Abstract
In this present study, we examined the anti-inflammatory and anti-oxidative properties of alcoholic lemon myrtle extract (LME). The total polyphenol and flavonoid content of LME were determined as 118.77 and 14.53 mg/g extract, respectively. LME showed anti-oxidative properties, such as 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) and 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) radical scavenging activity. The anti-inflammatory activities of LME were investigated using the lipopolysaccharide-stimulated murine macrophage RAW 264.7 cells. Pretreatment with LME was performed at non-cytotoxic concentrations of 10–100 μg/mL. LME inhibited the production of inflammatory mediators such as nitric oxide (NO). Enzyme-linked immunosorbent assay and reverse-transcriptase polymerase chain reaction (RT-PCR) revealed that pretreatment with LME suppressed the protein expression and mRNA levels of pro-inflammatory cytokines such as interleukin IL-6, and tumor necrosis factor (TNF)-α in a concentration-dependent manner, respectively. These results suggest that LME could be used as a potential therapeutic agent having potent anti-inflammatory effects that could be used to treat inflammatory bowel disease.
Keywords: Lemon myrtle, ABTS, DPPH, IL-6, TNF-α, RAW 264.7
1. Introduction
Due to increased health awareness, consumers are increasingly demanding functional health products. This has led to an expansion of the health food market and research; and food industry is actively searching for new functional materials from natural resources [1]. Research areas related to functional foods have also diversified. Among these areas, researchers have been focusing on the identification of diseases that can be caused by inflammation and the discovery of anti-inflammatory substances that could prevent or treat inflammatory diseases [2].
Oxidative stress, defined as the production of reactive oxygen species (ROS) and free radicals and an imbalance in antioxidant levels, is associated with various diseases, such as degenerative nervous system diseases, aging, and diabetes [[3], [4], [5], [6]]. Continuous and excessive oxidative stress in cells leads to an increase in gene expression in specific cells, which in turn, induces degenerative diseases as well as an increase in apoptosis leading to a chronic inflammatory response [7,8]. Inflammation is a repair mechanism that regenerates the damaged area following an invasion that causes physical changes due to physical stimulation, chemical substances, and bacterial infection [2]. However, persistent inflammation can lead to inflammatory diseases, rheumatoid arthritis, arteriosclerosis, gastritis, and asthma [9].
Macrophages play a pivotal role in the host immune defense system as effector cells and are activated by stimuli that include bacterial lipopolysaccharides (LPS) [10,11]. LPS is the major component of gram-negative Escherichia coli cell wall [12] and stimulates the production of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, and inflammatory mediators, such as nitric oxide (NO). These cytokines may contribute to the progression of several inflammatory diseases involving inflammatory bowel diseas (IBD), such as atherosclerosis, cancer, inflammatory arthritis [[13], [14], [15], [16], [17], [18]].
Lemon Myrtle, a shrub of the family Myrtaceae is a species native to tropical rainforests in the Queensland coastal region of Australia, and is found 50–800 m above sea level [19,20]. It is rich in lemon fragrance, contains citral, and is used as a traditional spice in Australia. The Australian Aborigines have used ‘Lemon Myrtle’ for both cooking and healing. The leaves are often used as dried flakes, or in the form of an encapsulated flavor essence that enhances shelf-life and is used for flavoring in vegetable oils, pasta, and shortbread. However, it is mainly used in teas, mostly as mixtures. In addition, ‘Lemon Myrtle’ is used as a lemon flavor replacement in milk-based foods, such as cheesecake or ice-cream to avoid curdling associated with the acidity of lemon fruits [21,22]. Studies on lemon myrtle have reported cytotoxic effects due to presence of essential oils [22], skin toxicity [23], antimicrobial and antifungal activity [24], and effects on bone formation [20]. However, the anti-inflammatory effects of lemon myrtle leaves have not been explored completely.
In this study, we investigated the total polyphenol content and flavonoid content, and ABTS and DPPH radical scavenging activity by using 70 % ethanol extract of Lemon myrtle leaves, and its anti-inflammatory effects on RAW 264.7 macrophages induced by LPS.
2. Material and methods
2.1. Extract preparation
The dried lemon myrtle leaves was purchased in August 2018 from Natureteamall (Gyeonggi, Korea). For extraction, 10 volumes of 70 % ethanol (50 mL) were added to the powered Lemon myrtle leaves (5 g). The supernatant of the mixture was condensed in a vacuum, and lyophilized. The Lemon myrtle leaves extract was stored at −20 °C and dissolved in dimethyl sulfoxide (DMSO) before the use.
2.2. Total polyphenol content
Total polyphenol content was determined by modifying the Folin-Denis method [25]. After mixing 25 μL of sample solution and 500 μL of 10 % Folin-Ciocalteau's phenol reagent (Sigma-Aldrich, Co., St. Louis, Mo., USA) for 5 min at room temperature, they were reacted with 500 μL of 10 % sodium carbonate (Junsei Chemical Co. Ltd., Tokyo, Japan) at 30 °C incubator for 90 min. And then, the absorbance was measured three times at 725 nm, and the average value was listed. Gallic acid (Sigma-Aldrich Co.) was used as a standard substance.
2.3. Total flavonoid content
Total flavonoid content was determined by the modified method of Moreno et al. [26] Samples were mixed with distilled water containing 10 % aluminum nitrate (Sigma-Aldrich Co.) and 1 M potassium acetate (Sigma-Aldrich Co.), incubated at 30 °C for 40 min and then, absorbance was measured at 415 nm. Quercetin (Sigma-Aldrich Co.) was used as a standard substance.
2.4. Antioxidant assay
2.4.1. DPPH radical scavenging activity
The radical scavenging effect of DPPH (1,1-diphenyl-β-picrylhydrazine, Sigma-Aldrich, Co.) was measured by modifying the method of Blois [27]. 100 μL of 0.2 mM DPPH solution was added to 100 μL sample on 96 well plates, mixed for 5 s, and then, reacted for 30 min after shading the light. Absorbance was measured at 517 nm using a microplate spectrophotometer (Epoch, Biotek Instruments, Inc., VT, USA), and ascorbic acid (Sigma-Aldrich Co.) was used as a positive control.
2.4.2. ABTS radical scavenging activity
The ABTS + radical scavenging activity were measured by modifying the method of Kim, & Han [[28]]. Seven millmoles 2,2′-Azobis (2-aminopropane) dihydrochlordie (Sigma-Aldrich, Co.) was mixed with 2.45 mM ABTS and then, reacted for 16 h at 23 °C. Fifty microliters of sample and 100 μL of ABTS solution were reacted at 23°C for 20 min after adding on a 96well-plate and then, the absorbance was measured at 734 nm. As a positive control, ascorbic acid (Sigma-Aldrich Co.) was used.
2.5. Anti-inflammatory assay
2.5.1. Cell culture and cytotoxicity determination
Murine macrophage, RAW 264.7 cell line was obtained from the Korean Cell Line Bank. Cells were maintained as a monolayer culture in Dulbecco’s Modified Eagle Medium (DMEM) (HyClone, Logan, UT, USA) supplemented with 10 % heat-inactivated FBS (HyClone), and 100 IU/mL penicillin and 100 μg/mL streptomycin solution (HyClone) and maintained at 37 °C in a humidified atmosphere of 5 % CO2. The cells were pretreated in serum-free DMEM medium with various concentration of extract for 1 h and then stimulated with LPS (1 g/mL) for 24 h. The cell viability was evaluated by MTS assay kit (Promega, Madison, WI, USA) according to the manufacture’s protocol. All experiments were carried out using 1 g/mL of LPS for the induction of inflammation.
2.5.2. Measurement of NO production
The cells were treated with LME for 1 h, and induced by stimulating them with LPS for 24 h. The culture media was mixed with an equal amount of Griess reagent, reacted at room temperature for 15 min, and measured at 550 nm using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). Serum free culture medium was used as the blank in all experiments.
2.5.3. Measurement of cytokines production in culture medium
The levels of pro-inflammatory cytokines involving IL-6 and TNF-α in the culture media produced RAW 264.7 cells were measured by enzyme-linked immunosorbent assay (ELISA) kits (BD OptEIA TM, San Diego, CA, USA) according to manufacturer’s instruction.
2.5.4. Analysis of mRNA levels of pro-inflammatory cytokines
The mRNA levels of IL-6 and TNF-α were assessed by Reverse-transcriptase polymerase chain reaction (RT-PCR) using specific primers (Table 1). RAW 264.7 cells (1 × 106 cells/ml) were pretreated with various concentration of LME for 2 h and stimulated with LPS for 24 h. Total RNA was extracted with an RNeasy mini kit (Qiagen, Hilden, Germany) and 1 mg of total RNA was reverse-transcribed into complementary DNA (cDNA) by Superscript III First-Strand synthesis kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The result cDNA samples were subjected to PCR amplification in the presence of specific sense and antisense primers. Human glyceraldehyde-3-phaosphate dehydrogenase (GAPDH) was used as an internal control, and the primer sequences are provided in Table 1. The PCR was conducted as follows: 94℃, 30 s, denaturing; 55℃, 30 s, annealing; 72℃, 1 min extension: and subjected to 19 cycles for IL-6 and TNF-α genes. The amplified PCR products were visualized via 1 % agarose gel electrophoresis and HiQ Blue Mango Dye (Bio-D, Gwangmyeong-Si, Korea) staining, and then analyzed using a gel documentation system (KoreaBiotek, Korea) quantitative analysis.
Table 1.
Primer sequences used in this study.
| IL-6 | Forward Reverse |
5′-GCCTTCTTGGGACTGATGCT-3′ 5′-TGGAAATTGGGGTAGGAAGGAC-3′ |
|---|---|---|
| TNF - α | Forward Reverse |
5′-TAGCCCACGTCGTAGCAAAC-3′ 5′-ACCCTGAGCCATAATCCCCT-3′ |
| GAPDH | Forward Reverse |
5′-ACCACAGTCCATGCCATCAC-3′ 5′-CCACCACCCTGTTGCTGTAG-3′ |
2.6. Statistical analysis
Data are expressed as means ± standard deviation (SD) of at least three independent experiments. Significant differences between the control and LME groups were determined by Student’s t-test at p < 0.05.
3. Results and discussion
3.1. Analysis of total polyphenol and flavonoid content
Phenolic compounds widely distributed in nature have various structures and molecular weights. The phenolic hydroxyl groups in these compounds impart physiological properties, such as anti-oxidative, anticancer, and antibacterial activity by binding to macromolecules such as proteins [29]. Several studies report an interaction between antioxidant activity and the amount of phenolic compounds [[30], [31], [32]]. Therefore, based on reported data, the antioxidant activity can be quantified by measuring the amount of phenolic compounds in the plant [33]. Antioxidants widely distributed in nature include flavonoids, tocopherols, polyphenols, and carotenoids. Among them, flavonoids are mainly present in the fruits, stem, and flowers and are compounds with C6-C3-C6 structure, the basic structure of flavone [34]. Total polyphenol content of the LME sample was 118.77 ± 0.34 mg/g extract. Cheon [20] reported that the total polyphenol content of hot water and ethanol extracts from Korean lemon myrtle were 207.7 and 246.73 μg GAE (gallic acid equivalents)/mg, respectively, and the total phenol content of ethanol extracts was higher than that of hot water extracts. However, in the study by Kim et al. [35], the total polyphenol contents of hot water and ethanol extracts were 331.54 and 204.74 μg TAE (tannic acid equivalents)/mg. And water extracts were reported to contain higher total phenol content. This difference between ethanolic and aqueous extracts could be due to difference in the extraction method and in the geographical location from which the plant was obtained. Total flavonoid content from the LEM sample was estimated at 14.53 ± 1.88 mg/g extract. In the study by Taghizadeh et al. [36], effects of three types of solvents namely, polar protic solvents (i.e. ultrapure water, methanol and ethanol), polar aprotic solvents (i.e. acetone and ethyl acetate) and a non-polar solvent (i.e. hexane), determinate of total flavonoids content. Results showed that total flavonoid levels significantly vary among different extracts. Based on results, the highest values for total flavonoid in pistachio extracts were obtained following extraction using ethanol. In terms of total flavonoids content, these results were showed that the efficiency of polar protic solvents for extraction of the flavonoids compounds, was more pronounced than that of polar aprotic solvents and non-polar solvent.
3.2. DPPH and ABTS radical scavenging activities
The antioxidant activity of a substance can be measured by various methods with varying sensitivities. ABTS is a relatively stable free radical and is widely used for measuring antioxidant activity in combination with DPPH, which can measure both lipophilic and hydrophilic substances. ABTS produces a cationic radical and DPPH produces an anionic radical for the detection of antioxidant activity. Therefore, it is known that these two methods are different from each other in the degree of binding between the substrates and the reactant, and therefore, the measurement results may be different depending on the method used.
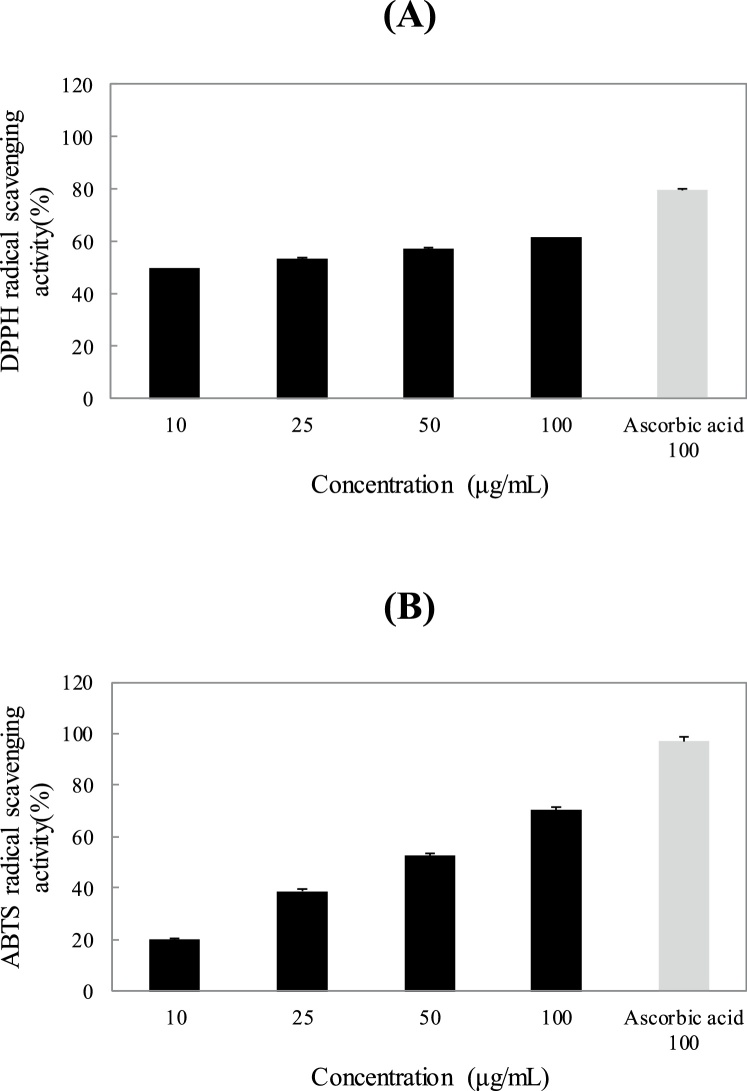
The DPPH scavenging activity of LME with different concentrations was 49.54, 53.41, 57.21, and 61.45 % at concentrations of 10, 25, 50, and 100 μg/mL, respectively (Fig. 1A). The antioxidant activity of 100 μg/mL ethanolic LME was lower than that of 100 μg/mL ascorbic acid, the positive control. Kean et al. [37] showed that both lemon myrtle leaf and stem volatile oils showed high antioxidant properties. DPPH scavenging activity for both the leaf and stem parts were 83.72 % and 84.46 %, respectively. As for the xanthine/xanthine oxidase superoxide scavenging assays, both the lemon myrtle leaf and stem volatile oils showed high superoxide scavenging activity, at 83.72 % and 84.46 %, respectively. The ABTS radical scavenging activity was similar to DPPH radical scavenging activity (Fig. 1B). The ABTS scavenging activity was 19.95, 38.88, 52.47, and 70.30 % at concentrations of 10, 25, 50 and 100 μg/mL, respectively. In the present study, the ABTS radical scavenging activity was relatively higher than the DPPH radical scavenging activity. A similar observation was made by Re et al. [38] who suggested that the difference in sensitivity likely arises because the ABTS method can measure both hydrogen-donating antioxidant levels and the chain-breaking antioxidant levels and can be applied to both the aqueous phase and the organic phase with higher sensitivity.
Fig. 1.
2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging activities (A) and 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) radical scavenging activities (B) of Lemon myrtle extract (LME) and ascorbic acid. Each Values are expressed as Mean ± standard deviation (SD). (n = 3).
3.3. In vitro cytotoxicity
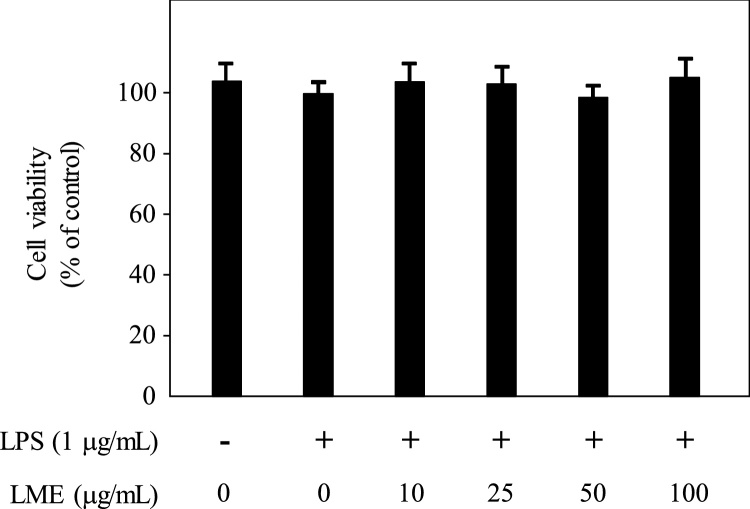
Oxidative stress promotes the occurrence of various chronic diseases such as aging, cancer, inflammation, neurodegeneration, and type 2 diabetes [39,40]. Therefore, many plant extracts have been used as traditionally prescribed herbal remedies for the treatment of these diseases worldwide. RAW 264.7 cells are a functional macrophage cell line transformed by Abelson leukemia virus and require stimuli such as LPS for activation [41]. The present study was undertaken to evaluate the potential in vitro anti-inflammatory activity of LME using the LPS-induced RAW model system. The viability of the RAW 264.7 cells was assessed using MTS assay to precisely determine the toxicity of LME. Cells were treated with various concentrations of LME (10–100 μg/mL) for 24 h followed by LPS stimulation. LME treatment did not exhibit any cytotoxic effects on RAW 264.7 cells at concentrations up to 100 μg/mL after treatment for 24 h (Fig. 2).
Fig. 2.
Effects of LME on cytotoxicity in lipopolysaccharide (LPS)-induced RAW 264.7 cells. Cells were pretreated with LME for 1 h and then stimulated with LPS (1 mg/mL) for 24 h under serum-free conditions. Cell viability were determined using MTS assay. Each determination was made in triplicate. Each determination was made in triplicates. Data are presented as means ± SD.
3.4. Effects on NO production
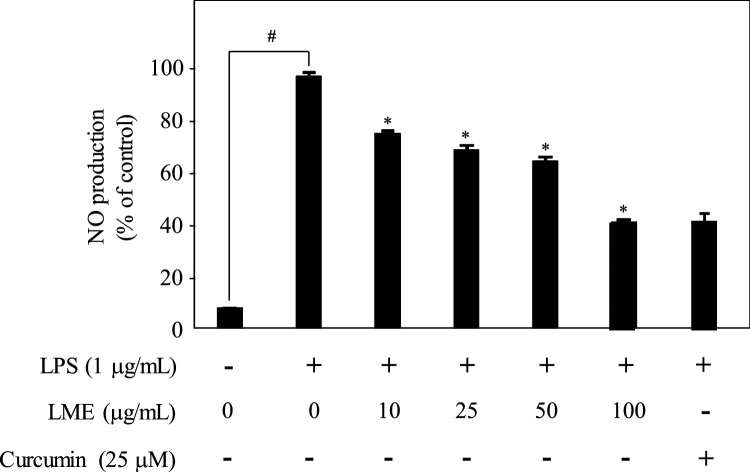
NO is released by activated macrophages and is an important molecule that regulates a range of physiological and pathological inflammatory responses. To investigate the effect of LME treatment on NO production, RAW264.7 cells were pretreated with LME for 1 h followed by stimulation with LPS (1 g/mL) for 24 h. Curcumin treatment was used as positive control for NO production, which was measured using Griess reagent. As shown in Fig. 3, NO production was induced by LPS (compared to vehicle control). However, LME pretreatment significantly decreased the levels of LPS-induced NO production in a concentration-dependent manner (P < 0.05).
Fig. 3.
Effects of LME on nitric oxide (NO) production in LPS-induced RAW 264.7. Cells were pretreated with LME for 1 h and then stimulated with LPS (1 mg/mL) for 24 h under serum-free conditions. NO production was determined using Griess assay. Each determination was made in triplicates. Data are presented as means ± SD. # P < 0.05 vs. control; * P < 0.05 vs. LPS-treated group.
3.5. Effects on pro-inflammatory cytokine production
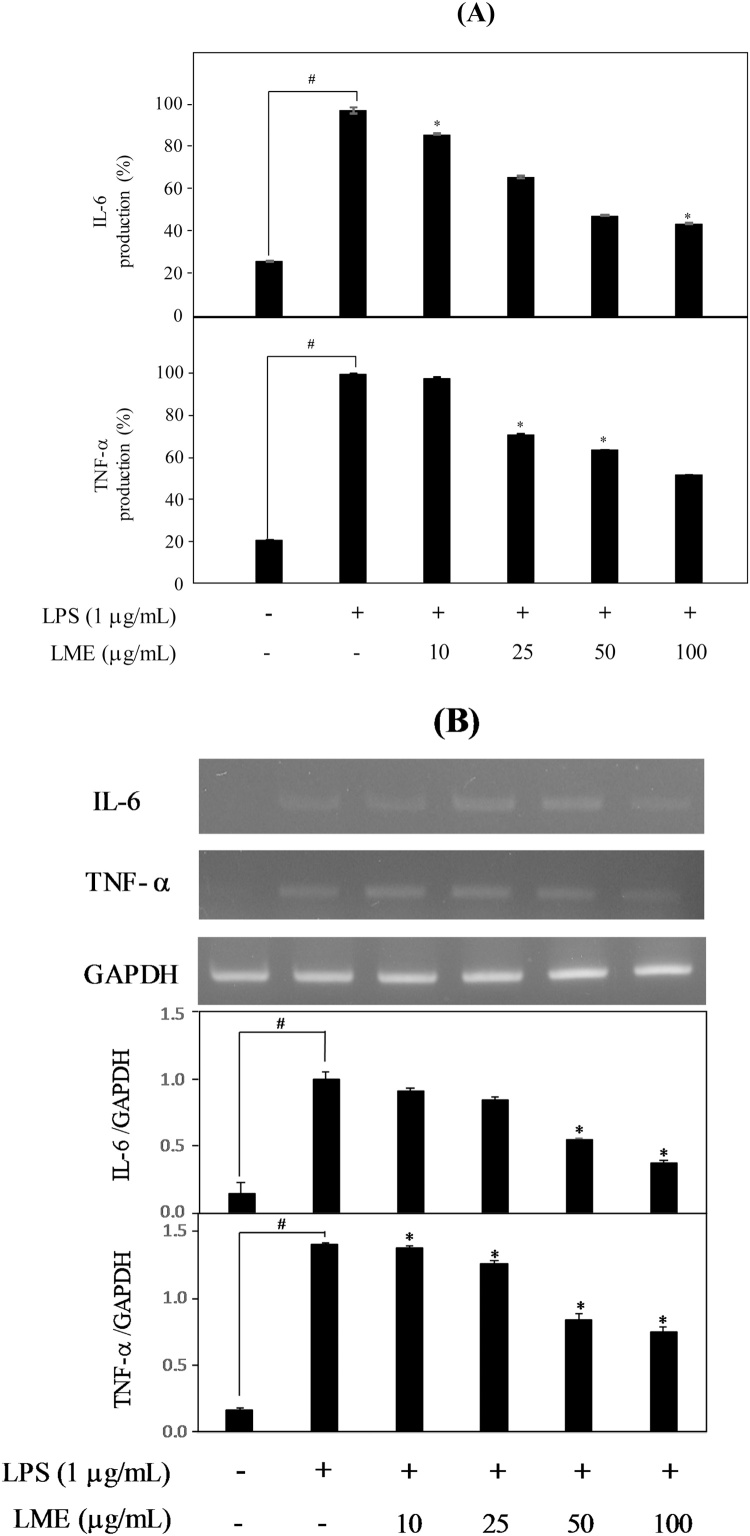
The cellular exposure to LPS results in an inflammatory response due to the secretion of pro-inflammatory cytokines. To assess the inhibitory effect of LME treatment on the secretion of proinflammatory cytokines in LPS-stimulated RAW 264.7 cells, the levels of these cytokines were measured by ELISA. Results indicated that LME treatment significantly suppressed the protein expression of IL-6 and TNF-α in a concentration-dependent manner in LPS-stimulated RAW 264.7 cells (P < 0.05, Fig. 4A). Moreover, to determine the inhibitory effects of LME on IL-6 and TNF-α gene expression, the mRNA levels of the IL-6 and TNF-α were measured via RT-PCR using total cellular RNA prepared from LPS-stimulated RAW 264.7 cells pretreated with different concentrations of LME. The mRNA levels for the IL-6 and TNF-α of non-treated cells was clearly detected; however, the corresponding mRNA of the LME-treated cells was shown to be significantly reduced with concentration-dependent manner (P < 0.05, Fig. 4B).
Fig. 4.
Effects of LME on production of pro-inflammatory cytokines. Cells were pretreated with LME for 1 h and then stimulated with LPS (1 μg/mL) for 24 h. Protein expression (A) and mRNA level (B) of interleukin (IL)-6 and tumor necrosis factor (TNF)-α were determined by enzyme-linked immunosorbent assay (ELISA) and reverse-transcriptase polymerase chain reaction (RT-PCR), respectively. Each determination was made in triplicates. Data are presented as means ± SD. #P < 0.05 vs. control; * P < 0.05 vs. LPS-treated group.
NO and pro-inflammatory cytokines such as IL-6 and TNF-α, which is an inflammatory regulatory mediator in the physiological and pathological processes of inflammation and is produced mainly by activated macrophages, was suppressed by LME. Our results shown that LME inhibited the pro-inflammatory mediators in a LPS-induced condition. Further studies on the protective action of active compounds in the molecular mechanism and the dextran sodium sulfate-induced colitis model from lemon myrtle would be necessary to confirm their potential therapeutic application in the treatment of IBD.
4. Conclusion
In the present study, we show that LME had anti-oxidative activities as confirmed by ABTS and DPPH radical scavenging. Moreover, LME negatively regulated the levels of the inflammatory mediator, NO, and pro-inflammatory cytokines, IL-6 and TNF-α. Further studies investigating anti-oxidative and anti-inflammatory mechanisms of active compounds isolated from lemon myrtle at the molecular level are required.
Authors’ contributions
Sun-Yup Shim and Ji-Hyun Kim designed and performed the experimental analysis and drafted the manuscript. Sun-Yup Shim, Kang-Hee Kho, and Mina Lee conceived the study and were also involved in the coordination of the study, and interpretation of the data.
Conflict of interest
The authors have no conflict of interest to disclose.
CRediT authorship contribution statement
Sun-Yup Shim: Investigation, Writing - original draft, Writing - review & editing, Supervision. Ji-Hyun Kim: Investigation, Writing - original draft. Kang-Hee Kho: Data curation, Project administration. Mina Lee: Writing - review & editing, Project administration, Funding acquisition.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2017R1C1B2005934).
References
- 1.Lee S.E., Lee J.H., Kim J.K., Kim G.S., Kim Y.O., Soe J.S., Choi J.H., Lee E.S., Noh H.J., Kim S.Y. Anti-inflammatory activity of medicinal plant extracts. Kor. J. Med. Crop Sci. 2011;19:217–226. [Google Scholar]
- 2.Jeoung Y.J., Choi S.Y., An C.S., Jeon Y.H., Park D.K., Lim B.O. Comparative effects on anti-inflammatory activity of the Phellinus linteus and Phellinus linteus grown in germinated brown rice extracts in murine macrophage RAW264.7 cells. Kor. J. Med. Crop Sci. 2009;17:97–101. [Google Scholar]
- 3.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked. Free Rad. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 5.Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidant: a review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 6.Uttara B., Singh A.V., Zamboni P., Mahajan R. Oxidative stress and neurodegenerative diseases; a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang H.W. Antioxidant and anti-inflammatory effect of extracts from Flammulina velutipes (Curtis) singer. J. Kor. Soc. Food Sci. Nutr. 2012;41:1072–1078. [Google Scholar]
- 8.Lee S.G., Jeong H.J., Lee B.J., Kim J.B., Choi S.W. Antioxidant and anti-inflammatory activities of ethanol extracts from medicinal herb mixtures. Kor. J. Food Sci. Technol. 2011;43:200–205. [Google Scholar]
- 9.Kaplanski G., V Marin, F Montero-Jukian, A Mantovani, C Farnarier. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z., Tang L., Zou P., Zhang Y., Zhe W., Fang Q., Jiang L., Chen G., Xu Z., Zhang H., Liang G. Synthesis and biological evaluation of allylated and prenylated mono‑carbonyl analogs of curcumin as anti‑inflammatory agents. Eur. J. Med. Chem. 2014;74:671–682. doi: 10.1016/j.ejmech.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce G.F. Macrophages: Important physiologic and pathologic sources of polypeptide growth factors. Am. J. Respir. Cell Mol. Biol. 1990;2:233–234. doi: 10.1165/ajrcmb/2.3.233. [DOI] [PubMed] [Google Scholar]
- 13.Boscá L., Zeini M., Traves P.G., Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–258. doi: 10.1016/j.tox.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Kim K.N., Ko Y.J., Kang M.C., Yang H.M., Roh S.W., Oda T., Kim D. Anti-inflammatory effects of trans-1,3-diphenyl-2,3-epoxypropane-1-one mediated by suppression of inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2013;53:371–375. doi: 10.1016/j.fct.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R., Janeway C.A. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 16.Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nature Immunol. 2005;6:1991–1997. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 17.Vezza T., Rodríguez-Nogales A., Algieri F., Utrilla M.P., Rodriguez-Cabezas M.E., Galvez J. Flavonoids in IBD: a review. Nutrients. 2016;8:1–22. doi: 10.3390/nu8040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Qw., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon-gamma and bacterial lipopolysaccharide. J. Exp. Med. 1993;106:1–12. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchaillot A., Caffin N., Bhandari B. Drying of lemon myrtle (Backhousia citriodora) leaves: retention of volatiles and color. Dry. Technol. 2009;27:445–450. [Google Scholar]
- 20.Cheon J.H. Silla University; Busan, Korea: 2015. Effects of Backhousia Citriodora Extracts on Antioxidant Activity and Bone Formation. Thesis Master. [Google Scholar]
- 21.Horn T., Barth A., Rühle M., Häser A., Jürges G., Nick P. Molecular diagnostics of Lemon Myrtle (Backhousia citriodora versus Leptospermum citratum) Eur. Food Res. Technol. 2012;234:853–861. [Google Scholar]
- 22.Hayes A.J., Markovic B. Toxicity of australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem. Toxicol. 2002;40:535–543. doi: 10.1016/s0278-6915(01)00103-x. [DOI] [PubMed] [Google Scholar]
- 23.Hayes A.J., Markovic B. Toxicity of australian essential oil Backhousia citriodora (Lemon myrtle). Part 2. Absorption and histopathology following application to human skin. Food Chem. Toxicol. 2003;41:1409–1416. doi: 10.1016/s0278-6915(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson J.M., Hipwell M., Ryan T., Cavanagh H.M.A. Bioactivity of Backhousia citriodora; Antibacterial and antifungal activity. J. Agric. Food Chem. 2003;51:76–81. doi: 10.1021/jf0258003. [DOI] [PubMed] [Google Scholar]
- 25.Biglari F., Alkarkhi A.F.M., Easa A.M. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008;107:1636–1641. [Google Scholar]
- 26.Moreno M.I.N., Isla M.I., Sampietro A.R., Vattuone M.A. Comparison of the free radical scavenging activity of propolis from several region of Argentina. J. Enthropharmacol. 2000;71:109–114. doi: 10.1016/s0378-8741(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 27.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 28.Kim J.Y., Han Y.S. Influence of roasting time on antibacterial and antioxidative effects of coffee extract. Kor. J. Food Cookery Sci. 2009;25:496–505. [Google Scholar]
- 29.Park C.S. Component and quality characteristics of powdered green tea cultivated in Hwagae area. Kor. J. Food Preserv. 2005;12:36–42. [Google Scholar]
- 30.Verzelloni E., Tagliazucchi D., Conte A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007:564–571. [Google Scholar]
- 31.Paixao N., Perestrelo R., Marques J.C., Câmara J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007:204–214. [Google Scholar]
- 32.Djeridane A., Yousfi M., Nadjemi B., Vidal N., Lesgards J.F., Stocker P. Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. Eur. Food Res.Technol. 2007:801–809. [Google Scholar]
- 33.Choi S.Y., Lim S.H., Kim J.S., Ha T.Y., Kim S.R., Kang K.S., Hwang I.K. Evaluation of the estrogenic and antioxidant activity of some edible and medicinal plants. Kor. J. Food Sci. Technol. 2005;37:549–556. [Google Scholar]
- 34.Vijaya K., Ananthan S., Nalini R. Antibacterial effect of theaflavin, polyphenon 60 (Camellia sinensis) and euphorbia hirta on shigella spp.--a cell culture study. J. Ethnopharmacol. 1995;49:115–118. doi: 10.1016/0378-8741(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 35.Kim P.K., Jung K.I., Choi Y.J., Gal S.W. Anti-inflammatory effects of lemon myrtle (Backhousia citriodora) leaf extracts in LPS-induced RAW 264.7 cells. J. Life Sci. 2017;27:986–993. [Google Scholar]
- 36.Taghizadeh S.F., Rezaee R., Davarynejad G., Karimi G., Nemati S.H., Asili J. Phenolic profile and antioxidant activity of Pistacia vera var. Sarakhs hull and kernel extracts: the influence of different solvents. J. Food Meas. Charact. 2018;12:2138–2144. [Google Scholar]
- 37.Kean O.B., Yusoff N., Ali N.A.M., Subramaniam V., Yee S.K. Chemical composition and antioxidant properties of Backhousia Citriodora volatile oil. Open Conference Proccedings J. 2013;4:194. [Google Scholar]
- 38.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 39.Bjelakovic G., Nikolova D., Gluud C. Antioxidant supplements and mortality. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:40–44. doi: 10.1097/MCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic.Biol. Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raschke W.C., Baird S., RalpH P., Nakoinz I. Functional macrophage cell lines transformed by Abelson Leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]