Abstract
In this data we report on conductors polymers nanocomposites synthesized by in situ polymerization of aniline (ANI) and/or 4-aminobenzylamine (4-ABA) in presence of chromium montmorillonite (MMT-Cr+3) and ammonium persulfate as oxidizing agent. Homopolymers and copolymers (PANI-co-4-ABA) were prepared at various initial monomer composition and were characterized by Fourier transform Infrared (FT-IR) and UV–vis spectroscopy, X-ray diffraction (XRD) and cyclic voltammeter. The data describes the behavior of the corresponding homopolymers Poly (4-ABA) and (PANI) and showed that the in-situ polymerization produced real nanocomposites containing aniline and 4-aminobenzylamine units and films of products exhibit good electrochemical properties.
Keywords: Polyaniline, Nanocomposites, In situ polymerization, Poly (4-aminobenzylamine), Montmorillonite clay
Specifications Table
| Subject | Polymer chemistry |
| Specific subject area | Synthesis and characterization of nanocomposites catalyzed by maghnite-H+(Algerian MMT) via in situ polymerization |
| Type of data | Table, Image and Figure |
| How data were acquired | SEM, NMR, FTIR, XRD,TGA,UV, Cyclic Voltammogram |
| Data format | Raw and analyzed |
| Experimental factors | Synthesis and characterization of new nanocomposites under effect of heterogeneous catalyst called maghnite-H+ (Algerian MMT) exchanged with chromium (III) via in situ polymerization. The obtained nanocomposite was characterized and discussed by several methods such as (XRD, FTIR, Electrical and electrochemical conductivity, SEM, HNMR). |
| Experimental features | Maghnite (Algerian MMT) was used as heterogeneous catalyst for synthesis of organic and polymeric materials. |
| Data source location | Republic algerian democratic and popular |
| Data accessibility | Data are supplied with this article |
| Related research article | A. Rahmouni and M. Belbachir. Molecular structure of PANI and its homologue PANI–PEO2000 catalyzed by Maghnite-H+ (Algerian MMT): Synthesis, characterization and physical and chemical properties. Polymer Bulletin (2019) 76:4677–4701.https://doi.org/10.1007/s00289-018-2620-7. |
Value of the Data
|
1. Data
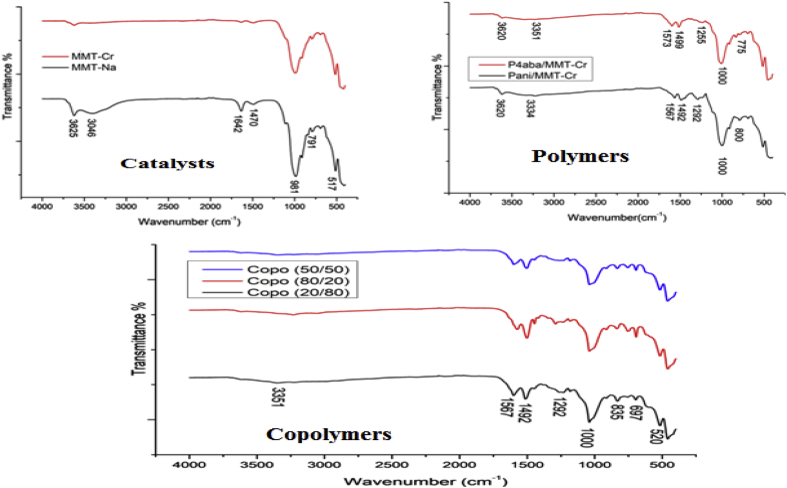
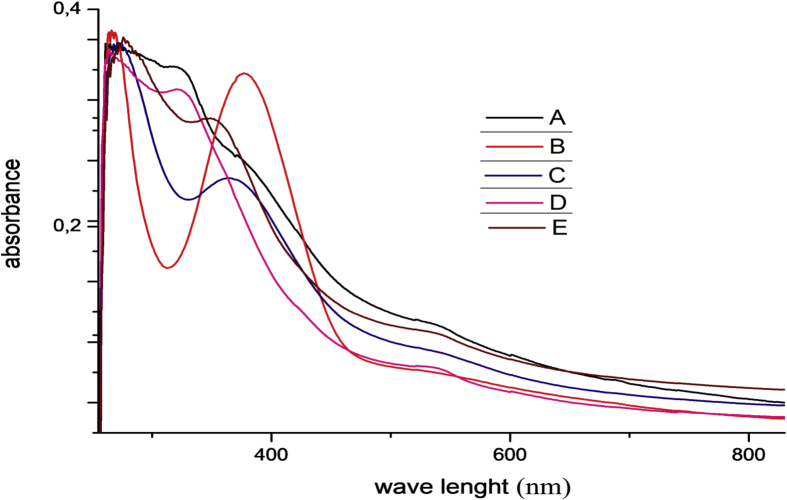
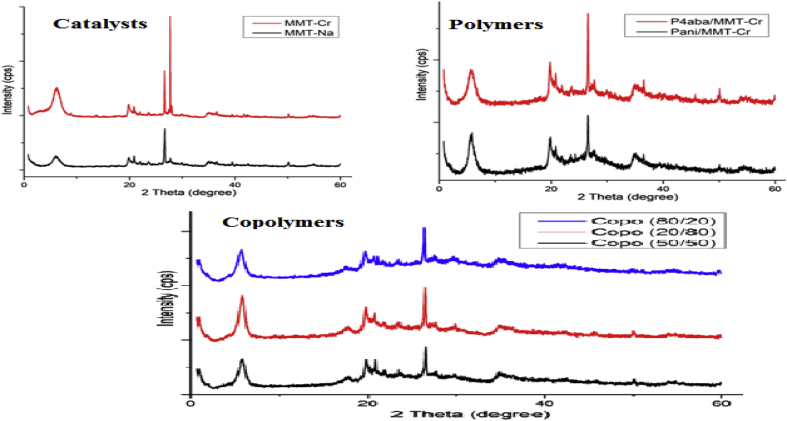
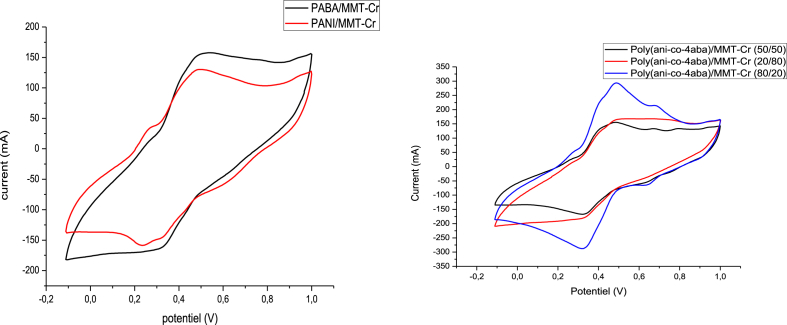
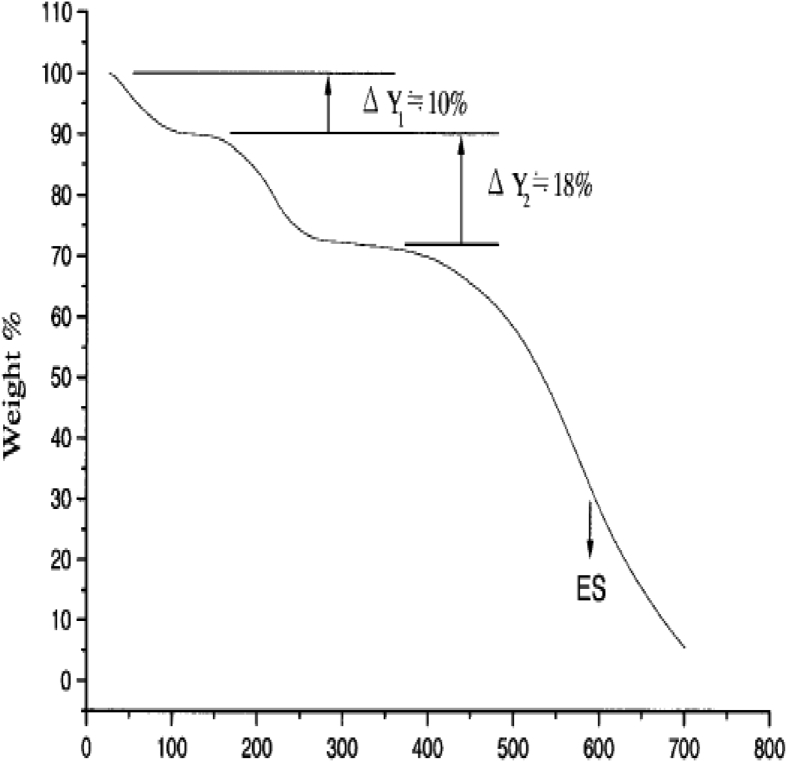
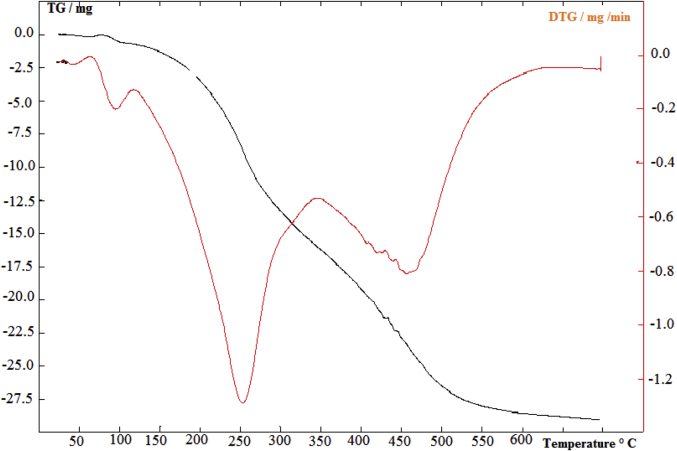
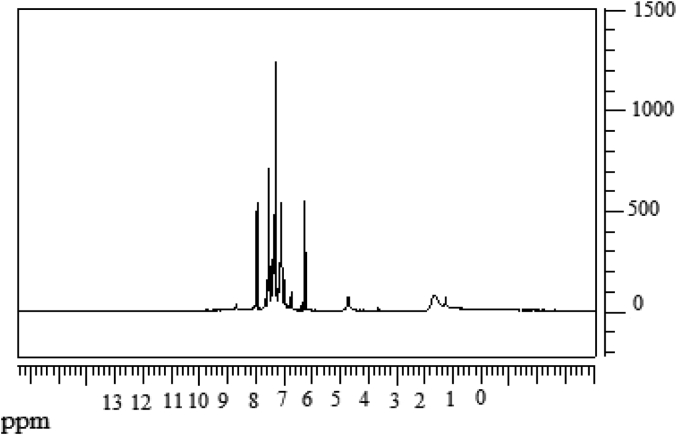
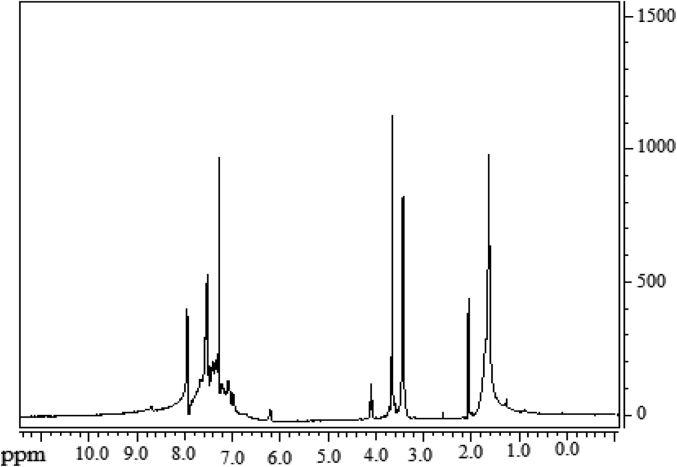
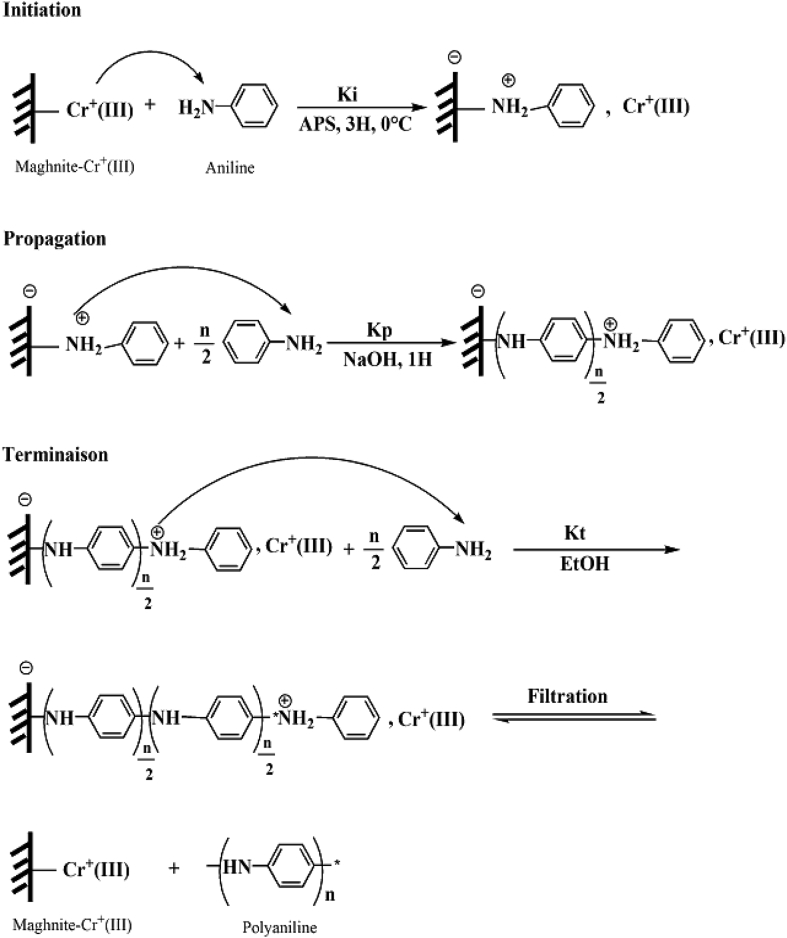
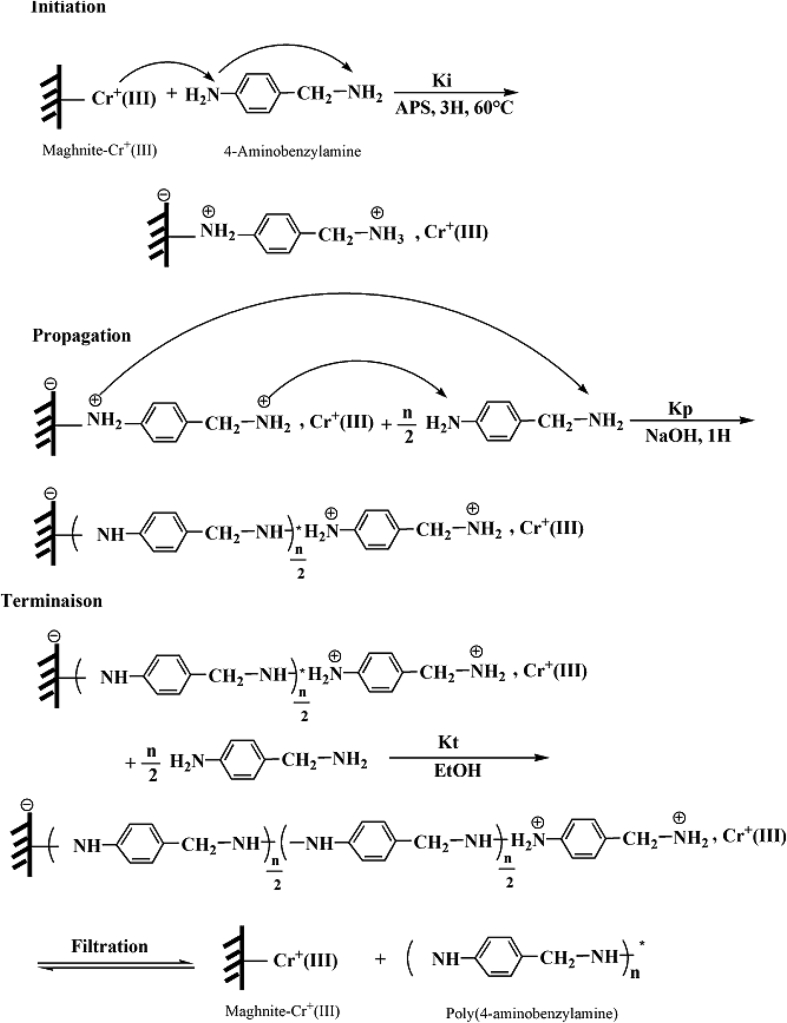
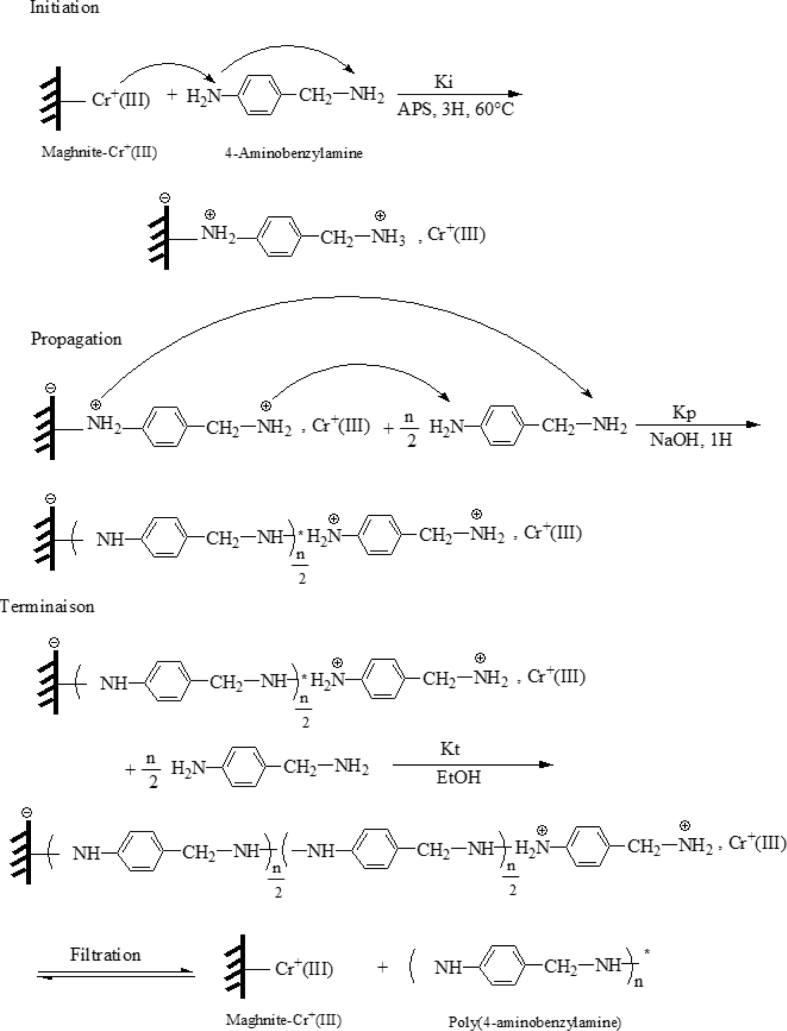
The data described in this paper provides formation of nanocomposite structures used in electronic domain catalyzed by maghnite-Cr3+. PANI/MMT-Cr3+, Poly (4-ABA/MMT-Cr3+) and Poly (ANI-co-4-ABA)/MMT-Cr3+ nanocomposites were successfully synthesized under effect of modified clay layered called maghnite-Cr3+ (Algerian MMT-Cr3+) by in situ polymerization route in the presence of oxidizing agent. The formation of polymers and copolymers was confirmed by FTIR, XRD, 1HNMR, ATG, electrical conductivity and Uv–Visible measurements [1,2]. Table 1 describes elementary compositions wt. % of chromium (Cr3+) and sodium (Na+) exchanged sample raw-maghnite (Algerian MMT). Table 2 describes Peak maximum and d-spacing of protonated and the nanocomposites intercalated into sodium montmorillonite. Fig. 1 FT IR-spectra of the MMT-Na, MMT-Cr and the nanocomposites poly (4-ABA/MMT-Cr), PANI/MMT-Cr and their copolymers Poly (4-ABA-co-ANI/MMT-Cr3+). Fig. 2 describes UV–Vis spectra of the homo and copolymers nanocomposites doped with MMT-Cr3+, A: Poly (4-ABA/MMT-Cr), B: Poly (ANI/MMT-Cr), C: Poly (4-ABA-co-ANI)/MMT-Cr3+: 80/20, D: Poly (4-ABA-co-ANI)/MMT-Cr3+: 20/80, E: Poly (4ABA-co-ANI)/MMT-Cr3+: 50/50). Fig. 3 describes X-ray diffraction patterns of two montmorillonite (MMT-Na and MMT-Cr), and the nanocomposites (PANI/MMT-Cr, Poly (ani-co-4aba)/MMT-Cr, P4ABA/MMT-Cr). Fig. 4 describes cyclic voltammogram recorded of polymer and copolymer films formed in 1.0 M HClO4 on graphite carbon electrode. Fig. 5 describes TGA curves of PANI prepared in the presence of Maghnite-H+ (0.25 M). Fig. 6 describes TGA curves of Poly (4-ABA-co-ANI/MMT-Cr) synthesized in the presence of Maghnite-H+ (0.25 M). Fig. 7 describes 1H-NMR spectra of (PANI) obtained by the intercaled method between Aniline and Maghnite-Cr3+ (black powder). Fig. 8 describes 1H-NMR spectra of the block copolymer poly (aniline)-b-poly (4-aminobenzylamine) catalyzed by Maghnite-Cr3+ by in situ polymerization. Fig. 9 describe proposed mechanism of homopolymer (PANI) catalyzed by Maghnite-H+ by in situ polymerization. Fig. 10 describe proposed mechanism of homopolymer poly (4-aminobenzylamine) catalyzed by Maghnite-H+ by in situ polymerization. Fig. 11 describe proposed mechanism block copolymer poly (aniline)-b-poly (4-aminobenzylamine).
Table 1.
Elementary compositions wt. % of chromium (Cr3+) and sodium (Na+) exchanged sample raw-maghnite (Algerian MMT).
| Compositions wt.% | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | MgO | K2O | TiO2 | Cr2O3 | Pert in fire |
|---|---|---|---|---|---|---|---|---|---|---|
| Raw-MMT | 69.39 | 14.67 | 1.16 | 0.30 | 0.50 | 1.07 | 0.79 | 0.16 | 0.00 | 11.96 |
| MMT-Na | 70.75 | 14.46 | 1.05 | 0.19 | 2.61 | 1.01 | 0.78 | 0.14 | 0.00 | 09.01 |
| MMT-Cr | 71.01 | 14.06 | 1.00 | 0.14 | 0.15 | 0.98 | 0.71 | 0.15 | 2.61 | 09.35 |
Table 2.
Peak maximum and d-spacing of protonated and the nanocomposites intercalated into sodium montmorillonite.
| Samples | Peak maximum |
Basal spacing |
Interlayer spacing |
|---|---|---|---|
| 2ϴ max (deg) | d(001) (A°) | Δd (A°) | |
| MMT-Na | 6.96 | 12.94 | – |
| MMT-Cr | 6.06 | 14.67 | 1.73 |
| P(4aba_co-ani)/MMT-Cr (20/80) | 5.65 | 15.63 | 2.69 |
| P(4aba_co-ani)/MMT-Cr (80/20) | 5.52 | 16.04 | 3.1 |
| P(4aba_co-ani)/MMT-Cr (50/50) | 5.64 | 15.71 | 2.77 |
| P4ABA/MMT-Cr | 5.56 | 15.88 | 2.94 |
| PANI/MMT-Cr | 5.64 | 15.71 | 2.77 |
Fig. 1.
FT IR-spectra of the MMT-Na, MMT-Cr and the nanocomposites poly (4-ABA/MMT-Cr), PANI/MMT-Cr and their copolymers Poly (4-ABA-co-ANI/MMT-Cr).
Fig. 2.
UV–Vis spectra of the homo and copolymer nanocomposites doped with MMT-Cr, A: Poly (4-ABA/MMT-Cr), B: Poly (ANI/MMT-Cr), C: Poly (4-ABA-co-ANI)/MMT-Cr; 80/20, D: Poly (4-ABA-co-ANI)/MMT-Cr; 20/80, E: Poly (4ABA-co-ANI)/MMT-Cr; 50/50).
Fig. 3.
X-ray diffraction patterns of two montmorillonite (MMT-Na and MMT-Cr), and the nanocomposites (PANI/MMT-Cr, Poly (ani-co-4aba)/MMT-Cr, P4ABA/MMT-Cr).
Fig. 4.
Cyclic voltammogram recorded of polymer and copolymer films formed in 1.0 M HClO4 on graphite carbon electrode.
Fig. 5.
TGA curves of PANI prepared in the presence of Maghnite-H+ (0.25 M).
Fig. 6.
TGA curves of Poly (4-ABA-co-ANI/MMT-Cr) prepared in the presence of Maghnite-H+ (0.25 M).
Fig. 7.
1H-NMR spectra of (PANI) obtained by the intercaled method between Aniline and Maghnite-Cr3+ (black powder).
Fig. 8.
1H-NMR spectra of the block copolymer poly (aniline)-b-poly (4-aminobenzylamine) catalyzed by Maghnite-Cr3+ by in situ polymerization.
Fig. 9.
Proposed mechanism of homopolymer (PANI) catalyzed by Maghnite-H+ by in situ polymerization.
Fig. 10.
Proposed mechanism of homopolymer poly (4-aminobenzylamine) catalyzed by Maghnite-H+ by in situ polymerization.
Fig. 11.
Proposed mechanism block copolymer poly (aniline)-b-poly (4-aminobenzylamine) catalyzed by Maghnite-H+ by in situ polymerization.
2. Experimental design, materials, and methods
2.1. Preparation of Maghnite-Cr+3 (MMT-Cr+3)
The raw-clay sample (Raw-MMT) was washed with distilled water to remove impurity [3,4]. The obtained montmorillonite (10 g) was crushed for 20 min using a Prolabo ceramic balls grinder. The greatest proton saturation of the <2 mm fractions of clay were obtained by first saturating with Na + ions using 1 M NaCl solution and to confirm the absence of chloride we use the silver nitrate [5,6]. To obtain MMT-Na+ with chromium intercalated (MMT-Cr+3), the MMT-Na+ was dispersed into a 1 M CrNO3 solution and stirred for 24 h and then the solid was recovered by centrifugation and washed with abundant water [7,8]. The catalyst composition was determined by X-ray fluorescence, the obtaining data are listed in Table 1 [9,10].
2.2. Synthesis of polymers/Maghnite-Cr+3
Polymer/MMT-Cr+3 nanocomposites have been prepared by In-Situ process and the synthesis procedure is briefly described as follows:
The monomers were added by various feed mole fractions. In all cases, the mole ratio of oxidant to the total monomer was defined.
Acknowledgments
This work was supported by the Directorate General of Scientific Research and Technological Development (DGRSDT) of Algeria, and was carried out within the State Program of second national forum.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2020.105161.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Stilwell D.E., Park S.M. Electrochemistry of conductive polymers. III. Some physical and electrochemical properties observed from electrochemically grown polyaniline. J. Electrochem. Soc. 1988;135:2491–2496. [Google Scholar]
- 2.Dominis A.J., Spinks G.M., Wallace G.G. Comparison of polyaniline primers prepared with different dopants for corrosion protection of steel. Prog. Org. Coating. 2003;48:43–49. [Google Scholar]
- 3.Abdel Salam M., Al-Juaid S.S., Qusti A.H., Hermas A.A. Electrochemical deposition of a carbon nanotube-poly(O-phenylenediamine) composite on a stainless steel surface. Synth. Met. 2011;161:153–157. [Google Scholar]
- 4.Shinde V., Patil P.P. Evaluation of corrosion protection performance of poly(O-EthylAniline) coated copper by electrochemical impedance spectroscopy. Mater. Sci. Eng. B. 2010;58:142–150. [Google Scholar]
- 5.Hasanov R., Bilgic S., Gece G. Experimental and theoretical studies on the corrosion properties of some conducting polymer coatings. J. Solid State Electrochem. 2011;15:1063–1070. [Google Scholar]
- 6.Alvi F., Ram M.K., Basnayaka P.A., Stefanakos E., Goswami Y., Kumar A. Graphene polyethylenedioxythiophene conducting polymer nanocomposite based supercapacitor. Electrochim. Acta. 2011;56:9406–9412. [Google Scholar]
- 7.Zhang Y., Shao Y., Zhang T., Meng G., Wang F. High corrosion protection of a polyaniline/organophilic montmorillonite coating for magnesium alloys. Prog. Org. Coating. 2013;76:804–811. [Google Scholar]
- 8.Bereket G., Hur E., Sahin Y. Electrochemical synthesis and anti-corrosive properties of polyaniline, poly(2-anisidine) and poly(aniline-Co-2-Anisidine) films on stainless steel. Prog. Org. Coating. 2005;54:63–72. [Google Scholar]
- 9.Chaudhari S., Patil P.P. Corrosion protective Bi-layered composites of polyaniline and poly(O-anisidine) on low carbon steel. J. Appl. Polym. Sci. 2008;109:2546–2561. [Google Scholar]
- 10.Yeh J.M., Chin C.P. Structure and properties of poly(O-Methoxyaniline)/Clay nanocomposite materials. J. Appl. Polym. Sci. 2003;88:1072–1080. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.