshold). Gene lists were evaluated for enrichment of transcription factor signatures and binding sites in the PPs and distant regulatory elements using iRegulon and DiRE http://dire.code.org with default analysis settings.
2.7. RNA isolation, PCR, and quantitative RT-PCR
RNA was isolated from frozen tissue or cells using TRIzol (Invitrogen). RNA (1 μg) treated with DNase I was reverse-transcribed using the High Capacity Reverse Transcription Kit (Applied Biosystems). cDNA was quantified using SYBR Green PCR master mix (Bioline) and presented as either “relative mRNA expression” (ΔΔCt threshold cycle method with normalization to Hypoxanthine-guanine phosphoribosyltransferase (Hprt) and expressed relative to the control/basal condition) or “2ˆ-ΔCt’’ (with data expressed relative to Hprt). The presence or absence of PGC-1α variants was confirmed using isoform-specific primers by conventional polymerase–chain reaction (PCR) and sequencing (Supplementary Tables 2 and 3).
2.8. Statistical analysis
Sample sizes were based on previous experience with assays and knowledge of expected variance. Normal distribution and homoscedasticity of data were tested by the Shapiro–Wilks and Bartlett tests, respectively. Parametric tests were used if distributions and normal variances were equal. One-way or two-way analysis of variance were followed by the Tukey test (one-way) or the Dunnett multiple comparisons (two-way) post-hoc test using GraphPad Prism software. Data are expressed as mean ± standard deviation (SD) for cell experiments and standard error of the mean (SEM) for in vivo experiments, unless otherwise indicated.
3. Results
3.1. PGC-1α1 and PGC-1α4 are expressed in primary mouse hepatocytes
PGC-1α mRNA expression is induced in a stimulus- and context-dependent manner [17,19,[38], [39], [40], [41], [42]], leading to expression of multiple PGC-1α variants whose pattern is tissue-specific [16,18,20,23,24]. Due to high homology, splice variants of this protein cannot be fully distinguished by quantitative PCR (qPCR) [43]. Using variant-specific non-quantitative PCR, we detected transcripts for NT-Pgc-1α-a and Pgc-1α1 of the proximal promoter (exon 1a), Pgc-1α-b and Pgc-1α4 of the alternative promoter (exon 1b), and an uncharacterized transcript of different size from the alternative promoter (exon 1b’) when primary mouse hepatocytes were treated with the adenylyl cyclase activator, forskolin (Fk), activating both promoters [17] (Supplementary Fig 2). Interestingly, bands corresponding to Pgc-1α-c, Pgc-1α2 and Pgc-1α3 were not detected in hepatocytes. PGC-1α4 undergoes splicing similar to NT-PGC-1α-a to create a truncated protein, but these two forms have different first exons. NT-PGC-1α-a has overlapping functions with PGC-1α1 in muscle, liver, and brown fat cells with respect to mitochondrial metabolism [19,22,44]. Whereas, PGC-1α4 uniquely regulates myocyte hypertrophy and does not appear to impact mitochondrial metabolism, suggesting that its expression from the alternative promoter and unique N-terminal structure make it different from other truncated forms of PGC-1α [16]. As full-length PGC-1α1 can modulate tissue responses to inflammation, we sought to determine whether PGC-1α4 also influences inflammatory signaling and determine whether there are functional consequences of this in hepatocytes.
3.2. PGC-1α1 and PGC-1α4 have distinct roles in the hepatic response to TNFα
We first compared the transcriptome of primary mouse hepatocytes by microarray following over-expression of PGC-1α1, PGC-1α4, or vector alone in the absence or presence of the inflammatory cytokine TNFα, a cytokine commonly associated with hepatic inflammation (see Supplementary Fig 3 for levels of PGC-1α over-expression). More than 1000 genes changed by two-fold or greater following PGC-1α1 over-expression compared with vector alone (adj. p-value < 0.01), whereas only 24 were changed by PGC-1α4 and only four genes overlapped between the two lists (Figure 1B, Supplementary File 1). Following TNFα treatment, more than 4500 genes were changed two-fold or greater in hepatocytes over-expressing PGC-1α1 and more than 3000 for PGC-1α4, with 36% of the genes shared between isoforms (Figure 1B, Supplementary File 2). Clustering of PGC-1α4-modulated genes and comparison to levels in vector- or PGC-1α1-expressing hepatocytes suggested that the activity of PGC-1α4 relied heavily on the presence of TNFα (Figure 1B). Within this inflammatory context, PGC-1α4 had both overlapping and distinct activity from PGC-1α1. Of the 2051 genes shared by the variants in TNFα-treated cells, the majority (91.5%) were regulated in the same manner (positively or negatively, Supplementary File 2). However, there were notable differences in some effector pathways for the two variants.
To gain a global impression of biological process regulated by the PGC-1α variants in hepatocytes, we performed gene set enrichment analysis (GSEA). Gene sets related to mitochondrial respiration and substrate metabolism were statistically enriched by both PGC-1α1 and PGC-1α4. PGC-1α1 predominantly regulated mitochondrial respiration, and glucose, amino acid, and fatty acid metabolism, regardless of TNFα treatment (Supplementary File 3). This is consistent with known roles of PGC-1α1 in mitochondrial metabolism and supported by qPCR (Supplementary Fig 4A-D). Although we saw no effect of PGC-1α4 on these PGC-1α1 target genes, PGC-1α1 and PGC-1α4 shared many overlapping gene sets (Supplementary File 3). GSEA for PGC-1α4 in untreated primary mouse hepatocytes centered on lipid metabolism (fatty acids and triglycerides), sterol metabolism, and mitochondrial respiration, but individual gene effects were mild and most did not reach the two-fold cutoff. However, with TNFα present, PGC-1α4-enriched pathways included regulation of transcription factor transport to the nucleus, innate immunity, responses to interferon/PAMP, TLR signaling, acute inflammation, and apoptosis. Overall, TNFα signaling showed isoform-specific responses and highlighted processes related to the innate immune response and cell death unique to PGC-1α4.
To explore differential effects of the isoforms on inflammation, we performed gene ontology (GO) analysis on gene changes occurring only in the presence of TNFα. The top 10 GO pathways unique to each variant, or shared, are shown in Figure 1D. All of the top PGC-1α1-regulated processes focused on energy metabolism and were shared with PGC-1α4. However, six of the top pathways for PGC-1α4 were unique to this variant, including 6-carbon metabolism, proteolysis, immune signaling in response to pathogens, and regulation of cell death (Figure 1D). Interestingly, GO terms associated with the 175 shared genes regulated in an opposite manner by the variants (Supplementary File 4) centered mainly on cell death and apoptosis (Figure 1E). These data suggest that apoptosis is an important effector pathway differentially regulated by these two PGC-1α protein variants.
3.3. PGC-1α1 and PGC-1α4 differentially regulate TNFα-induced apoptosis
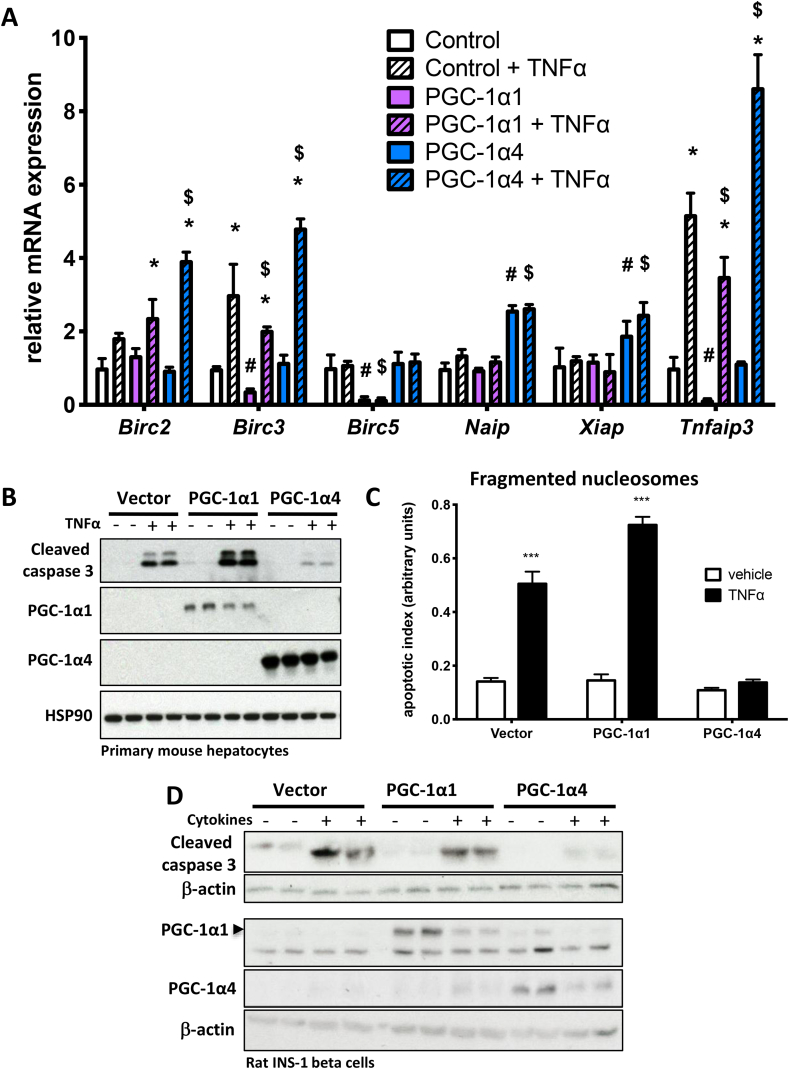
Upon searching the microarray for candidate genes involved in apoptosis downstream of PGC-1α4, we identified Birc2 (Ciap1) and Tnfaip3 (also known as A20), two anti-apoptotic proteins that prevent cell death downstream of inflammatory signaling. In a separate experiment, we confirmed that their transcript levels were significantly higher in mouse primary hepatocytes over-expressing PGC-1α4 only in the presence of TNFα (Figure 2A). Related Birc3 (Ciap2) was also increased by TNFα/PGC-1α4, whereas Birc5 (Survivin) expression did not change. In addition, transcripts for apoptosis inhibitors Naip and Xiap were significantly increased by PGC-1α4, regardless of TNFα treatment. In contrast, over-expression of PGC-1α1 decreased expression of Birc3, Birc5, and Tnfaip3 and had no effect on Naip and Xiap (Figure 2A). These data confirmed the array and GO analysis (Figure 1E), illustrating that PGC-1α1 and PGC-1α4 have differential effects on apoptotic gene programs in response to TNFα. The predicted consequences of this were then demonstrated in primary mouse hepatocytes, where over-expression of PGC-1α1 increased cleaved caspase 3 (Figure 2B) and nucleosome fragmentation (Figure 2C) in response to TNFα treatment, whereas over-expression of PGC-1α4 almost completely blocked apoptosis compared with vector and PGC-1α1 (Figure 2B,C). We obtained a similar decrease in cytokine-induced caspase 3 cleavage by PGC-1α4 using the rat pancreatic INS-1 beta cell line (Figure 2D), suggesting that the unique anti-apoptotic role of this PGC-1α isoform was not cell-type specific.
Figure 2.
Over-expression of PGC-1α4 attenuates apoptosis induced by inflammatory signals. A) mRNA expression in wild type primary mouse hepatocytes over-expressing PGC-1α1, PGC-1α4, or control vector alone following 2-hour treatment with 2 ng/mL TNFα or vehicle (n = 3). *p < 0.05 effect of TNFα within each genotype compared to untreated cells. #p < 0.05 effect of genotype alone compared with control. $p < 0.05 TNFα effect compared with control + TNFα. B) Western blot and C) fragmented nucleosomes (n = 4) in wild type primary mouse hepatocytes over-expressing either PGC-1α1, PGC-1α4, or vector control by adenoviral infection, treated with or without 20 ng/mL TNFα for 8 h. ***p < 0.001 versus vehicle. D) Western blot of protein from rat INS-1 β-cells over-expressing either PGC-1α1, PGC-1α4, or vector control by adenoviral infection, treated with or without cytokines (TNFα: 50 ng/mL, IFNγ: 50 ng/mL, IL-1β: 10 ng/mL) for 18 h. Western data are biological replicates representative of three independent experiments.
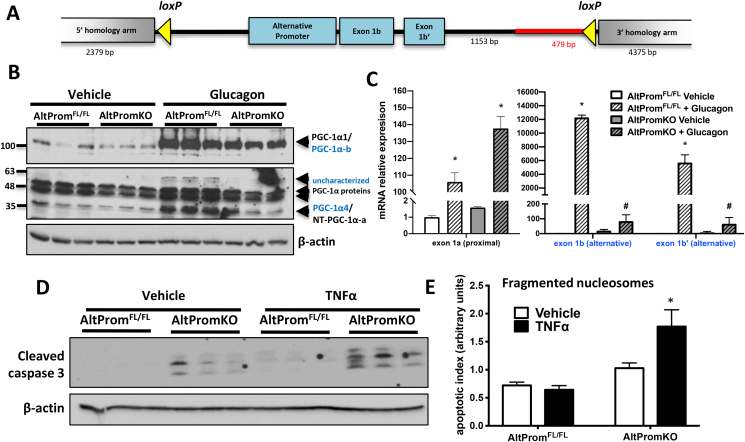
We next sought to determine whether PGC-1α4 played an essential role in the hepatic apoptotic response. The commonly used PGC-1α floxed mouse model [33] ablates all Ppargc1a transcripts from both promoters, making it impossible to discern roles for any specific isoform. Thus, we created a model where only the altervative promoter was disrupted (AltPromFL/FL), blunting expression of variants containing exon 1b and 1b’ (including PGC-1α4) (Figure 3A). Efficiency of the promoter knockout was confirmed using glucagon, which robustly induced expression of PGC-1α proteins (Figure 3B) and transcripts (Figure 3C) from both the proximal promoter (black) and alternative promoter (blue) in control (AltPromFL/FL) cells. Ablation of the alternative promoter by crossing floxed mice with Alb-CreTg mice (AltPromKO) effectively blunted induction of transcripts only from the upstream promoter, whereas increases in proximal transcripts (which include Pgc-1α1 and NT-Pgc-1α-a) remained similar to, or even higher than, control cells (Figure 3C; Supplementary Fig 5A), demonstrating specificity of the knockout to only alternative transcripts. It is noteworthy that although absolute levels of alternative promoter transcripts are much lower than proximal promoter transcripts (Supplementary Fig 5), these differences are not necessarily reflected at the protein level. Differential protein stability and post-translational modification greatly affect the abundance of PGC-1α proteins [21,25]. For example, despite having very low transcript levels, the half-life of PGC-1α4 protein is at least 10 times longer than PGC-1α1 (>6 h vs. 40 min) [25]. We also detected additional PGC-1α proteins at higher molecular weights (Figure 3B) that did not correspond to transcripts of known isoforms amplified by conventional PCR (Supplementary Fig 2). Some of these were glucagon-responsive and completely dependent on the alternative promoter (60 kDa), and others were a mix of proteins derived from the two promoters that did not change with glucagon (45–55 kDa) (Figure 3B). These could represent novel PGC-1α isoforms, or shifts in the molecular weight of known isoforms caused by post-translational modification.
Figure 3.
Loss of PGC-1α4 expression enhances apoptosis in response to TNFα. A) Targeting construct for creation of mouse allowing tissue-specific ablation of the alternative Ppargc1a promoter (AltPromFL/FL). B) Western blot of PGC-1α proteins and C) mRNA of proximal and alternative Pgc-1α transcripts from primary mouse hepatocytes treated with 50 nM glucagon or vehicle. *p < 0.05 versus AltPromFL/FL Vehicle. #p < 0.05 versus AltPromKO Vehicle, n = 3. D) Western blot and E) fragmented nucleosomes from primary mouse hepatocytes treated with 20 ng/mL TNFα or vehicle for 8 h *p < 0.05 versus AltPromFL/FL Vehicle, n = 3. Bars are mean ± SEM of biological replicates in one experiment. Data are representative of two independent experiments.
As our focus was on PGC-1α4, we noted the 37kD PGC-1α protein induced by glucagon was almost completely ablated by knockout of the alternative promoter identifying PGC-1α4 as the short variant most responsive to this treatment (Figure 3C). Consistent with PGC-1α4 being an important mediator of apoptosis, primary hepatocytes from AltPromKO mice had higher basal and TNFα-induced cleaved caspase 3 levels (Figure 3D) and increased fragmented nucleosomes in response to the cytokine (Figure 3E) compared with cells from littermate controls. Although this loss-of-function model could not isolate effects of specific alternative promoter variants, taken together with gain-of-function data (Figure 2), PGC-1α4 appears to have the unique ability to prevent inflammatory-mediated apoptosis in hepatocytes.
3.4. TNFα signaling influences localization of PGC-1α4 within hepatocytes
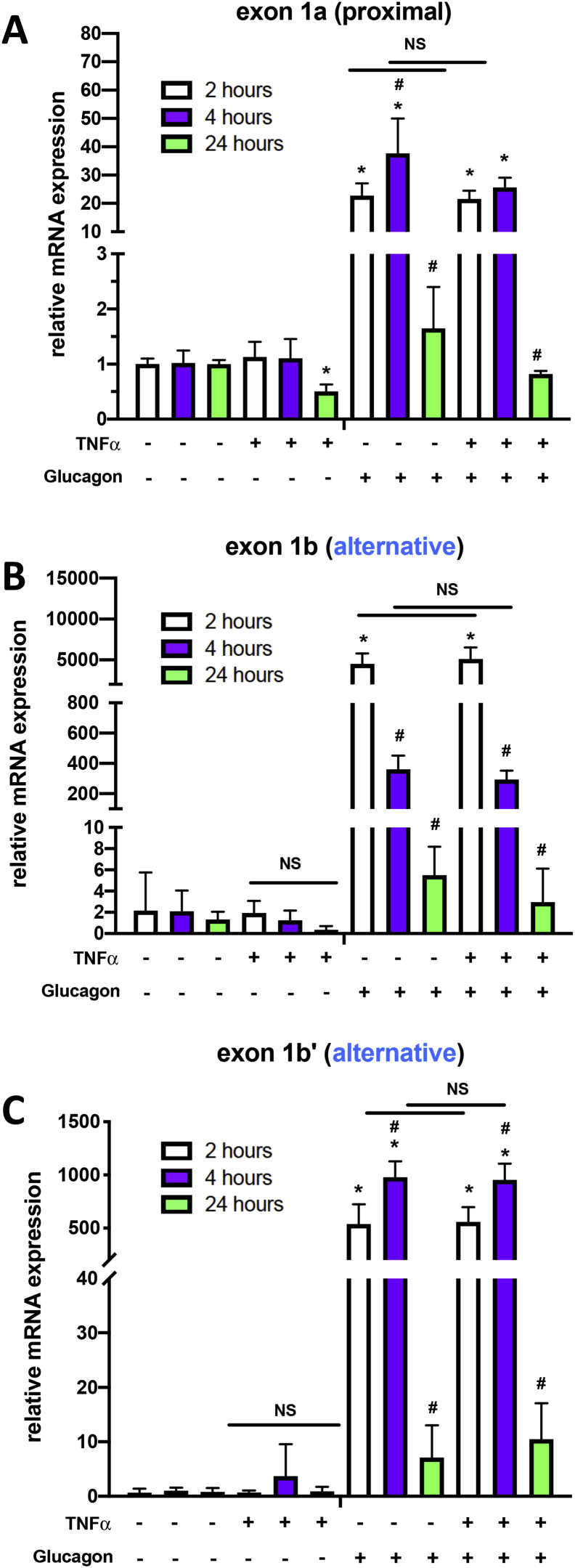
We were curious about why the activity of PGC-1α4 was dependent on activation of TNFα signaling. We first investigated whether TNFα itself regulates transcription from either Ppargc1a promoter, on its own or in conjunction with other signals. Unlike glucagon treatment, which activates cAMP:CREB and acutely induced both promoters, TNFα treatment decreased PGC-1α transcripts derived from proximal promoter transcripts (Pgc-1α1 or NT-Pgc-1α-a) after 24 h (Figure 4A), but there were no significant effects on alternative promoter transcripts (Pgc-1α-b or Pgc-1α4) at any time point (Figure 4B,C; Supplementary Fig 5B). TNFα also had no impact on the capacity of glucagon to induce either promoter.
Figure 4.
PPARGC1A proximal and alternative transcripts are differentially regulated by TNFα and glucagon. A-C) mRNA levels of transcripts expressing either exon 1a of the proximal promoter, exon 1b or exon 1b′ of the alternative promoter from wild type primary mouse hepatocytes treated with vehicle (PBS), glucagon (50 nM), or TNFα (20 ng/mL) for indicated times. *p < 0.05 effect of treatment compared to individual controls at the same time point, #p < 0.05 effect of time within the same treatments. Data are representative of two independent experiments.
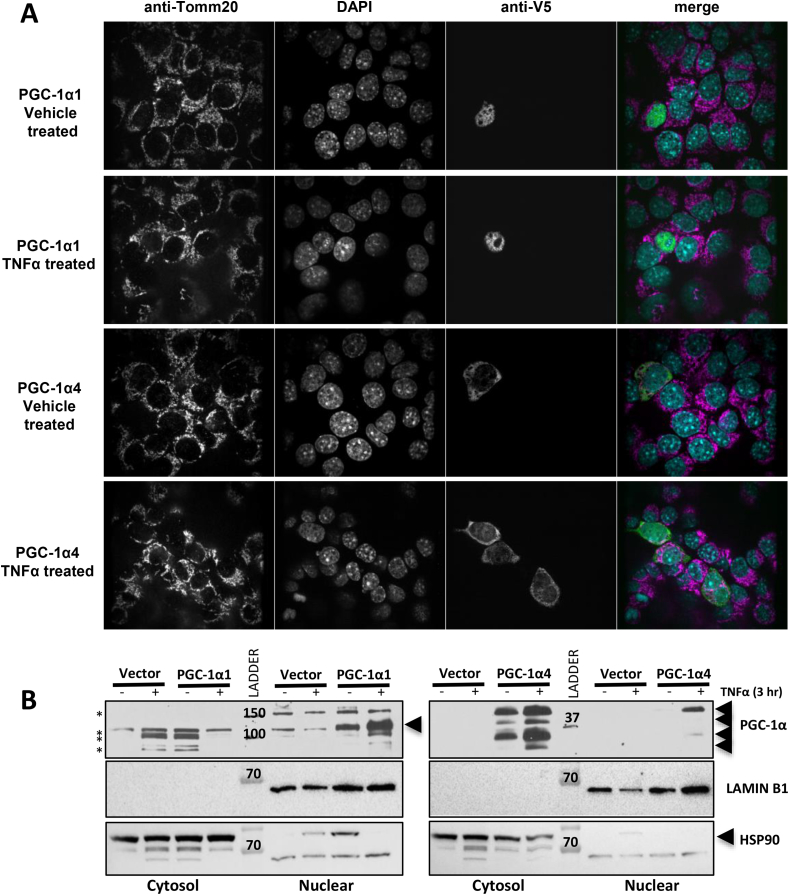
Since TNFα treatment substantially increased the number of PGC-1α4 gene targets in our microarray (Figure 1B), we proposed that inflammatory signals might regulate the PGC-1α proteins, or their activity, in other ways. Using immunofluorescence, we noted that over-expressed PGC-1α4 localized almost primarily to the cytoplasm of hepatocyte cell lines (H2.35 and IHH cell lines) (Figure 5A; Supplementary Fig 6). Following the addition of TNFα, a significant proportion of PGC-1α4 was detected in the nuclear compartment colocalized with DAPI (Figure 5A; Supplementary Fig 6). Since the related NT-PGC-1α-a was shown to colocalize with mitochondria [45], we also co-stained a component of the outer mitochondrial membrane with Tom20. However, we observed no obvious overlap in the PGC-1α4 and Tomm20 signals under basal or TNFα-treated conditions for either hepatocyte cell line. Cell fractionation confirmed that a fraction of the PGC-1α4 protein was detected in the nuclear pellet only following TNFα treatment. In contrast, PGC-1α1 localized exclusively to the nucleus regardless of treatment conditions (Figure 5B). PGC-1α4 migrated at a variety of molecular weights, but the highest molecular weight form was predominant in the nuclear fraction. Thus, limitation of nuclear PGC-1α4 localization may be a mechanism to control PGC-1α4 activity in the absence of inflammatory stimuli.
Figure 5.
TNFα signaling promotes mobilization of cytoplasmic PGC-1α4 to the nucleus. A) Confocal imaging of H2.35 mouse hepatocytes transfected with plasmids expressing V5-tagged PGC-1α1 or PGC-1α4 treated with 20 ng/mL TNFα or vehicle (PBS) for 3 h. Images are representative of n = 15–20 cells per condition. B) Western blots after cell fractionation of H2.35 mouse hepatocytes transduced with adenovirus expressing control vector, PGC-1α1, or PGC-1α4 and treated with 50 ng/mL TNFα or vehicle (PBS) for 3 h. *non-specific bands. Data are representative of two independent experiments.
3.5. PGC-1α4 does not repress the pro-inflammatory gene program in hepatocytes
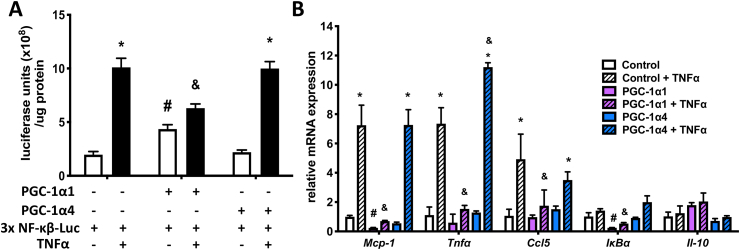
Since anti-apoptotic gene programs are often regulated by NF-κB, we hypothesized that PGC-1α1 and PGC-1α4 might have different effects on NF-κB activity. Basal expression of a 3x NF-κB response element reporter was increased in primary mouse hepatocytes when co-expressed with PGC-1α1 (Figure 6A). However, consistent with previous findings [29,35], TNFα-induced activity of the reporter was significantly blunted by high PGC-1α1 (Figure 6A) as well as basal and/or TNFα-induced levels of pro-inflammatory genes Mcp-1, Tnfα, Iκbα, and Ccl5 in primary hepatocytes (Figure 6B). This confirmed a strong inhibitory role for PGC-1α1 on NF-κB signaling in hepatocytes. In contrast, PGC-1α4 had no effect on NF-κB reporter activity and little impact on target pro-inflammatory genes, except to potentiate expression of Tnfα (Figure 6B).
Figure 6.
PGC-1α1, but not PGC-1α4, represses NF-κB activity and pro-inflammatory gene expression. A) Luciferase activity in wild type primary mouse hepatocytes treated with 2 ng/mL TNFα or vehicle (PBS) 48 h following transfection with a 3x NF-κB reporter and constructs for PGC-1α1 or PGC-1α4 (or vector alone, mean ± SD, n = 3). *p < 0.05 TNFα response compared to vehicle treatment, #p < 0.05 genotype effect compared to vector, &p < 0.05 genotype effect compared with vector + TNFα. B) mRNA expression of wild type primary mouse hepatocytes over-expressing PGC-1α1, PGC-1α4, or vector control following 2-hour treatment with 2 ng/mL TNFα or vehicle (n = 3). *p < 0.05 effect of TNFα within each genotype compared with untreated cells. #p < 0.05 effect of genotype alone compared with control. $p < 0.05 TNFα effect compared with control + TNFα. Data are representative of three independent experiments.
In an attempt to identify transcription factors with links to apoptosis and cell death downstream of PGC-1α variants, we probed array data for transcription factor motifs enriched in genes: changed by PGC-1α1 or PGC-1α4 alone; oppositely regulated by the two variants; or shared when TNFα was present using iRegulon (Supplementary Table 4) and DiRE (Supplementary Fig 7). Many motifs not previously associated with PGC-1α were identified including: ETV6 (only PGC-1α1); SP4, the NF-Y complex, IRF6, GM7148, TGIF2, HSF4, and E2F1DP1 (only PGC-1α4); and IRF4, LK4, NR1H2 (LXRβ), ZBTB33 (KAISO), ZFP143, and PITX2 (shared). Among the 175 genes oppositely regulated by the variants, binding sites for STAT, SPIB, NFATC2, and KLF4 were found. ST18 (also known as MYT3 or NZF3) was enriched when we focused on the gene list implicated in cell survival (Figure 1E). Narrowing our focus to transcription factors implicated in cell death and inflammation, we evaluated whether over-expression of the PGC-1α variants modulated expression of their target genes. PGC-1α4 had no significant effects on mRNA expression of any target genes, whereas PGC-1α1 repressed SP4, NF-Y, and STAT targets (Supplementary Fig 8,B,C) and increased IRF4 targets Tnfrsf17 and Nip3 (Supplementary Fig 8D). In summary, PGC-1α1 generally repressed NF-κB activity and the transcription of genes involved in inflammation and cell death. In contrast, PGC-1α4 differentially enhanced a distinct program of anti-apoptotic factors in hepatocytes only in the presence of TNFα signaling, but this did not appear to be via enhancement of NF-κB.
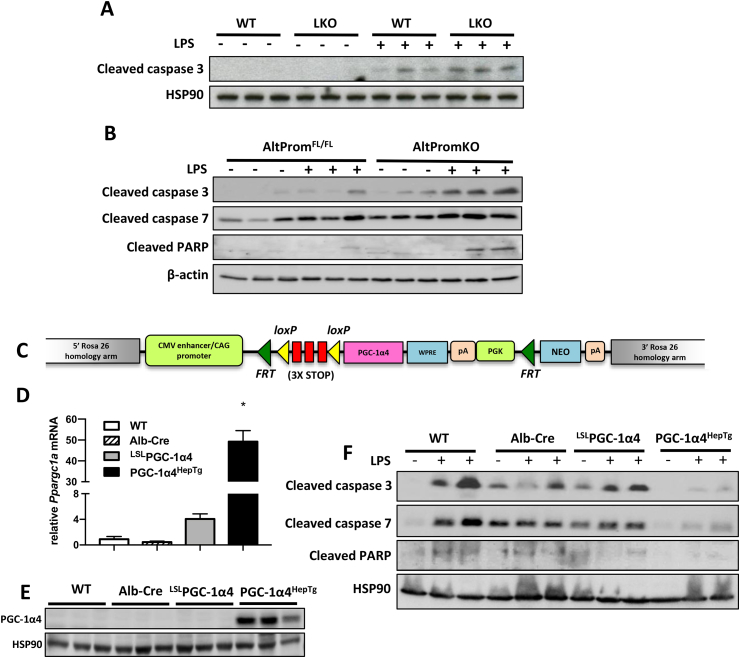
3.6. PGC-1α4 prevented lipopolysaccharide (LPS)-induced hepatocyte cell death in vivo
Finally, we investigated whether PGC-1α4 was important for regulating hepatocyte apoptosis downstream of inflammatory signals in vivo. Mice lacking all isoforms of PGC-1α in the liver had increased cleaved caspase 3 levels when exposed to LPS (Figure 7A). When we ablated transcription only from the alternative Ppargc1a promoter, protein extracts from livers of AltPromKO mice treated with LPS also had increased cleaved caspase 3, cleaved caspase 7, and cleaved PARP compared with littermate AltPromFL/FL control livers (Figure 7B). To determine whether increases in PGC-1α4 would conversely prevent LPS-induced apoptosis, we created a novel ROSA26 knockin mouse with PGC-1α4 cDNA positioned downstream of a Lox-Stop-Lox cassette (Figure 7C). There was a small increase in PGC-1α4 transcripts in the absence of Cre-recombinase (LSLPGC-1α4), suggesting a low level of leaky transgene expression (Figure 7D). However, upon crossing with Albumin-CreTg mice, PGC-1α4HepTg mice over-expressed PGC-1α4 only in hepatocytes approximately 50-fold higher than controls (Figure 7E). Supporting the anti-apoptotic role for hepatic PGC-1α4, there were decreased levels of cleaved caspase 3, cleaved caspase 7, and cleaved PARP in livers of both male and female PGC-1α4HepTg mice following injection of LPS (Figure 7F; Supplementary Fig 9). In line with published findings [30] and our finding in vitro with TNFα, LPS treatment decreased expression of the proximal promoter (including Pgc-1α1 and NT-Pgc-1α-a). However, LPS did not significantly influence levels of PGC-1α transcripts derived from the alternative promoter (Pgc-1α-b and Pgc-1α4) (Supplementary Fig 10).
Figure 7.
PGC-1α4 is necessary and sufficient to prevent LPS-induced hepatocyte apoptosis. A,B) Western blots of liver protein from male wild type littermate control, LKO, or AltPromKO mice (n = 3 mice) 6 h following tail-vein injection of LPS (2 mg/kg) or vehicle (PBS). C) Targeting construct for transgenic mouse allowing tissue-specific over-expression of PGC-1α4. D) mRNA and E) protein from livers of mice (n = 3) following breeding of LSLPGC-1α4 mice with Albumin-CreTg mice to drive PGC-1α4 expression only in hepatocytes (PGC-1α4HepTg). *p < 0.05 versus WT control. F) Western blot of liver protein from male mice 6 h following tail-vein injection of 2 mg/kg LPS (n = 6) or vehicle (PBS) (n = 2). Similar results were obtained in female mice.
4. Discussion
In the current study, we found that various non-canonical PGC-1α protein variants are expressed in the murine liver and that these differentially regulate metabolism and hepatic inflammatory signaling. Gene set enrichment analysis showed that in the presence of the inflammatory cytokine TNFα, PGC-1α4 influences innate immunity and cell death, whereas PGC-1α1 remains primarily associated with mitochondrial function and metabolic processes. Gene ontology (GO) analysis and qPCR illustrated that genes implicated in cell death and apoptosis are often oppositely regulated by these two variants. In primary mouse hepatocytes, PGC-1α4 has the unique ability to blunt apoptosis in response to TNFα, a function that may be controlled by shuttling PGC-1α4 between the cytoplasm and nucleus. We conclude that alternative forms of PGC-1α are inducible and co-expressed in the liver, giving rise to isoforms that coordinate the cellular response to inflammatory signaling. These findings give mechanistic insight into how PGC-1α, as a family of proteins facilitate parallel adaptation to metabolic demand and mitigation of inflammatory damage in cells.
Immune responses to danger signals are metabolically challenging and can lead to a trade-off between maintaining highly energy-demanding processes of nutrient metabolism versus adaptation to inflammatory stimuli [2]. Inflammation itself often inhibits metabolism and impedes mitochondrial function. Here, we show that signaling via TNFα or LPS leads to a shift in the PGC-1α gene program downstream of PGC-1α1 and PGC-1α4. PGC-1α1 maintains activation of metabolic gene programs and has a strong repressive role on NF-kB gene pathways, whereas PGC-1α4 becomes activated and potentiates the anti-apoptotic program. Concurrent activity of these two isoforms in periods when the liver is both metabolically challenged and exposed to inflammatory stimuli may help to ensure that inflammatory signaling does not impede the ability of hepatocytes to respond to the metabolic demand. This mechanism represents an additional layer by which the family of PGC-1α proteins help balance an integrated metabolic response that is modulated by the inflammatory status of the liver.
It is now well established that PGC-1α is a family of proteins created by alternative splicing of the PPARGC1A gene in many tissues including skeletal muscle [16,24,43], brown adipose tissue [20,46], and liver [23]. However, a functional role for many of the alternative isoforms remains unknown. While some PGC-1α variants share overlapping functions with canonical PGC-1α1 [18,19,21,41,47], PGC-1α4 has distinct effector pathways in muscle and brown adipose tissue [15,16]. We show here that PGC-1α1 and PGC-1α4 have differential effects on cell death downstream of inflammatory signals. PGC-1α4 almost completely blocks apoptosis in vitro and in vivo. Although PGC-1α1 decreased the expression of a broad program of inflammatory genes, it did not inhibit cell death in response to TNFα in primary mouse hepatocytes. In fact, PGC-1α1 has been shown to induce apoptosis through PPARγ, TFAM, generation of reactive oxygen species, or Ca2+ signaling [[48], [49], [50], [51], [52]], but may also attenuate cell death by activation of the p38/GSK3B/Nrf-2 axis or p53 [53,54]. Newly identified PGC-1α splice variants from differentially regulated promoters add a layer of complexity, but also may explain conflicting data in previous reports.
An obvious candidate effector in inflammation-mediated apoptosis is NF-κB. Consistent with previous studies [55,56], we show that PGC-1α1 represses NF-κB activity. However, unlike PGC-1α1, our evidence suggests no impact of PGC-1α4 on this transcription factor. Although PGC-1α4 shares the complete activation domain of PGC-1α1, its alternative exon 1 and significant C-terminal truncation may lead PGC-1α4 to regulate a different set of DNA-binding proteins. Our microarray identifies multiple pathways differentially regulated by the two variants, including those targeted by NF-κB, SP4, NF-Y, STAT, and IRF4. However, in our model system, PGC-1α4 did not behave as a traditional transcriptional coregulator for many of their gene targets. One possible explanation could be that PGC-1α4 instead promotes novel splicing events to create alternative gene products, similar to the function of related PGC-1α2 and PGC-1α3 variants [25]. Aberrant alternative splicing can substantially affect cellular function and is associated with disease. For example, alternative splicing of TNFα-regulated genes (such as Tnfaip3/A20) produces protein variants with distinct roles in cell death and cell survival [57].
While the exclusive nuclear localization of PGC-1α1 supports its function as a transcriptional coactivator, the ability of PGC-1α4 to shuttle between compartments suggests that it might interact with transcription factors in the cytoplasm and/or regulate their entry into the nucleus, a possibility also supported by our GSEA analysis. Interferon (INF) regulatory factors (IRFs) are well-known transcription factors that shuttle in response to inflammatory stimuli [58], and our data suggest that both PGC-1α1 and PGC-1α4 converge on INF signaling in hepatocytes. Canonical PGC-1α1 has been associated with INF response in the contexts of hepatitis C virus (HCV) infection and thermogenesis [59,60]. Interestingly, three IRFs (IRF1, IRF4, IRF6) were identified in our motif enrichment analysis, and numerous studies implicate interferons as critical regulators of apoptosis [61,62]. Although we focused on TNF signaling, our data suggest that PGC-1α1 and PGC-1α4 might also regulate the INF response; however, further studies are necessary to confirm this hypothesis. Overall, mechanisms underlying the anti-apoptotic role of hepatic PGC-1α4 appear complex, possibly involving interaction with cytoplasmic proteins, dominant-negative effects on other PGC-1α variants, or regulation of alternative splicing of genes implicated in apoptosis.
PGC-1α4 shares many similarities to another isoform, NT-PGC-1α-a, which is transcribed from the proximal promoter. Both have two N-terminal nuclear exclusion signals and three putative phosphorylation (S190, S237, and T252) sites, which regulate nuclear shuttling of NT-PGC-1α-a [46]. Our data are consistent with reports describing cytoplasmic-to-nuclear movement of other truncated variants of PGC-1α [20,46]. Given similarities between these proteins, it is possible that NT-PGC-1α-a localization is also regulated by inflammation similar to PGC-1α4; and while likely, it remains to be seen whether PGC-1α4 and NT-PGC-1α-a have overlapping functions in terms of inflammation and apoptosis. However, NT-PGC-1α-a expression is maintained (and possibly increased) in the AltPromKO mouse line, but these mice had increased susceptibility to apoptosis induced by inflammation. This suggests that NT-PGC-1α-a cannot compensate for loss of PGC-1α4 in this biological role.
5. Conclusion
In conclusion, coordinated activity of PGC-1α isoforms allows fine-tuning of metabolic and inflammatory networks, supporting efficient adaptation to energy demand within the highly dynamic and inflammatory environment of the liver. Our data imply that boosting expression of specific PGC-1α isoforms could allow hepatocytes to more efficiently respond to energy demand when faced with both high metabolic and inflammatory challenges associated with metabolic disease. Offsetting this balance could result in inefficient nutrient metabolism and/or inappropriate responses to inflammatory stimuli, which may play a role in inflammatory liver diseases such as non-alcoholic steatohepatitis (NASH) or cirrhosis.
Transcript profiling
GEO accession number GSE132458
Author contribution
ML, ABP, NJ, SJ, JLR and JLE designed concept and experiments. ML, ABP, NJ, AG, SJ, NPK, SS, CB, AD, JC, JB and PJ performed and analyzed experiments. ML, ABP, NJ, SJ, JLR and JLE wrote the manuscript. All authors reviewed the manuscript.
Grant support
Research was supported by grants from the CIHR (PJT-148771) and IDRC (108591-001) to JLE, and the Swedish Research Council and Karolinska Institutet to JLR. ML received a doctoral scholarship and JLE a Chercheur-boursier from the FRQS. SJ and NJ are supported by post-doctoral fellowships from Diabetes Canada and the Montreal Diabetes Research Centre, respectively.
Acknowledgments
We thank Dr Bruce Spiegelman for generating the AltPromFL/FL mouse line and members of the IRCM animal, microscopy, and molecular biology core facilities for invaluable technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.01.004.
Conflict of interest
The authors have none to declare.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bogdanos D.P., Gao B., Gershwin M.E. Liver immunology. Comparative Physiology. 2013;3(2):567–598. doi: 10.1002/cphy.c120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganeshan K., Nikkanen J., Man K., Leong Y.A., Sogawa Y., Maschek J.A. Energetic trade-offs and hypometabolic states promote disease tolerance. Cell. 2019;177(2):399–413 e312. doi: 10.1016/j.cell.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 4.Kelly D.P., Scarpulla R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes & Development. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 5.Baar K., Wende A.R., Jones T.E., Marison M., Nolte L.A., Chen M. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. The FASEB Journal. 2002;16(14):1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 6.Handschin C., Kobayashi Y.M., Chin S., Seale P., Campbell K.P., Spiegelman B.M. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes & Development. 2007;21(7):770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J., Wu H., Tarr P.T., Zhang C.Y., Wu Z., Boss O. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 8.Arany Z., Foo S.Y., Ma Y., Ruas J.L., Bommi-Reddy A., Girnun G. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 9.Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 10.Estall J.L., Kahn M., Cooper M.P., Fisher F.M., Wu M.K., Laznik D. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. Diabetes. 2009;58(7):1499–1508. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estall J.L., Ruas J.L., Choi C.S., Laznik D., Badman M., Maratos-Flier E. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besse-Patin A., Jeromson S., Levesque-Damphousse P., Secco B., Laplante M., Estall J.L. PGC1A regulates the IRS1:IRS2 ratio during fasting to influence hepatic metabolism downstream of insulin. Proceedings of the National Academy of Sciences of the United States of America. 2019 Mar 5;116(10):4285–4290. doi: 10.1073/pnas.1815150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leone T.C., Lehman J.J., Finck B.N., Schaeffer P.J., Wende A.R., Boudina S. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biology. 2005;3(4) doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers K.T., Chen Z., Lai L., Leone T.C., Towle H.C., Kralli A. PGC-1beta and ChREBP partner to cooperatively regulate hepatic lipogenesis in a glucose concentration-dependent manner. Molecular Genetics and Metabolism. 2013;2(3):194–204. doi: 10.1016/j.molmet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang J.S., Fernand V., Zhang Y., Shin J., Jun H.J., Joshi Y. NT-PGC-1alpha protein is sufficient to link beta3-adrenergic receptor activation to transcriptional and physiological components of adaptive thermogenesis. Journal of Biological Chemistry. 2012;287(12):9100–9111. doi: 10.1074/jbc.M111.320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruas J.L., White J.P., Rao R.R., Kleiner S., Brannan K.T., Harrison B.C. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151(6):1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinsomboon J., Ruas J., Gupta R.K., Thom R., Shoag J., Rowe G.C. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura S., Kai Y., Kamei Y., Ezaki O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to beta2-adrenergic receptor activation and exercise. Endocrinology. 2008;149(9):4527–4533. doi: 10.1210/en.2008-0466. [DOI] [PubMed] [Google Scholar]
- 19.Wen X., Wu J., Chang J.S., Zhang P., Wang J., Zhang Y. Effect of exercise intensity on isoform-specific expressions of NT-PGC-1 alpha mRNA in mouse skeletal muscle. BioMed Research International. 2014;2014:402175. doi: 10.1155/2014/402175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Huypens P., Adamson A.W., Chang J.S., Henagan T.M., Boudreau A. Alternative mRNA splicing produces a novel biologically active short isoform of PGC-1alpha. Journal of Biological Chemistry. 2009;284(47):32813–32826. doi: 10.1074/jbc.M109.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang J.S., Jun H.J., Park M. Transcriptional coactivator NT-PGC-1alpha promotes gluconeogenic gene expression and enhances hepatic gluconeogenesis. Physics Reports. 2016;4(20) doi: 10.14814/phy2.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J., Park M.S., Ha K., Park C., Lee J., Mynatt R.L. NT-PGC-1alpha deficiency decreases mitochondrial FA oxidation in brown adipose tissue and alters substrate utilization in vivo. The Journal of Lipid Research. 2018;59(9):1660–1670. doi: 10.1194/jlr.M085647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felder T.K., Soyal S.M., Oberkofler H., Hahne P., Auer S., Weiss R. Characterization of novel peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) isoform in human liver. Journal of Biological Chemistry. 2011;286(50):42923–42936. doi: 10.1074/jbc.M111.227496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshioka T., Inagaki K., Noguchi T., Sakai M., Ogawa W., Hosooka T. Identification and characterization of an alternative promoter of the human PGC-1alpha gene. Biochemical and Biophysical Research Communications. 2009;381(4):537–543. doi: 10.1016/j.bbrc.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Redondo V., Jannig P.R., Correia J.C., Ferreira D.M., Cervenka I., Lindvall J.M. Peroxisome proliferator-activated receptor gamma coactivator-1 alpha isoforms selectively regulate multiple splicing events on target genes. Journal of Biological Chemistry. 2016;291(29):15169–15184. doi: 10.1074/jbc.M115.705822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan M.C., Rowe G.C., Raghuram S., Patten I.S., Farrell C., Arany Z. Post-natal induction of PGC-1alpha protects against severe muscle dystrophy independently of utrophin. Skeletal Muscle. 2014;4(1):2. doi: 10.1186/2044-5040-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinulovic I., Furrer R., Di Fulvio S., Ferry A., Beer M., Handschin C. PGC-1alpha modulates necrosis, inflammatory response, and fibrotic tissue formation in injured skeletal muscle. Skeletal Muscle. 2016;6:38. doi: 10.1186/s13395-016-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisele P.S., Furrer R., Beer M., Handschin C. The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochemical and Biophysical Research Communications. 2015;464(3):692–697. doi: 10.1016/j.bbrc.2015.06.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisele P.S., Salatino S., Sobek J., Hottiger M.O., Handschin C. The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. Journal of Biological Chemistry. 2013;288(4):2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schilling J., Lai L., Sambandam N., Dey C.E., Leone T.C., Kelly D.P. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor gamma coactivator-1 signaling. Circulation Heart Failure. 2011;4(4):474–482. doi: 10.1161/CIRCHEARTFAILURE.110.959833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran M., Tam D., Bardia A., Bhasin M., Rowe G.C., Kher A. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. Journal of Clinical Investigation. 2011;121(10):4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sczelecki S., Besse-Patin A., Abboud A., Kleiner S., Laznik-Bogoslavski D., Wrann C.D. Loss of Pgc-1alpha expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(2):E157–E167. doi: 10.1152/ajpendo.00578.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besse-Patin A., Leveille M., Oropeza D., Nguyen B.N., Prat A., Estall J.L. Estrogen signals through peroxisome proliferator-activated receptor-gamma coactivator 1alpha to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology. 2017;152(1):243–256. doi: 10.1053/j.gastro.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Buler M., Aatsinki S.M., Skoumal R., Komka Z., Toth M., Kerkela R. Energy-sensing factors coactivator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha) and AMP-activated protein kinase control expression of inflammatory mediators in liver: induction of interleukin 1 receptor antagonist. Journal of Biological Chemistry. 2012;287(3):1847–1860. doi: 10.1074/jbc.M111.302356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Guardia D., Palomer X., Coll T., Davidson M.M., Chan T.O., Feldman A.M. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovascular Research. 2010;87(3):449–458. doi: 10.1093/cvr/cvq080. [DOI] [PubMed] [Google Scholar]
- 36.Matheoud D., Sugiura A., Bellemare-Pelletier A., Laplante A., Rondeau C., Chemali M. Parkinson's disease-related proteins PINK1 and Parkin repress mitochondrial antigen presentation. Cell. 2016;166(2):314–327. doi: 10.1016/j.cell.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 37.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norrbom J., Sallstedt E.K., Fischer H., Sundberg C.J., Rundqvist H., Gustafsson T. Alternative splice variant PGC-1alpha-b is strongly induced by exercise in human skeletal muscle. American Journal of Physiology. Endocrinology and Metabolism. 2011;301(6):E1092–E1098. doi: 10.1152/ajpendo.00119.2011. [DOI] [PubMed] [Google Scholar]
- 39.Popov D.V., Bachinin A.V., Lysenko E.A., Miller T.F., Vinogradova O.L. Exercise-induced expression of peroxisome proliferator-activated receptor gamma coactivator-1alpha isoforms in skeletal muscle of endurance-trained males. The Journal of Physiological Sciences. 2014;64(5):317–323. doi: 10.1007/s12576-014-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadaishi M., Miura S., Kai Y., Kawasaki E., Koshinaka K., Kawanaka K. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1alpha mRNA: a role of beta(2)-adrenergic receptor activation. American Journal of Physiology. Endocrinology and Metabolism. 2011;300(2):E341–E349. doi: 10.1152/ajpendo.00400.2010. [DOI] [PubMed] [Google Scholar]
- 41.Thom R., Rowe G.C., Jang C., Safdar A., Arany Z. Hypoxic induction of vascular endothelial growth factor (VEGF) and angiogenesis in muscle by truncated peroxisome proliferator-activated receptor gamma coactivator (PGC)-1alpha. Journal of Biological Chemistry. 2014;289(13):8810–8817. doi: 10.1074/jbc.M114.554394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ydfors M., Fischer H., Mascher H., Blomstrand E., Norrbom J., Gustafsson T. The truncated splice variants, NT-PGC-1alpha and PGC-1alpha4, increase with both endurance and resistance exercise in human skeletal muscle. Physics Reports. 2013;1(6) doi: 10.1002/phy2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Redondo V., Pettersson A.T., Ruas J.L. The hitchhiker's guide to PGC-1alpha isoform structure and biological functions. Diabetologia. 2015;58(9):1969–1977. doi: 10.1007/s00125-015-3671-z. [DOI] [PubMed] [Google Scholar]
- 44.Chang J.S., Ghosh S., Newman S., Salbaum J.M. A map of the PGC-1alpha- and NT-PGC-1alpha-regulated transcriptional network in brown adipose tissue. Scientific Reports. 2018;8(1):7876. doi: 10.1038/s41598-018-26244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang J.S., Ha K. An unexpected role for the transcriptional coactivator isoform NT-PGC-1alpha in the regulation of mitochondrial respiration in brown adipocytes. Journal of Biological Chemistry. 2017;292(24):9958–9966. doi: 10.1074/jbc.M117.778373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang J.S., Huypens P., Zhang Y., Black C., Kralli A., Gettys T.W. Regulation of NT-PGC-1alpha subcellular localization and function by protein kinase A-dependent modulation of nuclear export by CRM1. Journal of Biological Chemistry. 2010;285(23):18039–18050. doi: 10.1074/jbc.M109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadaishi M., Miura S., Kai Y., Kano Y., Oishi Y., Ezaki O. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bianchi K., Vandecasteele G., Carli C., Romagnoli A., Szabadkai G., Rizzuto R. Regulation of Ca2+ signalling and Ca2+-mediated cell death by the transcriptional coactivator PGC-1alpha. Cell Death & Differentiation. 2006;13(4):586–596. doi: 10.1038/sj.cdd.4401784. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Ba Y., Liu C., Sun G., Ding L., Gao S. PGC-1alpha induces apoptosis in human epithelial ovarian cancer cells through a PPARgamma-dependent pathway. Cell Research. 2007;17(4):363–373. doi: 10.1038/cr.2007.11. [DOI] [PubMed] [Google Scholar]
- 50.Onishi Y., Ueha T., Kawamoto T., Hara H., Toda M., Harada R. Regulation of mitochondrial proliferation by PGC-1alpha induces cellular apoptosis in musculoskeletal malignancies. Scientific Reports. 2014;4:3916. doi: 10.1038/srep03916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adhihetty P.J., Uguccioni G., Leick L., Hidalgo J., Pilegaard H., Hood D.A. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. American Journal of Physiology - Cell Physiology. 2009;297(1):C217–C225. doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- 52.D'Errico I., Salvatore L., Murzilli S., Lo Sasso G., Latorre D., Martelli N. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6603–6608. doi: 10.1073/pnas.1016354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi H.I., Kim H.J., Park J.S., Kim I.J., Bae E.H., Ma S.K. PGC-1alpha attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3beta inactivation mediated by activated p38 in HK-2 Cells. Scientific Reports. 2017;7(1):4319. doi: 10.1038/s41598-017-04593-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sen N., Satija Y.K., Das S. PGC-1alpha, a key modulator of p53, promotes cell survival upon metabolic stress. Molecular Cell. 2011;44(4):621–634. doi: 10.1016/j.molcel.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 55.Eisele P.S., Handschin C. Functional crosstalk of PGC-1 coactivators and inflammation in skeletal muscle pathophysiology. Seminars in Immunopathology. 2014;36(1):27–53. doi: 10.1007/s00281-013-0406-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y., Chen C., Jiang Y., Wang S., Wu X., Wang K. PPARgamma coactivator-1alpha (PGC-1alpha) protects neuroblastoma cells against amyloid-beta (Abeta) induced cell death and neuroinflammation via NF-kappaB pathway. BMC Neuroscience. 2017;18(1):69. doi: 10.1186/s12868-017-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez-Urrutia E., Campos-Parra A., Herrera L.A., Perez-Plasencia C. Alternative splicing regulation in tumor necrosis factor-mediated inflammation. Oncology Letter. 2017;14(5):5114–5120. doi: 10.3892/ol.2017.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reich N.C. Nuclear/cytoplasmic localization of IRFs in response to viral infection or interferon stimulation. Journal of Interferon and Cytokine Research. 2002;22(1):103–109. doi: 10.1089/107999002753452719. [DOI] [PubMed] [Google Scholar]
- 59.Shlomai A., Rechtman M.M., Burdelova E.O., Zilberberg A., Hoffman S., Solar I. The metabolic regulator PGC-1alpha links hepatitis C virus infection to hepatic insulin resistance. Journal of Hepatology. 2012;57(4):867–873. doi: 10.1016/j.jhep.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 60.Kong X., Banks A., Liu T., Kazak L., Rao R.R., Cohen P. IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell. 2014;158(1):69–83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chattopadhyay S., Marques J.T., Yamashita M., Peters K.L., Smith K., Desai A. Viral apoptosis is induced by IRF-3-mediated activation of Bax. The EMBO Journal. 2010;29(10):1762–1773. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim P.K., Armstrong M., Liu Y., Yan P., Bucher B., Zuckerbraun B.S. IRF-1 expression induces apoptosis and inhibits tumor growth in mouse mammary cancer cells in vitro and in vivo. Oncogene. 2004;23(5):1125–1135. doi: 10.1038/sj.onc.1207023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.