Highlights
-
•
Reward anticipation under threat-of-shock was explored in healthy volunteers with and without increased familial vulnerability to depression.
-
•
Offspring of depressed parents showed lower activation of the dorsal striatum across all conditions, possibly promoting decreased goal-oriented behaviors.
-
•
Offspring of depressed parents demonstrated lower ventral striatum reactivity under stress, in particular when the task was less demanding.
-
•
Stress amplified reward-related putamen reactivity when the task was more demanding in offspring of depressed parents, reflecting a potential neural signature of increased sensitivity to negative feedback.
-
•
Stress and attentional resources might interact to modulate the sensitivity to negative feedback, the ability to encode incentive value and to engage in motivated behaviors, specifically in at-risk individuals.
Keywords: Vulnerability, Major depressive disorder, Reward, Stress, Striatum, fMRI
Abstract
Introduction
Anhedonia, a core symptom of Major Depressive Disorder (MDD), manifests as a lack or loss of motivation as reflected by decreased reward responsiveness, at both behavioral and neural (i.e., striatum) levels. Exposure to stressful life events is another important risk factor for MDD. However, the mechanisms linking reward-deficit and stress to MDD remain poorly understood. Here, we explore whether the effects of stress exposure on reward processing might differentiate between Healthy Vulnerable adults (HVul, i.e., positive familial MDD) from Healthy Controls (HCon). Furthermore, the well-described reduction in cognitive resources in MDD might facilitate the stress-induced decrease in reward responsiveness in HVul individuals. Accordingly, this study includes a manipulation of cognitive resources to address the latter possibility.
Methods
16 HVul (12 females) and 16 gender- and age-matched HCon completed an fMRI study, during which they performed a working memory reward task. Three factors were manipulated: reward (reward, no-reward), cognitive resources (working memory at low and high load), and stress level (no-shock, unpredictable threat-of-shock). Only the reward anticipation phase was analyzed. Imaging analyses focused on striatal function.
Results
Compared to HCon, HVul showed lower activation in the caudate nucleus across all conditions. The HVul group also exhibited lower stress-related activation in the nucleus accumbens, but only in the low working memory (WM) load condition. Moreover, while stress potentiated putamen reactivity to reward cues in HVul when the task was more demanding (high WM load), stress blunted putamen reactivity in both groups when no reward was at stake.
Conclusion
Findings suggest that HVul might be at increased risk of developing anhedonic symptoms due to weaker encoding of reward value, higher difficulty to engage in goal-oriented behaviors and increased sensitivity to negative feedback, particularly in stressful contexts. These findings open new avenues for a better understanding of the mechanisms underlying how the complex interaction between the systems of stress and reward responsiveness contribute to the vulnerability to MDD, and how cognitive resources might modulate this interaction.
1. Introduction
Major Depressive Disorder (MDD) is a prevalent mental disorder affecting worldwide more than 4.4% of the population (World Health Organization, 2017). According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (American Psychiatric Association, 2013), long-lasting depressed mood and anhedonia are core symptoms of MDD. At the neural level, anhedonia is underpinned by a dysfunction of the reward circuitry, which is thought to constitute a major biological marker of MDD as well as a predisposition for increased vulnerability to MDD (Hasler et al., 2004; Martin-Soelch et al., 2009). The presence of anhedonic symptoms has been robustly associated with a dysregulation of reward processing in healthy adults (Chung and Barch, 2015; Harvey et al., 2007), in MDD patients (Epstein et al., 2006; Pizzagalli et al., 2009), and in unaffected offspring of MDD patients (Liu et al., 2016). Among MDD patients, a wealth of data provides strong evidence for impaired reward processes during the anticipation of a pleasurable event (Hägele et al., 2015; for a review see: Zhang et al., 2013). With the aim of understanding how reduced reward responsiveness might confer risk for major depression, recent studies have explored reward processes in first-degree relatives of MDD patients. Emerging findings show diminished striatal reactivity in unaffected offspring of MDD patients during the anticipation of potential rewards compared to healthy controls (Olino et al., 2014). With this in mind, the purpose of our study is to explore how both stress exposure and different levels of cognitive demands interact to modulate reward processing in individuals at increased familial risk for MDD, i.e., offspring of parents with a history of MDD.
Extensive research has demonstrated that stressful life events are intimately linked to depressive vulnerability by increasing the likelihood of the onset of the first depressive episode (Hammen, 2005; Kendler and Gardner, 2016), as well as to relapse and recurrence of MDD (Beshai et al., 2011; for a review see: Buckman et al., 2018). Diathesis-stress models (Ingram and Luxton, 2005; Monroe and Simons, 1991) propose that depressive symptoms result from an interaction between premorbid risk factors (e.g., abnormal reward function) and exposure to stressors. These models are consistent with the report of risk of developing a first depressive episode in individuals with a recent significant life stressor increased by factors of between 4 and 6.5 (Paykel, 1978). In line with this notion, a disrupted reward system (risk factor) combined with a strong stress response might precipitate the emergence of MDD. In fact, findings have evidenced an association between stress exposure (e.g., threat-of-shock, negative performance feedback, or stressful life events) and both reward hypo-responsivity (Berenbaum and Connelly, 1993; Bogdan and Pizzagalli, 2006; Pizzagalli et al., 2007) and negative affect (Bogdan and Pizzagalli, 2006).
Cognitive deficit, such as working memory, constitutes another consistent symptom in depression (American Psychiatric Association, 2013; Beevers, 2005; Clark and Beck, 2010). Such deficit can involve a reduction in cognitive resources or impaired inhibitory control over negative information which might give rise to negative cognitive biases underpinning subsequent depressive symptoms (Everaert et al., 2015, 2017; Gohier et al., 2009; Rose and Ebmeier, 2006). Of particular significance, reward is known to modulate cognitive performance by enhancing motivation to engage in cognitive effort, resulting in higher performance (Berridge, 2004; Niv et al., 2007; Pessoa, 2013). When reward receipt is contingent upon instrumental performance, people are more willing to work harder (e.g., Manohar et al., 2017; Savine et al., 2010; Yee and Braver, 2018). Operationally, the amount of effort that individuals are willing to exert reflects their motivation to achieve a goal (Ernst, 2014). In other words, individuals’ willingness to increase their attentional deployment to enhance performance exhibits their degree of motivation to engage in the task (Ernst, 2014; Westbrook and Braver, 2015; Yee and Braver, 2018). However, motivation that energizes behaviors is driven by a cost-benefit estimation, in which costs such as effort and risk are weighted against benefits such as reward (Apps et al., 2015). For instance, studies evidenced that a higher amount of cognitive effort modulated by the difficulty of the task reduces the value attached to a reward, a principle also known as effort-discounting effect (e.g., Botvinick et al., 2009; Krigolson et al., 2015). Nevertheless, few data exists so far on how cognitive effort modulates the effect of stress exposure on the reward function.
These three factors, reward dysfunction, hypersensitivity to stress, and low cognitive resources, could have critical synergistic influences on the development of MDD. To our knowledge, this question has not yet been examined. The present work is a first step to start addressing this possibility. To this goal, healthy vulnerable individuals with parental history of major depression (HVul) and healthy controls (HCon) will be compared on reward responsivity (neural and behavioral), while manipulating stress (threat of electrical shocks) and cognitive resources, i.e., low and high working memory (WM) load. Based on the above background, the following two hypotheses are tested. (1) Stress exposure will decrease striatal reactivity in response to reward in a larger extent in HVul relative to HCon individuals (e.g., Choi et al., 2014; Hanson et al., 2015; Kumar et al., 2014; Porcelli et al., 2012). (2) High cognitive load, vs. low cognitive load, will further increase the gap between HVul and HCon individuals regarding the effect of stress on reward responses.
2. Methods and material
2.1. Participants
Thirty-two healthy, non-smoking, and right-handed participants aged 20–36 years (M = 24.2; SE = 0.68) were recruited from the local community through advertisements, and from psychology courses at the University of Fribourg. General inclusion criteria encompassed being aged between 18 and 40 years old, right-handed, non-smoking and having a good command of French. Among the participants, 16 healthy adults (12 women) without any past or current mental disorder presented increased familial vulnerability to MDD (healthy vulnerable, HVul), characterized by having a biological parent with a history of MDD. Sixteen healthy controls (12 women) without any past or current mental disorder and without increased familial vulnerability to MDD were age- and gender-matched (healthy control, HCon). As reported in Table 1, groups did not significantly differ on age, gender, socio-demographic status, and depressive symptomatology. Parental MDD was evaluated with the family history method with the participant as an informant (Andreasen et al., 1977) using the Family Interview for Genetic Studies (FIGS; Maxwell, 1992). Eleven HVul reported having a mother, 3 HVul a father, and 1 HVul both parents with a history of MDD. Fifteen out of the 16 HVul cohabitated with their parents at the time of parental MDD history, with length of cohabitation ranging from 1 to 19 years. General exclusion criteria comprised current or past neurological disorder, brain injury, endocrinological condition, mental disorder, and use of psychotropic drugs including alcohol, nicotine, medicines. Among HCon, any individual who reported a first-degree relative with a history of any psychiatric disorders was excluded. Moreover, general exclusion criteria related to the participation in a study including resonance imaging measures included pregnancy, having a pacemaker, a mechanical heart valve or metal implant. With the aim of estimating the minimal number of participants required to detect small-sized (f = 0.15; eta2 = 0.02), medium-sized (f = 0.25; eta2 = 0.06) and large-sized (f = 0.35; eta2 = 0.11) effects (Cohen, 1988), we performed an a-priori power analysis using the statistical package GPower (Faul et al., 2007) with alpha threshold set to 0.05, power to 0.95 and correlations among repeated measures to 0.50 (see Figure A.1 in Appendix). The projected sample size needed with a repeated measures design including two (no-shock vs threat-of-shock) by two (reward vs no-reward) by two (high vs low cognitive load) within-subject variables and two groups was calculated as a function of effect size (Cohen, 1988), with a small-sized (N = 60), medium-sized (N = 24) and large-sized effects (N = 12), respectively (Cohen, 1988).
Table 1.
Group demographics and psychological measures of depressive symptoms.
| HCon (4 males, 12 females) | HVul (4 males, 12 females) | Group difference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SE | SD | Range | M | SE | SD | df | T-value | p-value | Mann–Whitney U | p-value | |
| Age | 24.1 | 0.9 | 3.7 | – | 24.3 | 1.0 | 4.1 | 30 | −0.18 | 0.86 | 119.5 | 0.75 |
| IPSE | 57.1 | 4.0 | 15.9 | – | 58.2 | 4.3 | 17.1 | 30 | −0.19 | 0.85 | 102.0 | 0.33 |
| Age at parental MDD onset | – | – | – | 0 to 25 | 11.8 | 2.4 | 8.3 | – | – | – | – | – |
| MADRS mean scores | 4.3 | 1.1 | 4.4 | – | 3.8 | 0.7 | 2.8 | 30 | 0.39 | 0.70 | 121.5 | 0.81 |
| BDI-II mean scores | 5.1 | 1.4 | 5.4 | – | 6.8 | 1.7 | 6.8 | 30 | −0.74 | 0.46 | 107.5 | 0.43 |
| Shock intensity level | 102.8 | 9.9 | 39.5 | – | 99.6 | 6.1 | 24.4 | 30 | 0.28 | 0.79 | – | – |
| Length (years) of cohabitation with a depressed parent | – | – | – | 1 to 19 | 7.6 | 1.9 | 6.5 | – | – | – | – | – |
Note Healthy control individuals (HCon) without increased familial risk for major depressive disorder (MDD); healthy adults with increased familial risk for MDD (HVul, healthy vulnerable individual); N, number; M, mean; SD, standard deviation; SE, standard error; df, degree of freedom; T-value, Student's t-test; IPSE, Index of Economic Status Position according to the Swiss population; BDI-II, Beck Depression Inventory-II; MADRS, Montgomery-Asberg Depression Rating Scale; MDD, Major Depressive Disorder.
2.2. Clinical measures
Presence and history of mental disorders among participants were tested using the French version (Lecrubier et al., 1998) of the Mini-International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 1998). None of the participants met criteria for past or current neurological, mental, or hormonal conditions. Additionally, depressive symptoms among participants were assessed using the Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979; French version: Pellet et al., 1980) and the Beck Depression Inventory-II (BDI-II; Beck et al., 1996, French version: 1998). The MADRS scale includes 10 items coded from 0 to 6, with a total score ranging from 0 to 60. A score of 15 or above indicates the presence of a major depressive episode (Bouvard and Cottraux, 2010). The BDI-II is a standardized and widely used scale to evaluate the intensity and severity of depressive symptoms over the two weeks preceding the measurements. Depressive symptoms are reported using 21 items rated on a 4-point Likert-like scale ranging from 0 to 3. The total score for all 21 items ranges from 0 to 63. As guidance, thresholds for the French version specify that total scores ranging from 0 to 11 correspond to the absence of major depressive episode, from 12 to 19 to a mild depressive episode, from 20 to 27 to a moderate depressive episode, and above 27 to a severe depressive episode (Bouvard and Cottraux, 2010). In our sample, 2 HCon and 3 HVul reported BDI-II scores between 12 and 19 indicating mild depressive episode. Psychometric properties have been widely validated with high reliability and internal consistency in clinical samples and in the general population (Wang and Gorenstein, 2013), as reported in a study including healthy young adults (Cronbach's α = 0.89) (Whisman et al., 2000). In our sample including 32 participants, the internal consistency was high with Cronbach's α equal to 0.91.
2.3. General procedure
All recruitment and testing procedures were approved by the local ethical review boards of Vaud and Fribourg region (Commission cantonale d’éthique de la recherche sur l’être humain (CER-VD), study number 261/14) as well as Bern region (Kantonale Ethikkommission Bern (KEK BE), study number 337/14). This study comprised an experimental task with fMRI measurements followed by the completion of self-reported questionnaires. The fMRI session was performed at the Department of Diagnostic and Interventional Neuroradiology of the University Hospital of Bern, Switzerland. During scanning, participants completed two blocks of the same experimental task, one without and one with administration of experimental stress.
2.4. Fribourg reward task
Adapted by Gaillard et al. (2019), this event-related fMRI task was used to assess how the neural responses to monetary reward is modulated by stress exposure (unpredictable threat-of-shock) and by variable levels of WM load (low and high) during the anticipation phase. Each of the 96 trials, 48 in each block, started by a visual cue (1500 ms) to inform the subjects of the level of cognitive effort to exert as a function of the WM load (low and high) and the amount of monetary reward associated with the performance (“blank screen” for no-reward trials; “$$” for rewarded trials). Here, motivation is operationalized as the amount of cognitive effort that the subjects are willing to exert to perform the WM task as a function of the expected reward and WM load required in the task. A fixation cross (500 ms) preceded the presentation of an array of yellow circles (3 or 7 circles, 1500 ms). A second fixation cross (3000 ms) was displayed during memorization, followed by the visual target (1500 ms). The visual target consisted in a green circle presented at any position on the screen. The participant was instructed to indicate as quickly and accurately as possible whether this green circle appeared at the same position as one of the yellow circles previously presented. Afterwards, a variable jittered inter-stimulus-interval (ISI; 0 ms or 2000 ms) occurred, followed by two feedback screens (2000 ms). A first feedback screen informed the participants of the monetary gain (“blank screen” for no-reward trials; “1 CHF” for rewarded trials; 1000 ms). It was followed by a second screen (1000 ms) with the cumulative amount of monetary reward (rewarded trials) or a blank screen (no-reward trials). At the end of every four trials, participants rated their mood level (max. 20 s). Correct response was associated with monetary gain (1 CHF) in the rewarded trials, whereas correct response was not associated with monetary gain (0 CHF) in the no-reward trials. In this version of the Fribourg reward task, participants performed the same reward task in two blocks of 20 min each. The first block was devoid of experimental stressor (i.e. no-shock), while the second block included stressor manipulation (i.e. stress condition) consisting of the administration of unpredictable mild electric shocks. All four types of trials (reward × load) were randomly distributed within each block. Prior to the scanning session, each subject was trained on the task outside the scanner. Furthermore, participants were told that they would receive, in cash, the total amount of earned money at the end of the scanning session. Fig. 1 details the timing of a trial in the rewarded and no-reward trials. The task was implemented using E-Prime Professional (Version 2.0.10.353, Psychology Software Tools, Inc.). Stimuli were presented via goggles (VisualStimDigital MR-compatible video goggles; Resonance Technology Inc., Northridge, CA, USA) with a visual angle of 60°, a resolution of 800 × 600 pixels and 60 Hz refresh rate.
Fig. 1.
Fribourg reward task. Illustration of the four types of trials (reward × load) randomly distributed in the no-shock and unpredictable threat-of-shock conditions. The anticipation phase corresponds to the presentation of the reward-cue (1500 ms).
2.5. Experimental stress induction
Before entering the scanner, participants were informed that mild electrical shocks would be delivered unpredictably during the second block (stress condition) of the Fribourg reward task, while the first block (no-shock) would be devoid of stressor. Shocks were delivered on the external side of the participants’ non-dominant left hand via 6-mm Ag/AgCl electrodes, using a non-ferromagnetic shock box (Psychlab system, Contact Precision Instruments, London, UK) positioned on a table next to the scanner. Prior to the MRI data acquisition, the individual shock intensity was titrated for each participant by administering a standard shock work-up procedure to determine an intensity rated as “ aversive, but not painful ” by the participant (Robinson et al., 2011). During the standard workup procedure, intensity of the shock could range from 0 to 5 mA with shock intensity characterized by a number ranging from 0 to 255 (M = 101.2 ± 5.7). The duration of the mild electrical shock delivery was constantly set at 0.1 second. The effectiveness of this experimental manipulation has been well-demonstrated as a way to induce a stress response characterized by increased arousal, cortisol concentrations, negative mood and state of anxiety (for a review see: Grillon and Baas, 2003).
2.6. Effect of the experimental acute stressor on self-reported mood
At the end of every four trials of the Fribourg reward task, participants reported their mood using a Visual Analog Mood Scale presented on the screen (scaled from 0 ‘very negative mood’ to 9 ‘very positive mood’) adapted from Nyenhuis and colleagues (1997).
2.7. MR data acquisition
Scanning was performed on a Siemens TrioTim syngo 3.0-Tesla whole-body scanner (Erlangen, Germany) equipped with a radio frequency 32-channel head coil. MRI acquisition included 3D T1-weighted (MPRAGE) images, collected with the following settings: sagittal slices: 176; slice thickness: 1 mm; FOV: 256 × 256 mm2; matrix size: 256 × 256; voxel size: 1 × 1 × 1 mm3; TR: 1950 ms; TE: 2.2 ms; flip angle: 9° The functional event-related task-based MRI acquisition was collected using EPI pulse sequence with the following settings: interleaved ascending slices: 38; slice thickness: 3 mm; FOV: 230 × 230 mm2; matrix size: 64 × 64; voxel size: 3.6 × 3.6 × 3 mm3; TR: 2000 ms; TE: 30 ms; flip angle: 90°
2.8. Behavioral data analyses
2.8.1. Working memory performance
A 2 × 2 × 2 × 2 repeated measures ANOVA with group (HCon vs HVul) as between-subject factor, and stress (unpredictable threat-of-shock vs no-shock), reward (reward vs no-reward), and WM load (high vs low) as within-subject factors was conducted on response accuracy scores and reaction times. Further, a 2 × 2 × 2 × 2 repeated measures ANCOVA with the individuals’ BDI-II score (grand-mean centered) as covariate was carried out in order to determine the effects of mild subclinical depressive symptoms experienced by the participants at the time of the study on response accuracy scores and reaction times. Both analyses were performed using SPSS (IBM SPSS Statistics, Version 25.0, Armonk, NY, USA). In order to correct for the multiple comparisons conducted in the repeated measures ANOVA and ANCOVA, a Bonferroni's approach was applied.
2.8.2. Self-reported mood ratings during the Fribourg reward task
A 2 × 2 × 2 × 2 repeated measures ANOVA was conducted on participants’ mood ratings during the Fribourg reward task, with group (HCon vs HVul) as between-subject factor and stress (unpredictable threat-of-shock vs no-shock), reward (reward vs no-reward), and WM load (high vs low) as within-subject factors. Additionally, a 2 × 2 × 2 × 2 repeated measures ANCOVA with individuals’ BDI-II score (grand-mean centered) as covariate was carried out in order to determine the effects of mild subclinical depressive symptoms experienced by the participants at the time of the study on participants’ mood ratings. Both analyses were performed using SPSS (IBM SPSS Statistics, Version 25.0, Armonk, NY, USA). In order to correct for the multiple comparisons conducted in the repeated measures ANOVA and ANCOVA, a Bonferroni's approach was applied. With the aim of further examining the relationship between self-reported mood ratings and mild subclinical depressive symptoms experienced by the participants at the time of the study, a Spearman correlation was conducted between self-reported mood ratings in both the no-shock and threat-of-shock conditions and individuals’ BDI-II scores.
2.9. fMRI data analysis
The preprocessing and statistical analyses of the structural and functional MRI data were performed with AFNI software package (Cox, 1996).
2.9.1. Task-based fMRI data preprocessing
T1-weighted (MPRAGE) images were first processed with the standard FreeSurfer (version 6.0.0.) pipeline (Fischl, 2004) to obtain segmentation masks corresponding to the brain (skull-stripped), white matter, and ventricles. The preprocessing was performed on the EPI data using the AFNI afni_proc.py script with the following steps: despiking the time-series (despike), correcting for slice timing (tshift), volume co-registering to the participants’ corresponding anatomical (3D T1-weighted) image (align), volume registration across the timeseries (volreg), blurring within the whole-brain mask (blur), normalization (scale), and regressors modeled (regress). The EPI data were corrected for motion (averaged motion per volume: 0.049 mm ± 0.015) by censoring EPI volumes and their preceding volume where the derivative of the motion regressors from 3dvolreg had a Euclidean norm above 0.3 mm. Volumes with more than 10% voxel outliers were censored as well. Over the initial 32 age- and gender-matched participants selected, none reached these exclusion criteria. The preprocessed EPI timeseries were then warped to MNI space using the ICBM 2009a Nonlinear Symmetric atlas (Fonov et al., 2009), and spatially smoothed using an isotropic 6 mm FWHM Gaussian filter. Lastly, a group-level gray matter mask was created by averaging and thresholding binary masks at 0.95 overlap (Torrisi et al., 2018).
2.9.2. Task-based fMRI data analysis
Individual subject regressions were performed within the framework of the general linear model (GLM) implemented in the AFNI program 3dDeconvolve. Regressors of interest included in our model comprised events modeling anticipation during the cue presentation (1500 ms), working memory including the stimulus presentation, cross fixation and target presentation (6000 ms), feedback delivery events during the feedback and balance account presentation (2000 ms), and self-reported ratings event including self-reported mood (variable duration up to 20′000 ms). Anticipation, working memory, and feedback delivery events were modeled for the four conditions combining reward and load modalities, that are (i) no-reward/low load, (ii) no-reward/high load, (iii) reward/low load, (iv) reward/high load for both blocks (i.e., the no-shock and unpredictable threat-of-shock conditions). However, statistical analyses were exclusively focused on the anticipation phase during cue presentation. Further, six motion parameters were modeled as nuisance variables for both blocks and consisted of three rotational (roll, yaw, pitch) and three translational (x, y, z) variables. All the events defined from the experimental design and the six residual motion parameters for each block (no-shock and unpredictable threat-of-shock conditions) were regressed on the processed time series at the subject level. The events were coded by onset time. An incomplete gamma function was convolved with a boxcar function beginning at stimulus onset and having the duration of the corresponding event.
For the group-level analyses, the GLM included group (HCon vs HVul) as between-subject fixed factor, stress (unpredictable threat-of-shock vs no-shock), reward (reward vs no-reward), and WM load (high vs low) as within-subject fixed factors, and subjects as random factor. A 2 × 2 × 2 × 2 repeated measures ANOVA was run using 3dMVM program implemented in AFNI to determine the effect of the (i) group, (ii) unpredictable stressor, (iii) monetary reward, and (iv) WM load on the BOLD signal. These analyses were focused on a-priori striatal regions during the anticipation phase, i.e., on the bilateral NAcc, caudate nucleus, and putamen. These striatal regions of interest (ROIs) were defined using the Desai DKD maximum probability atlas implemented in FreeSurfer (Desikan et al., 2006; Destrieux et al., 2010; Fischl, 2004). From the three ROI contrast maps, individual parameter estimates were extracted by averaging the activation of all voxels located in each ROI for each participant and condition. Next, parameter estimates were entered into SPSS. A 2 × 2 × 2 × 2 repeated measures ANOVA was conducted for each of the three ROIs to evaluate the effects of the (i) group, (ii) unpredictable stressor, (iii) monetary reward, and (iv) WM load on the brain activation in the three ROIs. Additionally, a 2 × 2 × 2 × 2 repeated measures ANCOVA with the individuals’ BDI-II score (grand-mean centered) as covariate was carried out in order to determine the effects of mild subclinical depressive symptoms experienced by the participants at the time of the study on the BOLD signal in the three striatal ROIs. A Bonferroni's approach was applied to correct for multiple comparisons conducted in each repeated measures ANOVA and ANCOVA. Further, an additional correction using the Bonferroni-Holm's approach was applied in order to correct for multiple comparisons performed on the three striatal ROIs.
3. Results
3.1. Behavioral results
3.1.1. Working memory performance: response accuracy
Response accuracy was analyzed using a 4-way repeated measures ANOVA with group (HCon vs HVul), stress (unpredictable threat-of-shock vs no-shock), reward (reward vs no-reward), and WM load (high vs low) as factors. Group showed no main and interaction effects on response accuracy as a function of stress, reward, cognitive load, and their interactions. However, a trend toward increased response accuracy in rewarded trials (M = 82.1%; SE = 1.9%) compared to no-reward trials (M = 79.8%; SE = 2.1%) emerged, F(1,30) = 3.2, pone-tailed ≤ 0.05, η2 = 0.10 (see Panel A in Fig. 2). Moreover, a significant main effect of WM load indicated decreased response accuracy in the high WM load condition (M = 74.1%; SE = 2.1%) compared to the low WM load condition (M = 87.8%; SE = 1.9%), F(1,30) = 78.5, ptwo-tailed ≤ 0.001, η2 = 0.72, Bonferroni-corrected. Interestingly, a significant main effect of stress indicated increased response accuracy in the unpredictable threat-of-shock condition (M = 83.4%; SE =2.0%) compared to the no-shock condition (M = 78.5%; SE = 2.0%), F(1,30) = 8.0, ptwo-tailed ≤ 0.01, η2 = 0.21, Bonferroni-corrected). Further, a significant twofold interaction effect (reward × load) emerged (F(1,30) = 5.1, ptwo-tailed < 0.05, η2 = 0.15, Bonferroni-corrected). Post-hoc analyses showed diminished response accuracy in the no-reward trials (M = 71.7%; SE = 2.3%) compared to the rewarded trials (M = 76.5%; SE = 2.3%) in the high WM load condition (t(31) = −2.38, ptwo-tailed < 0.05, Bonferroni-corrected), while response accuracy did not differ significantly in the low WM load condition between the no-reward trials (M = 87.9%; SE = 2.2%) and the rewarded trials (M = 87.7%; SE = 1.9%) (t(31) = 0.20, ptwo-tailed > 0.05, Bonferroni-corrected). The additional 4-way repeated measures ANCOVA with individual's BDI-II scores included as covariate showed no significant effect of the covariate and no interaction effect between the covariate and any within-subject factors on response accuracy, indicating that the mild subclinical depressive symptoms observed in our sample did not influence significantly response accuracy.
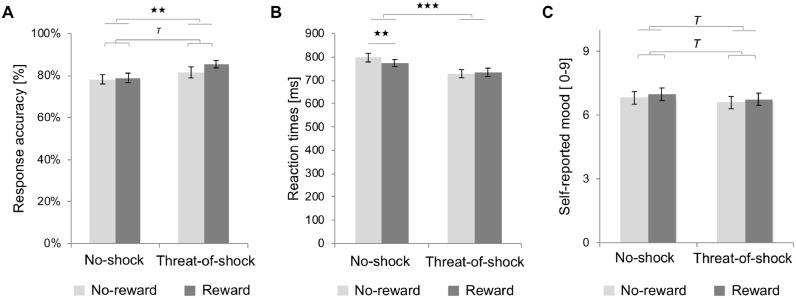
Fig. 2.
Effect of stress induction and reward on the working memory performance and self-reported mood ratings during the Fribourg reward task across groups. Mean and standard error as a function of stress induction (unpredictable threat-of-shock vs no-shock) and reward (reward vs no-reward) for the (A) response accuracy, (B) reaction times, and (C) self-reported mood scaled from 0 ‘very negative mood’ to 9 ‘very positive mood’. Lines with brackets above the data-mean indicate a main effect of reward (i.e., significant differences between the reward vs not-reward trials, represented by a dark gray bar and a light gray bar, respectively. Line without brackets above the data-mean indicate a main effect of stress (i.e., unpredictable threat-of-shock vs no-shock). Tpone-tailed < 0.05, ★★p < 0.01, ★★★p < 0.001.
3.1.2. Working memory performance: reaction times (RT)
The fourfold (group × stress × reward × load) repeated measures ANOVA on RT showed no main and interaction effects of group on reaction times as a function of stress, reward, cognitive load, and their interactions. However, a significant main effect of load emerged, with slower RT in the high WM load condition (M = 804.0 ms; SE = 15.7 ms) compared to the low WM load condition (M = 711.6 ms; SE = 16.0 ms), F(1,30) = 112.9, ptwo-tailed ≤ 0.001, η2 = 0.79 (see Panel B in Fig. 2). A significant main effect of stress indicated faster RT during the unpredictable threat-of-shock condition (M = 730.7 ms; SE = 16.7 ms) than during the no-shock condition (M = 784.8 ms; SE = 16.4 ms), F(1,30) = 17.9, ptwo-tailed ≤ 0.001, η2 = 0.37, Bonferroni-corrected). Additionally, a significant twofold interaction effect (stress × reward) occurred (F(1,30) = 4.21, ptwo-tailed < 0.05, η2 = 0.12, Bonferroni-corrected). Post-hoc analyses demonstrated that RTs were faster in the rewarded trials (M = 773.1 ms; SE = 15.2 ms) compared to the no-reward trials (M = 796.5 ms; SE = 18.4 ms) in the no-shock condition (t(31) = 2.8, ptwo-tailed ≤ 0.01, Bonferroni-corrected), whereas this enhancing effect of reward disappeared in the unpredictable threat-of-shock condition (t(31) = −0.5, ptwo-tailed ≥ 0.05, Bonferroni-corrected), in which RT did not differ between rewarded (M = 733.8 ms; SE = 18.1 ms) and no-reward trials (M = 727.7 ms; SE = 17.6 ms). Further, the additional 4-way repeated measures ANCOVA with individual's BDI-II scores included as covariate showed no significant effect of the covariate and no interaction effect between the covariate and any within-subject factors on reaction times, indicating that the mild subclinical depressive symptoms observed in our sample did not influence significantly reaction times. The panel A and panel B in Fig. 2 describe the main and interaction effects of stress and reinforcement on response accuracy and reaction times, respectively.
3.1.3. Self-reported mood ratings during the Fribourg reward task
Next, we assessed whether self-reported mood ratings were influenced by stress (unpredictable threat-of-shock vs no-shock), reward (reward vs no-reward), and WM load (high vs low), and whether these factors affected differently self-reported mood ratings in HVul compared to HCon. In accordance with our hypotheses, the fourfold repeated measures ANOVA showed a trend effect induced by threat-of-shock (F(1,30) = 3.3, pone-tailed < 0.05, η2 = 0.10, Bonferroni-corrected), with decreased positive mood in the unpredictable threat-of-shock condition (M = 6.7; SE = 0.3) compared to the no-shock condition (M = 6.9; SE = 0.3). As expected, a trend effect of reward occurred (F(1,30) = 4.11, pone-tailed < 0.05, η2 = 0.12, Bonferroni-corrected), with increased positive mood in the rewarded trials (M = 6.9; SE = 0.3) compared to the no-reward trials (M = 6.7; SE = 0.3) (see Panel C in Fig. 2). However, neither threefold interaction effect (group × stress × reward) nor fourfold interaction effect (group × stress × reward × load) were detected on self-reported mood ratings, suggesting that groups did not differ regarding regulation of mood. Nevertheless, the additional 4-way repeated measures ANCOVA with individual's BDI-II scores included as covariate showed an interaction effect between load and individual's BDI-II scores (F(1,29) = 5.3, ptwo-tailed ≤ 0.05, η2 = 0.16, Bonferroni-corrected) as well as a main effect of individual's BDI-II scores (F(1,29) = 6.2, ptwo-tailed ≤ 0.05, η2 = 0.18, Bonferroni-corrected) on self-reported mood ratings. These effects indicated that self-reported mood decreased in individuals with higher BDI-II scores. This negative correlation between self-reported mood ratings and the individual's BDI-II scores was stronger following trials with high compared to low cognitive load (see Figure A.2 in Appendix). Additionally, self-reported mood ratings during both the no-shock (rS = −0.46, p ≤ 0.01) and threat-of-shock (rS = −0.48, p ≤ 0.01) conditions were negatively associated with individual's BDI-II scores.
3.2. fMRI results: activations in striatal ROIs
fMRI results presented here are focused on a-priori striatal regions (i.e., bilateral NAcc, caudate nucleus, and putamen) during the anticipation phase.
3.2.1. Group differences in striatal reactivity
A significant main effect of group was found in the bilateral caudate nucleus (F(1,30) = 6.1, ptwo-tailed ≤ 0.05, η2 = 0.17, Bonferroni-corrected), indicating significantly lower recruitment of the bilateral nucleus caudate in HVul compared to HCon, irrespective of unpredictable threat-of-shock, reward or WM load (see Fig. 3). Also, a significant threefold interaction effect (group × stress × load) emerged in the bilateral NAcc (F(1,30) = 7.1, ptwo-tailed < 0.05, η2 = 0.19, Bonferroni-corrected). Post-hoc analyses demonstrated a significant reduction in bilateral NAcc reactivity in the unpredictable threat-of-shock condition compared to the no-shock condition in HVul, but only in the low WM load condition (t(15) = 2.89, ptwo-tailed < 0.05, Bonferroni-corrected). There was no difference in the high WM load, t(15) = −0.3, ptwo-tailed ≥ 0.05, Bonferroni-corrected.
Fig. 3.
Illustration of the main effect of group comparing the healthy adults without (HCon, healthy control) and with (HVul, healthy vulnerable) increased familial risk for major depression, and threefold interaction effect (group × stress × load). (A) Significant reduced recruitment of the bilateral caudate nucleus in the HVul across conditions, irrespective of stress, reward and WM load. (B) Significant reduced activation in the bilateral nucleus accumbens in the HVul during the unpredictable threat-of-shock condition vs no-shock condition, but only in the low load compared to high load conditions. Parameter estimates (βeta weights) mean with standard errors and ROI's masks from which parameter estimates were extracted are presented at the top of the figure. Statistical parametric maps corresponding to the contrasts of interest are presented below. These whole-brain activations are corrected for multiple comparisons, but thresholded here at 0.05 for visualization purpose. ★p < 0.05.
Moreover, a significant fourfold interaction effect (group × reward × stress × load) was found in the bilateral putamen, F(1,30) = 4.7, ptwo-tailed ≤ 0.05, η2 = 0.13, Bonferroni-corrected (see Fig. 4). In HCon, post-hoc analyses indicated a significant decrease in the bilateral putamen activation in response to no-reward cues in the unpredictable threat-of-shock compared to the no-shock conditions (t(15) = 2.33, ptwo-tailed ≤ 0.05, Bonferroni-corrected), with this stress-induced effect occurring exclusively in the low cognitive load condition. In HVul, stress heightened the reactivity of the bilateral putamen in response to reward compared to no-reward cues, in particual when the task involved higher cognitive load, t(15) = 4.26, ptwo-tailed ≤ 0.001, Bonferroni-corrected. In the low cognitive load condition, the effect of stress resulted in bilateral putamen deactivation in response to both reward and no-reward cues, with stronger deactivation in response to no-reward than reward cues (t(15) = 2.84, ptwo-tailed ≤ 0.05, Bonferroni-corrected).
Fig. 4.
Illustration of the fourfold interaction effect (group × stress × reward x load) in the bilateral putamen. (A) Post-hoc comparisons evidenced a significant stress-induced reduction in the bilateral putamen activation in response to no-reward cues in the unpredictable threat-of-shock compared to no-shock conditions in healthy control (HCon) adults without increased familial risk for major depression, but exclusively in the low cognitive load condition. (B) In healthy adults with increased familial risk for major depression (HVul, healthy vulnerable), threat-of-shock potentiated the bilateral putamen reactivity in response to reward compared to no-reward cues, in particular when the task was more demanding (i.e., high working memory load). In turn, threat-of-shock resulted in a deactivation in response to both reward and no-reward cues in the low cognitive load condition, with stronger deactivation in response to no-reward compared to reward cues. Parameter estimates (βeta weights) mean with standard errors and ROI's masks from which parameter estimates were extracted are presented at the top of the figure. Statistical parametric map corresponding to the fourfold interaction effect (group × stress × reward × load) is presented below These whole-brain activations are corrected for multiple comparisons, but thresholded here at 0.05 for visualization purpose. ★p ≤ 0.05, ★★p ≤ 0.01, ★★★p ≤ 0.001.
3.2.2. Stress-induced effect in striatal reactivity across groups
Irrespective of groups, a significant twofold interaction effect (stress × reward) was found in the bilateral putamen (F(1,30) = 13.6, ptwo-tailed < 0.001, η2 = 0.31, Bonferroni-corrected). Post-hoc analyses showed a stress-induced decrease of activation in the bilateral putamen in response to no-reward compared to reward cues in the unpredictable threat-of-shock condition (t(31) = 4.86, ptwo-tailed < 0.001, Bonferroni-corrected), whereas no significant difference occurred in the no-shock condition (t(31) = 0.28, ptwo-tailed > 0.05, Bonferroni-corrected) (see Fig. 5).
Fig. 5.
Illustration of the main effect of reward and the twofold interaction effect (stress × reward) that occurred in striatal ROIs during the anticipation phase in the healthy adults without (HCon, healthy control) and with (HVul, healthy vulnerable) increased familial risk for major depression. Significant increased reactivity to cued rewards (reward vs no-reward) in the bilateral (A) nucleus accumbens, (B) caudate nucleus, and (C) putamen. (D) Significant reduced activation in the bilateral putamen during the unpredictable threat-of-shock condition vs no-shock condition, but only in no-reward trials. Parameter estimates (βeta weights) mean with standard errors and ROI's masks from which parameter estimates were extracted are presented at the top of the figure. Statistical parametric maps corresponding to the contrasts of interest during anticipation are presented below. These whole-brain activations are corrected for multiple comparisons, but thresholded here at 0.05 for visualization purpose. ★★p < 0.01, ★★★p < 0.001.
3.2.3. Reward-enhancing effect in striatal reactivity across groups
Irrespective of groups, a main effect of reward showed greater activation in reward than no-reward trials in the bilateral NAcc (F(1,30) = 23.2, ptwo-tailed < 0.001, η2 = 0.44, Bonferroni-corrected), bilateral caudate nucleus (F(1,30) = 14.3, ptwo-tailed < 0.001, η2 = 0.32, Bonferroni-corrected), and bilateral putamen (F(1,30) = 11.8, ptwo-tailed < 0.01, η2 = 0.28, η2 = 0.28, Bonferroni-corrected) (see Fig. 5).
However, the additional 4-way repeated measures ANCOVA carried out to examine the effect of individual's BDI-II scores included as covariate on a-priori striatal regions (i.e., bilateral NAcc, bilateral caudate nucleus, and bilateral putamen) did not show any significant main effect of mild subclinical depressive symptoms or interaction effect with stress induced by threat-of-shock, reward or cognitive load on striatal reactivity during the anticipation phase. Table 2 presents a comprehensive overview of all main and interactions effects for the within- and between-subject contrasts in the NAcc, caudate nucleus, and putamen. The results of the repeated-measure ANCOVA carried out to explore the main effect of mild subclinical depressive symptoms experienced by the participants at the time of the study as well as their potential interaction effect with threat-of-shock, reward and cognitive load on the BOLD signal in the three striatal regions are presented in Table 2. Significant whole-brain clusters for all contrasts (p < 0.05, cluster-wise corrected) are presented in appendix (see Table A.1).
Table 2.
Main and interaction effects for the within- and between-subject contrasts in the bilateral nucleus accumbens (NAcc), caudate nucleus, and putamen.
| Within-subjects contrasts | Stress | Reward | WM load | NAcc | Caudate nucleus | Putamen | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(1,30) | p | η2 | F(1,30) | p | η2 | F(1,30) | p | η2 | ||||
| Stress | Threat-of-shock vs No-shock | 3.05 | 0.09 | 0.09 | 1.92 | 0.18 | 0.06 | 4.50 | 0.04 | 0.13 | ||
| Stress × BDI-II | Threat-of-shock vs No-shock | 0.13 | 0.72 | 0.01 | 0.23 | 0.64 | 0.01 | 0.53 | 0.47 | 0.02 | ||
| Reward | R vs NR | 23.15 | 0.00 | 0.44 | 14.3 | 0.00 | 0.32 | 11.80 | 0.00 | 0.28 | ||
| Reward × BDI-II | R vs NR | 0.42 | 0.52 | 0.01 | 3.50 | 0.07 | 0.11 | 0.00 | 0.96 | 0.00 | ||
| Load | High vs Low | 0.00 | 0.99 | 0.00 | 2.2 | 0.15 | 0.07 | 2.23 | 0.15 | 0.07 | ||
| Load × BDI-II | High vs Low | 0.28 | 0.60 | 0.01 | 0.07 | 0.80 | 0.00 | 0.65 | 0.43 | 0.02 | ||
| Stress × Group | Threat-of-shock vs No-shock | 0.21 | 0.65 | 0.00 | 2.7 | 0.11 | 0.08 | 0.77 | 0.39 | 0.03 | ||
| Reward × Group | R vs NR | 0.00 | 0.96 | 0.00 | 0.02 | 0.88 | 0.00 | 0.003 | 0.96 | 0.00 | ||
| Load × Group | High vs Low | 0.36 | 0.55 | 0.01 | 0.02 | 0.89 | 0.00 | 0.31 | 0.58 | 0.01 | ||
| Stress × Reward | Threat-of-shock vs No-shock | R vs NR | 0.15 | 0.70 | 0.01 | 0.43 | 0.52 | 0.01 | 13.63 | 0.00 | 0.31 | |
| Stress × Reward × BDI-II | Threat-of-shock vs No-shock | R vs NR | 0.70 | 0.41 | 0.02 | 1.64 | 0.21 | 0.05 | 0.02 | 0.88 | 0.00 | |
| Stress × Load | Threat-of-shock vs No-shock | High vs Low | 3.16 | 0.09 | 0.10 | 2.77 | 0.11 | 0.08 | 1.63 | 0.21 | 0.05 | |
| Stress × Load × BDI-II | Threat-of-shock vs No-shock | High vs Low | 0.10 | 0.76 | 0.00 | 0.06 | 0.81 | 0.00 | 0.00 | 0.99 | 0.00 | |
| Reward × Load | R vs NR | High vs Low | 0.32 | 0.58 | 0.01 | 0.13 | 0.72 | 0.00 | 0.02 | 0.90 | 0.00 | |
| Reward × Load × BDI-II | R vs NR | High vs Low | 0.52 | 0.48 | 0.02 | 0.03 | 0.86 | 0.00 | 0.20 | 0.66 | 0.01 | |
| Stress × Reward × Group | Threat-of-shock vs No-shock | R vs NR | 0.90 | 0.35 | 0.03 | 0.83 | 0.37 | 0.03 | 2.67 | 0.11 | 0.08 | |
| Stress × Load × Group | Threat-of-shock vs No-shock | High vs Low | 7.09 | 0.01 | 0.19 | 0.10 | 0.75 | 0.00 | 0.01 | 0.92 | 0.00 | |
| Reward × Load × Group | R vs NR | High vs Low | 0.00 | 1.00 | 0.00 | 0.03 | 0.86 | 0.00 | 0.48 | 0.49 | 0.02 | |
| Stress × Reward × Load | Threat-of-shock vs No-shock | R vs NR | High vs Low | 0.96 | 0.33 | 0.03 | 5.23 | 0.03 | 0.15 | 0.30 | 0.59 | 0.01 |
| Stress × Reward × Load × BDI-II | Threat-of-shock vs No-shock | R vs NR | High vs Low | 0.12 | 0.73 | 0.00 | 0.78 | 0.38 | 0.03 | 0.47 | 0.50 | 0.02 |
| Stress × Reward × Load × Group | Threat-of-shock vs No-shock | R vs NR | High vs Low | 1.12 | 0.30 | 0.04 | 0.65 | 0.43 | 0.02 | 4.68 | 0.04 | 0.14 |
| Between-subjects contrasts | ||||||||||||
| Group | HCon vs HVul | 0.98 | .330 | 0.03 | 6.14 | 0.019 | 0.17 | 1.199 | 0.28 | 0.04 | ||
| BDI-II scores | 0.94 | 0.34 | 0.03 | 1.66 | 0.21 | 0.05 | 0.15 | 0.70 | 0.01 | |||
Note. A four-fold repeated measures ANOVA was conducted to determine the effect of group as between-subject factor as well as the effect of stress, reward and load as the within-subject factors. Additionally, a four-fold repeated measures ANCOVA was conducted to evaluate the main effect of subclinical depressive symptoms assessed with the scores at the Beck Depression inventory-II (BDI-II; Beck et al., 1996, French version: 1998) scores (grand-mean centered) as well as its interactions with the within-subject factors. Four-fold repeated measures ANOVA and ANCOVA were corrected for multiple comparisons by applying a Bonferroni correction. Further, the significant p-value was adjusted using the Bonferroni-Holm's approach in order to take into account the analyses performed on three different regions-of-interest. Partial eta squared (η2) represents the proportion of total variance accounted for by the factor, while excluding other factors from the total explained variance (i.e. nonerror variation) in the repeated measures ANOVA (Pierce et al., 2004). Partial eta squared (η2) values range from 0 to 1. Abbreviations: BDI-II, Beck Depression Inventory-II, F, F-statistic with degrees of freedom for effect and error; HCon, healthy control; HVul, healthy vulnerable; η2, partial eta squared; NR, no-reward; R, reward; WM, working memory.
4. Discussion
This study investigated the effect of stress exposure on reward anticipation as a potential vulnerability factor for MDD. Specifically, we explored whether stress exposure differentially affects striatal reactivity in HVul compared to HCon. Additionally, we examined, in an exploratory way, whether the level of cognitive effort required in the task modulates differentially the effect of stress exposure on reward processing in HVul compared to HCon. Of clinical importance, HVul presented a lower dorsal striatum recruitment compared to the HCon across all experimental conditions, i.e. irrespective of stress, reward and cognitive load. Stress reduced the ventral striatal responses in HVul, regardless of the reward condition. This effect was modulated by cognitive load, with stronger stress-induced effect on the ventral striatum in the low compared to high cognitive load conditions. Furthermore, stress potentialized reward reactivity in the putamen among HVul, specifically when the task was more demanding. Across groups, stress blunted the activation of the putamen when no reward was at stake. At the behavioral level, stress exposure was associated with enhanced performance, with higher response accuracy and faster reaction times. In line with fMRI findings, which evidenced significant activation to reward cues (vs. no-reward cues) in the ventral and dorsal striatum in both groups, both groups showed better performance in the rewarded than no-reward trials. As expected, stress induction successfully amplified self-reported negative mood in participants (Bogdan and Pizzagalli, 2006; Grillon and Ameli, 1998; Torrisi et al., 2016), while rewarded trials were associated with significantly increased positive mood and enhanced behavioral performance. Moreover, negative mood was associated with mild subclinical depressive symptoms experienced by the participants at the time of the study, such that individuals with higher intensity and severity of mild subclinical depressive symptoms experienced more negative mood during the experimental task. Surprisingly, groups did not differ in reaction times or response accuracy as a function of reward, stress exposure or cognitive load.
However, behavioral differences are far more difficult to detect in the MRI context, more particularly in non-clinical samples (e.g., Dienes et al., 2013; Kumar et al., 2014; Lewis et al., 2014; Oei et al., 2014; Ossewaarde et al., 2011; Treadway et al., 2013). Despite the lack of group differences in behavioral measures, our study demonstrated that individuals at increased familial risk for MDD differed significantly from healthy controls at the neural level. One possible mechanistic interpretation relies on the idea that, among at-risk individuals, a brain compensatory process might take place to maintain homeostasis and prevent behavioral changes. We now discuss in more detail the neural changes that differentiated individuals at increased familial risk for MDD from healthy controls.
4.1. Group differences in striatal reactivity
In MDD patients, behavioral and neuroimaging studies evidenced (i) higher difficulty to evaluate potential gains (Eshel and Roiser, 2010; Pizzagalli, 2014), (ii) higher difficulty to modulate behaviors as a function of reward magnitude and reinforcement history (Pechtel et al., 2013; Pizzagalli et al., 2008; Treadway et al., 2012), (iii) lower willingness to exert effort to obtain a predicted reward (Vrieze et al., 2013), and (iv) blunted responsiveness of the ventral striatum to cues predicting rewards (Stringaris et al., 2015; Ubl et al., 2015). Partly converging with evidences in both MDD patients and their offspring, our study extends previous findings by showing how the effect of stress exposure and its modulation by the amount of cognitive resources available might increase the risk for the development of depressive symptoms in individuals at increased familial vulnerability to MDD.
Supporting previous evidence indicating a diminished recruitment of the caudate nucleus in MDD patients (for a review see: Dillon et al., 2014), HVul showed reduced caudate nucleus activation compared to HCon, irrespective of stress, reward or the cognitive effort to exert. Accordingly, the existing literature has evidenced a reduced recruitment of the caudate nucleus in depressed young adults during the anticipation phase (Olino et al., 2011) and lower caudate reactivity to reward cues in depressed patients (Pizzagalli et al., 2009; Stoy et al., 2012; Tricomi and Fiez, 2012). This is in line with findings that demonstrated a diminished caudate volume in MDD patients, with a lower volume associated with stronger anhedonic symptoms (Pizzagalli et al., 2009). In humans, the caudate nucleus is notably involved in feedback-driven contingency learning (Tricomi and Fiez, 2012) and in goal-directed behaviors (Grahn et al., 2008; Schwabe and Wolf, 2010). A reduced reactivity in the caudate nucleus might give rise to an impaired ability to learn action-reward and stimulus-reward associations, and therefore might cause an increased difficulty to engage in motivated behaviors (Dillon et al., 2014). However, contrary to our hypotheses and to clinical data in MDD patients, HVul showed no difference in their neural and behavioral reactivity to monetary rewards, nor in the stress-induced effects on reward reactivity in the caudate nucleus.
Convergent with the pattern observed in MDD patients (Berghorst et al., 2013; Bogdan and Pizzagalli, 2006), exposure to unpredictable acute stress in our study reduced the ventral striatum activation in HVul, irrespective of reward condition. Of primary importance, cognitive load modulated the effect of stress on the ventral striatal responses. When the cue indicated low cognitive load to exert, stress exposure induced stronger decreased activation in the ventral striatum of the HVul. This is in line with previous findings in healthy adults showing that stress induced through threat-of-shock impairs WM performance under low load, but is reduced when task performance requires more cognitive engagement from the subjects (Vytal et al., 2012, 2013). Based on these preceding results, one hypothesis that might explain increased stress-induced effects preceding low-demanding cognitive effort in the ventral striatum in our study is that under stress exposure, and in particular when less cognitive effort is required during the task, attentional resources are more prone to be captured by stress-related stimuli, decreasing therefore the striatal activation in response to incentives. This might potentially generate increased threat-related processing resulting in worries and physiological arousal (for reviews see: Bourke et al., 2010; Peckham et al., 2010). The ventral striatum is particularly implicated in encoding the valence of incentives and in reward learning (Kable and Glimcher, 2007). Through its projections, the ventral striatum informs the dorsal striatum about the motivational value of potential outcomes (Hassan and Benarroch, 2015). Therefore, individuals at increased vulnerability for MDD might have a reduced ability to evaluate the motivational valence of incentives, resulting in increased difficulty to implement and to maintain motivated behaviors, specifically in contexts in which more cognitive resources are available to process threat-related information (O'Doherty et al., 2004; Schonberg et al., 2007).
The induction of stress strengthened the putamen reactivity to reward cues in vulnerable individuals. Specifically, this stress-induced potentiation was modulated by the cognitive load imposed by the task, with higher stress-induced reactivity to reward cues when the task was more demanding (i.e., when it was less likely to obtain the predicted reward). Notably, the putamen has been implicated in the planning and implementation of actions (Grahn et al., 2008; Schwabe and Wolf, 2010). One hypothesis is that stress-induced increased activation of the putamen in individuals at risk for MDD might reflect the expression of active coping strategies generated by stress exposure (Cabib and Puglisi-Allegra, 2012), possibly to avoid punishment (i.e., not receiving a predicted reward). This assumption is in line with the dysfunctional response to negative feedback evidenced in depressed patients (for a review see: Martin-Soelch, 2009) as well as the presence of elevated punishment sensitivity in depressed patients (Hevey et al., 2017; for a review see: Starcke and Brand, 2012). In other words, stress might trigger a stronger willingness to avoid negative feedback (i.e., failing to obtain a reward due to a wrong response) in individuals at increased familial risk for MDD, notably due to the heightened sensitization of the biological stress system in at-risk individuals compared to healthy controls (Dienes et al., 2013). This hypothesis is also convergent with a study demonstrating a stress-induced increase of putamen reactivity in response to conditioned stimuli predicting rewards of higher magnitude, specifically in subjects exhibiting higher stress sensitivity (Lewis et al., 2014). Altogether, our results suggest that stress might amplify the propensity to engage in active strategies to avoid penalties, in particular in individuals with increased familial risk for MDD. Amplified stress-induced putamen reactivity in response to rewards might constitute a neural signature of increased risk for the development of maladaptive coping strategies, disrupted reward learning and impaired decision making.
4.2. Stress-induced effect in striatal reactivity across groups
In line with numerous studies showing an abnormal processing of anticipated rewards among depressed patients (for a meta-analytic review see: Keren et al., 2018), our study demonstrated a stress-induced blunting effect on the putamen reactivity in both groups when no reward was at stake. As discussed above, this effect might be more salient in at-risk individuals. Stress is known as one of the most important environmental risk factors for depression onset, in particular when it interacts synergistically with existing vulnerability traits (e.g., Dienes et al., 2013; Hasler and Northoff, 2011). Based on the predominant role played by stress exposure in MDD, this blunting effect of stress on the putamen responsiveness in absence of reward might constitute a biological marker of increased risk for the emergence of depressive symptoms. This is in accordance with previous findings demonstrating the unique role played by the putamen in the parental transmission of reward learning and reward responsiveness (Colich et al., 2017). Notably, offspring of parents with a history of MDD exhibited smaller putamen volume which might increase risk for MDD by altering reward learning processes (Pagliaccio et al., 2019). An abnormal engagement of the putamen might be a critical factor underpinning amotivation in MDD, as evidenced by a study showing the relationship linking lower effort-related activation in the putamen to the severity of amotivation in MDD (Park et al., 2017).
However, these findings are also consistent with prior research pointing to the stress-induced amplification of “incentive-triggered motivation” (Kumar et al., 2014; Pool et al., 2015). Accordingly, an other hypothesis is that the heightened reward reactivity of the putamen under stress exposure might reflect a similar mechanism evidenced in the development of compulsive-like seeking behaviors toward rewards (Koob, 2013; Koob and Le Moal, 2001; Nikolova and Hariri, 2012; Volkow and Morales, 2015). Stress exposure might increase arousal and trigger a transition from voluntary to compulsive-like seeking behaviors, indicated by a shift in the striatal regions engaged in the processing of rewarding stimuli, from the ventral striatum involved in reward valuation to the dorsal striatum implicated in the implementation of actions and habit formation (Everitt and Robbins, 2013; Malvaez and Wassum, 2018). Altogether, our findings suggest that stress exposure might result in dysfunctional reward seeking behaviors and reward learning processes. Stress-induced reduction in the putamen reactivity might reflect a critical biological marker of increased risk for the development of amotivation.
4.3. Limitations
Several limitations are noted. First, although the negative mood ratings in response to the stress manipulation were in line with expectations, no physiological measures supported the subjective reports to confirm that the stress manipulation induced an increased reactivity of the biological stress system. Second, due to our within-subject design and to the completion of the two blocks (i.e., no-shock and unpredictable threat-of-shock conditions) on the same day, the two blocks were not randomized in order to avoid potential bleeding of the stress-related negative affect into the no-shock condition. However, the strength of this design relies on the within-subject manipulation of the stressor with the avoidance of methodological concerns raised by scanning on different days. Third, the small sample size is an important limitation to take into account when interpreting the present results and suggests that our study was possibly underpowered to detect small effect sizes. Due to the unexpected replacement of the scanner used to perform our fMRI measurements, we were forced to interrupt prematurely our study, before reaching the projected number of participants (N = 60) necessary to detect small-sized effects. The power of our study to detect smaller-sized effect is therefore a critical limitation to consider. Due to the small sample size, an ultimate limitation was the inability to assess how the offspring's age at the onset of parental MDD, and the duration of the parent's depressive episodes might have a moderation effect. Altogether, our results should be regarded as preliminary, with a need for replication.
4.4. Conclusion
The results of this study indicate that stress exposure might have adverse effects on the ability of individuals at increased risk for MDD to evaluate the motivational value of incentives, to learn from rewards and to make adaptive decisions. In particular, the effect of stress exposure on reward valuation might be amplified when more cognitive resources are at disposal to process threat-related informations. In turn, at-risk individuals may exhibit increased propensity to avoid negative feedback and penalties in threatening contexts, resulting in impaired decision making and reward learning. In addition, caudate recruitment across all conditions in HVul compared to HCon suggests that at-risk individuals might be proner to have increased difficulties to engage in goal-oriented behaviors. Altogether, higher difficulty to evaluate reward value, to engage in motivated behaviors combined with increased sensitivity to negative feedback might result in stronger risk for developing anhedonic symptoms. These findings may open new avenues to build up a better understanding of the role played by stress exposure in the vulnerability for major depression. Stress might precipitate reward dysfunctions which manisfest as anhedonic symptoms. Further investigation is needed to disentangle the complex relationship linking reward processing to stress reactivity and cognitive processes in the etiology of MDD.
Funding
This study was funded by the Research Pool of the University of Fribourg, Fribourg, Switzerland (578) and by the Gottfried und Julia Bangerter-Rhyner-Stiftung, Switzerland (8472). This study was also supported by a Doc.Mobility grant (P1FRP1_174818) from the Swiss National Science Foundation (SNSF) awarded to Claudie Gaillard.
CRediT authorship contribution statement
Claudie Gaillard: Investigation, Software, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Matthias Guillod: Investigation, Methodology, Writing - review & editing. Monique Ernst: Supervision, Writing - review & editing. Andrea Federspiel: Validation. Dominik Schoebi: Writing - review & editing, Methodology. Romina Evelyn Recabarren: Investigation. Xinyi Ouyang: Software. Christoph Mueller-Pfeiffer: Writing - review & editing. Antje Horsch: Writing - review & editing. Philipp Homan: Software, Methodology, Writing - review & editing. Roland Wiest: Resources. Gregor Hasler: Writing - review & editing. Chantal Martin-Soelch: Supervision, Conceptualization, Methodology, Project administration, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
None
Acknowledgments
We are grateful to the Research Pool of the University of Fribourg, Fribourg, Switzerland (578), to the Gottfried und Julia Bangerter-Rhyner-Stiftung, Switzerland (8472) and to the Swiss National Science Foundation (SNSF; P1FRP1_174818) for enabling this research. We thank the MRI technicians at the Department of Diagnostic and Interventional Neuroradiology at the University Hospital of Bern, Switzerland, and all the participants for making this study possible. We also thank Richard Reynolds and Salvatore Torrisi for their precious suggestions and guidance during the preparation of the analyses as well as Madeleine Viviani for the provision of language revisions to this manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102193.
Appendix. Supplementary materials
References
- American Psychiatric Association . American Psychiatric Publishing; Arlington: 2013. Diagnostic and Statistical Manual of Mental Disorders (5th ed.) [Google Scholar]
- Andreasen N.C., Endicott J., Spitzer R.L., Winokur G. The family history method using diagnostic criteria. reliability and validity. Arch. Gen. Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Apps M.A.J., Grima L.L., Manohar S., Husain M. The role of cognitive effort in subjective reward devaluation and risky decision-making. Sci. Rep. 2015;5(16880):1–11. doi: 10.1038/srep16880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G. TX: Psychological Corporation; San Antonio: 1996. Manual For the Beck Depression Inventory-II. [Google Scholar]
- Beck A.T., Steer R.A., Brown G. Editions du Centre de psychologie appliquée; Paris: 1998. Inventaire De Dépression De Beck. [Google Scholar]
- Beevers C. Cognitive vulnerability to depression : a dual process model. Clin. Psychol. Rev. 2005;25(7):975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Berenbaum H., Connelly J. The effect of stress on hedonic capacity. J. Abnorm. Psychol. 1993;102(3):474–481. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- Berghorst L.H., Bogdan R., Frank M.J., Pizzagalli D.A. Acute stress selectively reduces reward sensitivity. Front. Hum. Neurosci. 2013;7(133):1–15. doi: 10.3389/fnhum.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. Motivation concepts in behavioral neuroscience. Physiol. Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Beshai S., Dobson K.S., Bockting C.L.H., Quigley L. Relapse and recurrence prevention in depression : current research and future prospects. Clin. Psychol. Rev. 2011;31(8):1349–1360. doi: 10.1016/j.cpr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Bogdan R., Pizzagalli D.A. Acute stress reduces reward responsiveness : implications for depression. Biol. Psychiatry. 2006;60(10):1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Huffstetler S., McGuire J.T. Effort discounting in human nucleus accumbens. Cognitive Affect. Behav. Neurosci. 2009;9(1):16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke C., Douglas K., Porter R. Processing of facial emotion expression in major depression : a review. Aust. N Z J Psychiatry. 2010;44(8):681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Bouvard M., Cottraux J. Elsevier Masson; Paris: 2010. Protocoles Et échelles D’évaluation En Psychiatrie Et Psychologie. [Google Scholar]
- Buckman J.E.J., Underwood A., Clarke K., Saunders R., Hollon S.D., Fearon P., Pilling S. Risk factors for relapse and recurrence of depression in adults and how they operate : a four-phase systematic review and meta-synthesis. Clin. Psychol. Rev. 2018;64:13–38. doi: 10.1016/j.cpr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S., Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012;36(1):79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Choi J.M., Padmala S., Spechler P., Pessoa L. Pervasive competition between threat and reward in the brain. Soc. Cogn. Affect. Neurosci. 2014;9(6):737–750. doi: 10.1093/scan/nst053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.S., Barch D. Anhedonia is associated with reduced incentive cue related activation in the basal ganglia. Cogn Affect. Behav. Neurosci. 2015;15(4):749–767. doi: 10.3758/s13415-015-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Beck A.T. Cognitive theory and therapy of anxiety and depression : convergence with neurobiological findings. Trends Cogn. Sci. (Regul. Ed.) 2010;14(9):418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed). Hillsdale, N.J: L. Erlbaum Associates.
- Colich N.L., Ho T.C., Ellwood-Lowe M.E., Foland-Ross L.C., Sacchet M.D., LeMoult J.L., Gotlib I.H. Like mother like daughter : putamen activation as a mechanism underlying intergenerational risk for depression. Soc Cogn. Affect. Neurosci. 2017;12(9):1480–1489. doi: 10.1093/scan/nsx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI : software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D.…Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes K.A., Hazel N.A., Hammen C.L. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology. 2013;38(6):927–940. doi: 10.1016/j.psyneuen.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon D.G., Rosso I.M., Pechtel P., Killgore W.D.S., Rauch S.L., Pizzagalli D.A. Peril and pleasure : an RDOC-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety. 2014;31(3):233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J., Pan H., Kocsis J.H., Yang Y., Butler T., Chusid J.…Silbersweig D.A. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am. J. Psychiatry. 2006;163(10):1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N., Roiser J.P. Reward and punishment processing in depression. Biol. Psychiatry. 2010;68(2):118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Everaert J., Duyck W., Koster E.H.W. Emotionally biased cognitive processes : the weakest link predicts prospective changes in depressive symptom severity. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0124457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert J., Grahek I., Koster E.H.W. Individual differences in cognitive control over emotional material modulate cognitive biases linked to depressive symptoms. Cognition and Emotion. 2017;31(4):736–746. doi: 10.1080/02699931.2016.1144562. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. From the ventral to the dorsal striatum : devolving views of their roles in drug addiction. Neurosci. Biobeh. Rev. 2013;37(9):1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-.G., Buchner A. G*Power 3 : a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fischl B. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fonov V., Evans A., McKinstry R., Almli C., Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102. [Google Scholar]
- Gaillard C., Guillod M., Ernst M., Torrisi S., Federspiel A., Schoebi D.…Martin‐Soelch C. Striatal responsiveness to reward under threat‐of‐shock and working memory load : a preliminary study. Brain Behav. 2019:e01397. doi: 10.1002/brb3.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohier B., Ferracci L., Surguladze S.A., Lawrence E., El Hage W., Kefi M.Z.…Le Gall D. Cognitive inhibition and working memory in unipolar depression. J Affect Disord. 2009;116(1–2):100–105. doi: 10.1016/j.jad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Grahn J.A., Parkinson J.A., Owen A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008;86(3):141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Grillon C., Ameli R. Effects of threat of shock, shock electrode placement and darkness on startle. Int. J. Psychophysiol. 1998;28(3):223–231. doi: 10.1016/s0167-8760(97)00072-x. [DOI] [PubMed] [Google Scholar]
- Grillon C., Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. 2003;114(9):1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hägele C., Schlagenhauf F., Rapp M., Sterzer P., Beck A., Bermpohl F.…Heinz A. Psychopharmacology (Berl.) 2015;232(2):331–341. doi: 10.1007/s00213-014-3662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C.L. Stress and depression. Annu. Rev. Clin. Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Hariri A.R., Williamson D.E. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry. 2015;78(9):598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.-.O., Pruessner J., Czechowska Y., Lepage M. Individual differences in trait anhedonia : a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol. Psychiatry. 2007;12(8):767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Hasler G., Drevets W.C., Manji H.K., Charney D.S. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasler G., Northoff G. Discovering imaging endophenotypes for major depression. Mol. Psychiatry. 2011;16(6):604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Hassan A., Benarroch E.E. Heterogeneity of the midbrain dopamine system : implications for parkinson disease. Neurology. 2015;85(20):1795–1805. doi: 10.1212/WNL.0000000000002137. [DOI] [PubMed] [Google Scholar]
- Hevey D., Thomas K., Laureano-Schelten S., Looney K., Booth R. Clinical depression and punishment sensitivity on the bart. Front Psychol. 2017;8 doi: 10.3389/fpsyg.2017.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram R.E., Luxton D.D. Vulnerability-Stress Models. In: Hankin B.L., Abela J.R.Z., editors. Development of psychopathology: A vulnerability-stress perspective. Sage Publications; 2005. pp. 32–46. [Google Scholar]
- Kable J.W., Glimcher P.W. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Gardner C.O. Depressive vulnerability, stressful life events and episode onset of major depression : a longitudinal model. Psychol. Med. 2016;46(09):1865–1874. doi: 10.1017/S0033291716000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H., O'Callaghan G., Vidal-Ribas P., Buzzell G.A., Brotman M.A., Leibenluft E.…Stringaris A. Reward processing in depression : a conceptual and meta-analytic review across fMRI and eeg studies. Am. J.Psychiatry. 2018;175(11):1111–1120. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Addiction is a reward deficit and stress surfeit disorder. Front. Psychiatry. 2013;4:72–100. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Krigolson O.E., Hassall C.D., Satel J., Klein R.M. The impact of cognitive load on reward evaluation. Brain Res. 2015;1627:225–232. doi: 10.1016/j.brainres.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Kumar P., Berghorst L.H., Nickerson L.D., Dutra S.J., Goer F.K., Greve D.N., Pizzagalli D.A. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y., Weiller E., Hergueta T., Amorim P., Bonora L.I., Lépine J.P. Mini International Neuropsychiatric Interview French Version 5.0.0. INSERM; Paris: 1998. [DOI] [PubMed] [Google Scholar]
- Lewis A.H., Porcelli A.J., Delgado M.R. The effects of acute stress exposure on striatal activity during pavlovian conditioning with monetary gains and losses. Front. Behav. Neurosci. 2014;8(179):1–11. doi: 10.3389/fnbeh.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Roiser J.P., Wang L., Zhu Y., Huang J., Neumann D.L.…Chan R.C.K. Anhedonia is associated with blunted reward sensitivity in first-degree relatives of patients with major depression. J. Affect. Disord. 2016;190:640–648. doi: 10.1016/j.jad.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M., Wassum K.M. Regulation of habit formation in the dorsal striatum. Curr. Opin. Behav. Sci. 2018;20:67–74. doi: 10.1016/j.cobeha.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S.G., Finzi R.D., Drew D., Husain M. Distinct motivational effects of contingent and noncontingent rewards. Psychol Sci. 2017;28(7):1016–1026. doi: 10.1177/0956797617693326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem. Soc. Trans. 2009;37(1):313–317. doi: 10.1042/BST0370313. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C., Kobel M., Stoecklin M., Michael T., Weber S., Krebs B., Opwis K. Reduced response to reward in smokers and cannabis users. Neuropsychobiology. 2009;60(2):94–103. doi: 10.1159/000239685. [DOI] [PubMed] [Google Scholar]
- Maxwell M.E. MD: National Institute of Health; Bethesda: 1992. Manual For the Family Interview For Genetic Studies (FIGS) [Google Scholar]
- Monroe S.M., Simons A.D. Diathesis-stress theories in the context of life stress research : implications for the depressive disorders. Psychol. Bull. 1991;110(3):406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nikolova Y.S., Hariri A.R. Neural responses to threat and reward interact to predict stress-related problem drinking : a novel protective role of the amygdala. Biol. Mood Anxiety Disord. 2012;2(19):1–3. doi: 10.1186/2045-5380-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y., Daw N.D., Joel D., Dayan P. Tonic dopamine : opportunity costs and the control of response vigor. Psychopharmacology (Berl.) 2007;191(3):507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P., Dayan P., Schultz J., Deichmann R., Friston K., Dolan R.J. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Oei N.Y.L., Both S., van Heemst D., van der Grond J. Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology. 2014;39:111–120. doi: 10.1016/j.psyneuen.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Olino T.M., McMakin D.L., Dahl R.E., Ryan N.D., Silk J.S., Birmaher B.…Forbes E.E. « i won, but I'm not getting my hopes up » : depression moderates the relationship of outcomes and reward anticipation. Psychiatry Res. 2011;194(3):393–395. doi: 10.1016/j.pscychresns.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino T.M., McMakin D.L., Morgan J.K., Silk J.S., Birmaher B., Axelson D.A.…Forbes E.E. Reduced reward anticipation in youth at high-risk for unipolar depression: a preliminary study. Dev. Cogn. Neurosci. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L., Qin S., Van Marle H.J.F., van Wingen G.A., Fernández G., Hermans E.J. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage. 2011;55(1):345–352. doi: 10.1016/j.neuroimage.2010.11.068. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D., Alqueza K.L., Marsh R., Auerbach R.P. Brain volume abnormalities in youth at high risk for depression : adolescent brain and cognitive development study. J. Am. Acad. Child Adolesc. Psychiatr. 2019 doi: 10.1016/j.jaac.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.H., Lee B.C., Kim J.-.J., Kim J.I., Koo M.-.S. Effort-based reinforcement processing and functional connectivity underlying amotivation in medicated patients with depression and schizophrenia. J.Neurosci. 2017;37(16):4370–4380. doi: 10.1523/JNEUROSCI.2524-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paykel E.S. Contribution of life events to causation of psychiatric illness. Psychol. Med. 1978;8(2):245–253. doi: 10.1017/s003329170001429x. [DOI] [PubMed] [Google Scholar]
- Pechtel P., Dutra S.J., Goetz E.L., Pizzagalli D.A. Blunted reward responsiveness in remitted depression. J. Psychiatr. Res. 2013;47(12):1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham A.D., McHugh R.K., Otto M.W. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety. 2010;27(12):1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Pellet J., Bobon D.P., Mormont I., Lang F., Massardier A. Présenté à Congrès de Psychiatrie Et De Neurologie De Langue Française. Reims; France: 1980. Étude Princeps de validation française de la MADRS, sous-échelle dépression de la CPRS. [Google Scholar]
- Pessoa L. MIT Press; Cambridge: 2013. The Cognitive-Emotional brain : From interactions to Integration. [Google Scholar]
- Pierce C.A., Block R.A., Aguinis H. Cautionary note on reporting eta-squared values from multifactor anova designs. Educ. Psychol. Meas. 2004;64(6):916–924. [Google Scholar]
- Pizzagalli D.A. Depression, stress, and anhedonia : toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10(1):393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Bogdan R., Ratner K.G., Jahn A.L. Increased perceived stress is associated with blunted hedonic capacity : potential implications for depression research. Behav Res Ther. 2007;45(11):2742–2753. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Bogdan R.…Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Iosifescu D., Hallett L.A., Ratner K.G., Fava M. Reduced hedonic capacity in major depressive disorder : evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool E., Brosch T., Delplanque S., Sander D. Stress increases cue-triggered “wanting” for sweet reward in humans. J. Exp. Psychol. 2015;41(2):128–136. doi: 10.1037/xan0000052. [DOI] [PubMed] [Google Scholar]
- Porcelli A.J., Lewis A.H., Delgado M.R. Acute stress influences neural circuits of reward processing. Front. Neurosci. 2012;6(157):1–9. doi: 10.3389/fnins.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson O.J., Letkiewicz A.M., Overstreet C., Ernst M., Grillon C. The effect of induced anxiety on cognition : threat of shock enhances aversive processing in healthy individuals. Cognitive Affect. Behav. Neurosci. 2011;11(2):217–227. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E.J., Ebmeier K.P. Pattern of impaired working memory during major depression. J. Affect. Disord. 2006;90(2–3):149–161. doi: 10.1016/j.jad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Savine A.C., Beck S.M., Edwards B.G., Chiew K.S., Braver T.S. Enhancement of cognitive control by approach and avoidance motivational states. Cogn. Emot. 2010;24(2):338–356. doi: 10.1080/02699930903381564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T., Daw N.D., Joel D., O'Doherty J.P. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J. Neurosci. 2007;27(47):12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Learning under stress impairs memory formation. Neurobiol. Learn Mem. 2010;93(2):183–188. doi: 10.1016/j.nlm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E.…Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(20) doi: 10.1016/j.dcn.2013.11.005. 22–33, 34–57. doi: [DOI] [PubMed] [Google Scholar]
- Starcke K., Brand M. Decision making under stress : a selective review. Neurosci. Biobehav. Rev. 2012;36(4):1228–1248. doi: 10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Stoy M., Schlagenhauf F., Sterzer P., Bermpohl F., Hägele C., Suchotzki K.…Ströhle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J. Psychopharmacol. 2012;26(5):677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Stringaris A., Vidal-Ribas Belil P., Artiges E., Lemaitre H., Gollier-Briant F., Wolke S., Vulser H., Miranda R., Penttilä J., Struve M., Fadai T.… The brain's response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am. J. Psychiatry. 2015;172(12):1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S., Gorka A.X., Gonzalez-Castillo J., O'Connell K., Balderston N., Grillon C., Ernst M. Extended amygdala connectivity changes during sustained shock anticipation. Transl. Psychiatry. 2018;8(1):1–12. doi: 10.1038/s41398-017-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S., Robinson O.J., O'Connell K., Davis A., Balderston N., Ernst M., Grillon C. The neural basis of improved cognitive performance by threat of shock. Soc. Cogn. Affect. Neurosci. 2016;11(11):1677–1686. doi: 10.1093/scan/nsw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Bossaller N.A., Shelton R.C., Zald D.H. Effort-based decision-making in major depressive disorder : a translational model of motivational anhedonia. J. Abnorm. Psychol. 2012;121(3):553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Buckholtz J.W., Zald D.H. Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Front. Hum. Neurosci. 2013;7(180):1–10. doi: 10.3389/fnhum.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E., Fiez J.A. Information content and reward processing in the human striatum during performance of a declarative memory task. Cognitive Affect. Behav. Neurosci. 2012;12(2):361–372. doi: 10.3758/s13415-011-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl B., Kuehner C., Kirsch P., Ruttorf M., Diener C., Flor H. Altered neural reward and loss processing and prediction error signalling in depression. Soc. Cogn. Affect. Neurosci. 2015;10(8):1102–1112. doi: 10.1093/scan/nsu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Morales M. The brain on drugs : from reward to addiction. Cell. 2015;162(4):712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Vrieze E., Pizzagalli D.A., Demyttenaere K., Hompes T., Sienaert P., de Boer P., … Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry. 2013;73(7):639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K.E., Cornwell B.R., Arkin N., Grillon C. Describing the interplay between anxiety and cognition : from impaired performance under low cognitive load to reduced anxiety under high load: anxiety and cognition. Psychophysiology. 2012;49(6):842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K.E., Cornwell B.R., Letkiewicz A.M., Arkin N.E., Grillon C. The complex interaction between anxiety and cognition : insight from spatial and verbal working memory. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-.P., Gorenstein C. Psychometric properties of the Beck Depression Inventory-II : a comprehensive review. Revista Brasileira de Psiquiatria. 2013;35(4):416–431. doi: 10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- Westbrook A., Braver T.S. Cognitive effort : a neuroeconomic approach. Cognitive Affect. Behav. Neurosci. 2015;15(2):395–415. doi: 10.3758/s13415-015-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman M.A., Perez J.E., Ramel W. Factor structure of the Beck Depression Inventory-Second Edition (BDI-II) in a student sample. J. Clin. Psychol. 2000;56(4):545–551. doi: 10.1002/(sici)1097-4679(200004)56:4<545::aid-jclp7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yee D.M., Braver T.S. Interactions of motivation and cognitive control. Curr. Opin. Behav. Sci. 2018;19:83–90. doi: 10.1016/j.cobeha.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.-.N., Chang S.-.H., Guo L.-.Y., Zhang K.-.L., Wang J. The neural correlates of reward-related processing in major depressive disorder : a meta-analysis of functional magnetic resonance imaging studies. J. Affect. Disord. 2013;151(2):531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2017). Depression and other common mental disorders : global health estimates (No WHO/MSD/MER/2017.2; p. 24). Retrieved from: https://https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.