Abstract
Objective
Deposit contracts, where participants “bet” on achieving a goal and get their money back only if successful, have been shown to be effective for short-term weight-loss. This pilot study examined their effect on weight-loss maintenance.
Methods
From 2016 to 2018, we conducted a pilot, 50-week randomized controlled trial among 42 hospital employees (19 intervention and 23 control), in Boston, Massachusetts, who lost ≥10 lb (4.5 kg) in the two years prior to enrollment. Participants were recruited primarily in-person. Both control and intervention participants were asked to attend a weigh in weekly and received weekly email communication. Intervention participants also entered into a deposit contract to maintain baseline weight within ≤2 lb (0.9 kg). We examined weight change from baseline to 50 weeks (primary outcome) and maintenance of baseline weight at 50 weeks (secondary outcome; binary – yes v. no). Participants completed baseline and follow-up surveys and received incentives for completion.
Results
At baseline, mean (SD) weight was 83.2 (15.5 kg) among intervention and 80.7 (14.5 kg) among control participants. After 50 weeks, intervention participants had slightly less but non-significant weight gain (adjusted β −1.12 kg; 95% CI −5.28, 3.05) than control participants; 73.7% of intervention v. 39.1% of control participants met their weight-loss maintenance goal by study end (adjusted OR 4.78; 95% CI 1.01, 22.71).
Conclusions
A deposit contract was not associated with differences in weight but led to more participants meeting their weight-loss maintenance goals; a deposit contract for weight-loss maintenance should be tested in a full-scale intervention. Most intervention participants viewed the deposit contract as acceptable.
Keywords: Obesity, Weight-loss maintenance, Deposit contract
1. Introduction
Almost seven in 10 adults in the US have overweight or obesity (Ogden et al., 2014). Obesity is one of the top causes of preventable mortality and morbidity, and weight-loss can reduce these risks (Haslam and James, 2005, Poobalan et al., 2007). Although research has identified strategies that are effective for short-term weight-loss, weight-loss maintenance is difficult (Anderson et al., 2001, Dombrowski et al., 2014, Franz et al., 2007). Weight-loss often plateaus after six months of a behavioral intervention and is typically followed by a period of gradual weight regain (Avenell et al., 2004, Dombrowski et al., 2010). Even in an intensive weight-loss study of more than 800 subjects who received 72 counseling sessions over two years, participants had an average weight-loss of 13 lb (6 kg) at six months but only 6–8 lb (3–4 kg) after two years (Katan, 2009, Sacks et al., 2009). Weight regain is even more common in less intensive interventions and among populations with lower education and lower income (Franz et al., 2007, Katan, 2009). Myriad factors, including declining resting and total energy expenditure, difficulty adhering to lifestyle changes over time, and hormonal changes promoting hunger may counteract weight-loss maintenance (Anderson et al., 2001, Leibel and Hirsch, 1984, Leibel et al., 1995). Prevention of weight regain is key to ensuring long-lasting health benefits from initial weight-loss (Penn et al., 2013).
While workplaces seem like ideal settings to promote health because Americans spend many of their waking hours at work, recent findings show that current workplace wellness initiatives are not generally effective in improving health outcomes (Song and Baicker, 2019). Employers have powerful economic incentives to promote healthy weight because of higher health care costs and absenteeism among employees with obesity (Trogdon et al., 2008, Trogdon et al., 2009). New approaches to workplace wellness, like deposit contracts, have been used to encourage behavior change among employees, and further research is needed to measure their impact (Kullgren et al., 2016, Halpern et al., 2018, Halpern et al., 2015). In a deposit contract, subjects “bet” on achieving a specific goal (e.g. losing or maintaining weight, taking medications) and get their money back only if they successfully meet that goal. The behavioral economic theory of loss aversion motivates the use of deposit contracts. While any financial incentive might promote behavior change, the strongest financial motivator appears to be the natural inclination to avoid losses rather than respond to a potential financial reward (Connolly and Butler, 2006, Kahneman and Tversky, 1979). Loss aversion might be particularly important when a behavior change is difficult, as with weight-loss maintenance.
A recent study of lotteries and direct financial incentives found no effect on weight-loss maintenance (Yancy et al., 2018). In contrast, previous research has found some success with deposit contracts for short-term weight-loss (10 and 16 weeks, respectively) (Jeffery et al., 1978, Volpp et al., 2008). A longer-term study examined the effect of a deposit contract over 32 weeks and demonstrated successful weight-loss, but over the subsequent 36 weeks after the completion of the study, subjects regained weight (John et al., 2011). A cluster randomized controlled trial (RCT) of 32 workplaces in 1993 found no effect of two-year deposit contracts on weight-loss; however, that study included employees with healthy weights on average (mean BMI of 24.5 kg/m for intervention women), and higher participation in the incentive program was associated with greater weight-loss (Jeffery et al., 1993). We are aware of no studies that have examined a deposit contract solely for the purpose of weight-loss maintenance, and a recent review of financial incentives identified maintenance incentives as an important research gap (Ananthapavan et al., 2018).
We conducted a pilot, randomized controlled trial of a deposit contract specifically targeting 1-year weight-loss maintenance among hospital employees who had lost at least 10 lb (4.5 kg) in the two years prior to enrollment.
2. Methods
2.1. Design
We conducted a 50-week randomized control trial among Brigham and Women’s Hospital employees, from June 2016 through June 2018. Forty-two participants were randomly assigned to a control or a deposit contract arm with the goal of maintaining their baseline weight (as measured at enrollment), defined in this study as ≤2 lb (0.9 kg) above baseline weight (equivalent to 20% of the minimum weight-loss for enrollment). In the deposit contract, subjects “bet” on achieving weight-loss maintenance and received their money back only if they met their goal.
2.2. Study eligibility
Eligible participants were ≥18 years old and full or part-time employees of Brigham and Women’s Hospital, Brigham and Women’s Faulkner Hospital, or Dana Farber Cancer Institute who had objective documentation of ≥10 lb (4.5 kg) weight-loss in the two years prior to enrollment. Participants were required to have overweight or obesity (BMI ≥ 25 kg/m2) before weight-loss. Prior evidence shows strong health benefits (i.e. lower blood pressure, glucose, and LDL cholesterol) for 5% body weight-loss (Williamson et al., 2015). To make it easier for employees to identify if they would be eligible for the study, we chose a fixed weight loss amount of 10 lb (4.5 kg) as an inclusion criteria, equivalent to 5% body weight-loss for an individual weighing 200 lb, rather than percentage of body weight lost. We verified prior weight-loss in the electronic medical record of Partners Healthcare, a large healthcare system based in Eastern Massachusetts, including Brigham and Women’s and Massachusetts General Hospitals among others, for patients receiving care there; from letters by participants’ physicians; or in documentation from a weight-loss program such as Weight Watchers. Exclusion criteria included weight-loss surgery in the three years before enrollment, a medical diagnosis (e.g. cancer, mental illness, eating disorder) that would substantially affect weight, and a planned or current pregnancy during the expected study period.
We recruited participants through employee newsletters, bulletin boards, and in-person at high foot traffic areas in the hospitals; prior to in-person meetings for enrollment, we did preliminary eligibility screening over the phone or in-person. The Institutional Review Board (IRB) of Harvard Pilgrim Health Care approved this study. Prior to initiation, we registered the study with ClinicalTrials.gov (NCT02709642).
2.3. Study procedures for control and intervention participants
A computerized routine randomly assigned 60 participant IDs to a treatment condition (half intervention, half control) before we started recruitment. Using results from a prior study of deposit contracts for weight loss by Volpp et al (which found a difference of 10 lb between intervention and control arms), we conservatively estimated that we would need 26 participants per arm to achieve 80% power to detect an expected lower difference of 7 lb between arms at 1 year (Volpp et al., 2008). At enrollment, a research assistant measured each participant’s height and weight using a Seca 213 stadiometer and Seca scale. Following this, the research assistant opened the randomization assignment (control vs. deposit contract) and provided each participant a study ID number.
For both control and intervention participants, we requested that all participants attend a weekly weigh-in for the entire 50-week study period, using study provided Seca scales, at designated locations at the Brigham and Women’s and Brigham and Women’s Faulkner Hospitals, including in Occupational Health and Nutrition Services offices. Staff in these offices recorded weights and transmitted them to the study team. Participants received a weekly email with information regarding their weight and how much they had gained/lost in the prior week, noting when they missed a weigh-in, and their baseline weight. At study completion, participants received a $40 prepaid cash card incentive if they attended at least half of the weekly weigh-ins during the 50-week period.
All participants completed a baseline survey and were asked to complete a follow-up survey 50 weeks after their enrollment date (Appendix 1). Surveys queried use of weight-loss methods, motivation for weight loss, confidence in maintaining their weight, and demographic information; many of the questions were taken from previously validated surveys (Wing and Phelan, 2005, Seward et al., 2018, Hunt et al., 2007). Participants received a $30 incentive for both the enrollment and follow-up survey. We also asked participants, including those who had withdrawn from the active study, to attend a final weigh-in with a research assistant at the end of the study period. If an in-person follow-up weight measure was not possible, we accessed participants’ electronic health records, with their signed consent, for the most recent weight measurement available.
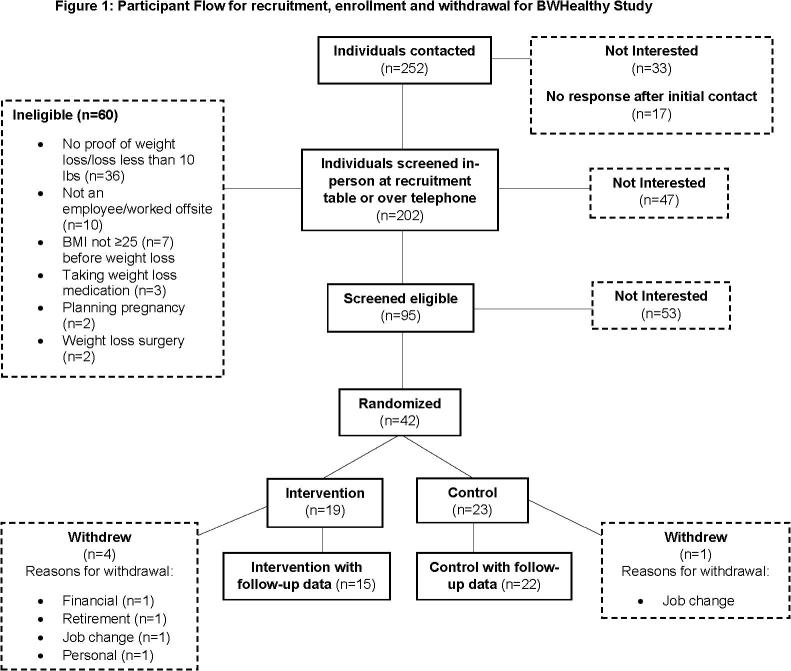
We contacted 252 individuals and screened 202 for eligibility by phone or in person at a recruiting table in the hospitals. During this screening, 47 were not interested in continuing and 60 were ineligible, mostly because they could not provide objective documentation of a ≥10-pound weight loss in the prior two years, were not an employee, or worked offsite at a location where weigh-ins were not available (Fig. 1). Of the 95 who were deemed eligible and expressed a willingness to participate, 53 later indicated they were not interested in enrolling, and 42 were randomized.
Fig. 1.
Participant Flow for recruitment, enrollment and withdrawal for BWHealthy Study. This figure shows the individuals contacted for enrollment in the BWHealthy Weight study in Boston, MA between 2016 and 2018. It follows the flow of individuals’ eligibility status, interest in participation, randomization and withdrawals.
2.4. Intervention
Those assigned to the deposit contract intervention were asked to complete the same activities as control participants (surveys, weekly weigh-ins with follow-up emails, end of study weigh-in). In addition, they were required to deposit at least $100 with the study team. Participants could elect to deposit more, but no one did so. For intervention participants, we divided the 50 weeks of participation into five 10-week periods. For each period, each participant’s goal was to maintain their baseline weight within no more than 2 lb (0.9 kg) gained. We set this as the threshold to allow for some margin above baseline weight, in this case 20% above the minimum weight loss required for eligibility. For any week during the trial that participants did not maintain their weight at ≤2 lb (0.9 kg) gained, they forfeited $10 of their $100 contribution. They also forfeited $10 if they missed more than two consecutive weigh-ins; we set this requirement to encourage consistent measures while also allowing participants to skip a weigh-in if they were out of work or did not want a recorded weight in a given week (e.g., because of a natural weight fluctuation).
The weekly email sent to intervention participants was the same as for control participants, except that we additionally reported the amount of money that participants had been awarded back (from their initial deposit) or forfeited. At the end of each 10-week period, the funds that any participants had forfeited for that period were distributed as a bonus among intervention participants who were successful in maintaining their weight for that 10-week period. For each consecutive 10-week period, participants were asked to make an additional deposit to maintain the $100 deposit balance if they had forfeited money during any weeks of the previous 10-week period. If participants were not willing to deposit any more money, we asked them to continue in the study regardless, continuing to put their remaining deposit balance at risk.
A committee of study and independent investigators biannually reviewed the masked (assigned groups were not disclosed) weight trajectories for all participants to monitor unsafe weight-loss, defined as 20 lb (9.1 kg) lost in a month. While protocols were in place to report unsafe weight-loss as adverse events to the IRB, no study participants met these criteria.
2.5. Analysis
To assess the primary outcome of differential weight change, intervention vs. control, from baseline to 50 weeks, we used multivariable linear regression models, adjusted for age, sex, race/ethnicity, baseline weight, amount of weight loss in the prior two years, days since maximum weight in the last two years, and time elapsed since enrollment. We used logistic regression models to analyze successful weight-loss maintenance at 50 weeks (≤2 lb (0.9 kg) above baseline weight) calculated as the odds ratio for meeting the goal, intervention vs. control. Our main analyses included all participants including the five who withdrew from the study before completion; for those five, we carried forward their last measured weight. As a sensitivity analysis, we included only completers. As a secondary analysis, we examined effect modification by baseline weight status by running stratified models (<30 vs. ≥30 kg/m2) and computed interaction p-values.
3. Results
Most participants were female (69%), non-Hispanic white (86%), had annual household income over $70,000 (81%), had college or greater education (93%), and worked full-time (88%) (Table 1); baseline characteristics were similar between intervention and control. At baseline, mean (SD) age was 50.7 (11.7) and 46.0 (10.2) years, weight was 83.2 (15.5) and 80.7 (14.5) kg, and BMI was 29.9 (4.6) and 29.0 (4.3) kg/m among intervention and control participants, respectively. Most participants (intervention 63%; control 52%) were very or somewhat dissatisfied with their weight at baseline (Table 1). In total five participants withdrew from the study (4 intervention; 1 control) for a variety of reasons including financial, retirement, job change and personal (Fig. 1). Due to lower recruitment levels than expected, planned for up to 60 people, we had more participants randomized, by chance, into the control group (23 control; 19 intervention).
Table 1.
Participant baseline characteristics according to intervention among 42 participants in the BWHealthy Weight Study.†
| Baseline Characteristics | Intervention | Control |
|---|---|---|
| n = 19 | n = 23 | |
| N (%) | ||
| Female | 13 (68.4) | 16 (69.6) |
| Race/ethnicity | ||
| Hispanic | 2 (10.5) | 2 (8.7) |
| Non-Hispanic Black | 1 (5.3) | 1 (4.3) |
| Non-Hispanic White | 16 (84.2) | 20 (87.0) |
| Annual household income | ||
| $20,001 to $40,000 | 2 (10.5) | 1 (4.3) |
| $40,001 to $70,000 | 4 (21.1) | 1 (4.3) |
| $70,001 to $150,000 | 7 (36.8) | 11 (47.8) |
| More than $150,000 | 6 (31.6) | 10 (43.5) |
| Education | ||
| High school graduate or some college | 1 (5.3) | 2 (8.7) |
| College graduate | 6 (31.6) | 9 (39.1) |
| Graduate school | 12 (63.2) | 12 (52.2) |
| Full-time worker | 17 (89.5) | 20 (87.0) |
| How satisfied with current weight at baseline | ||
| Very or somewhat dissatisfied | 12 (63.2) | 12 (52.2) |
| Neither dissatisfied nor satisfied | 2 (10.5) | 3 (13.0) |
| Somewhat or very satisfied | 5 (26.3) | 8 (34.8) |
| How satisfied with current weight at follow-up | ||
| Very or somewhat dissatisfied | 10 (71.4) | 14 (66.7) |
| Neither dissatisfied nor satisfied | 1 (7.1) | 2 (9.5) |
| Somewhat or very satisfied | 3 (21.4) | 5 (23.8) |
| Mean (SD) | ||
| Age at baseline, years | 50.7 (11.7) | 46.0 (10.2) |
| Highest weight ever, kg | 97.5 (20.6) | 94.7 (16.3) |
| Highest weight in year prior to enrollment, kg | 92.8 (19.2) | 91.9 (15.5) |
| Height, meters | 1.7 (0.1) | 1.7 (0.1) |
| Weight-loss in the prior 2 years, kg | 8.3 (4.6) | 11.1 (5.5) |
| Time elapsed from max weight in prior 2 years to enrollment date, days* | −366 (1 4 5) | −315 (1 8 0) |
| Baseline measures | ||
| Weight, kg | ||
| BMI, kg/m2 | 83.2 (15.5) | 80.7 (14.5) |
†Data collected from Brigham and Women’s Hospital employees in Boston, MA between 2016 and 2018.
*One participant was missing max weight loss in the prior two years, so we used the self-reported max weight loss in one year prior.
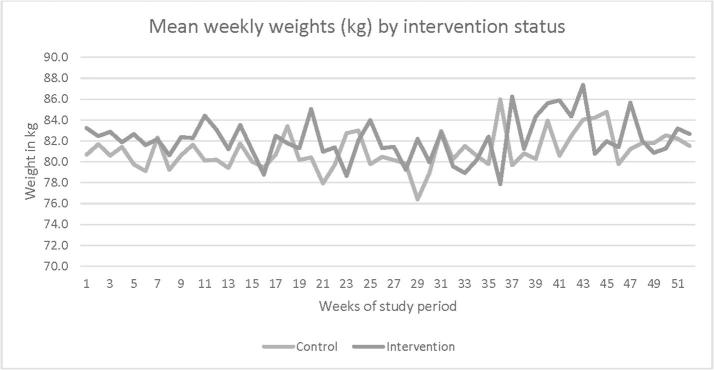
After a mean of 48.9 weeks after enrollment, using all participants (N = 42) with last weight carried forward for the five who withdrew from the study, change in weight from baseline was 0.22 (5.51) kg for intervention and 1.06 (5.56) kg for control participants (Table 2 and Fig. 2). For completers only, mean (SD) change in weight was −0.03 (5.94) and 0.83 (5.75) kg (N = 37). Using data from all participants, 73.7% (N = 14) and 39.1% (N = 9) of intervention and control participants met their goal of weight-loss maintenance (≤2 lb gained from baseline) after 50 weeks from enrollment; for completers only, 75% and 43% met their goal. In multivariable linear regression, compared with controls, intervention participants had non-significant but slightly less weight gain (adjusted β −1.12 kg; 95% CI −5.28, 3.05) by the end of study (Table 3, Model 4). For intervention participants, the odds of meeting the weight-loss maintenance goal by study completion was 4.78 (95% CI 1.01, 22.71; p = 0.05) compared to control participants (Table 4, Model 4). When assessing potential effect modification by baseline weight status (<30 vs. ≥ 30 kg/m2), we found larger differences among participants with baseline BMI < 30. However, the sample size was small, and all interaction p-values were non-significant (Supplemental Table 1).
Table 2.
Weight outcomes according to intervention among 42 participants in the BWHealthy Weight Study†
| Weight Outcomes | Intervention | Control |
|---|---|---|
| n=19 | n=23 | |
| Change baseline to follow-up* | Mean (SD) | |
| Time elapsed, weeks | 46.6 (15.5) | 50.8 (6.7) |
| Change in weight, kg | 0.22 (5.51) | 1.06 (5.56) |
| Change in BMI, kg/m2 | 0.04 (1.78) | 0.38 (2.03) |
| N (%) | ||
| Met goal (≤2 lb from baseline to follow-up), % | 14 (73.7) | 9 (39.1) |
| Sensitivity** | Mean (SD) | |
| Time elapsed, weeks | 51.8 (9.6) | 51.8 (5.9) |
| Change in weight, kg | −0.03 (5.94) | 0.83 (5.75) |
| Change in BMI, kg/m2 | −0.06 (1.92) | 0.29 (2.11) |
| N (%) | ||
| Met goal (≤2 lb from baseline to follow-up), % | 12 (75.0) | 9 (42.9) |
†Data collected from Brigham and Women’s Hospital employees in Boston, MA between 2016 and 2018.
*For participants who withdrew from the study before the completion of 50 weeks, the last weigh-in value for each participant was carried forward (N = 5, 4 intervention and 1 control participants). Differences in time elapsed were due to 4 people lost to follow-up in the intervention arm (including 1 who dropped out after only 9 weeks) vs. only 1 lost to follow-up in the control.
**Excluded participants who withdrew from the study before the completion of 50 weeks (N = 5).
Fig. 2.
BWHealthy Study participant mean weekly weights by intervention status over 50 weeks.
Table 3.
Difference in weight and BMI from baseline to ~ 50-week follow-up among intervention vs. control participants in the BWHealthy Weight Study†
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| Outcome | ||||||||
| Difference baseline to follow-up, for intervention vs. control participants* | ||||||||
| Difference in weight, kg | −0.84 (−4.31, 2.63) | 0.63 | −1.17 (−4.97, 2.63) | 0.54 | −1.25 (−5.10, 2.61) | 0.52 | −1.12 (−5.28, 3.05) | 0.59 |
| Difference in BMI, kg/m2 | −0.34 (−1.55, 0.87) | 0.57 | −0.46 (−1.77, 0.86) | 0.49 | −0.46 (−1.79, 0.88) | 0.49 | −0.48 (−1.93, 0.96) | 0.50 |
| Sensitivity** | ||||||||
| Difference in weight, kg | −0.86 (−4.79, 3.06) | 0.66 | −0.93 (−5.27, 3.41) | 0.66 | −0.95 (−5.36, 3.46) | 0.66 | −0.58 (−5.39, 4.22) | 0.81 |
| Difference in BMI, kg/m2 | −0.36 (−1.72, 1.01) | 0.60 | −0.38 (−1.88, 1.13) | 0.61 | −0.38 (−1.91, 1.15) | 0.62 | −0.35 (−2.04, 1.33) | 0.67 |
*For participants who withdrew from the study before the completion of 50 weeks, the last weigh-in value for each participant was carried forward (N = 5; 4 intervention, 1 control).
**Excluded participants who withdrew from the study before the completion of 50 weeks (N = 5).
Model 1. Unadjusted linear regression
Model 2. Adjusted for age, sex, race/ethnicity, and time elapsed from baseline to follow-up (weeks)
Model 3. Model 2 additionally adjusted for baseline weight (for weight outcomes) or baseline BMI (for BMI outcomes)
Model 4. Model 3 additionally adjusted for weight loss in the prior 2 years and time elapsed from max weight in prior 2 years to enrollment date. One participant was missing max weight loss in the prior two years; we used the validated max weight loss in one year prior.
†Data collected from Brigham and Women’s Hospital employees in Boston, MA between 2016 and 2018.
Table 4.
Odds of meeting weight maintenance goal (≤2 lb gained from baseline to ~ 50-week follow-up) among intervention vs. control participants in the BWHealthy Weight Study†
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Outcome | ||||||||
| Met goal = yes* | 4.36 (1.16, 16.31) | 0.03 | 4.73 (1.07, 20.91) | 0.04 | 4.79 (1.08, 21.14) | 0.04 | 4.78 (1.01,22.71) | 0.05 |
| Sensitivity** | ||||||||
| Met goal = yes | 4.00 (0.96, 16.61) | 0.06 | 3.86 (0.82, 18.06) | 0.09 | 3.86 (0.82, 18.14) | 0.09 | 3.84 (0.76,19.46) | 0.10 |
*For participants who withdrew from the study before the completion of 50 weeks, the last weigh-in value for each participant was carried forward (N = 5; 4 intervention, 1 control).
**Excluded participants who withdrew from the study before the completion of 50 weeks (N = 5).
Model 1. Unadjusted logistic regression
Model 2. Adjusted for age, sex, race (white yes/no), and time elapsed from baseline to follow-up (weeks)
Model 3. Model 2 additionally adjusted for baseline weight
Model 4. Model 3 additionally adjusted for weight loss in the prior 2 years and time elapsed from max weight in prior 2 years to enrollment date
†Data collected from Brigham and Women’s Hospital employees in Boston, MA between 2016 and 2018.
At the time of enrollment, in responses to surveys, all participants reported being confident in their ability to maintain their current weight in the next year. Similarly, 95% (N = 18/19) of intervention participants and 96% (N = 22/23) of control participants reported being confident that they would lose more weight during the next year. At follow up, 91% (N = 30/33) reported confidence in their ability to maintain their weight in the next year. Fewer intervention (62%, N = 8/13) than control participants (85%, N = 17/20) reported confidence in their ability to lose more weight at the end of the study.
At baseline, all control participants reported that in the past 24 months they had dieted; 74% (N = 17) reported exercising, and 78% (N = 18) reported using other weight-loss maintenance strategies, including: weighing themselves more frequently, working to reduce stress, or maintaining a consistent eating pattern throughout the week. Among intervention participants 95% (N = 18/19) were dieting before enrollment, 37% (N = 7) reported exercising, and 89% (N = 17) used other weight-loss maintenance strategies. At follow-up, all participants were generally less adherent to the weight-loss strategies they had noted when the study began. More than two-thirds (71%, N = 15/21) of control participants reported dieting, 62% (N = 13) reported exercising, and 67% (N = 14) modified other behaviors during the study, whereas 64% (N = 9/14) of intervention participants reported dieting, 43% (N = 6) reported exercising, and 57% (N = 8) modified other behaviors during their 12-month participation in the study.
At follow-up, 71% of intervention participants agreed or strongly agreed that a deposit contract was an acceptable strategy for weight-loss maintenance, compared to 33% of controls. On average, intervention participants lost $60.95 (range $0 to $340) and gained $66.85 from bonuses (range $0, $183.57), an average net gain of $5.90. Among intervention participants, 84% received at least one bonus payment during the study period. The mean bonus payment was $24.40 (SD $14.58). Of the four participants that withdrew from the deposit contract arm, one lost their entire $100 contribution and cited financial constraints as a reason for not continuing; the other three lost $10, $10, and $20 before cashing out upon withdrawal.
4. Discussion
This workplace-based, pilot, randomized control trial examined the extent to which a deposit contract helped individuals to maintain their weight after previously losing at least 10 lb (4.5 kg). In models of all participants, intervention participants had higher odds (OR 4.78) of meeting their end of study weight goal than control participants; however, differences between intervention and control participants’ weight change by the end of the study did not meet statistical significance. Weight differences during the trial were very small when comparing intervention and control participants; control participants gained a mean of 2.3 lb (1.06 kg) and intervention participants 0.5 lb (0.22 kg). However, because the control participants gained on average just above the goal of ≤2 lb from their starting weight, most of the control participants failed to officially meet their goal. This led to substantial differences in that outcome comparing intervention to control participants. Based on survey responses regarding how intervention participants responded to engaging in a deposit contract, most intervention participants agreed that the deposit contract was an acceptable strategy for weight-loss maintenance.
Weight regain is almost ubiquitous among people who have previously lost weight. Numerous weight-loss studies have charted this phenomenon over one to two years of follow-up, with all showing at least some mean weight regain over time (Sacks et al., 2009, Wadden et al., 2011, Orchard et al., 2013). Bariatric surgical studies show the same, albeit from a lower nadir of weight (Sarzynski et al., 2011, Karlsson et al., 2007). Employers, clinicians, and patients are in need of evidence-based approaches to help mitigate the inevitable struggle with regaining weight. With the modest success of a deposit contract in this study, such an approach might be one option for helping patients over the long term. Weight-loss maintenance is particularly important for patients who achieve modest weight loss when they are actively trying to lose weight. Numerous studies have demonstrated health improvements with as little as 5% of baseline body weight loss (Magkos et al., 2016, Blackburn, 1995). However, those benefits will only accrue if maintained over the long term. Participants in this study had lost 8.8% (intervention) and 12.1% (control) of body weight prior to starting the study; maintaining a large portion of this weight loss, as was more likely in the intervention arm, would allow for long-term benefits. Because we found such limited differences in weight after 50 weeks, it is possible that weight tracking alone would be sufficient to achieve weight-loss maintenance.
Our results are comparable to prior studies of deposit contracts used for weight loss. In a large analysis of individuals who participated in online commitment contracts (of which deposit contracts are one type), Lesser et al. found an average of −0.39% weight loss per week, with perhaps selection bias driving some of the loss (Lesser et al., 2018). Kullgren et al. found in a workplace-based intervention that while participation was lower in a deposit contract arm of a weight-loss intervention, weight loss among that group was significantly more than the control group, particularly in the first 26 weeks of the study (Kullgren et al., 2016). Not all studies on deposit contracts or similar incentives have shown benefit. In a 2018 systematic review, Ananthapavan et al. found that programs with positive incentives were more effective and more popular than those using negative incentives (like deposit contracts) or a combination of negative and positive incentives (Ananthapavan et al., 2018). They also found that negative incentive programs had a higher attrition rate than those using positive incentives. Several other studies have had null results or postulated that positive outcomes may be driven or enhanced by other factors associated with use of a deposit contract (Thirumurthy et al., 2019). For example, participants assigned to a deposit contract arm may be more motivated to weigh-in regularly, a behavior which has been shown to support weight-loss maintenance (Wing and Phelan, 2005). Our results show that on average intervention participants attended slightly more weigh-ins (69.8% vs 64.8%) than control participants, 2.5 more weigh-ins over 50 weeks. We found this small difference even though intervention participants were penalized financially if they missed two consecutive weigh-ins. Individuals volunteering for deposit contract studies may already be highly motivated toward achieving the outcome for the study, leading to more successful weight loss or maintenance in both the intervention and control groups, diluting the effect of the intervention while showing more successful results compared to non-volunteers (Thirumurthy et al., 2019). Success outside of clinical trials is always hard to predict. However, our study may be closer to the real-world effect. We incorporated elements of a pragmatic trial; the study was low-cost and conducted within a workplace, utilizing existing resources to facilitate study activities. More and larger studies will be necessary to determine the role of deposit contracts, especially in weight-loss maintenance.
This study has several strengths and limitations. This was the first study to explicitly examine the effect of deposit contracts on weight-loss maintenance, and it continued for one year of follow-up, longer than the vast majority of prior studies of financial incentives on weight outcomes (Ananthapavan et al., 2018). Participants were generally positive about the study and the use of a deposit contract, and we demonstrated that such an intervention could be carried out in a pragmatic way using resources available in a large workplace. Sample size was limited to 42 participants, however, because recruitment was more challenging than expected, particularly due to the eligibility requirement to document prior weight-loss within the specific 2-year window time-period. The $100 required deposit contract may have been a barrier to enrollment, especially for lower-income participants, leading to higher income and more educated participants than the overall hospital workforce. Even without financial barriers, some potential participants may not have wanted to enter into a deposit contract. Acceptability of deposit contracts was likely higher among study participants than in the general population. For example, in one large, employer-based randomized controlled trial of smoking cessation by Halperin and colleagues, deposit contracts were more effective than a pure reward program for which there was no possible financial penalty when failing to meet a goal (Halpern et al., 2015). However, many fewer participants (13.7% vs. 90%) were willing to enroll in the deposit contract compared to the reward program. In this study, most of the intervention participants rated the intervention as acceptable. However, four of the deposit contract participants dropped out of the study, and another four stayed in the study but chose not to add additional funds to their account when their financial loss was more than $100.
We also learned several important logistical lessons from this study. Enrollment might be improved with less restrictive criteria, such as allowing self-reported information for documentation of prior weight loss. The financial management of this study was laborious, requiring frequent financial transactions to deposit contributions from participants and pay out bonuses. Using a less cumbersome technological solution for managing the financial aspects of a deposit contract, such as PayPal or Venmo, would make the process more efficient. In this study, we only returned money to participants at the end of the study, to ease processing burden. Participants might be motivated by immediate feedback or rewards. To simulate this, we notified participants weekly about the amount of money they earned. However, receiving the money immediately after weigh-ins, via electronic transfer, could be a more powerful motivation. Requiring participants to get weigh-ins at the workplace can also introduce a burden that could be alleviated with web-enabled home scales. Alternative funding structures could be explored such as employees setting their own dollar amount for the deposit contract, with weekly contributions allowed rather than a larger sum at the onset. The optimal amount for a deposit contract remains uncertain and could be explicitly tested in a study with varying contributions (Lewis and Block, 2015). These additional innovations could make a workplace-based deposit contract intervention more straightforward to implement.
5. Conclusion
A workplace deposit contract intervention over 50 weeks was associated with higher success in achieving weight-loss maintenance but did not show significant differences in overall weight change compared to a control group. This was a low-cost intervention for employers that was popular among participants. With several administrative changes to enhance recruitment, ease administrative burdens, increase accessibility of the program for lower-income individuals, and make weigh-ins less burdensome, this type of intervention could be a useful workplace wellness offering to employees.
Data sharing statement
We posted the study protocol and analysis plan on clinicaltrials.gov. We are willing to share data upon request and as approved by the Institutional Review Board of Harvard Pilgrim Health Care.
Clinical trial registration
This study is registered with www.ClinicalTrials.gov under identifier: NCT02709642
Final disclosure
This study was funded primarily with a career development award from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (K23 HL111211, PI: Block), and Jason P. Block, Michael W. Seward, Lauren P. Cleveland, and Denise Simon received funding from this award. Support from a gift of the McLaughlin Family Foundation (PI: Block) also was used for this study, with funding going to Michael W. Seward and Lauren P. Cleveland. These sponsors were not involved in the study design, collection, analysis, and interpretation of data, the writing of the manuscript, or the decision to submit for publication. Florencia Halperin reports receiving salary and stock options from Form Health, Inc.
Author contributions
JB conceived of and designed the study, interpreted the data and drafted the manuscript. LC collected the data, interpreted the data, and drafted the manuscript. DS was involved with analyzing the data, interpreting the data and drafting the manuscript. MS was involved with collecting the data, interpreting the data and drafting the manuscript. SRS was involved in analyzing the data, interpreting the data and drafting the manuscript. KL was involved in critically reviewing the manuscript for important intellectual content. CBR, FH, KDM were involved in the design of the study and in critically reviewing the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Acknowledgments
Acknowledgements
We thank the participants and the Occupational Health and Nutritional Services staff at Brigham and Women’s Hospital.
Funding
This work was supported by the National Institutes of Health [grant number K23 HL111211] and a gift from the McLaughlin Family Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2020.101061.
Contributor Information
Lauren P. Cleveland, Email: lauren_cleveland@harvardpilgrim.org.
Jason P. Block, Email: lauren_cleveland@harvardpilgrim.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam D.W., James W.P. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Poobalan A.S., Aucott L.S., Smith W.C., Avenell A., Jung R., Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes. Rev. 2007;8(6):503–513. doi: 10.1111/j.1467-789X.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- Anderson J.W., Konz E.C., Frederich R.C., Wood C.L. Long-term weight-loss maintenance: a meta-analysis of US studies. Am. J. Clin. Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- Dombrowski S.U., Knittle K., Avenell A., Araujo-Soares V., Sniehotta F.F. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348 doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M.J., VanWormer J.J., Crain A.L. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Avenell A., Broom J., Brown T.J. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol. Assess. 2004;8(21):1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- Dombrowski S.U., Avenell A., Sniehott F.F. Behavioural interventions for obese adults with additional risk factors for morbidity: systematic review of effects on behaviour, weight and disease risk factors. Obesity Facts. 2010;3(6):377–396. doi: 10.1159/000323076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M.B. Weight-loss diets for the prevention and treatment of obesity. N Engl. J. Med. 2009;360(9):923–925. doi: 10.1056/NEJMe0810291. [DOI] [PubMed] [Google Scholar]
- Sacks F.M., Bray G.A., Carey V.J. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl. J. Med. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel R.L., Hirsch J. Diminished energy requirements in reduced-obese patients. Metabolism. 1984;33(2):164–170. doi: 10.1016/0026-0495(84)90130-6. [DOI] [PubMed] [Google Scholar]
- Leibel R.L., Rosenbaum M., Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Penn L., White M., Lindstrom J. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Baicker K. Effect of a workplace wellness program on employee health and economic outcomes: a randomized clinical trial. JAMA. 2019;321(15):1491–1501. doi: 10.1001/jama.2019.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trogdon J.G., Finkelstein E.A., Hylands T., Dellea P.S., Kamal-Bahl S.J. Indirect costs of obesity: a review of the current literature. Obes. Rev. 2008;9(5):489–500. doi: 10.1111/j.1467-789X.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- Trogdon J., Finkelstein E.A., Reyes M., Dietz W.H. A return-on-investment simulation model of workplace obesity interventions. J. Occup. Environ. Med. 2009;51(7):751–758. doi: 10.1097/JOM.0b013e3181a86656. [DOI] [PubMed] [Google Scholar]
- Kullgren J.T., Troxel A.B., Loewenstein G. A randomized controlled trial of employer matching of employees' monetary contributions to deposit contracts to promote weight loss. Am. J. Health Promot. 2016;30(6):441–452. doi: 10.1177/0890117116658210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern S.D., Harhay M.O., Saulsgiver K., Brophy C., Troxel A.B., Volpp K.G. A pragmatic trial of E-cigarettes, incentives, and drugs for smoking cessation. N Engl. J. Med. 2018;378(24):2302–2310. doi: 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- Halpern S.D., French B., Small D.S. Randomized trial of four financial-incentive programs for smoking cessation. N Engl. J. Med. 2015;372(22):2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Butler D. Regret in economic and psychological theories of choice. J. Behav. Decis. Making. 2006;19:139–158. [Google Scholar]
- Kahneman D., Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Yancy W.S., Jr., Shaw P.A., Wesby L. Financial incentive strategies for maintenance of weight loss: results from an internet-based randomized controlled trial. Nutr. Diabetes. 2018;8(1):33. doi: 10.1038/s41387-018-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery R.W., Thompson P.D., Wing R.R. Effects on weight reduction of strong monetary contracts for calorie restriction or weight loss. Behav. Res. Ther. 1978;16(5):363–369. doi: 10.1016/0005-7967(78)90005-0. [DOI] [PubMed] [Google Scholar]
- Volpp K.G., John L.K., Troxel A.B., Norton L., Fassbender J., Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John L.K., Loewenstein G., Troxel A.B., Norton L., Fassbender J.E., Volpp K.G. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. 2011;26(6):621–626. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery R.W., Forster J.L., French S.A. The Healthy Worker Project: a work-site intervention for weight control and smoking cessation. Am. J. Public Health. 1993;83(3):395–401. doi: 10.2105/ajph.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthapavan J., Peterson A., Sacks G. Paying people to lose weight: the effectiveness of financial incentives provided by health insurers for the prevention and management of overweight and obesity – a systematic review. Obes. Rev. 2018;19(5):605–613. doi: 10.1111/obr.12657. [DOI] [PubMed] [Google Scholar]
- Williamson D.A., Bray G.A., Ryan D.H. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring, Md). 2015;23(12):2319–2320. doi: 10.1002/oby.21358. [DOI] [PubMed] [Google Scholar]
- Wing R.R., Phelan S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005;82(1 Suppl):222s–225s. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- Seward M.W., Simon D., Richardson M., Oken E., Gillman M.W., Hivert M.F. Supporting healthful lifestyles during pregnancy: a health coach intervention pilot study. BMC Pregnancy Childbirth. 2018;18(1):375. doi: 10.1186/s12884-018-2010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M.K., Barbeau E.M., Lederman R. Process evaluation results from the Healthy Directions-Small Business study. Health Educ. Behav. 2007;34(1):90–107. doi: 10.1177/1090198105277971. [DOI] [PubMed] [Google Scholar]
- Wadden T.A., Volger S., Sarwer D.B. A two-year randomized trial of obesity treatment in primary care practice. N Engl. J. Med. 2011;365(21):1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard T.J., Temprosa M., Barrett-Connor E. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet. Med. 2013;30(1):46–55. doi: 10.1111/j.1464-5491.2012.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzynski M.A., Jacobson P., Rankinen T. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int. J. Obes. (Lond). 2011;35(5):676–683. doi: 10.1038/ijo.2010.166. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Taft C., Ryden A., Sjostrom L., Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int. J. Obes. (Lond). 2007;31(8):1248–1261. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- Lewis K.B., Block J.P. Incentivizing Health Behaviors. In: Roberto C., Kawachi I., editors. Behavioral Economics and Public Health. Oxford University; 2015. [Google Scholar]
- Magkos F., Fraterrigo G., Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3(Suppl 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Lesser L.I., Thompson C.A., Luft H.S. Association between monetary deposits and weight loss in online commitment contracts. Am J Health Promot. 2018;32(1):198–204. doi: 10.1177/0890117116661157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumurthy H., Asch D.A., Volpp K.G. The uncertain effect of financial incentives to improve health behaviors. JAMA. 2019 doi: 10.1001/jama.2019.2560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.