Highlights
-
•
Patients with major depression underwent two-stage 16-week antidepressant trial.
-
•
Whole-brain cortical thickness (CT) was measured in patients and healthy controls.
-
•
Site (N = 6) differences were corrected at each vertex using ComBat.
-
•
Patients exhibited thinner cortex in left rostral middle frontal cortex at baseline.
-
•
There were no baseline CT features associated with antidepressant response.
Keywords: Major depressive disorder, Cortical thickness, Structural neuroimaging, Antidepressant response, Clinical trial
Abstract
Major depressive disorder (MDD) is considered a highly heterogeneous clinical and neurobiological mental disorder. We employed a novel layered treatment design to investigate whether cortical thickness features at baseline differentiated treatment responders from non-responders after 8 and 16 weeks of a standardized sequential antidepressant treatment. Secondary analyses examined baseline differences between MDD and controls as a replication analysis and longitudinal changes in thickness after 8 weeks of escitalopram treatment. 181 MDD and 95 healthy comparison (HC) participants were studied. After 8 weeks of escitalopram treatment (10–20 mg/d, flexible dosage), responders (>50% decrease in Montgomery-Åsberg Depression Scale score) were continued on escitalopram; non-responders received adjunctive aripiprazole (2–10 mg/d, flexible dosage). MDD participants were classified into subgroups according to their response profiles at weeks 8 and 16. Baseline group differences in cortical thickness were analyzed with FreeSurfer between HC and MDD groups as well as between response groups. Two-stage longitudinal processing was used to investigate 8-week escitalopram treatment-related changes in cortical thickness. Compared to HC, the MDD group exhibited thinner cortex in the left rostral middle frontal cortex [MNI(X,Y,Z=−29,9,54.5,−7.7); CWP=0.0002]. No baseline differences in cortical thickness were observed between responders and non-responders based on week-8 or week-16 response profile. No changes in cortical thickness was observed after 8 weeks of escitalopram monotherapy. In a two-step 16-week sequential clinical trial we found that baseline cortical thickness does not appear to be associated to clinical response to pharmacotherapy at 8 or 16 weeks.
(Registration: ClinicalTrials.gov identifier NCT01655706)
1. Introduction
Major depressive disorder (MDD) affects up to 300 million people worldwide and is one of the most prevalent causes of disability globally (“WHO | Depression” n.d.). First-line antidepressants have limited efficacy (Cipriani et al., 2018), often necessitating additional treatment courses that can prolong or worsen the patient's distress. Moreover, antidepressants are prescribed based on group average responses from clinical trials, rather than on objective individual characteristics derived from clinical or neurobiological data. The high degree of heterogeneity among individuals meeting diagnostic criteria for MDD likely underlie a wide range of neurobiological subtypes (Drysdale et al., 2017). Clinical- and biomarker-informed treatment selection is the ultimate goal of precision medicine, where accurate subtyping would help clinicians discern whether certain medications are differentially effective in a subtype-dependent manner (Paris, 2014; Young et al., 2016; Kennedy and Ceniti, 2018). The Canadian Biomarker Integration Network in Depression (CAN-BIND) is a multi-site clinical treatment trial involving several major research centres in Canada (Kennedy and Ceniti, 2018; Lam et al., 2016). Clinical, molecular and neuroimaging data were collected from over 300 participants, including MDD patients and healthy comparison (HC) participants.
Neuroimaging has emerged as a promising approach in the search for biomarkers, including anatomical magnetic resonance imaging (MRI), which makes possible the visualization and quantification of the structure of the brain at millimeter resolution. One such parameter is cortical thickness, defined as the distance from the pial boundary to the gray matter (GM)/white matter (WM) boundary comprising the cell bodies of the cerebral cortex as well as intracortical myelin (Narr et al., 2007; Seldon, 2007; Shaw et al., 2008). There are various factors that could lead to cortical thinning, including reduction of synapses, atrophy of dendritic trees or reduced vascularization (Lyttle et al., 2015; Schüz and Palm, 1989). Cortical thinning, independent of aging processes, has been demonstrated in samples of MDD participants and has been replicated in previous studies, albeit not without ambiguity regarding the specific subregions affected by cortical thinning (Schmaal et al., 2017; Pink et al., 2017; Suh et al., 2019b). Moreover, cortical thickening has also been observed, particularly in medication-naïve, first-episode MDD patients (Philip van Eijndhoven et al., 2013; Fonseka et al., 2016; Peng et al., 2015; Qiu et al., 2014; Yang et al., 2015), which has been hypothesized to reflect glial hypertrophy as an immune response to initial excitotoxic injury during the first episode (Dowlati et al., 2010).
Until recently (Schmaal et al., 2017; Perlman et al., 2017), studies on cortical thickness were hampered by small-to-moderate sample sizes (N<100) and therefore low power, especially when the statistical considerations that must be made for multi-dimensional neuroimaging data are taken into account (Cremers et al., 2017). Under-powered neuroimaging studies suffer from effect size inflation and have low replicability (Button et al., 2013), often constraining statistical analyses to regions of interest that may not fully capture the whole-brain signature of the associated disorder. Another limitation is the paucity of longitudinal studies assessing patient response to a given antidepressant over time (Suh et al., 2019). The few longitudinal studies that have tracked changes in cortical thickness over time are smaller than most cross-sectional studies (N<30) (Eijndhoven et al., 2016; Gryglewski et al., 2018; Koenig et al., 2018; Phillips et al., 2015; Pirnia et al., 2016; Sartorius et al., 2016; Suh et al., 2019). A recent consortium study has addressed several of these gaps in the literature, including a large MDD sample for sufficient power and a longitudinal design within a treatment paradigm that includes a placebo arm (Bartlett et al., 2018). Examining average values from 5 a priori selected regions (rostral and caudal anterior cingulate cortex (ACC), lateral orbitofrontal cortex, rostral middle frontal cortex and hippocampus), they found that only early thickening in the rostral ACC during the first week of treatment was associated with SSRI response at week 8 (Bartlett et al., 2018). No significant associations between pre-treatment cortical thickness and week-8 response were observed. This study, however, did not employ a whole-brain approach to capture information on cortical thickness in regions outside the pre-selected regions of interest (ROI).
We used a vertex/surface-based method to calculate cortical thickness with FreeSurfer, which utilizes a triangulated mesh to model the two surfaces that delineate the cerebral cortex: the pial boundary separating GM and cerebrospinal fluid (CSF) and the WM boundary that lies below cortical GM. There are several advantages to vertex-based methods when compared to conventional voxel-based morphometry, including sub-voxel accuracy, topological continuity, robustness to varying acquisition and scanner parameters and decreased susceptibility to partial volume effects (Clarkson et al., 2011; Fischl, 2012).
Multi-site, multi-scanner effects are known to be a complex issue among the increasing number of large multi-site neuroimaging studies, which are necessary for increasing sample sizes conducive to reliably detecting effects (Jahanshad et al., 2019; Hawco et al., 2018; Tozzi et al., 2019). In recent years, the ComBat algorithm (Fortin et al., 2017; Johnson et al., 2007), originally developed for correcting batch effects in genomics, has been applied to correcting site- and scanner-associated variation (Bartlett et al., 2018; Yu et al., 2018). Here we have taken a similar approach, applying this algorithm to vertex-wise datapoints in our sample to accommodate our whole-brain analyses.
We present a study novel in its simultaneous whole-brain approach, large sample of MDD and HC participants and 16-week sequential treatment designed to investigate associations with antidepressant response. Our primary objectives were to investigate vertex-wise pre-treatment features of cortical thickness associated with antidepressant response at 8 weeks and 16 weeks of treatment. Specifically, we aimed to determine whether MDD participants who achieved clinical response at 8 and/or 16 weeks had differences in cortical thickness at baseline compared to those who did not achieve clinical response. We also aimed to identify differences between MDD and HC participants at baseline. We hypothesized that thinner cortex at baseline, which has been associated with increased vulnerability to MDD (Hao et al., 2017; Papmeyer et al., 2015), would be associated with worse response to treatment. A final aim of the study was to determine whether there were measurable changes in cortical thickness over the 8-week course of treatment with escitalopram. It has been suggested that antidepressant treatment may cause thickening of the cortex (Koenig et al., 2018; Phillips et al., 2015), but this hypothesis has not been confirmed in larger trials or in whole-brain analyses and is contradicted by preclinical findings, in which stress-related decreases in cortical thickness are not normalized following SSRI administration (Lyttle et al., 2015). To our knowledge, this is the first treatment study to examine whole-brain group differences and longitudinal changes in cortical thickness with a sample size that can support this statistical approach (Pardoe et al., 2013). This last point in particular, combined with a reliable site correction method, is key to interrogating the often contradictory and inconclusive cortical thickness findings in recent years (Suh et al., 2019).
2. Materials and methods
Full details of the clinical, neuroimaging and biomarker protocols and patient outcomes are available elsewhere (Kennedy et al., 2019; Lam et al., 2016; MacQueen et al., 2019). The protocol was approved by the Research Ethics Boards at each institution. Information pertaining to inclusion/exclusion criteria and MRI acquisition parameters for this cohort can be found in Supplementary Materials. The CONSORT diagram outlining the flow of participants throughout the 16-week clinical trial can be found in (Kennedy et al., 2019). MDD participants were aged 18–60 meeting DSM-IV-TR criteria for a major depressive episode (duration > 3 months) and HC subjects were matched for age and sex.
2.1. Treatment protocol
At the baseline visit (week 0), all MDD participants started treatment with escitalopram 10 mg daily, flexible-dosage, with a maximum dose of 20 mg per day. At week 8, responders (defined as a greater than 50% reduction in MADRS score (Lam et al., 2016)) continued to receive escitalopram for a further eight weeks. Participants who did not respond received aripiprazole at 2–10 mg per day (flexible-dosage), a first-line adjunctive agent chosen based on clinical guidelines set out by the Canadian Network for Mood and Anxiety Treatments (CANMAT) (Kennedy et al., 2016).
2.2. Imaging processing for cortical thickness analysis
We obtained T1-weighted images at weeks 0 (baseline), 2 and 8. Raw images were pre-processed using the fully automated pipeline from FreeSurfer (version 6.0) (https://surfer.nmr.mgh.harvard.edu/) (Fischl. 2012). After motion correction and averaging (Reuter et al., 2010), surrounding non-brain tissue is removed (Ségonne et al., 2004) and the images then undergo a transformation to standard Talairach space and intensity normalization (Sled et al., 1998). The boundary between GM and WM undergoes tessellation to a triangular mesh and topological corrections are made. The GM/WM and pial surfaces are then deformed to certain locations where the greatest shifts in intensity occur and which indicate boundaries between different tissue compartments (WM/GM/cerebrospinal fluid) (Dale et al., 1999). These surfaces are then inflated to a spherical model and registered to the MNI atlas. For each participant, cortical thickness values are measured for each vertex on the cortical surface mesh as the shortest distance between the reconstructed pial and GM/WM surfaces.
For longitudinal analyses, all images completed the FreeSurfer two-stage longitudinal processing stream (Reuter et al., 2012). The first step is the creation of a “base” unbiased template for each subject based on images from all timepoints (Reuter et al., 2010). Cross-sectional images are then processed for longitudinal analysis using the base template for initialization for skull stripping, transformation and registration, in order to minimize random error and preserve stable within-subject features across all timepoints (Reuter et al., 2012). First-pass quality assurance following all FreeSurfer pre-processing involved checking for correct skull strips and registration to Talairach space.
Quality assurance protocols from the Enhancing Neuroimaging Genetics through Meta-analysis (ENIGMA) consortium (http://enigma.ini.usc.edu/; April 2017) were used to assess results from the automatic cortical segmentation procedure outlined above. These protocols comprise an outlier detection analysis for parcellated ROIs as per the Desikan-Killiany atlas, visual inspection of internal segmentation (using sampled coronal and axial slices) and external surface reconstruction for each subject. Participants whose images failed at least one of these three steps were flagged for further inspection and manual edits of the main structural volume and white matter volume were made as necessary (254/795 total images over 3 timepoints). Edits were confined to cleaning up the pial boundary and on the white matter mask in the temporal lobes to improve segmentation of the gray matter from CSF on the superior aspect and gray matter from white matter in the temporal regions of the cortex, respectively. The resulting cortical maps were smoothed with a Gaussian kernel of 15 mm full width at half-maximum in preparation for statistical group analyses.
2.3. Site effect corrections
Following FreeSurfer pre-processing and the completion of final quality checks, the Python implementation of the ComBat algorithm was used to correct for site effects on a vertex-wise basis (GitHub repository: https://github.com/ncullen93/neuroCombat). Full details can be found in Supplementary Materials.
2.4. Statistical analyses
All statistical analyses for demographics were performed in the open-source software Python (version 3.6) using the scipy library. MDD and HC groups were compared in terms of age, years of education and baseline MADRS using the Student's t-test. Age, years of education, age of illness onset, MADRS scores at the three timepoints, number of previous major depressive episodes and duration of illness were compared between the response groups using analysis of variance. Proportions of females/males were compared using a chi-square test (see Table 1).
Table 1.
Demographic and clinical information for the MDD and HC samples.
| MDD Patients |
Healthy Controls |
|||||
|---|---|---|---|---|---|---|
| N | Mean/ frequency | SD | N | Mean/ frequency | SD | |
| Age (1) | 181 | 35.03 | 12.56 | 95 | 32.99 | 10.87 |
| Sex% F (1) | 181 | 64.84 | – | 95 | 63.54 | – |
| Years of Education (2) | 181 | 16.87 | 2.08 | 95 | 18.40 | 2.23 |
| Baseline MADRS (2) | 181 | 29.84 | 5.65 | 95 | 0.82 | 1.73 |
| Age of Illness Onset | 175 | 20.38 | 10.26 | – | – | – |
| Number of previous MDEs | 132 | 4.02 | 2.64 | – | – | – |
| % Change MADRS (8-week) | 159 | −45.79 | 32.43 | – | – | – |
| % Change MADRS (16-week) | 141 | −65.71 | 27.70 | – | – | – |
| % Responders @ 8 weeks | 159 | 47.17 | – | – | – | – |
| % Responders @ 16 weeks | 141 | 75.20 | – | – | – | – |
| % Family history of psychiatric illness | 180 | 77.78 | – | – | – | – |
Superscripts indicate the significance of the test statistic comparing patient and healthy control samples. ‘1′ – p > 0.05, no significant differences between samples. ‘2′ – p < 0.005. The N indicates the number of participants for which the corresponding information is available.
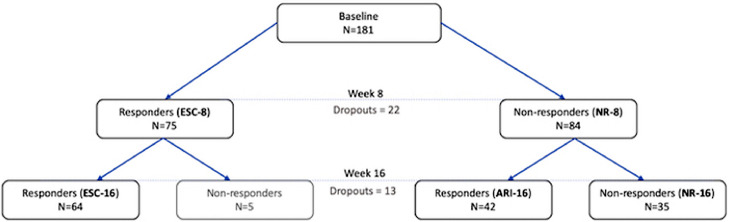
At week 8, MDD participants were either escitalopram responders (‘ESC-8′) or non-responders (‘NR-8′). At the week 16 endpoint, there were three groups based on response: those who continued to respond to escitalopram throughout the 16 weeks (‘ESC-16′), participants who responded to adjunctive aripiprazole (‘ARI-16′) and patients who continued to be non-responders by week 16 despite the addition of aripiprazole (‘NR-16′). In a secondary analysis, ESC-16 and ARI-16 groups were combined to define overall response at week 16. A small sample of five MDD participants who were in the ESC-8 group but were no longer responding by week 16 were excluded only in subsequent analyses (Fig. 1).
Fig. 1.
Number of participants in response subgroups at week 8 and week 16. 22 participants were lost to attrition at week 8 and an additional 13 had dropped out by week 16.
To test for changes in vertex-wise cortical thickness as a result of antidepressant treatment over 8 weeks, longitudinal analyses were performed using repeated-measures ANCOVA for the following groups to test for any changes, controlled for sex, age and age2: the HC group, the MDD group and the three week-16 response subgroups. We also tested for differences in longitudinal changes from baseline to week 8 between ESC-8 and NR-8 groups to assess any between-group effects of escitalopram treatment. We measured longitudinal change using symmetrized percent change (SPC), the rate of change with respect to average thickness over the two timepoints. Using the average thickness renders SPC particularly robust to noise effects (Reuter et al., 2012; Tamnes et al., 2017).
Cross-sectional analyses consisted of whole-brain, vertex-wise comparisons between the aforementioned groups at baseline, and pairs of timepoints were used for longitudinal analyses (baseline to week 2, week 2 to week 8, baseline to week 8). General linear modelling (ANCOVA) was used for both between- and within-group analyses using FreeSurfer statistical tools, incorporating age, age2 and sex as covariates (as per Perlman et al., 2017; Bartlett et al., 2018).
All vertex-wise results were corrected for multiple comparisons and separate hemisphere testing using Monte Carlo simulation (10,000 iterations) with a vertex-wise threshold of p = 0.01 and cluster thresholding at p = 0.05 (Hagler et al., 2006). Briefly, this technique involves indexing based on a lookup table provided by FreeSurfer that has tabulated p-values corresponding to various cluster sizes at different smoothing levels. These values are derived from Gaussian Monte Carlo simulations of a z-field synthesized onto the cortical surface that is then thresholded to extract the largest cluster at a range of p-value thresholds, repeated 10,000 times. An additional Bonferroni correction was applied to take into account multiple exploratory cross-sectional contrasts (9 in total) for a post-correction cluster p-value threshold of p = 0.0056 (family-wise threshold of 0.05 divided by the number of tests), set as the display threshold for all figures.
To examine in more detail how cortical thickness is related to the extent of improvement following 8 and 16 weeks of pharmacotherapy, we tested the relationship between percent change in MADRS score at 8 and 16 weeks and baseline cortical thickness within the MDD group using multiple linear regression (controlling for sex, age and age2). Significant clusters from the whole-brain analysis were chosen as regions of interest, and a mask was created to extract average thickness values for each participant. Scatterplots were constructed to display the distributions and curves of best fit for each group in R version 3.4.1 (https://www.r-project.org/), using the package ggplot2.
3. Results
Baseline neuroimaging data were available for a total of 308 participants. Following the systematic quality screen as described above, we obtained FreeSurfer outputs for baseline images of usable quality from 181 MDD and 95 HC participants. 32 images were excluded on the basis of poor overall segmentation that could not be manually corrected. There were no significant differences between MDD and HC on mean age or female:male ratio, although there was a significant difference in years of education (t = 5.67; p = 0.000) and baseline MADRS score (t=−63.88; p = 0.000) (Table 1). By week 8, 22 participants had dropped out to give a sample size of 159 MDD participants, with a further reduction to 141 participants at week 16 (see Fig. 1 for final MDD subgroup sample sizes). As indicated by omnibus tests, differences in age, sex, years of education, age of onset, illness duration, baseline severity and number of previous episodes were not significant between week-16 subgroups (Supplementary Table 1).
3.1. Baseline cross-sectional analyses between groups
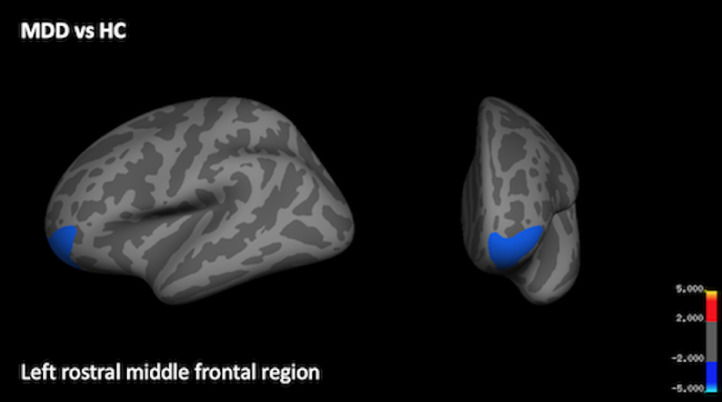
Whole-brain analyses revealed that the MDD group exhibited thinner cortex at baseline in the left rostral middle frontal cortex (Fig. 2; Table 2). There were no statistically significant differences at baseline in cortical thickness across the whole brain between the ESC-8 and NR-8 groups, between the ESC-16, ARI-16 and NR-16 groups, nor between the combined week-16 response group (ESC-16+ARI-16) and NR-16.
Fig. 2.
Region of thinner cortex at baseline in MDD as compared to HC. A significant cluster indicating thinner cortex in the left rostral middle frontal region is displayed from the lateral and frontal views. Scale bar shows max -log(p) values, following corrections for multiple comparisons using Monte Carlo thresholding.
Table 2.
Cortical region exhibiting greater thinning in MDD group compared to HC.
| Cluster # | Max -log(p) | Annotation | Size(mm^2) | MNIX | MNIY | MNIZ | CWP |
|---|---|---|---|---|---|---|---|
| Left hemisphere | |||||||
| 1 | −3.389 | rostral middle frontal | 1599.21 | −29.9 | 54.5 | −7.7 | 0.0002 |
Max -log(p) indicates the maximum -log(p) value among the vertices in the cluster. CWP = cluster-wise p-value. The Annotation heading refers to the location of the peak voxel as per the Desikan-Killiany atlas. The MNI coordinates indicate the location of the peak vertex.
3.2. 8-week longitudinal changes in cortical thickness
There were no significant longitudinal changes in cortical thickness within the HC group, the pooled MDD group or week-16 subgroups from baseline to week 8. Trajectories of cortical thickness change over the course of escitalopram treatment between ESC-8 and NR-8 groups were not found to be different.
3.3. Relationship between baseline cortical thickness and improvement in symptom severity
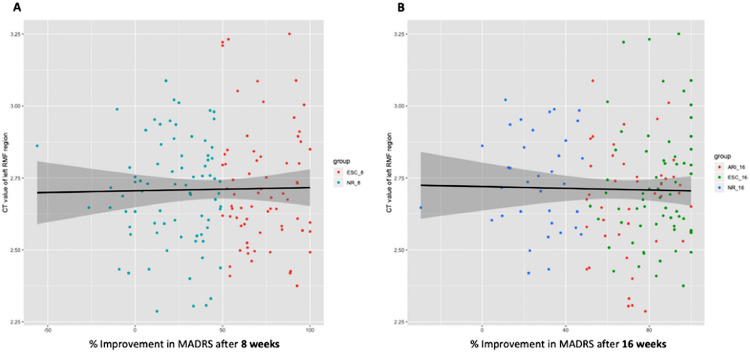
There were no significant relationships between baseline cortical thickness and extent of treatment response (percent improvement in MADRS scores) at either 8 or 16 weeks within the MDD group (Fig. 3).
Fig. 3.
Scatterplots and regression lines depicting the relationship between RMF thickness and % improvement in MADRS scores, grouped by response. Thickness values over the significant cluster in the RMF cortex at baseline were extracted for all MDD participants and plotted over % improvement in MADRS score at A) 8 weeks of escitalopram treatment and B) 16 weeks of escitalopram or adjunctive treatment with aripiprazole for each participant. Datapoints have been grouped by colour based on response group at the respective treatment timepoints.
4. Discussion
In a large sample, with the novel combination of robust site effect correction and a vertex-based whole-brain approach, we found no significant differences at baseline between week-8 or week-16 responders and non-responders. Therefore, we were unable to confirm the hypothesis that non-responders to pharmacotherapy would exhibit thinner cortex at baseline and subsequently, the notion that cortical thickness might be a useful biomarker for treatment response. We found that the MDD group exhibited thinner cortex in the left rostral middle frontal (RMF) cortex as compared to HC. This result is a replication of findings from previous studies. Two studies found thinner cortex in the RMF region in MDD compared to HC, although one found a bilateral effect (Zhao et al., 2017) whereas the other also observed thinning in only the left region (Peng et al., 2015). Abnormalities in structural asymmetry in the RMF cortex has been reported in treatment-naïve MDD (Zuo et al., 2019). We note that these three studies also found additional effects of thinner cortex in other regions in MDD that we did not replicate. Additionally, increased thickness in the RMF region over the course of treatment has been found to be indicative of remission in two separate studies (Phillips et al., 2015; Saricicek Aydogan et al., 2019)– however, in the current study we did not find significant changes in thickness within any response group on a whole-brain basis. Interestingly, in one study thinner bilateral RMF at baseline was correlated to better response in the placebo group (Bartlett et al., 2018) and increased thickness in this region was negatively correlated to symptom severity in MDD (Qiu et al., 2014). It appears that even for one region that is implicated relatively often in the literature, previous reports are mixed and are often accompanied by other findings that have not been replicated.
Although our primary analyses yielded largely null results as far as the association between cortical thickness and antidepressant response, they are fairly consistent with recent studies that have been published on the topic of cortical thickness as a neuroimaging biomarker in MDD. Despite some positive preliminary results, pre-treatment cortical thickness predictors of treatment response that are robust and replicable have yet to be discovered (Phillips et al., 2015; Suh et al., 2019; Bartlett et al., 2018). Similarly, studies on diagnostic group differences in cortical thickness, although more numerous, have yet to converge on a set of replicable differences (Suh et al., 2019), finding both thinner and thicker regions among disparate regions in MDD. The largest cortical thickness analysis to date from the ENIGMA consortium, with 1902 MDD patients and 7658 HC participants drawn from 20 cohorts, found small absolute effect sizes of thinning in MDD (Cohen's d no larger than 0.14) despite its robust statistical power. Studies with sample sizes ranging from 50 to >100 per group tend towards null results for statistical testing of the MDD vs HC comparison (Perlman et al., 2017; Saricicek Aydogan et al., 2019). This indicates that smaller samples, particularly in the context of cortical thickness analysis (Button et al., 2013), may run an increased risk of false positive results. Inconsistent methods for multiple comparison corrections and variable utilization of region-based vs. vertex-wise approaches have also contributed to the considerable heterogeneity among studies. Other clinical characteristics of the MDD sample may also influence the final results, such as varying definitions of response/remission, medication status and disease chronicity.
When the extant literature and the current results are taken together, it seems unlikely that cortical thickness alone could be used as a reliable predictor of short-term treatment response to pharmacotherapy. However, it has been shown to be useful for mapping and predicting clinical response to electroconvulsive therapy in a regionally consistent manner in several studies (Eijndhoven et al., 2016; Pirnia et al., 2016; Sartorius et al., 2016; Schmitgen et al., 2019; Wade et al., 2017). Effectively, cortical thickness represents a totality of numerous microscopic properties; shown to be a relatively stable measure over the lifespan (Storsve et al., 2014; Hogstrom et al., 2013), it appears likely that any subtle structural characteristics predictive of the extent of short-term response to pharmacotherapy, if they exist, are not well-reflected in this measure.
We found no significant longitudinal changes in cortical thickness following 8 weeks of escitalopram treatment, nor any differences between responders and non-responders. Most studies reporting longitudinal changes in cortical thickness (Bartlett et al., 2018; Koenig et al., 2018; Phillips et al. 2015) have focused on pre-determined regions of interest for analysis, lending them the advantage of increased statistical power. Other studies have found no change in either cortical volume nor thickness within several weeks of SSRI or SNRI treatment (Fu et al., 2015; Lyttle et al., 2015). It is also conceivable that 8 weeks might be too short a period of time to detect cortical thickness changes via MRI.
This study addressed a gap in the literature regarding identification of baseline features associated with differential treatment response (Phillips et al., 2015), particularly being the first neuroimaging study to incorporate sequential adjunctive treatment following lack of response to a first-line antidepressant medication (Kennedy et al., 2016). Given the variety of cortical regions that have been identified as being altered or abnormal in MDD, we opted for a whole-brain, exploratory approach, taking advantage of the relatively large sample size afforded by the multi-site effort of the CAN-BIND trial. Other advantages of the current study include controlling for heterogeneity introduced by different MRI scanners and varying acquisition parameters by correcting for these effects on a vertex-wise basis using the ComBat algorithm. We also carried out blinded systematic manual correction of FreeSurfer outputs, which has been shown to provide more accurate segmentations when compared to uncorrected outputs (Popescu et al., 2016).
There are also several limitations associated with the study. First, although we have a relatively large sample size overall for both the MDD and HC groups, this advantage is reduced once we subdivide the MDD group based on week-16 response. Second, our age range was relatively large, possibly obscuring potential age-related trajectories and mechanisms of disease progression. Although we attempted to mitigate this limitation by controlling for both age and age2 in all neuroimaging analyses, it is possible that age-specific relationships may emerge in studies with narrower age ranges or greater sample sizes. Third, we excluded subcortical and cerebellar regions in the analysis, as we focused only on cerebral cortical thickness, and therefore could not ascertain any longitudinal changes or baseline associations with treatment response in these areas. Moreover, we did not control for the potential confounding variable of cigarette smoking status (Zorlu et al., 2017). Finally, mass univariate analyses are not sufficient to discriminate structural features that could aid in informing individualized treatment.
In conclusion, we show that cortical thickness in MDD at baseline was not associated to antidepressant response at 8 or 16 weeks, nor was it shown to change over an 8-week period of escitalopram treatment. We did replicate previous findings of cortical thinning in the left RMF cortex in MDD. Future studies might investigate not only univariate approaches to isolating potential biomarkers, but also multivariate methods incorporating multiple measures, an approach requiring a sufficient number of participants and clearly defined patient samples (Kim and Na, 2018; Raamana et al., 2014). Another promising approach is the extraction of advanced multi-variate network-level features from whole-brain thickness features to assess their utility in predicting response to treatment (Raamana and Strother, 2018). The emergence of larger consortia with sufficient power to identify subgroups based on biomarkers is a promising sign for the field (Brunoni et al., 2015; Lam et al., 2016; Schmaal et al., 2017; Trivedi et al., 2016). Methods range from retrospective grouping of subjects based on some outcome parameter (as shown here with ‘response’) to using unsupervised, data-driven machine learning algorithms to model underlying patterns of variability (Drysdale et al., 2017).
Declaration of Competing Interest
Dr. Frey has received a research grant from Pfizer.
Dr. Strother is the Chief Scientific Officer of ADMdx, Inc., which receives NIH funding, and currently has research grants from Brain Canada, CIHR, the Ontario Brain Institute in Canada.
Dr. Milev is a member of an Advisory Boards and/or Speaker's Bureau member for the following organizations: Lundbeck, Pfizer, Shire, Sunovion, Janssen, Allergan, BMS, Otsuka; and has received research grants and participated in clinical trials for: Lundbeck, Merck, Pfizer, BI, Janssen, CIHR, OBI, CANBIND, OMHF; and received presenter honorariums from: Lundbeck, Pfizer, Shire, Sunovion, Allergan, BMS, Otsuka, Janssen.
Dr. Lam has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from: Akili, Allergan, Asia-Pacific Economic Cooperation, BC Leading Edge Foundation, Brain Canada, Canadian Institutes of Health Research, Canadian Depression Research and Intervention Network, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, CME Institute, Hansoh, Janssen, Lundbeck, Lundbeck Institute, Medscape, Mind Mental Health Technologies, Otsuka, Pfizer, St. Jude Medical, University Health Network Foundation, and VGH Foundation.
Dr. Kennedy has received research funding or honoraria from the following sources:
Abbott, Alkermes, Allergan, BMS, Brain Canada, Canadian Institutes for Health Research (CIHR), Janssen, Lundbeck, Lundbeck Institute, Ontario Brain Institute, Ontario Research Fund (ORF), Otsuka, Pfizer, Servier, Sunovion and Xian-Janssen.
The remaining authors have to conflict of interest to declare.
Acknowledgements
This study was supported in part by the Ontario Ministry of Research and Innovation (Early Research Award – Dr. Frey). JS was supported by the Canadian Institutes for Health Research (CIHR; CGS-Master's). PRR is supported by Ontario Neurodegenerative Disease Research Initiative (ONDRI) and CAN-BIND, which are two Integrated Discovery Programs carried out in partnership with, and partially sponsored by the Ontario Brain Institute, an independent non-profit corporation, funded partially by the Ontario government. The neuroimaging platform was supported in part by a CIHR grant (Co-PIs: Drs. Kennedy and MacQueen, MOP 125880). CAN-BIND is an Integrated Discovery Program carried out in partnership with, and financial support from, the Ontario Brain Institute, an independent non-profit corporation, funded partially by the Ontario government. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Additional funding is provided by CIHR, Lundbeck, Bristol-Myers Squibb, Pfizer, and Servier. Funding and/or in-kind support is also provided by the investigators’ universities and academic institutions. All study medications are independently purchased at wholesale market values.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102178.
Appendix. Supplementary materials
References
- Bartlett E., DeLorenzo C., Sharma P., Yang J., Zhang M., Petkova E., Weissman M. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology. 2018;43(11):2221–2230. doi: 10.1038/s41386-018-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A.R., Sampaio-Junior B., Sampaio-Junior B., Henrique Moffa A., Borrione L., Schwair Nogueira B., Vanessa Marotti Aparicio L., Veronezi B. The escitalopram versus electric current therapy for treating depression clinical study (ELECT-TDCS): rationale and study design of a non-inferiority, triple-arm, placebo-controlled clinical trial. Sao Paulo Med. J. 2015;133(3):252–263. doi: 10.1590/1516-3180.2014.00351712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cipriani A., Furukawa T.A., Salanti G., Chaimani A., Atkinson L.Z., Ogawa Y., Leucht S. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet North Am. Ed. 2018;391(10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson M.J., Jorge Cardoso M., Gerard R.R., Modat M., Kelvin K.L., Jonathan D.R., Nick C.F., Ourselin S. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage. 2011;57(3):856–865. doi: 10.1016/j.neuroimage.2011.05.053. [DOI] [PubMed] [Google Scholar]
- Cremers H.R., Wager T.D., Yarkoni T. The relation between statistical power and inference in FMRI. PLoS ONE. 2017;12(11) doi: 10.1371/journal.pone.0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis - I. segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Elyse K.R., Krista L.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Drysdale A.T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y., Robert N.F. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijndhoven P., Mulders P., Kwekkeboom L., van Oostrom I., van Beek M., Janzing J., Schene A., Tendolkar I. Bilateral ECT induces bilateral increases in regional cortical thickness. Transl. Psychiatry. 2016;6(August):e874. doi: 10.1038/tp.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijndhoven P.van, Guido van W., Maartje K., Wouter G., Ralf T., Guillen F., Jan B., Indira T. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am. J. Psychiatry. 2013;170(12):1477–1486. doi: 10.1176/appi.ajp.2013.12121504. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseka B.A., Jaworska N., Courtright A., MacMaster F.P., MacQueen G.M. Cortical thickness and emotion processing in young adults with mild to moderate depression: a preliminary study. BMC Psychiatry. 2016;16(February):38. doi: 10.1186/s12888-016-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, Jean-Philippe, Nicholas Cullen, Yvette I.Sheline, Warren D.Taylor, Irem Aselcioglu, Phil Adams, Crystal Cooper, et al. 2017. “Harmonization of cortical thickness measurements across scanners and sites.” BioRxiv, June. 10.1101/148502. [DOI] [PMC free article] [PubMed]
- Fu C.H.Y., Sergi G.C., Anjali S., Tracey M.A., Mark M.R., Peng L., Robert D., Luigi A.M., Paul H., Lauren B.M. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry. 2015;15(1):82. doi: 10.1186/s12888-015-0457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski G., Baldinger-Melich P., Seiger R., Godbersen G.M., Michenthaler P., Klöbl M., Spurny B. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br. J. Psychiatry. 2018:1–9. doi: 10.1192/bjp.2018.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Saygin A.P., Sereno M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of FMRI data. Neuroimage. 2006;33(4):1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X., Talati A., Stewart A.S., Jun L., Jurgen K., Craig E.T., Virginia W. Stability of cortical thinning in persons at increased familial risk for major depressive disorder across 8 years. Biologic. Psychiat: Cognit. Neurosci. Neuroimaging. 2017;2(7):619–625. doi: 10.1016/j.bpsc.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawco C., Viviano J.D., Chavez S., Dickie E.W., Calarco N., Kochunov P., Argyelan M. A longitudinal human phantom reliability study of multi-center T1-Weighted, DTI, and resting state FMRI data. Psychiat. Res. Neuroimaging. 2018;282(December):134–142. doi: 10.1016/j.pscychresns.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom L.J., Westlye L.T., Walhovd K.B., Fjell A.M. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb. Cortex. 2013;23(11):2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- Jahanshad, Neda, Joshua I. Faskowitz, Gennady Roshchupkin, Derrek Hibar, Boris A. Gutman, Nicholas J. Tustison, Hieab H.H. Adams, et al. 2019. “Multi-site meta-analysis of morphometry.” IEEE/ACM Trans. Comput. Biol. Bioinf., May. 10.1109/TCBB.2019.2914905. [DOI] [PMC free article] [PubMed]
- Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Kennedy S.H., Ceniti A.K. Unpacking major depressive disorder: from classification to treatment selection. Can. J. Psychiatry. 2018;63(5):308–313. doi: 10.1177/0706743717748883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S.H., Lam R.W., McIntyre R.S., Valérie Tourjman S., Bhat V., Blier P., Hasnain M. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can. J. Psychiatry. 2016;61(9):540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S.H., Lam R.W., Rotzinger S., Milev R.V., Blier P., Downar J., Evans K.R., Farzan F., Foster J.A., Frey B.N. Symptomatic and functional outcomes and early prediction of response to escitalopram monotherapy and sequential adjunctive aripiprazole therapy in patients with major depressive disorder: a CAN-BIND-1 report. J. Clin. Psychiatry. 2019;80(2) doi: 10.4088/JCP.18m12202. 0–0. [DOI] [PubMed] [Google Scholar]
- Kim Y.-K., Na K.-S. Application of machine learning classification for structural brain MRI in mood disorders: critical review from a clinical perspective. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;80(January):71–80. doi: 10.1016/j.pnpbp.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Koenig J., Schreiner M.W., Klimes-Dougan B., Ubani B., Bryon A.M., Kelvin O.L., Michael K., Kathryn R.C. Increases in orbitofrontal cortex thickness following antidepressant treatment are associated with changes in resting state autonomic function in adolescents with major depression – Preliminary Findings from a pilot study. Psychiat. Res. Neuroimaging. 2018;281(November):35–42. doi: 10.1016/j.pscychresns.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam R.W., Milev R., Rotzinger S., Andreazza A.C., Blier P., Brenner C., Daskalakis Z.J. Discovering biomarkers for antidepressant response: protocol from the Canadian biomarker integration network in depression (CAN-BIND) and clinical characteristics of the first patient cohort. BMC Psychiatry. 2016;16(April):105. doi: 10.1186/s12888-016-0785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttle K., Ohmura Y., Konno K., Yoshida T., Izumi T., Watanabe M., Yoshioka M. Repeated fluvoxamine treatment recovers juvenile stress-induced morphological changes and depressive-like behavior in rats. Brain Res. 2015;1616:88–100. doi: 10.1016/j.brainres.2015.04.058. August. [DOI] [PubMed] [Google Scholar]
- MacQueen G.M., Hassel S., Arnott S.R., Jean A., Bowie C.R., Bray S.L., Davis A.D. The Canadian biomarker integration network in depression (CAN-BIND): magnetic resonance imaging protocols. J. Psychiat. Neurosci. JPN. 2019;44(3):1–14. doi: 10.1503/jpn.180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr K.L., Woods R.P., Thompson P.M., Szeszko P., Robinson D., Dimtcheva T., Gurbani M., Toga A.W., Bilder R.M. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb. Cortex. 2007;17(9):2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Papmeyer M., Giles S., Jessica E.S., Shauna K., Tiffany S., Stephen M.L., Heather C.W., Andrew M.M. Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biol. Psychiatry. 2015;78(1):58–66. doi: 10.1016/j.biopsych.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Pardoe H.R., Abbott D.F., Jackson G.D. Sample size estimates for well-powered cross-sectional cortical thickness studies. Hum. Brain Mapp. 2013;34(11) doi: 10.1002/hbm.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J. The mistreatment of major depressive disorder. Can. J. Psychiatry. 2014;59(3):148–151. doi: 10.1177/070674371405900306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D., Shi F., Li G., Fralick D., Shen T., Qiu M., Liu J., Jiang K., Shen D., Fang Y. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G., Bartlett E., DeLorenzo C., Weissman M., McGrath P., Ogden T., Jin T. Cortical thickness is not associated with current depression in a clinical treatment study. Hum. Brain Mapp. 2017;38(9):4370–4385. doi: 10.1002/hbm.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.L., Batten L.A., Tremblay P., Aldosary F., Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int. J. Neuropsychopharmacolog. 2015;18(8) doi: 10.1093/ijnp/pyv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Chase H.W., Sheline Y.I., Etkin A., Almeida J.R.C., Deckersbach T., Trivedi M.H. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am. J. Psychiatry. 2015;172(2):124–138. doi: 10.1176/appi.ajp.2014.14010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink A., Przybelski S.A., Krell-Roesch J., Stokin G.B., Roberts R.O., Mielke M.M., Knopman D.S., Jack C.R., Petersen R.C., Geda Y.E. Cortical thickness and depressive symptoms in cognitively normal individuals: the mayo clinic study of aging. J. Alzheimer’s Dis. JAD. 2017;58(4):1273–1281. doi: 10.3233/JAD-170041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirnia T., Joshi S.H., Leaver A.M., Vasavada M., Njau S., Woods R.P., Espinoza R., Narr K.L. Electroconvulsive therapy and structural neuroplasticity in neocortical, limbic and paralimbic cortex. Transl. Psychiatry. 2016;6(June):e832. doi: 10.1038/tp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu V., Klaver R., Versteeg A., Voorn P., Jos W., Twisk R., Frederik B., Jeroen J., Geurts G., Hugo V. Postmortem validation of MRI cortical volume measurements in MS. Hum. Brain Mapp. 2016;37(6):2223–2233. doi: 10.1002/hbm.23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., Lui S., Kuang W., Huang X., Li J., Li J., Zhang J., Chen H., Sweeney J.A., Gong Q. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl. Psychiatry. 2014;4(April):e378. doi: 10.1038/tp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raamana P.R., Stephen C.S. Graynet: single-Subject morphometric networks for neuroscience connectivity applications. J. Open Source Softw. 2018;3(30):924. [Google Scholar]
- Raamana P.R., Wen W., Nicole A.K., Henry B., Perminder S.S., Lei W., Mirza F.B. The sub-classification of amnestic mild cognitive impairment using MRI-based cortical thickness measures. Front. Neurol. 2014;5:76. doi: 10.3389/fneur.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Diana Rosas H., Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Diana Rosas H., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saricicek Aydogan A., Oztekin A., E., Esen M.E., Dusmez S., Gelal F., Besiroğlu L., Zorlu N. Cortical thickening in remitters compared to non‐remitters with major depressive disorder following 8‐week antidepressant treatment. Acta Psychiatr. Scand. 2019;140(3):217–226. doi: 10.1111/acps.13065. [DOI] [PubMed] [Google Scholar]
- Sartorius A., Traute D., Andreas B., Christian C.von H., Suna S.A., Jan M.B., Laura K., Gabriele E. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur. Neuropsychopharmacol. 2016;26(3):506–517. doi: 10.1016/j.euroneuro.2015.12.036. [DOI] [PubMed] [Google Scholar]
- Schmaal L., Hibar D.P., Saemann P.G., Hall G.B., Baune B.T., Jahanshad N., Cheung J.W. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the enigma major depressive disorder working group. Mol. Psychiatry. 2017;22(6):900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitgen, Mike M., Katharina M. Kubera, Malte S. Depping, Henrike M. Nolte, Dusan Hirjak, Stefan Hofer, Julia H. Hasenkamp, et al. 2019. “Exploring cortical predictors of clinical response to electroconvulsive therapy in major depression.” Eur. Arch. Psychiatry Clin. Neurosci., July. 10.1007/s00406-019-01033-w. [DOI] [PubMed]
- Schüz A., Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J. Comp. Neurol. 1989;286(4):442–455. doi: 10.1002/cne.902860404. [DOI] [PubMed] [Google Scholar]
- Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seldon H.L. Extended neocortical maturation time encompasses speciation, fatty acid and lateralization theories of the evolution of schizophrenia and creativity. Med. Hypotheses. 2007;69(5):1085–1089. doi: 10.1016/j.mehy.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Storsve A.B., Fjell A.M., Tamnes C.K., Westlye L.T., Overbye K., Aasland H.W., Walhovd K.B. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J. Neurosci. 2014;34(25):8488–8498. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J.S., Minuzzi L., Cudney L.E., Maich W., Eltayebani M., Soares C.N., Frey B.N., Benicio N.F. Cerebral cortical thickness after treatment with desvenlafaxine succinate in major depressive disorder. NeuroReport. 2019;30(5):378. doi: 10.1097/WNR.0000000000001211. [DOI] [PubMed] [Google Scholar]
- Suh J.S., Schneider M.A., Minuzzi L., MacQueen G.M., Strother S.C., Kennedy S.H., Frey B.N. Cortical thickness in major depressive disorder: a systematic review and meta-analysis. Progr. Neuro-psychopharmacol. Biol. Psychiatry. 2019;88(January):287–302. doi: 10.1016/j.pnpbp.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.-L., Meuwese R., Blackmore S.-J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017;37(12):3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi, Leonardo, Lisa Garczarek, Deborah Janowitz, Dan J. Stein, Katharina Wittfeld, Henrik Dobrowolny, Jim Lagopoulos, et al. 2019. undefined/ed. “Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort.” Psychol. Med., 1–12. 10.1017/S003329171900093X. [DOI] [PMC free article] [PubMed]
- Trivedi M.H., McGrath P.J., Fava M., Parsey R.V., Kurian B.T., Phillips M.L., Oquendo M.A. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J. Psychiatr. Res. 2016;78(July):11–23. doi: 10.1016/j.jpsychires.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B.S.C., Sui J., Hellemann G., Leaver A.M., Espinoza R.T., Woods R.P., Abbott C.C., Joshi S.H., Narr K.L. Inter and intra-hemispheric structural imaging markers predict depression relapse after electroconvulsive therapy: a multisite study. Transl. Psychiatry. 2017;7(12):1270. doi: 10.1038/s41398-017-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “WHO | Depression.” n.d. WHO. Accessed December12, 2018. http://www.who.int/mental_health/management/depression/en/.
- Yang X., Wang Y., Huang J., Zhu C., Liu X., Eric F., Cheung C., Guang-rong X., Raymond C., Chan K. Increased prefrontal and parietal cortical thickness does not correlate with anhedonia in patients with untreated first-episode major depressive disorders. Psychiat. Res.-Neuroimaging. 2015;234(1):144–151. doi: 10.1016/j.pscychresns.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Young J.J., Silber T., Bruno D., Galatzer-Levy I.R., Pomara N., Charles R.M. Is there progress? An overview of selecting biomarker candidates for major depressive disorder. Front. Psychiatry. 2016;7:72. doi: 10.3389/fpsyt.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Linn K.A., Cook P.A., Phillips M.L., McInnis M., Fava M., Trivedi M.H., Weissman M.M., Shinohara R.T., Sheline Y.I. Statistical harmonization corrects site effects in functional connectivity measurements from multi-site FMRI data. Hum. Brain Mapp. 2018;39(11):4213–4227. doi: 10.1002/hbm.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chen L., Zhang W., Xiao Y., Shah C., Zhu H., Yuan M. Gray matter abnormalities in non-comorbid medication-naive patients with major depressive disorder or social anxiety disorder. EBioMedicine. 2017;21(July):228. doi: 10.1016/j.ebiom.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorlu N., Cropley V.L., Zorlu P.K., Delibas D.H., Adibelli Z.H., Baskin E.P., Esen O.S., Bora E., Pantelis C. Effects of cigarette smoking on cortical thickness in major depressive disorder. J. Psychiatr. Res. 2017;84(January):1–8. doi: 10.1016/j.jpsychires.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Zuo Zhiwei, Ran Shuhua, Wang Yao, Li Chang, Han Qi, Tang Qianying, Qu Wei, Li Haitao. Asymmetry in cortical thickness and subcortical volume in treatment-naïve major depressive disorder. NeuroImage : Clinical. 2019;21 doi: 10.1016/j.nicl.2018.101614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.