Highlights
-
•
Ultrasound produced significant mechanochemical effect on starch granules.
-
•
Starch granules went through three different stages during ultrasonic treatment.
-
•
Quality of OSA-modified starch prepared by ultrasonic-assisted treatment was improved.
-
•
Mechanism of preparing high quality modified starch was revealed.
Keywords: Ultrasound, OSA-modified starch, Quality, Mechanochemical effect, Influence mechanism
Abstract
The purpose of this study was to reveal the influence mechanism of preparing high quality modified starch by ultrasonic-assisted treatment. In this paper, ultrasonic modified starch and octenyl succinic anhydride (OSA) modified starch were prepared under ultrasonic conditions. The effect of ultrasound on the structure and properties of native starch were studied to see whether ultrasound could produce mechanochemical effect on starch granules. Then the mechanism of ultrasonic effect on the quality of OSA-modified starch was revealed by mechanochemical effect. The results showed that the morphology and crystalline regions of starch granules were destroyed after ultrasonic treatment, and the structure and properties of starch granules changed in different stages. These changes showed that ultrasonic treatment produced significant mechanochemical effect on starch granules. Thus the quality of OSA-modified starch prepared by ultrasonic-assisted treatment was improved significantly, and its influence mechanism was analyzed using the theory of mechanochemistry.
1. Introduction
Starch is widely used in various industries, however, the application of starch is severely limited by its native defects and properties such as poor solubility, poor heat resistance and so on (Shahbazi, Majzoobi, & Farahnaky, 2018). So it is necessary to modify starch to improve its quality and expand its application range. OSA-modified starch is a kind of esterified starch with excellent amphiphilic character and interfacial properties (Altuna, Herrera, & Foresti, 2018). It is a good emulsifying stabilizer and thickener, which is widely used in many food, cosmetics and pharmaceutical products. For example, Baranauskienė, Rutkaitė, Peciulyte, Kazernavičiūtė, and Venskutonis (2016) prepared modified potato starch derivatives suitable for the encapsulation of essential oils by hydroxypropylation with propylene oxide and esterification with OSA. Cheuk et al. (2015) used OSA-modified starch to encapsulate or entrap coenzyme Q10 (CoQ10) and characterise the product. The results showed that the current CoQ10 formulation was ideal not only for adding nutritional value to common beverages and fruit juices, but may also find use in raising CoQ10 levels in baked goods. Many studies on esterification of starch have shown that OSA-modified starch with low degree of substitution (DS) (0.010–0.045) is commonly used in food industry (Jyothi, Rajasekharan, Moorthy, & Sreekumar, 2010). Nevertheless, the quality of OSA-modified starch prepared by conventional methods is poor. Thus, how to prepare high quality of OSA-modified starch has become a hot research topic.
In recent years, ultrasonic modification being as an eco-friendly and high efficient method, is widely used in the production of modified starch and attains more attentions (Hu et al., 2015). Pamela et al. (2017) prepared corn modified starch by ultrasonic treatment, and found the resistant starch content increased from 2.1% to 4.0% and the rapidly digestible starch fraction showed an increase from 42.9% to 60.0%. Boufi et al. (2018) successfully disintegrated starch granules into nanoparticles by high-power ultrasonic treatment. Abral et al. (2018) have shown that ultrasonic treatment significantly improved the biocomposite properties of nanofiber fractions. The influence mechanism is attributed to the cavitation effect of ultrasound on materials (Yamakoshi & Miwa, 2011). Ultrasonic cavitation is a series of dynamic processes of bubbles in the liquid when they are exposed to ultrasonic field, and it can produce three kinds of mechanical forces, including local ultra-high pressure, high speed jet and high frequency vibration (Nguyen, Asakura, Koda, & Yasuda, 2017). Ultrasound is an effective treatment method, and the influence mechanism is expected to be explained using mechanochemical theory.
Mechanochemistry is the process of converting the energy of mechanical force into chemical energy (Takuma, Ryuji, & Masaki, 2018). The process of mechanical force is usually divided into three stages which are the stress stage, aggregation stage and agglomeration stage (Juhász and Opoczky, 1990, Saranu et al., 2011). Mechanochemistry has become one of the most active research fields. So far, researches on mechanochemistry mainly focused on the synthesis of crystalline materials. For example, Nada, Gillan, and Larsen (2019) identified the molecular level details of important mechanochemical pre-reaction steps in the solvent-free synthesis of crystalline ZSM-5. Taking recent background into consideration, starch is a renewable and important polycrystalline material for many products, but there are few researches on the mechanochemical effect about it.
If ultrasound could produce significant mechanochemical effect on starch granules, the structure and properties of starch would change greatly. Thereby the influence mechanism of preparing high quality of OSA-modified starch with low DS by ultrasonic-assisted treatment could be revealed. Thus, in this study, ultrasonic modified starch and OSA-modified starch with low DS were prepared under ultrasonic conditions, using corn starch as raw material. The effect of ultrasound on the structure and properties of native corn starch were studied to see whether ultrasound could produce mechanochemical effect on starch granules. Then the influence mechanism of ultrasound on the quality of OSA-modified starch was analyzed by mechanochemical effect.
2. Materials and methods
2.1. Materials
Corn starch was purchased from Shandong Zhucheng Xingmao Corn Development Co., Ltd. (Zhucheng City, Shandong Province, China). 8-Aminopyrene-1,3,6-trisulfonic acid trisodium salt (APTS) and sodium cyanoborohydride were purchased from Sigma-Aldrich (Shanghai, China). All other chemicals and reagents were of analytical grade.
2.2. Experimental methods
2.2.1. Preparation of ultrasonic modified corn starch
100.00 g (dry basis) of corn starch was weighed to prepare a 35% (w/v) starch suspension by adding distilled water. The suspension was stirred evenly and then placed in the ultrasonic processor (FS-600, Shanghai Shengxi Ultrasonic Instrument CO., Ltd, Shanghai City, China). The ultrasonic power was set to 500 W and the temperature was controlled at 35 °C. The suspensions were treated with 0, 5, 15, 30 and 60 min using the probe of ultrasonic processor, respectively. The ultrasound was stopped for 25 min after 5 min of each ultrasonic treatment, and it was continued when the stirring temperature was 35 °C. After ultrasonic treatments, these samples were dried in an oven at 45 °C for 48 h, crushed and obtained through a 100-mesh standard sieve.
2.2.2. Preparation of OSA-modified starch with low DS by ultrasonic-assisted treatment
100.00 g (dry basis) of corn starch was weighed to prepare a 35% (w/v) starch suspension by adding distilled water, the pH of suspension was adjusted to 8.0 with 1 M NaOH solution. The time of alkalization was 20 min, and the temperature was controlled at 35 °C. OSA (3.0%, based on dry starch basis) solution (diluted three times with anhydrous ethanol, v/v) was added and the pH was controlled at 8.0. Under the same ultrasonic conditions, the suspensions were also treated for 0, 5, 15, 30 and 60 min using the probe of ultrasonic processor, respectively. The ultrasound was stopped for 25 min after 5 min of each ultrasonic treatment, and it was continued when the stirring temperature was 35 °C. The total reaction time of esterification and ultrasonication was 6 h. After completion of the reaction, the pH of suspension was neutralized to 6.5 with 1 M HCl solution and vacuum-filtered through filter paper. Then each suspension was washed three times with distilled water and once with ethanol solution (95%, v/v) to remove the residual reagents (Chen, Huang, Fu, & Luo, 2014). Similarly, the samples were dried and crushed. Finally, OSA-modified starch was obtained through a 100-mesh standard sieve.
2.2.3. Determination of the structure and properties of ultrasonic modified corn starch
2.2.3.1. X-ray diffraction (XRD)
The crystalline characteristic of sample was obtained using an X-ray diffractometer (D8 ADVANCE, Bruker AXS GMBH, Karlsruhe, Germany) with CuKα X-ray source. The measured step of XRD was 0.02° and the scanning rate was 4°/min. The radiation parameters were set to 40 kV and 100 mA, and the XRD patterns were recorded for an angular range of 5-40° (2θ). The divergence slit width and scattering slit width were 1° and the receiving slit width was 0.16 mm.
2.2.3.2. Scanning electron microscopy (SEM)
The morphology of sample was observed by a scanning electron microscope (QUANTAFEG250, FEI, Portland Oregon, USA) at 2000× magnifications according to the method of Zhang et al. (2018).
2.2.3.3. Amylose content
Amylose content of sample was determined by dual wavelength spectrophotometry according to the method of Jialiexi, Jing, and Xuelaiti (2010).
2.2.3.4. Differential scanning calorimetry (DSC)
The thermal properties of sample were determined by a differential scanning calorimeter (200 PC, Netzsch, Germany) according to the method of Raguzzoni, Delgadillo, and da Silva (2016). 5.00 mg (dry basis) of sample was weighed and mixed with 15 μL distilled water in a aluminum pot, and then all samples were placed at 4 °C for 12 h. The samples were conducted from 20 to 100 °C at a rate of 10 °C/min with an empty pan as reference. Nitrogen was the protective gas and the flow rate was 60 mL/min.
2.2.3.5. Differential thermogravimetric (DTG)
DTG was measured by a thermal gravimetric analyzer (TA-60, Shimadzu, Kyoto, Japan). Dynamic measurements were carried out at the rate of 10 °C/min in the temperature range of 25–500 °C, and under a nitrogen atmosphere with a flow rate of 60 mL/mim.
2.2.3.6. Pasting properties
According to the method of Tong et al. (2014), the pasting properties of sample were determined using a Rapid Visco Analyzer (RVA Eritm, Perten, Stockholm, Sweden), and it was run using the Thermocline for Windows software (Version 1.2). 6.00 g (dry basis) of sample and 50.00 g distilled water were mixed evenly in the RVA sample can. The heating and cooling cycles of the program were set as follows: (1) holding at 50 °C for 1 min, (2) heated to 95 °C in 3.8 min, (3) holding at 95 °C for 2.5 min, (4) cooling to 50 °C in 3.8 min, (5) holding at 50 °C for 1.4 min. During the first 10 min of test, the RVA paddle speed was at 960 rpm. Then the speed was 160 rpm.
2.2.4. Determination of the quality of OSA-modified starch prepared by ultrasonic-assisted treatment
2.2.4.1. Degree of substitution (DS) and reaction efficiency (RE)
1.50 g (dry basis) of sample was weighed and dispersed in 50 mL ethanol solution (95%, v/v), and magnetic stirred for 10 min. Then 15 mL hydrochloric acid–ethanol solution (2 M) was added and stirred for 30 min. Each suspension was filtered through a glass filter and the residue was washed with ethanol solution (95%, v/v) until no Cl− could be detected (using 0.1 M AgNO3 solution). The sample was transfered into a 250 mL triangle bottle and 100 mL distilled water was added, and then the suspension was heated in a boiling water-bath for 30 min with stirring. In the meantime, two drops of phenolphthalein were added into the suspension, and then NaOH standard solution (0.1 M) was dropped into suspension until the suspension became pink. Unmodified starch was used as blank control.
The DS was calculated by the following equation (Chen et al., 2014):
| (1) |
In the equation, 0.162 is the molar mass of glucose residues, kg/mol; 0.210 is the molar mass of octenyl succinyl group, kg/mol; A is the titration volume of 0.1 M NaOH standard solution, mL; W is the dry basis mass of OSA-modified starch sample, g; M is the molarity of NaOH standard solution, M.
The RE was calculated by the following equation:
| (2) |
In the equation, C is the actual DS and D is the theoretical DS. The theoretical DS is calculated by assuming that all of the added OSA reacted with starch to form the ester derivative.
2.2.4.2. Solubility (S) and swelling power (SP)
10.00 g (dry basis) of sample was weighed and dispersed in distilled water to prepare a 2% (w/v) starch suspension. Each suspension was gelatinized at 85 °C for 30 min and then cooled to room temperature. Then the suspension was centrifuged at 3500 r/min for 30 min and the precipitate was weighed to obtain the weight (P) of swollen starch. After the supernatant was decanted, it was dried in an oven at 105 °C until a constant weight (A) was obtained. S and SP of samples were calculated by the following equations (Raguzzoni et al., 2016):
| (3) |
| (4) |
In the equations, A is the quality of supernatant after drying constant weight, g; W is the dry sample quality, g; P is the quality of sediment after centrifugation, g.
2.2.4.3. Enzymatic resistance
50 mL of distilled water was heated to 60 °C, and 5.00 g of sample was added for stirring. The pH was adjusted to 6.0–7.0, then 30.00 mg of calcium chloride and 25.00 mg of ɑ-amylase (Shanghai Yuanye Biotechnology Co., Ltd, Shanghai, China) were added. Suspension was continue heated to 95 °C and heated for 20 min. After heated, suspension was taken out for filtration, drying and weighing.
2.2.4.4. Freeze-thaw stability
The freeze–thaw stability is generally expressed by the water separating proportion. 1.50 g (dry basis) of sample was weighed and dispersed in distilled water to prepare a 3% (w/v) starch suspension. Each suspension was heated in a boiling water bath for 20 min and stirred continuously to make it gelatinized completely. After cooling to room temperature, the starch paste was put in the refrigerator at −18 °C for 24 h and then taken out. After thawing at room temperature, starch paste was centrifuged at 4500 r/min for 15 min, and the supernatant was discarded. The quality of the supernatant was measured and the water separating proportion was calculated (Pongsawatmanit & Srijunthongsiri, 2008).
| (5) |
In the equation, W1 is the quality of starch paste, g; W2 is the quality of sediment, g.
2.2.5. Statistical analysis
Statistical analysis was processed with ORIGIN 8.5 (Origin-Lab Inc., Northampton, MA, USA) and SPSS 19.0 (IBM Corporation, Armonk, NY, USA). Data were expressed as means ± standard deviations of at least three independent determinations on each sample.
3. Results and discussion
3.1. Effect of ultrasound on the structure and properties of native corn starch and its mechanochemical effect
3.1.1. Effect of ultrasound on crystalline structure of native corn starch
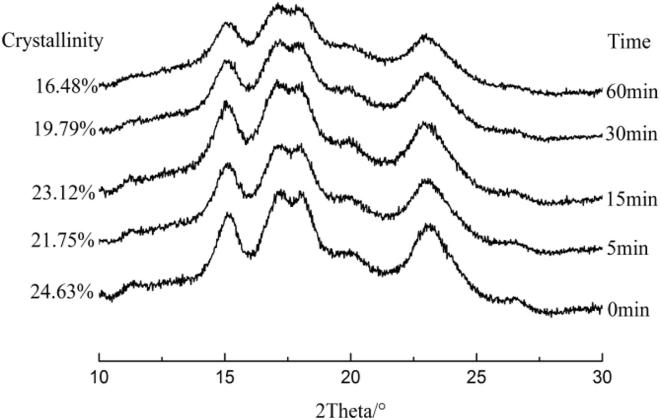
Fig. 1 showed that corn starch had obvious diffraction peaks at 15.3°, 17.1°, 18.3° and 23.1°, which was a typical A-type crystalline structure (Bettaieb, Jerbi, & Ghorbel, 2014). The shape and angular position of the different peaks remained unchanged after ultrasonic treatment, which indicated that ultrasonic treatment was not enough to change the crystalline type of corn starch.
Fig. 1.
XRD patterns of native corn starch treated at different ultrasonic time.
It could be seen from Fig. 1 that the relative crystallinity decreased from 24.63% to 21.75% after ultrasonic treatment for 5 min, which showed that ultrasonic treatment destroyed the structure. After ultrasonic treatment for 15 min, the relative crystallinity increased to 23.12%, which indicated that the internal structure of starch granules became orderly. It was obvious that ultrasonic treatment interrupted the starch molecular chains, which resulted in the rearrangement of starch molecules and promoted the crystallization of sub-microcrystals (Tian et al., 2014, Zhang et al., 2018). Zheng et al. (2013) found the structure of starch granules was seriously damaged with the prolongation of treatment time. Jambrak et al. (2010) reported that ultrasonic treatment increased the reactivity of water molecules, which enhanced the diffusion of water molecules into starch granules, especially into the amorphous regions. That was to say, the destruction of starch granules and the diffusion of water molecules led to the fracture of crystalline structure. Thus the relative crystallinity decreased significantly after ultrasonic treatments for 30–60 min.
3.1.2. Effect of ultrasound on morphology of native corn starch
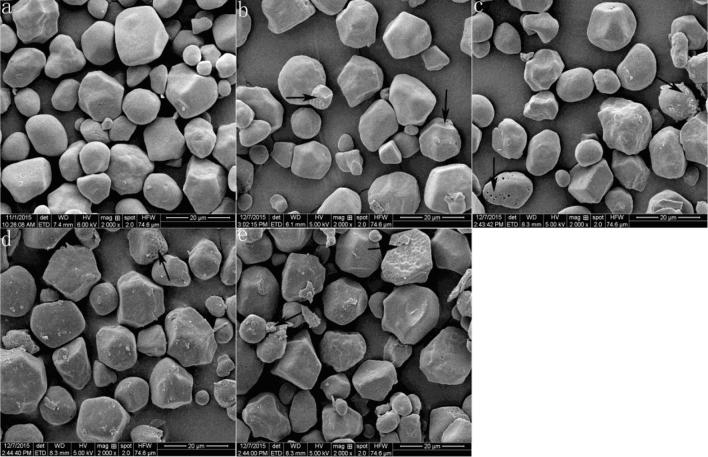
SEM images displayed that the shape of native corn starch granules was irregular, the surface was smooth and there was no fragments (Fig. 2a). The surface of some starch granules became rough and small fragments flaked off after 5 min of ultrasonic treatment. After ultrasonic treatment for 15 min, some obvious bulges and holes were formed on the surface of starch granules (Fig. 2c). Starch granules were severely deformed after 30–60 min of ultrasonic treatments. The reason was that the ultrasonic energy was trapped by the dispersed granules, which produced high-frequency vibrations and eventually destroyed starch granules (Monroy, Rivero, & García, 2018).
Fig. 2.
SEM of native corn starch treated at different ultrasonic time.
3.1.3. Effect of ultrasound on amylose content of native corn starch
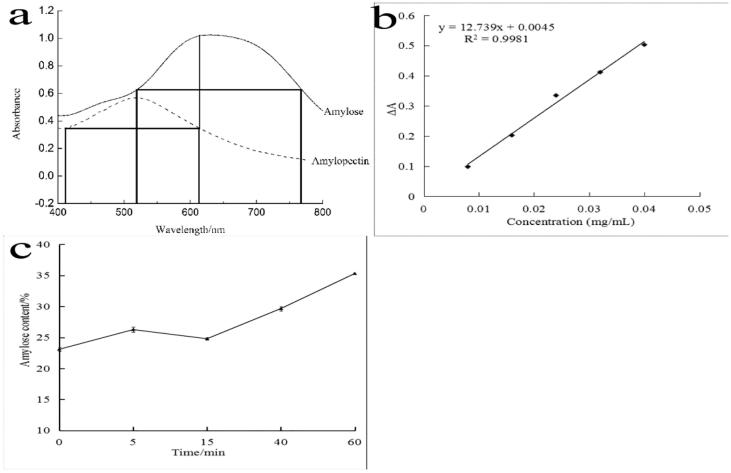
According to the method of isoabsorptive points, the regression equation was y = 12.739x + 0.0045, and the correlation coefficient of the regression equation was r = 0.9981 (Fig. 3b), which showed that there was a good linear relationship between amylose concentration and absorbance.
Fig. 3.
Standard curve of amylose and the amylose content of native corn starch treated at different ultrasonic time.
Fig. 3c showed that the treatment of different ultrasonic time had different effect on the amylose content of samples. The amylose content increased from 23.09% to 26.26% after 5 min of ultrasonic treatment. While the amylose content decreased after 15 min of ultrasonic treatment, which indicated that ultrasound promoted the crystallization of starch granules to a certain extent. Long-term ultrasonic treatment produced intense mechanical force on starch granules, which broke the C—C bond and degraded amylopectin to varying degrees (Jambrak et al., 2010). Therefore, the amylose content increased significantly after 30–60 min of ultrasonic treatment.
3.1.4. Effect of ultrasound on thermal properties of native corn starch
The gelatinization temperature (To, Tp and Tc) reflects the crystalline structure and the degree of crystallization of starch granules. The gelatinization enthalpy (ΔH) represents the energy required for the separation of double helix structure during gelatinization (Piecyk, Drużyńska, Worobiej, Wołosiak, & Ostrowska-Ligęza, 2013).
Table 1 showed that the gelatinization temperature and ΔH were lower than that of native corn starch after 5 min of ultrasonic treatment, which showed that the degree of crystallization of starch granules decreased and the gelatinization of starch granules required less energy. After ultrasonic treatment for 15 min, the gelatinization temperature and ΔH increased. This was due to the starch granules after treatment had relatively perfect and homogeneous structures in this stage, therefore starch granules needed more energy (high temperature) to break intermolecular bonds in starch molecules. This phenomenon was similar to the previous study (LeCorre, Bras, & Dufresne, 2012). After 30–60 min of ultrasonic treatments, starch granules were severely deformed and the intensity degree of crystalline structure decreased, thus gelatinization temperature dropped. Besides, some of the double helices presented in the crystalline and non-crystalline regions of starch granules were disrupted, so the energy required for gelatinization was decreased. The result was in consistent with the previous report by Jambrak et al. (2010).
Table 1.
Thermal properties of native corn starch treated at different ultrasonic time.
| Time (min) | To (°C) | Tp (°C) | Tc (°C) | ΔΗ (J/g) |
|---|---|---|---|---|
| 0 | 66.21 ± 0.21a | 72.25 ± 0.14a | 78.33 ± 0.15a | 12.17 ± 0.20a |
| 5 | 65.76 ± 0.13b | 71.52 ± 0.20b | 77.51 ± 0.23b | 10.78 ± 0.22c |
| 15 | 65.99 ± 0.22ab | 72.07 ± 0.09a | 77.89 ± 0.17b | 11.22 ± 0.13b |
| 30 | 64.43 ± 0.18c | 70.96 ± 0.11c | 76.18 ± 0.26c | 10.07 ± 0.30d |
| 60 | 63.06 ± 0.27d | 69.87 ± 0.14d | 75.07 ± 0.25d | 9.48 ± 0.24e |
To, onset temperature; Tp, peak temperature; Tc, conclusion temperature; ΔH, gelatinization enthalpy.
There was significant difference between different letters in the same column (P < 0.05).
3.1.5. Effect of ultrasound on thermal stability of native corn starch
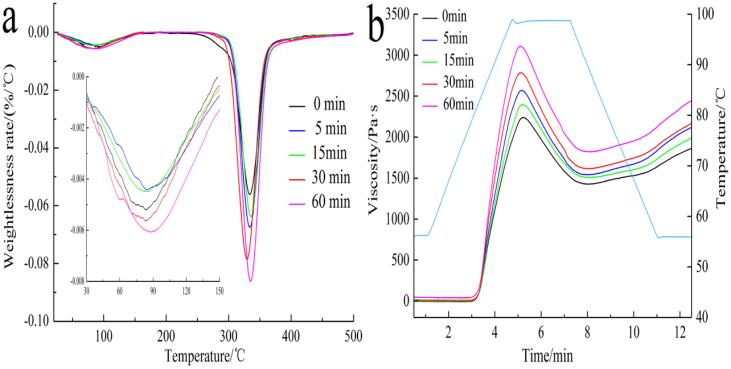
Fig. 4a showed that the thermal decomposition of corn starch granules had two stages. In the first stage (T ≤ 150 °C), the mass loss was mainly caused by the evaporation of water in starch granules. In the second stage (250 ≤ T ≤ 400 °C), the mass loss was mainly related to pyrolysis and dissipation of carbohydrate organic compounds (Zhang et al., 2018).
Fig. 4.
DTG and RVA of native corn starch treated at different ultrasonic time.
As could be seen from Fig. 4a, the thermal stability reduced and the decomposition rate increased after ultrasonic treatment for 5 min. While the thermal decomposition rate decreased to the lowest in the two stages after 15 min of ultrasonic treatment, which was ascribed to the increase of crystallinity and thermal stability. A similar phenomenon was also reported that compact structure led to the restriction of movement of the molecular chains and increased the resistance to decomposition (Xie, Zhang, Li, & Chen, 2019). When ultrasonic treatment time was up to 30–60 min, the crystallinity decreased (Fig. 1) and starch granules were destroyed seriously (Fig. 2), so the decomposition rates increased significantly at these stages.
3.1.6. Effect of ultrasound on pasting properties of native corn starch
When starch suspension was heated to a certain temperature, starch granules would expand and the hydrogen bonding force between starch granules would be destroyed. Thus starch granules would swell and absorb water during gelatinization, and starch suspension could become viscous.
The high temperature and high pressure environment caused by the mechanical force and cavitation effect of ultrasonic treatment for a long time resulted in the breakage of amylopectin and the degradation of molecular weight (Jambrak et al., 2010). Thus, the structure of starch granules became loose and the amylose content increased significantly, which was beneficial to the contact and movement of starch molecules (Hu et al., 2015). So the viscosity properties increased. In addition, the viscosity properties decreased after 15 min of ultrasonic treatment (Fig. 4b). This was due to the increase of crystallinity at this stage, and the order of internal molecular chains enhanced. Pamela et al. (2017) also found that higher crystallinity of starch granules reduced gelatinization viscosity.
3.1.7. Mechanochemical effect of ultrasound on native corn starch
Ultrasonic cavitation could exclude air in starch granules (Miano, Ibarz, & Augusto, 2017), which made water enter starch granules through the pores easily. In addition, ultrasonic treatment could produce strong mechanical forces on the interior of starch granules and make starch molecules hydrate. The similar phenomenon was also reported in precious studies (Boufi et al., 2018, HerCeg et al., 2010, Hu et al., 2015). After ultrasonic treatment for 5 min, a small amount of water entered the starch granules. In a short period of time, ultrasonic mechanical force destroyed amorphous regions and a part of crystalline regions, which made the structure loosen and disorderly arranged the destroyed molecules, and starch granules required less energy for gelatinization. After ultrasonic treatment for 15 min, some irregular starch molecules in crystalline regions were hydrated, and the amplitude of free vibration increased. After a long period of vibration, these molecules rearranged to form a more stable and ordered crystalline structure (Bernardo, Ascheri, Chávez, & Carvalho, 2018). Therefore, the crystallinity increased, the thermal decomposition rate decreased and the organization of double helix structure became better. In addition, the internal molecules of starch granules were rearranged and aggregated at this stage, so some bulges formed on the surfaces. After ultrasonic treatments for 30–60 min, strong mechanical force of ultrasonic cavitation seriously destroyed the morphology and crystalline structure of starch granules (Pamela et al., 2017). Thus, the crystallinity and the energy required for gelatinization decreased, and the thermal decomposition rates increased.
According to the theory of mechanochemistry, 5 min of ultrasonic treatment corresponded to the stress stage of starch granules, 15 min and 30–60 min of ultrasonic treatments corresponded to the aggregation stage and the agglomeration stage of starch granules, respectively. From the previous analysis and discussion, it could draw a conclusion that ultrasound produced significant mechanochemical effect on starch granules.
3.2. Effect of ultrasound on the quality of OSA-modified starch with low DS and its influence mechanism
3.2.1. Effect of ultrasound on DS, RE, S and SP of OSA-modified starch
The DS and RE reflected the chemical activity of starch (Tian, Zhao, Huang, & Tong, 2009). As shown in Table 2, although the DS of OSA-modified starch was 0.0134 at 0 min of ultrasound, the S and SP rose slightly compared with native corn starch. Compared with the OSA-modified starch prepared without ultrasonic treatment, the DS, RE, S and SP of OSA-modified starch markedly rose after ultrasonic treatment. These results showed that ultrasonic treatment significantly improved the chemical activity and quality characteristics of OSA-modified starch. However, OSA-modified starch showed poor quality and performance after 15 min of ultrasonic treatment.
Table 2.
Chemical activity and quality of OSA-modified starch prepared at different ultrasonic time.
| Time (min) | DS | RE(%) | S (%) | SP (g/g) | Enzyme resistant starch (%) |
|---|---|---|---|---|---|
| 0 | 0.0134 ± 0.0006d | 50.01 ± 0.09d | 12.75 ± 0.17d (11.38 ± 0.09de) | 10.64 ± 0.06e (9.82 ± 0.07d) | 20.65 ± 0.10e |
| 5 | 0.0146 ± 0.0008c | 54.50 ± 0.10c | 18.53 ± 0.09b (14.98 ± 0.10c) | 16.78 ± 0.09c (12.56 ± 0.04c) | 27.67 ± 0.05c |
| 15 | 0.0138 ± 0.0003d | 51.51 ± 0.07d | 15.64 ± 0.13c (12.55 ± 0.08d) | 14.27 ± 0.05d (11.58 ± 0.10 cd) | 25.98 ± 0.09d |
| 30 | 0.0159 ± 0.0005b | 59.35 ± 0.08b | 19.76 ± 0.16b (16.72 ± 0.12b) | 20.61 ± 0.11b (16.53 ± 0.03b) | 34.46 ± 0.03b |
| 60 | 0.0175 ± 0.0003a | 65.32 ± 0.04a | 22.46 ± 0.07a (19.44 ± 0.14a) | 28.69 ± 0.12a (22.79 ± 0.07a) | 42.93 ± 0.08e |
The values in parentheses are the S and SP of naive corn starch prepared at the same ultrasonic treatment time in absence of an esterification reaction.
There was significant difference between different letters in the same column (P < 0.05).
3.2.2. Effect of ultrasound on enzymatic resistance of OSA-modified starch
Enzyme resistant starch was a kind of starch derivative which could not be hydrolyzed by amylase in human body. Wolf et al. (2001) found that when people took OSA-modified starch, blood glucose concentration decreased significantly. This phenomenon indicated that OSA-modified starch contained a certain amount of resistant starch, which could promote intestinal digestion and effectively prevent the rise of blood sugar. Heacock, Hertzler, and Wolf (2004) also found that OSA-modified starch could promote digestion and absorption and reduce the risk of diabetes. As a pretreatment for the preparation of resistant starch, ultrasonic treatment could accelerate the enzymatic hydrolysis. In this experiment, the enzymatic resistance was only regarded as one of the properties of OSA-modified starch. The effect of different ultrasonic treatment time on the enzymatic resistance of OSA-modified starch was studied.
The resistance of starch to enzymatic hydrolysis was mainly related to the change of its crystalline regions. The enzymatic hydrolysis mainly occured in amorphous regions, less crystalline regions and loosely structured regions. It could be seen from Table 2 that the content of enzyme resistant starch increased after a certain time of ultrasonic treatment, which was due to the destruction of amorphous regions and less crystalline regions of OSA-modified starch. The content of enzyme resistant starch increased and the ability of enzymatic resistance decreased after 15 min of ultrasonic treatment. This might be due to the increase of crystallinity and the decrease of amylose content in this stage. After a long time of ultrasonic treatment, starch structure was seriously damaged, starch molecules were degraded and amylose content was significantly increased. Generally speaking, amylose was easier to aging than amylopectin, so the content of resistant starch increased. Therefore, the content of enzyme resistant starch were significantly improved after ultrasonic treatment for 30–60 min.
3.2.3. Effect of ultrasound on freeze–thaw stability of OSA-modified starch
Supplementary Fig. S1 showed that the more freeze–thaw times, the higher the water separating proportion. The water separating proportion reduced after a short period of ultrasonic treatment, which indicated that the freeze–thaw stability of OSA-modified starch was improved. This was due to the degradation of starch molecules and the increase of amylose content after ultrasonic treatment for a certain period of time. These starch molecules interacted with each other and formed stable structures (Lee, Kumar, Rozman, & Bmn, 2005). However, after a long time of ultrasonic treatment, a large number of starch molecules were degraded and amylose molecular chains became shorter, so water was easy to precipitate. On the whole, the freeze–thaw stability could be improved by appropriate ultrasonic treatment according to different requirements.
3.2.4. Influence mechanism of ultrasound on the quality of OSA-modified starch with low DS
OSA is an oily substance, so it is difficult to contact with starch suspension well. The reaction site is usually limited to the surface of starch granules (Shogren, Viswanathan, Felker, & Gross, 2000). In addition, starch is a kind of polycrystalline polymer with thick shell and compact crystalline structure. Under normal conditions, water and reagents are difficult to enter the starch granules, which results in the uneven distribution of OS groups in starch granules and the low chemical reaction efficiency (Segura-Campos, Chel-Guerrero, & Betancur-Ancona, 2008).
Ultrasound could produce significant effect on starch granules. On the one hand, ultrasonic cavitation eliminated the air inside starch granules, facilitated the entry of water molecules and reagents into the internal channels, which improved the reaction efficiency (Chan et al., 2010, Chen et al., 2014). On the other hand, ultrasound produced remarkable mechanochemical effect on starch granules, which promoted the production of free radicals, increased the free energy of starch molecules, and caused the increase and movement of crystalline dislocations (Jambrak et al., 2010). The increase of mechanochemical active sites in starch granules were conducive to the homogeneous reaction of OSA and starch molecules. This was consistent with the previous research by Chen et al. (2014).
When ultrasound acted on OSA, the high temperature and high pressure environment generated by ultrasonic cavitation could break and disperse OSA droplets floating on the suspension, which was beneficial to contact with starch granules (Chen et al., 2014, Montalbo-Lomboy et al., 2010). Additionally, ultrasonic treatment made OSA droplets smaller and further increased the solubility of droplets in starch suspension. The smaller droplets were easier to enter the starch granules (Kentish et al., 2008), so the reaction was not limited to the surface of starch granules, which improved the DS and RE. In general, the quality of OSA-modified starch prepared by ultrasonic-assisted treatment was improved.
When starch granules were in the aggregation stage, due to the aggregation and rearrangement of starch molecules, the structure of starch granules was tight. Thus water was difficult to enter the interior of starch granules, and the amyloses could hardly be dissolved. So the quality of OSA-modified starch decreased. Meanwhile, because of the aggregation effect, some substituent groups of starch chains filled in starch granules could hardly be determined, which might lead to the lower determination results than the actual values (Choi, Kim, Park, Kim, & Baik, 2009). According to the mechanochemical theory, some OSA entered the granules and were incapable of being determined due to the agglomeration effect of starch granules. Thus when starch granules were in the agglomeration stage, the determination results were also lower than the actual values. Nevertheless, the structure of starch granules was loose at this stage, water molecules were easy to enter the interior of granules, which dissolved more starch molecules, so the quality improved.
4. Conclusions
With the prolongation of ultrasonic treatment time, the structure and properties of corn starch changed constantly. According to these changes, it was concluded that ultrasound produced significant mechanochemical effect on starch granules, and starch granules went through the stress stage, aggregation stage and agglomeration stage in turn. The quality of OSA-modified starch prepared by ultrasonic-assisted treatment was significantly improved in the stress stage and agglomeration stage. This study could provide an effective method for the research of high quality modified starch, and lay a theoretical foundation for expanding the application of ultrasound in various fields.
CRediT authorship contribution statement
Yujie Zhang: Conceptualization, Methodology, Writing - original draft. Yangyong Dai: Validation, Writing - review & editing, Formal analysis. Hanxue Hou: Validation, Resources. Xiangyang Li: Visualization, Formal analysis. Haizhou Dong: Visualization, Investigation. Wentao Wang: Validation, Visualization. Hui Zhang: Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 31471619); and the Funds of Shandong “Double Tops” Program of China (grant number SYL2017XTTD01).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2020.100077.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abral H., Anugrah A.S., Hafizulhaq F., Handayani D., Sugiarti E., Muslimin A.N. Effect of nanofibers fraction on properties of the starch based biocomposite prepared in various ultrasonic powers. International Journal of Biological Macromolecules. 2018;116:1214–1221. doi: 10.1016/j.ijbiomac.2018.05.067. [DOI] [PubMed] [Google Scholar]
- Altuna L., Herrera M.L., Foresti M.L. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocolloids. 2018;80:97–110. [Google Scholar]
- Baranauskienė R., Rutkaitė R., Peciulyte L., Kazernavičiūtė R., Venskutonis P.R. Preparation and characterization of single and dual propylene oxide and octenyl succinic anhydride modified starch carriers for the microencapsulation of essential oils. Food and Function. 2016;7(8):3555–3565. doi: 10.1039/c6fo00775a. [DOI] [PubMed] [Google Scholar]
- Bernardo C.O., Ascheri J.L.R., Chávez D.W.H., Carvalho C.W.P. Ultrasound assisted extraction of yam (Dioscorea bulbífera) starch: Effect on morphology and functional properties. Starch-Stärke. 2018;70(5–6):1700185. [Google Scholar]
- Bettaieb N.B., Jerbi M.T., Ghorbel D. Gamma radiation influences pasting, thermal and structural properties of corn starch. Radiation Physics and Chemistry. 2014;103:1–8. [Google Scholar]
- Boufi S., Bel S.H., Magnin A., Pignon F., Impéror-Clerc M., Mortha G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrasonics Sonochemistry. 2018;41:327–336. doi: 10.1016/j.ultsonch.2017.09.033. [DOI] [PubMed] [Google Scholar]
- Chan H.T., Bhat R., Karim A.A. Effects of sodium dodecyl sulphate and sonication treatment on physicochemical properties of starch. Food Chemistry. 2010;120(3):703–709. [Google Scholar]
- Chen H.M., Huang Q., Fu X., Luo F.X. Ultrasonic effect on the octenyl succinate starch synthesis and substitution patterns in starch granules. Food Hydrocolloids. 2014;35:636–643. [Google Scholar]
- Cheuk S.Y., Shih F.F., Champagne E.T., Daigle K.W., Patindol J.A., Mattison C.P., Boue S.M. Nano-encapsulation of coenzyme q10 using octenyl succinic anhydride modified starch. Food Chemistry. 2015;174:585–590. doi: 10.1016/j.foodchem.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Choi H.S., Kim H.S., Park C.S., Kim B.Y., Baik M.Y. Ultra high pressure (UHP)-assisted acetylation of corn starch. Carbohydrate Polymers. 2009;78:862–868. [Google Scholar]
- Heacock P.M., Hertzler S.R., Wolf B. The glycemic, insulinemic, and breath hydrogen responses in humans to a food starch esterified by 1-octenyl succinic anhydride. Nutrition Research. 2004;24(8):581–592. [Google Scholar]
- HerCeg I.L., Jambrak A.R., ŠubArIć D., Brnčić M., Brnčić S.R., Badanjak M.…Herceg Z. Texture and pasting properties of ultrasonically treated corn starch. Czech Journal of Food Sciences. 2010;28(2):83–93. [Google Scholar]
- Hu A., Jiao S., Zheng J., Li L., Fan Y., Chen L., Zhang Z. Ultrasonic frequency effect on corn starch and its cavitation. LWT – Food Science and Technology. 2015;60(2):941–947. [Google Scholar]
- Jambrak A.R., Herceg Z., Šubarić D., Babić J., Brnčić M., Brnčić S.R.…Gelo J. Ultrasound effect on physical properties of corn starch. Carbohydrate Polymers. 2010;79(1):91–100. [Google Scholar]
- Jialiexi M., Jing W.W., Xuelaiti Z. Determination of amylose and amylopectin in grain and bean by dual-wavelength spectrophotometry. Xinjiang Agricultural Sciences. 2010;47(3):564–568. [Google Scholar]
- Juhász A.Z., Opoczky L. 1th ed. Halsted; New York: 1990. Mechanical activation of minerals by grinding pulverizing and morphology of particles. [Google Scholar]
- Jyothi A.N., Rajasekharan K.N., Moorthy S.N., Sreekumar J. Synthesis and characterization of low DS succinate derivatives of cassava (manihot esculenta crantz) starch. Starch-Stärke. 2010;57(7):319–324. [Google Scholar]
- Kentish S., Wooster T.J., Ashokkumar M., Balachandran S., Mawson R., Simons L. The use of ultrasonics for nanoemulsion preparation. Innovative Food Science and Emerging Technologies. 2008;9(2):170–175. [Google Scholar]
- LeCorre D., Bras J., Dufresne A. Influence of native starch's properties on starch nanocrystals thermal properties. Carbohydrate Polymers. 2012;87(1):658–666. doi: 10.1016/j.carbpol.2011.08.042. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Kumar R.N., Rozman H.D., Bmn A. Pasting, swelling and solubility properties of uv initiated starch-graft-poly(aa) Food Chemistry. 2005;91(2):203–211. [Google Scholar]
- Miano A.C., Ibarz A., Augusto P.E.D. Ultrasound technology enhances the hydration of corn kernels without affecting their starch properties. Journal of Food Engineering. 2017;197:34–43. [Google Scholar]
- Monroy Y., Rivero S., García M.A. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrasonics Sonochemistry. 2018;42:795–804. doi: 10.1016/j.ultsonch.2017.12.048. [DOI] [PubMed] [Google Scholar]
- Montalbo-Lomboy M., Khanal S.K., Leeuwen J.H.V., Raman D.R., Grewell D. Ultrasonic pretreatment of corn slurry for saccharification: A comparison of batch and continuous systems. Ultrasonics Sonochemistry. 2010;17(5):939–946. doi: 10.1016/j.ultsonch.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Nada M.H., Gillan E.G., Larsen S.C. Mechanochemical reaction pathways in solvent-free synthesis of ZSM-5. Microporous and Mesoporous Materials. 2019;276:23–28. [Google Scholar]
- Nguyen T.T., Asakura Y., Koda S., Yasuda K. Dependence of cavitation, chemical effect, and mechanical effect thresholds on ultrasonic frequency. Ultrasonics Sonochemistry. 2017;39:301–306. doi: 10.1016/j.ultsonch.2017.04.037. [DOI] [PubMed] [Google Scholar]
- Pamela C.F.S., César A.R.C., Gerardo C.E., Eduardo J.V.C., Luis A.B.P., Jose A.R. In vitro digestibility of ultrasound-treated corn starch. Starch-Stärke. 2017;69:9–10. [Google Scholar]
- Piecyk M., Drużyńska B., Worobiej E., Wołosiak R., Ostrowska-Ligęza E. Effect of hydrothermal treatment of runner bean (Phaseolus coccineus) seeds and starch isolation on starch digestibility. Food Research International. 2013;50(1):428–437. [Google Scholar]
- Pongsawatmanit R., Srijunthongsiri S. Influence of xanthan gum on rheological properties and freeze-thaw stability of tapioca starch. Journal of Food Engineering. 2008;88(1):137–143. [Google Scholar]
- Raguzzoni J.C., Delgadillo I., da Silva J.A.L. Influence of a cationic polysaccharide on starch functionality. Carbohydrate Polymers. 2016;150:369–377. doi: 10.1016/j.carbpol.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Saranu S., Selve S., Kaiser U., Han L., Wiedwald U., Ziemann P., Herr U. Effect of large mechanical stress on the magnetic properties of embedded Fe nanoparticles. Beilstein Journal of Nanotechnology. 2011;2:268–275. doi: 10.3762/bjnano.2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Campos M., Chel-Guerrero L., Betancur-Ancona D. Synthesis and partial characterization of octenylsuccinic starch from Phaseolus lunatus. Food Hydrocolloids. 2008;22(8):1467–1474. [Google Scholar]
- Shahbazi M., Majzoobi M., Farahnaky A. Impact of shear force on functional properties of native starch and resulting gel and film. Journal of Food Engineering. 2018;223:10–21. [Google Scholar]
- Shogren L., Viswanathan A., Felker F., Gross R.A. Distribution of octenyl succinate groups in octenyl succinic anhydride modified waxy maize starch. Starch-Stärke. 2000;52:196–204. [Google Scholar]
- Takuma S., Ryuji I., Masaki N. Noninvasive mechanochemical imaging in unconstrained caenorhabditis elegans. Materials. 2018;11(6):1034. doi: 10.3390/ma11061034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Li D., Zhao J., Xu X., Jin Z. Effect of high hydrostatic pressure (HHP) on slowly digestible properties of rice starches. Food Chemistry. 2014;152:225–229. doi: 10.1016/j.foodchem.2013.11.162. [DOI] [PubMed] [Google Scholar]
- Tian B.H., Zhao Y.L., Huang Z.Q., Tong Z.F. Synthetic technics of starch phosphate with mechanically activated cassava starch. Journal of Chemical Engineering of Chinese Universities. 2009;23:491–495. [Google Scholar]
- Tong C., Chen Y., Tang F., Xu F., Huang Y., Chen H., Bao J. Genetic diversity of amylose content and RVA pasting parameters in 20 rice accessions grown in Hainan, China. Food Chemistry. 2014;161:239–245. doi: 10.1016/j.foodchem.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Wolf B.W., Wolever T.M., Bolognesi C., Zinker B.A., Garleb K.A., Firkins J.L. Glycemic response to a food starch esterified by 1-octenyl succinic anhydride in humans. Journal of Agricultural and Food Chemistry. 2001;49(5):2674–2678. doi: 10.1021/jf0015017. [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhang B., Li M.N., Chen H.Q. Effects of cross-linking with sodium trimetaphosphate on structural and adsorptive properties of porous wheat starches. Food Chemistry. 2019;289:187–194. doi: 10.1016/j.foodchem.2019.03.023. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y., Miwa T. Effect of ultrasonic wave irradiation sequence in microhollow production produced by bubble cavitation. Japanese Journal of Applied Physics. 2011;50(7S):07HF01. [Google Scholar]
- Zhang K., Dai Y., Hou H., Li X., Dong H., Wang W., Zhang H. Preparation of high quality starch acetate under grinding and its influence mechanism. International Journal of Biological Macromolecules. 2018;120(Part B):2026–2034. doi: 10.1016/j.ijbiomac.2018.09.196. [DOI] [PubMed] [Google Scholar]
- Zheng J., Li Q., Hu A., Yang L., Lu J., Zhang X., Lin Q. Dual-frequency ultrasound effect on structure and properties of sweet potato starch. Starch-Stärke. 2013;65(7–8):621–627. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.