Abstract
Metabolic side effects such as weight gain and disturbed lipid metabolism are often observed in the treatment of atypical antipsychotic drugs (AAPDs), which contribute to an excessive prevalence of metabolic syndrome among schizophrenic patients. Great individual differences are observed but the underlying mechanisms are still uncertain. Research on pharmacogenomics indicates that gene polymorphisms involved in the pathways controlling food intake and lipid metabolism may play a significant role. In this review, relevant genes (HTR2C, DRD2, LEP, NPY, MC4R, BDNF, MC4R, CNR1, INSIG2, ADRA2A) and genetic polymorphisms related to metabolic side effects of AAPDs especially dyslipidemia were summarized. Apart from clinical studies, in vitro and in vivo evidence is also analyzed to support related theories. The association of central and peripheral mechanisms is emphasized, enabling the possibility of using peripheral gene expression to predict the central status. Novel methodological development of pharmacogenomics is in urgent need, so as to provide references for individualized medication and further to shed some light on the mechanisms underlying AAPD-induced lipid disturbances.
Keywords: atypical antipsychotic drugs, weight gain, metabolic syndrome, pharmacogenomics, single nucleotide polymorphisms, leptin, 5-HT2C receptor
Introduction
Schizophrenia is a severe mental disorder with a lifetime morbid risk of approximately 1% across the world (McGrath et al., 2008). Continuous treatment with sufficient dosage of antipsychotic drugs is essential in the therapy and management of schizophrenia (Emsley, 2018). Second-generation antipsychotics [also called atypical antipsychotic drugs (AAPDs)] are first-line antipsychotics with greater improvement of negative symptoms and fewer extrapyramidal symptoms than first-generation antipsychotics. However, metabolic side effects (e.g., weight gain, dyslipidemia, hyperglycemia, etc.) induced by AAPDs raise the risk of cardiovascular diseases, which results in patient noncompliance, relapse, and increased mortality (Mottillo et al., 2010; Mitchell et al., 2013; Ringen et al., 2014). Several studies have reported that antipsychotic-induced weight gain is reversible among pediatric and adult patients who discontinued treatment of antipsychotics (de Kuijper et al., 2013; Upadhyay et al., 2019). It is usually uneasy to make an optimum choice since benefits of these drugs have to be weighed against risks.
Although there have been tremendous reports on the metabolic side effects of atypical antipsychotic drugs, the mechanisms remain elusive (Reynolds and McGowan, 2017). Available evidence has suggested that the clinical responses to antipsychotics and related side effects could vary from patient to patient. The large variability can be attributed to a variety of complex factors, in which genetic factors may play a dominant role. Numerous studies on pharmacogenomics have been conducted to elucidate gene variants related to antipsychotic-induced weight gain or metabolic disturbances (Lett et al., 2011; Zhang et al., 2016; Zai et al., 2018). A meta-analysis has revealed that the genes of pharmacodynamic targets of antipsychotics like HTR2C, DRD2, ADRA2A and genes implicated in obesity such as MC4R, GNB3, FTO, LEP, LEPR, BDNF, and INSIG2 seem to be consistently relevant to antipsychotic-induced weight gain (Zhang et al., 2016). The current systematic review aims to provide an update on the gene polymorphisms related to lipid disturbances of AAPDs and to find the possible relations between central and peripheral pathways.
Methods
Literature research was conducted on PubMed (last: 31 October 2019) with the combinations of the key words: antipsychotic* neuroleptic*, gene, pharmacogen*, polymorphism, weight gain, metabolic, and dyslipidemia. Inclusion criteria were: 1) patients with mental illness; 2) under the treatment of atypical antipsychotic drugs; 3) specific gene polymorphisms were studied; 4) outcomes involved in lipid metabolism such weight, BMI, and percentage of metabolic syndrome, etc. Exclusion criteria were: 1) a review or letter; 2) studies on animals; 3) studies of genes not examined in other studies. Totally, 43 studies were selected for this review (see Figure 1 and Table 1 for details).
Figure 1.
Flowchart of study selection.
Table 1.
Demographic, treatment, and lipid parameters of mentioned studies.
| Reference | Genes (SNPs) | Functional SNPs | Risk allele | Period | APs (n) | % Caucasian | Sample size (M/F), %FEDN | Age | Diagnoses | Single/multi-center | Lipid parameters |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reynolds et al., 2002 | HTR2C rs3813929 (-759C/T) | Yes | C | 6, 10 wk | CP (69), RIS (46), CLO (4), FLU (3), SUL (1) | 0 (100% Asian) | 123 (61/62), 100% | 26.6 ± 7.7 | SCZ | Single | BMI change, >7% weight gain |
| Templeman et al., 2005 | HTR2C rs3813929 | – | C | 6 wk, 3 mo, 9 mo | RIS (26), OLZ (19), HAL (10), QUE (11), ZIP (6), AMI (1) | 100 | 73 (55/18), 100% | 25.2 ± 0.78 | Psychosis | Single | BMI change, >7% BMI increase |
| LEP rs7799039 (-2548A/G) | Yes | G | |||||||||
| Ryu et al., 2007 | HTR2C rs3813929 | – | C | 4 wk | RIS (53), OLZ (12), AMI (5), QUE (4), SGAs (10) | 0 (100% Asian) | 84 (39/45), 69% | 30.1 ± 7.4 | SCZ | Single | >5, >7% BMI increase |
| Mulder et al., 2007 | HTR2C rs3813929 | – | NS | Cross-sectional study | CLO, OLZ, RIS (> 80%); others | 88 | 112 (74/38), 0% | 36 ± 10 | SCZ (63%), SZA (25%), others (12%) | Single | Presence of MS, HDL-C, TG, Waist, BP |
| HTR2C rs518147 (-697G/C) | Yes | C | |||||||||
| Kuzman et al., 2008 | HTR2C rs3813929 | – | NS | 4 mo | OLZ (61), RIS (47) | 100 | 108 (0/108), 89% | 30.6 ± 11.5 | SCZ, SZA | Two sites | ≥7% weight gain |
| Sicard et al., 2010 | HTR2C rs3813929 | – | NS | 6 wk | CLO or OLZ (130), others | 68 | 205 (141/64), N/A | 35.9 ± 10.1 | SCZ, SZA | Multiple | >7% weight gain, % weight change |
| HTR2C rs518147 | – | G | |||||||||
| HTR2C rs6318 (Ser23Cys) | Yes | NS | |||||||||
| Kuzman et al., 2011 | HTR2C rs3813929 | – | T | 3 mo | OLZ (61), RIS (40) | 100 | 101 (0/101), 100% | 33.47 ± 10.62 | SCZ (76%), SZA (7%), delusional disorder (17%) | Single | FBG, TC, LDL, HDL, TG, BP, Waist, Hip; occurrence of MS |
| Hong et al., 2010 | DRD2 rs4436578 | N/A | C | 48.2 ± 27.8 mo | CLO (239), OLZ (70), RIS (170) | 0 (100% Asian) | 479 (292/187), 0% | 47.2 ± 13.2 | SCZ | Single | ≥7% weight gain |
| Lencz et al., 2010 | DRD2 rs1799732 (-141C Ins/Del) | Yes | Del | 6 wk | RIS (32), OLZ (26) | 28 (40% African-American) | 58 (44/14), 100% | 23.5 ± 4.9 | SCZ | Two sites | The log of the ratio of weight relative to baseline weight |
| Muller et al., 2012 | DRD2 rs6277 (-957C/T) | N/A | C | 6, 14 wk | CLO or OLZ (132), others | 62 (28% African American) | 206 (141/65), N/A | 35.69 ± 19.55 | SCZ, SZA | Three sites | ≥7% weight gain, % weight change |
| DRD2 rs1079598 | N/A | C | |||||||||
| DRD2 rs1800497 (TaqIA) | N/A | A1 (T) | |||||||||
| Tybura et al., 2014 | DRD2 rs1799732 | – | NS | 12 wk | ZIP (59), OLZ (82), perazine (60) | 100 | 191 (89/102), N/A | 36.1 ± 12.4 | SCZ | Two sites | Average weight change |
| DRD2 rs1800497 | – | NS | |||||||||
| Alladi et al., 2017 | DRD2 rs1799732 | – | NS | 4 wk | RIS | 100% South Indian | 289 (175/114), N/A | 35.3 ± 10.0 | SCZ | Single | >5% weight gain |
| HTR2C rs3813929 | – | NS | |||||||||
| de Leon et al., 2008 | NPY rs1468271 | N/A | G | Cross-sectional study | OLZ, QUE, or CP (165); others (192) | 88 | 357 (229/128), 0% | 37.6 ± 10.6 | Severe mental illnesses | Three sites | Serum glucose, TC, HDL-C, TG |
| Tiwari et al., 2013 | NPY rs10551063 | N/A | NS | 7.03 ± 3.4 wk | CLO (99), HAL (18), OLZ (37), RIS (40), others (32) | 70 (25% African American) | 226 (151/75), 58% | 35.78 ± 10.6 | SCZ, SZA | Three sites | ≥7% weight gain, % weight change |
| NPY rs16147 | Yes | C | |||||||||
| NPY rs5573 | Yes | A | |||||||||
| NPY rs5574 | Yes | T | |||||||||
| NPY rs16475 | Yes | NS | |||||||||
| Malhotra et al., 2012 | MC4R rs489693 | N/A | A | 12 wk | RIS (84), QUE (25), ARI (30) | 55.4 (23.0% African American) | 139 (81/58), 100% | 13.38 ± 3.75 | Various diagnoses (5% SCZ) | Multiple | BMI change, weight change, fat mass, TG, TC, HDL-C, LDL-C, glucose, insulin, HOMA-IR index, leptin |
| 6 wk | CLO | 70 | 73 (45/28), 0% | 33.48 ± 8.33 | SCZ | ||||||
| 6 wk | RIS (20), QUE (5), ARI (15) | 100 | 40 (22/18), 0% | 35.20 ± 11.33 | SCZ, SZA | ||||||
| 12 wk | HAL (31), AMI (21), QUE (25), ZIP (15) | 100 | 92 (53/39), 100% | 26.02 ± 5.17 | SCZ, SZA, SZP | ||||||
| Czerwensky et al., 2013a | MC4R rs17782313 | N/A | C | 4 wk | OLZ (135), CLO, RIS, PAL, QUE, AMI | 100 | 345 (138/207), 26% | 40.1 ± 14.7 | Various diagnoses | Single | % weight gain, % BMI increase |
| Czerwensky et al., 2013b | MC4R rs489693 | – | A | 4 wk | OLZ (135), CLO, RIS, PAL, QUE, AMI | 100 | 341 (143/198), 25% | 41.3 ± 15.0 | Various diagnoses | Single | % weight gain, % BMI increase |
| Chowdhury et al., 2013 | MC4R rs17782313 | – | NS | 6-14 wk | CLO (99), HAL (16), OLZ (36), RIS (40), others (33) | 70 (25% African American) | 224 (150/74), N/A | 35.63 ± 10.50 | SCZ, SZA | Three sites | % weight gain |
| MC4R rs11872992 | N/A | NS | |||||||||
| MC4R rs8087522 | N/A | A | |||||||||
| Zhang et al., 2019 | MC4R rs489693 | – | A | 6 wk | ZIP (330), ARI (313), OLZ (341), QUE (332), RIS (344), HAL (166), perphenazine (165) | 0 (100% Asian) | 1991 (999/992), 28% | 31.95 ± 7.93 | SCZ | Multiple | >7% BMI gain, % change of BMI, Waist, glucose, TG, HDL, LDL |
| MC4R rs17782313 | – | NS | |||||||||
| Zhang et al., 2008 | BDNF rs6265 (Val66Met) | Yes | Met | 18 ± 6 y | CLO (98), RIS (36), FGAs (62) | 0 (100% Asian) | 196 (130/66) patients, 0% | N/A | SCZ | Single | BMI gain |
| 50 (34/16) controls | N/A | ||||||||||
| Tsai et al., 2011 | BDNF rs6265 | – | NS | 49.2 ± 28.2 mo | CLO (266), OLZ (79), RIS (136) | 0 (100% Asian) | 481 (293/188), 0% | 43.9 ± 8.9 | SCZ | Single | % weight gain |
| BDNF rs11030101 | N/A | T | |||||||||
| BDNF rs12291186 | N/A | NS | |||||||||
| Zai et al., 2012 | BDNF rs6265 | – | NS | 6 wk | CLO or OLZ (207); others | 100 | 257 (188/69), 0% | 31.8 ± 7.9 | SCZ, SZA | Multiple | ≥7% weight gain |
| BDNF rs1519480 | N/A | A | |||||||||
| Zhang et al., 2013 | BDNF rs6265 | – | Met | Cross-sectional study | CLO | 0 (100% Asian) | 199 (143/56), 0% | 55.3 ± 6.9 | SCZ | Single | Presence of MS, individual parameters |
| Fonseka et al., 2015 | BDNF rs6265 | – | Val | 7.26 ± 3.5 | CLO (87), OLZ (32), RIS (31), HAL (15), others | 68 (27% African American) | 188 (126/62), 0% | 35.8 ± 10.0 | SCZ, SZA | Three sites | ≥7% weight gain, % weight change |
| Fang et al., 2016 | BDNF rs6265 | – | Met | Cross-sectional study | CLO (156), RIS (86), SGAs (66) | 0 (100% Asian) | 308 (205/103) patients, 0% | 44.6 ± 10.3 | SCZ | Single | BMI |
| 304 (180/124) controls | 44.5 ± 14.4 | ||||||||||
| Tiwari et al., 2010a | CNR1 rs806378 | N/A | T | 7.54 ± 3.55 wk | CLO (101), OLZ (34), HAL (12), RIS (36) | 64 (30% African-American) | 183 (124/59), 0% | 36.12 ± 10.17 | SCZ, SCA | Three sites | % weight gain |
| Monteleone et al., 2010 | CNR1 rs1049353 (-1359G/A) | N/A | NS | 24 wk | CLO (25), OLZ (22), RIS (14), QUE (6), ARI (6), HAL (10) | 100 | 83 (50/33) patients, 0% | 44.0 ± 10.5 | SCZ (86%) | Two sites | >7% weight gain |
| 80 (40/40) controls | 49.4 ± 12.9 | ||||||||||
| Park et al., 2011b | CNR1 rs1049353 | – | NS | Over 1 y | OLZ | 0 (100% Asian) | 78 (52/26), N/A | 46.4 ± 11.6 | SCZ | Three sites | ≥7% weight gain |
| CNR1 rs806368 | – | NS | |||||||||
| CNR1 rs4707436 | N/A | NS | |||||||||
| Yu et al., 2013 | CNR1 rs6928499 | N/A | G | Cross-sectional study | CLO or OLZ (197); others | 95.1 | 407 (274/133), 0% | 35.6 ± 11.0 | SCZ (69.3%), SZA (20.1%), SZP (10.6%) | Single | Presence of MS, individual parameters |
| CNR1 rs1535255 | N/A | T | |||||||||
| CNR1 rs2023239 | N/A | T | |||||||||
| Kang et al., 2008 | LEP rs7799039 (-2548A/G) | Yes | A | 453 ± 289 d | OLZ | 0 (100% Asian) | 74 (50/24), N/A | 47.2 ± 11.6 | SCZ | Three sites | ≥7% weight gain |
| Yevtushenko et al., 2008 | LEP rs7799039 | – | G | Cross-sectional study | CLO (21), OLZ (31), RIS (16) | 100 | 134 (87/47), 0% | 41.6 ± 11.8 | SCZ, SZA | Two sites | Presence of MS, Waist, BMI, BMI ≥30 kg/m2, presence of central obesity |
| HTR2C rs3813929 (-759C/T) | – | NS | |||||||||
| Gregoor et al., 2010 | LEP rs7799039 | – | A | Cross-sectional study | OLZ (99), CLO (86), QUE (56), RIS (46), ARI (14) | 100 | 353 (184/169), 0% | N/A | Psychotic disorder (50.7%) | Single | Serum TC/HDL |
| Gregoor et al., 2011 | LEP rs7799039 | – | NS | within 1 y | CLO (68), OLZ (54), QUE (31), ARI (23), RIS (30) | 100 | 141 (82/59), 0% | N/A | Psychotic disorder (63.1%) | Single | BMI >30, BMI change, BMI change per week |
| Brandl et al., 2012 | LEP rs7799039 | – | NS | 7.19 ± 3.47 wk | CLO (80), OLZ (28), RIS (32), HAL (16), others (24) | 70.2 (23.8% African-American) | 181 (118/63), N/A | 35.93 ± 10.94 | SCZ, SZA | Three sites | % weight change |
| LEP rs10954173 | N/A | NS | |||||||||
| LEP rs3828942 | N/A | NS | |||||||||
| Le Hellard et al., 2009 | INSIG2 rs17587100 | N/A | N/A | 12 ± 1.2 wk | CLO | 100 | 160 (97/63), 0% | 21.9 ± 8.9 | Schizophrenia spectrum disorders | Single | BMI change |
| INSIG2 rs10490624 | N/A | N/A | |||||||||
| INSIG2 rs17047764 | N/A | N/A | |||||||||
| Opgen-Rhein et al., 2010 | INSIG2 rs17587100 | – | NS | 6 wk | CLO, OLZ, RIS, AMI, QUE, SGAs | 100 | 128 (48/80), 17% | 38.63 ± 11.96 | SCZ, SZA | Three sites | ≥7% weight gain, % weight change |
| INSIG2 rs10490624 | – | NS | |||||||||
| INSIG2 rs17047764 | – | NS | |||||||||
| Tiwari et al., 2010b | INSIG2 rs17587100 | – | NS | 7.94 ± 3.74 wk | CLO (96), OLZ (34), RIS (12), HAL (12) | 57.8 (35.1% African-American) | 154 (110/44), 0% | 35.78 ± 9.82 | SCZ, SZA | Three sites | ≥7% weight gain, % weight change |
| INSIG2 rs10490624 | – | NS | |||||||||
| INSIG2 rs17047764 | – | NS | |||||||||
| Liou et al., 2012 | INSIG2 rs11123469 | N/A | C | Cross-sectional study | CLO (171), OLZ (91), RIS (194) | 0 (100% Asian) | 456 (369/157), 0% | N/A | SCZ | Single | Prevalence of MS |
| INSIG2 rs10185316 | N/A | NS | |||||||||
| INSIG2 rs1559509 | N/A | NS | |||||||||
| Wang et al., 2005 | ADRA2A rs1800544 (-1291C/G) | G | 14.0 ± 6.2 mo | CLO | 0 (100% Asian) | 93 (49/44), 0% | 38.4 ± 8.1 | SCZ | Single | Weight gain, >7% weight gain | |
| Park et al., 2006 | ADRA2A rs1800544 | – | G | Over 1 y | OLZ | 0 (100% Asian) | 62 (44/18), 0% | 46.5 ± 11.1 | SCZ | Two sites | >10% weight gain, % weight change |
| Sickert et al., 2009 | ADRA2A rs1800544 | – | C | 8.4 ± 3.6 wk | CLO (85), OLZ (20), RIS (13), HAL (11) | 50 (42% African-American) | 129 (96/33), 0% | 36.5 ± 9.0 | SCZ, SZA | Multiple | weight gain, % weight change |
| Risselada et al., 2010 | ADRA2A rs1800544 | – | NS | Cross-sectional study | OLZ (106), RIS (103), CLO (102), ARI (21), QUE (12), SGAs (69) | 94 | 470 (320/150), 0% | 38 ± 10 | SCZ (78%), SZA (17%) | Multiple | Presence of MS, HDL, TC, Waist, BP, glucose |
| De Luca et al., 2011 | ADRA2A rs1800544 | – | NS | 8.3 ± 3.7 wk | CLO (91), OLZ (22), HAL (12), RIS (14) | 51.8 (40.3% African-American | 139 (101/38), N/A | 36.2 ± 9.4 | SCZ | Three sites | % weight change, weight gain >8% |
SCZ, schizophrenia; SZA, schizoaffective disorder; SZP, schizophreniform disorder; FEDN, first-episode drug-naïve patients; SNPs, single nucleotide polymorphisms; N/A, not available; NS, not significant; APs, antipsychotics; FGAs, first-generation antipsychotics; FLU, fluphenazine; HAL, haloperidol; CP, chlorpromazine; CLO, clozapine; OLZ, olanzapine; RIS, risperidone; ARI, aripiprazole; QUE, quetiapine; AMI, amisulpride; ZIP, ziprasidone; PAL, paliperidone; SUL, sulpiride; d, days; wk, weeks; mo, months; y, years; M, male; F, female; MS, metabolic syndrome; HDL, high-density lipoprotein; HDL-C, HDL cholesterol; LDL, low-density lipoprotein; LDL-C, LDL cholesterol; FBG, fasting blood glucose; BP, blood pressure; TG, triglycerides; TC, total cholesterol; HOMA-IR index, the homeostasis model assessment insulin resistance index; Waist, waist circumference; Hip, hip circumference.
Gene Polymorphisms Related to Central Nervous System
The Serotonin 5-HT2c Receptor
Central serotonin system is associated with the modulation of feeding behavior (Lam et al., 2010). There are at least seven subtypes of 5-HT receptors, of which the 5-HT2 subtype is divided into 5-HT2A, 5-HT2B, and 5-HT2C. The serotonin 5-HT2C receptor has shown the most consistent findings in studies on atypical antipsychotic drug-induced lipid disturbances. The serotonin 5-HT2C receptor is present in hypothalamic nuclei such as the arcuate nucleus (ARC) and the ventral tegmental area (VTA) (Faton et al., 2018). Animal experiments have shown that 5-HT2C receptor agonists reduce feeding (Clifton et al., 2000), and the antagonists increase feeding and lead to weight gain (Bonhaus et al., 1997). The serotonin 5-HT2C receptor gene knockout mice ate more than the controls and became obesity (Tecott et al., 1995). Numerous studies have indicated that 5-HT2C receptor mediates leptin-induced anorexia, but reports regarding serotonin-leptin interactions are discrepant (von Meyenburg et al., 2003; Voigt and Fink, 2015; Wierucka-Rybak et al., 2016). Cannabinoid receptor 1 (CB1 receptor) stimulation could inhibit the secretion of cerebral serotonin in the mouse brain (Nakazi et al., 2000). Coupled with the inhibition of neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons by 5-HT1B receptor action, 5-HT2C receptor mediates the activation of pro-opiomelanocortin (POMC) neurons as a downstream pathway of serotonin controlling food intake (Lam et al., 2010). Showing the highest antagonizing affinity to 5-HT2C receptor among antipsychotic drugs, clozapine and olanzapine exert the most serious effects of weight gain in patients (Allison et al., 1999). It suggested that the antagonism against 5-HT2C receptor by antipsychotic drugs may lead to increased food intake and eventually weight gain. This is supported by the observation that olanzapine exerts its metabolic side effects by targeting 5-HT2C receptor in mouse model (Lord et al., 2017).
The promoter region of HTR2C gene is mainly influenced by -759C/T (rs3813929) and -697G/C (rs518147) polymorphisms. HTR2C -759C/T polymorphism was the first single nucleotide polymorphism (SNP) to be reported as an associated HTR2C polymorphism with antipsychotic drug-induced weight gain (Reynolds et al., 2002). Besides, it is the most replicated gene polymorphism related to AAPD-induced weight gain (Zhang et al., 2016). Various clinical studies suggested that HTR2C -759C allele was a risk allele of a substantial weight gain (over 7% than baseline) in patients treated with typical or atypical antipsychotic drugs (Reynolds et al., 2002; Templeman et al., 2005; Ryu et al., 2007). A study in first episode drug-naive female patients with schizophrenia showed that -759T is associated with an increase in waist circumference, fasting blood glucose, and blood triglyceride levels (Kuzman et al., 2011). However, associations between HTR2C -759C/T polymorphism and weight gain or presence of metabolic syndrome were reported to be nonsignificant in other studies (Mulder et al., 2007; Kuzman et al., 2008; Sicard et al., 2010; Alladi et al., 2017). Despite the negative results, haplotype analyses suggested that HTR2C 759C-697G-Cys23 haplotype was associated with the most percentage weight gain induced by various antipsychotics (Sicard et al., 2010).
Dopamine Receptor D2
Dopamine receptor D2 (DRD2) is the main target of antipsychotic drugs. An animal study found that the availability of striatal D2 receptor in obese rats was lower than that in lean controls (Hamdi et al., 1992). In a human study, the striatal dopamine transporter availability was negatively correlated with body mass index (BMI) in a group of healthy volunteers (Chen et al., 2008). It is postulated that it could be a mechanism of overeating that the neuropeptides regulating homeostatic energy balance also modulate the activity of dopamine neurons and the rewarding circuits underlying food intake (Volkow et al., 2011). Therefore, Blum et al. hypothesized that as dopamine D2 receptor antagonists, antipsychotic drugs cause a hypodopaminergic reward circuitry, leading to excessive food intake and ultimately obesity (Blum et al., 2014). Functional magnetic resonance imaging (fMRI) showed that an increased activity in striatal regions of the reward system was positively correlated with weight gain after 6-week amisulpride treatment (Nielsen et al., 2016).
The role of DRD2 rs4436578-C in weight gain induced by atypical antipsychotics is verified in 479 chronic schizophrenic patients under long-term treatment of clozapine, olanzapine, or risperidone (Hong et al., 2010). DRD2 promoter region polymorphism -141C Ins/Del (rs1799732) was also reported to be associated with weight gain in 58 first episode patients treated with randomly-assigned olanzapine or risperidone for 6 weeks (Lencz et al., 2010), whereas nonsignificant associations were found in later studies of larger samples (Tybura et al., 2014; Alladi et al., 2017). A systematic analysis of genetic polymorphisms spanning the five dopamine receptor genes (DRD1–DRD5) found only DRD2 rs6277 (C957T), rs1079598, and rs1800497 (TaqIA) to be significantly associated with antipsychotic-induced weight gain in chronic patients with schizophrenia or schizoaffective disorder (Muller et al., 2012). It was suggested that the C957T polymorphism would not change the amino acid sequence of the dopamine D2 receptor, but it was related to the stability of the striatum D2 receptor (Hirvonen et al., 2009) and the stability and half-life of the DRD2 messenger RNA (mRNA) (Duan et al., 2003). The TaqIA polymorphism is located in coding region of the adjacent ANKK1 gene and overlaps with the 3’ end region of the DRD2 gene. So, it may be in linkage disequilibrium with a functional polymorphism of DRD2, or may affect the dopamine signaling through the ANKK1 gene. Studies have shown that if the TaqIA site is A1, it may result in decreased expression of DRD2 and decreased dopaminergic activity (Giegling et al., 2013).
Neuropeptide Y
NPY is a 36-amino-acid peptide expressed in the central and peripheral nervous system. In the ARC neurons, NPY colocalizes with AgRP, which can antagonize α-melanocyte-stimulating hormones (α-MSH) binding to melanocortin-3 receptor (MC3R) and melanocortin-4 receptor (MC4R). Another group of neurons co-express POMC and cocaine and amphetamine-regulated transcript (CART) and inhibit food intake (Arora and Anubhuti, 2006). Low leptin levels can upregulate neuropeptide Y and exert orexigenic effects. Leptin directly and differentially regulates NPY and POMC neurons, and then controls feeding behavior and energy homeostasis (Elias et al., 1999). Outside the hypothalamus, NPY mainly exists in the brainstem and the catecholaminergic neurons in the sympathetic nervous system. Results in vitro indicate that NPY may inhibit lipolysis in murine adipocytes (Bradley et al., 2005). Transgenic mice overexpressing NPY showed significant obesity and the lipogenic effects as well as inhibition of catecholaminergic tone of NPY were suggested (Vahatalo et al., 2015). NPY and leptin are recognized to interact in a homeostatic loop to regulate energy balance not only in the brain, but also directly at the adipocyte level (Martinez et al., 2000).
Chronic treatment of atypical antipsychotic drugs increased NPY immunoreactivity and mRNA expression in the rat hypothalamus (Kirk et al., 2006; Weston-Green et al., 2012). NPY rs1468271 was associated with hypercholesterolemia in patients taking olanzapine, quetiapine, or chlorpromazine (de Leon et al., 2008). Significant associations between the SNPs rs16147, rs5573, and rs5574 in NPY and weight gain in clozapine or olanzapine-treated patients of European ancestry were reported (Tiwari et al., 2013). Compared with carriers of TT genotype at rs16147, individuals with the C allele showed a higher risk of weight gain probably due to increased NPY levels. Besides, genetic interaction between rs16147 in NPY and rs806378 in cannabinoid receptor 1 gene further supports their biological interaction (Tiwari et al., 2013).
The Melanocortin 4 Receptor
The MC4R is a transmembrane G protein-coupled receptor expressed in the hypothalamus and peripheral tissues. The central melanocortin system potently regulates feeding and directly controls lipid metabolism in liver and adipocytes (Nogueiras et al., 2007). MC4R plays a key role in suppressing food intake. MC4R gene is the most common single-gene effect of human obesity (Beckers et al., 2011). Rodent experiments showed that Mc4r knockout mouse exhibited hyperinsulinemia, hyperglycemia, and adult obesity syndrome (Srisai et al., 2011). Multiple pathways are involved in the regulation of central melanocortin system in energy homeostasis (Shen et al., 2017). MC4R signaling regulates energy balance through stimulating brain-derived neurotrophic factor (BDNF) expression in the ventromedial hypothalamus (VMH) (Xu et al., 2003). Central melanocortin pathway through MC4R is an indispensable downstream mediator of the anorexigenic effect of serotonin (Lam et al., 2008). The expression of MC4R in the rat hypothalamus is increased under the long-term treatment of antipsychotics probably through a compensatory mechanism (Rojczyk et al., 2015). A genome-wide association study found that MC4R rs489693 demonstrated consistent effects on weight gain, as well as on levels of triglycerides, leptin, and insulin, HOMA-IR index (the homeostasis model assessment insulin resistance index), and total fat mass (Malhotra et al., 2012). Afterward, the association between MC4R rs489693 A-allele and greater weight gain was confirmed (Czerwensky et al., 2013b). They also reported carriers of MC4R rs17782313 C-allele at risk of greater percentage weight gain after taking atypical antipsychotics (Czerwensky et al., 2013a). However, Chowdhury et al. didn’t replicate the significant association of rs17782313, but they reported that carriers of rs8087522-A gained significantly more weight than non-carriers in white Americans (Chowdhury et al., 2013). Located in the MC4R promoter region, rs8087522 A-allele may affect the gene expression of MC4R by binding to an unknown nuclear protein, while the G-allele has no effect. A large-scale pharmacogenetic study in Chinese schizophrenia patients reported the ubiquitous association between rs489693 and metabolic measures, while rs17782313 is less involved in antipsychotic-induced metabolic disturbances (Zhang et al., 2019).
Brain-Derived Neurotrophic Factor
The BDNF is a member of the neurotrophic factor family abundantly expressed in the hippocampus and hypothalamus. Beyond a fundamental role in the brain development and plasticity, BDNF is thought to play a major part in the regulation of food intake (Rosas-Vargas et al., 2011). It is reported that central infusion of BDNF can induce dose-dependent food restriction and weight loss, perhaps via its up-regulation of hypothalamic serotonin activity (Pelleymounter et al., 1995). BDNF was also observed to regulate food intake via its inhibitory effect on NPY and modulation of the dopamine system (Wang et al., 2007; Cordeira et al., 2010). Researchers have found reduced serum BDNF levels in first-episode drug naive psychosis patients and a trend of greater reductions in female patients (Jindal et al., 2010). Moreover, a significant increase in BDNF levels in prefrontal cortex and cerebrospinal fluid samples of postmortem schizophrenia patients was reported (Issa et al., 2010). Thus, alterations in BDNF may play a role in the pathophysiology of schizophrenia (Favalli et al., 2012). Both typical (haloperidol) and atypical antipsychotic drugs (clozapine, risperidone) decrease serum BDNF levels in schizophrenia patients (Xiu et al., 2009) and the expression of Bdnf mRNA in the hippocampus of rats (Angelucci et al., 2000; Lipska et al., 2001; Chlan-Fourney et al., 2002) although results are inconsistent in some studies (Bai et al., 2003; Park et al., 2011a). Further, reduced serum BDNF levels may be related with weight gain in female but not in male patients with schizophrenia under long-term antipsychotic treatment (Zhang et al., 2007). The effect of BDNF on weight gain induced by antipsychotics seems to be gender-specific but the results are inconsistent.
Human BDNF gene is located on chromosome 11p14.1. The Val66Met variant (rs6265) in the BDNF promoter region is the most investigated SNP of the gene, showing an association of cognitive impairment (Bath and Lee, 2006) and obesity (Skledar et al., 2012). It markedly alters the intracellular trafficking and packaging of pro-BDNF and impacts the activity-dependent secretion of the mature peptide (Egan et al., 2003). The Met/Met genotype of the BDNF Val66Met polymorphism has a significant effect on BMI gain and metabolic syndrome in male but not female schizophrenic patients treated with long-term antipsychotic drugs (Zhang et al., 2008; Zhang et al., 2013; Fang et al., 2016). However, these results are incompatible with some studies that indicate Val/Val was associated with greater weight gain induced by antipsychotics (Fonseka et al., 2015). Tsai et al. failed to replicate the relationship between the Val66Met polymorphism and body weight gain after long-term antipsychotic treatment, but they found a visible difference in percentage weight change in patients with different copies of haplotype GTA (rs6265-rs11030101-rs12291186) (Tsai et al., 2011). Additionally, a two-marker haplotype rs6265-rs1519480 was also reported to be associated with antipsychotic-induced weight change in European ancestry (Zai et al., 2012).
The Cannabinoid 1 Receptor
The endocannabinoid system is involved in modulating energy homeostasis by controlling food intake via central and peripheral pathways, as well as stimulating lipogenesis and fat accumulation (Di Marzo and Matias, 2005; Bluher et al., 2006). It may be negatively regulated by leptin in the neural circuitry (Di Marzo et al., 2001). In the hypothalamus, the interaction between the endocannabinoid and NPY systems appears to be bidirectional, and peripheral endocannabinoid levels are increased in obese mice induced by neuropeptide Y overexpression (Vähätalo et al., 2015). Encoding by the gene CNR1, the CB1 receptor mediates the effects of cannabinoid binding primarily in the brain and also presents in peripheral tissues, including adipocytes (Bensaid et al., 2003), hepatocytes (Osei-Hyiaman et al., 2005), pancreas (Nakata and Yada, 2008), muscle (Mendizabal-Zubiaga et al., 2016), and the gut (Coutts and Izzo, 2004). Cnr1 knockout mice experienced food restriction compared with wild-type littermates (Di Marzo et al., 2001). Selective CB1 receptor antagonist SR141716A (rimonabant) ameliorates diet-induced obesity of mice through enhancement of fatty acid oxidation and energy expenditure in white adipocytes (Jbilo et al., 2005), or modulating macrophage inflammatory mediators via gut microbiota alterations (Mehrpouya-Bahrami et al., 2017). Clinical trials proved that rimonabant decreased body weight and waist circumference in overweight or obese patients (Van Gaal et al., 2005; Pi-Sunyer et al., 2006). The 3813G allele at the exon 4 of CNR1 is associated with obesity-related phenotypes like waist circumference and subscapular skinfold thickness in adult men (Russo et al., 2007).
Evidence from pre-clinical, clinical, genetic, postmortem, and neuroimaging studies have indicated an important role of the endocannabinoid system and cannabinoid receptors in the pathophysiology of schizophrenia (Fakhoury, 2017). The G allele frequency of the CNR1 1359G/A gene polymorphism potentially relates to therapeutic response to atypical antipsychotics (Hamdani et al., 2008). Alterations in CB1 receptor-mediated G-protein signaling by antipsychotic treatment was different in a sex- and age-selective manner (Wiley et al., 2008). Chronic treatment with aripiprazole upregulated the gene expression of Cnr1 in the frontal cortex of rats (Cheng et al., 2008). Risperidone increased CB1 receptor binding in rat brain (Secher et al., 2010). Oral intake of haloperidol or olanzapine produces region-specific increase in cannabinoid receptor levels distinctly (Delis et al., 2017). Both CB1 receptor antagonist NESS06SM and inverse agonist rimonabant reduced food intake and weight gain and restored all blood parameters in a rat model treated with olanzapine (Lazzari et al., 2017). However, the results of the relationship between CNR1 polymorphisms and antipsychotic-induced lipid disturbances differ from various single nucleotide polymorphisms. A study of 20 tag SNPs found the rs806378 polymorphism to be associated with weight gain in European patients treated with clozapine or olanzapine (Tiwari et al., 2010a). CNR1 polymorphisms -1359 G/A (rs1049353, rs806368, and rs4707436) were not associated with antipsychotic-induced weight gain (Monteleone et al., 2010; Park et al., 2011b). A cross-sectional study of a naturalistic cohort of 407 patients with schizophrenia showed the minor alleles of rs6928499, rs1535255, and rs2023239 were associated with lower levels of high-density lipoprotein cholesterol and fasting glucose (Yu et al., 2013).
Gene Polymorphisms Related to Peripheral Tissues
Leptin
Leptin is a peptide hormone predominantly secreted by adipocytes, targeting hypothalamic nerve network to suppress appetite. At peripheral level, leptin is involved in the regulation of lipid and glucose metabolism in adipose tissue, liver, and skeletal muscle, as well as gastrointestinal nutrient absorption (Sáinz et al., 2015). Leptin resistance primarily takes responsibility for obesity in some studies (Sáinz et al., 2015). Serum leptin levels were elevated significantly after treatment of olanzapine, clozapine, and quetiapine, whereas haloperidol and risperidone produced nonsignificant leptin changes (Potvin et al., 2015). The significant association between leptin increases and BMI changes was observed across studies. Two hypotheses about the role played by leptin in antipsychotic-induced weight gain were proposed: leptin as an epiphenomenon of weight gain, or antipsychotic-induced leptin resistance causing weight gain (Panariello et al., 2012).
Therefore, the correlation between leptin gene (LEP) and lipid disturbances induced by atypical antipsychotics has been a research hotspot. Among the polymorphisms, LEP rs7799039 (-2548A/G) was verified to be associated with weight gain in many studies (Templeman et al., 2005; Kang et al., 2008; Shen et al., 2014). A cross-sectional study showed that serum total cholesterol (TC)/high-density lipoprotein (HDL) ratio in LEP -2548G male carriers was lower than that of non-carriers after taking AAPDs for more than 3 months, but not significant in female patients (Gregoor et al., 2010). However, a longitudinal study conducted by the same group found LEP -2548G was not significantly associated with BMI change during treatment of atypical antipsychotics (Gregoor et al., 2011). A haplotype of LEP rs7799039G-rs10954173G-rs3828942G showed a significant association with weight gain despite results of all the SNPs were not significant (Brandl et al., 2012). A meta-analysis indicated that the LEP -2548A allele was associated with an increased risk of antipsychotic-induced weight gain in Asian patients, while it seemed to decrease the risk in European populations (Shen et al., 2014). Combined genotype analysis revealed that gene-gene interaction between the LEP and HTR2C polymorphisms was highly significant in their associations with occurrences of metabolic syndrome, BMI, and waist circumference (Yevtushenko et al., 2008).
Insulin-Induced Gene 2
In the endoplasmic reticulum (ER), insulin-induced gene (INSIG) proteins form complexes with sterol-regulatory element-binding proteins (SREBPs) and SREBP cleavage activating proteins (SCAP), regulating cholesterol and lipid fatty acid biosynthesis (McPherson and Gauthier, 2004). There are two isoforms of INSIG proteins, INSIG1 and INSIG2. INSIG2 is not a transcriptional target of SREBPs as INSIG1, but it can also cause the retention of the SCAP/SREBP complex in the ER in a sterol dependent way and thereby blocks cholesterol synthesis (Yabe et al., 2002). The INSIG2/SCAP/SREBP signaling may be altered by various antipsychotic drugs. Both clozapine and haloperidol can activate the gene expressions of the SREBP system in human glioma cells, which may be a mechanism of therapeutic efficacy (Ferno et al., 2005). But the upregulation of the lipogenesis in peripheral tissues can be a cause of the metabolic side effects induced by antipsychotics. Clozapine and risperidone significantly reduced INSIG2 and activated the expression of SCAP/SREBP in rat liver (Cai et al., 2015). It is reported that AAPD treatment induces early-stage lipid biosynthesis in adipose-derived stem cells (ASCs) and such abnormal lipogenesis can be reversed when INSIG2 expression was increased (Chen et al., 2017). Three markers (rs17587100, rs10490624, and rs17047764) localized within or near the INSIG2 gene had a strong association with clozapine-induced BMI gain in German patients with schizophrenia (Le Hellard et al., 2009). However, significant associations of the three aforementioned SNPs weren’t replicated in other European patients (Opgen-Rhein et al., 2010; Tiwari et al., 2010b). Liou et al. demonstrated that the C-C-C haplotype of INSIG2 rs11123469-rs10185316-rs1559509 significantly elevated the risk of AAPD-induced metabolic syndrome (Liou et al., 2012). This association can be attributed to the action of INSIG2 independently or the gene-gene interaction with INSIG1.
Adrenergic Alpha-2a Receptor
The sympathetic nervous system regulated by the hypothalamus plays an important role in energy expenditure and lipolysis. Adrenergic α-2 receptors are classified into three subtypes, α2A, α2B, and α2C. Mice lacking α2A-adrenoceptors (ADRA2A) showed increased energy expenditure, lipolysis, and hyperinsulinemia (Ruohonen et al., 2018). Atypical antipsychotics have affinities for adrenergic receptors, including subtypes of α2A, α2B, α2C, α1A, α1B (Roth et al., 2004). The -1291 C/G promoter polymorphism (rs1800544) located in the regulatory promoter sequence of the ADRA2A gene may influence the transcription factor control. Association between ADRA2A rs1800544 polymorphism and schizophrenia was found in a study of Czech male patients with schizophrenia (Lochman et al., 2013). Carriers of ADRA2A 1291-GG gained more weight than the subjects with genotype 1291-CC in Asian patients after long-term treatment of clozapine or olanzapine (Wang et al., 2005; Park et al., 2006). But results of European-Americans showed the carriers of the ADRA2A -1291C allele gained more weight during treatment of 8.4 weeks on average (Sickert et al., 2009). The association between ADRA2A -1291C/G and the prevalence of metabolic syndrome wasn’t significant among white patients using antipsychotics (Risselada et al., 2010). No significant association between ADRA2A -1291C/G and weight gain could be detected in another study among complex ethnic subjects treated with different antipsychotic drugs (De Luca et al., 2011). Conflicting results might be attributed to ethnic differences and diverse observation periods.
Relations Between Genes in Central Nervous System and Peripheral Tissues
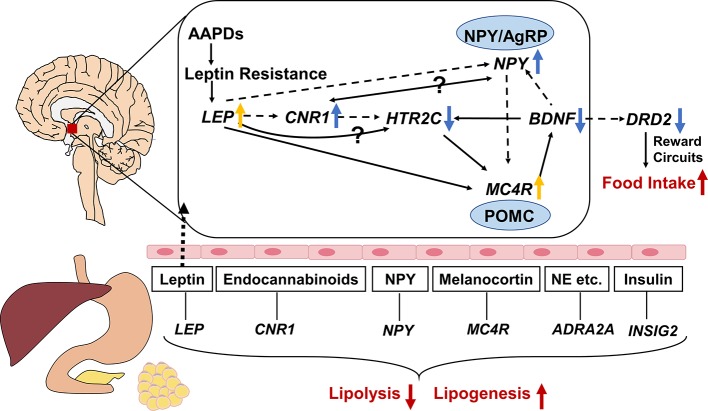
Gene expression in central nervous system is not readily available, therefore, it is of great importance to find equivalent evidence from the peripheral blood. It was reported that gene expression in peripheral blood mononuclear cells could be used as a fingerprint of central nervous system disease (Achiron and Gurevich, 2006). Thus, we focus on the network connections between the regulatory mechanisms of central nervous system pathways and peripheral pathways. The connection of the overall related genes mentioned in this review is shown in Figure 2 . It is obvious that antipsychotics might induce weight gain or metabolic syndrome through central and peripheral ways. In central nervous system, HTR2C, DRD2, LEP, NPY, MC4R, BDNF, CNR1 polymorphisms play an important role in regulating food intake, and they can be affected by AAPDs. Besides, the lipid metabolism in peripheral tissues may be altered by the SNPs of LEP, NPY, MC4R, CNR1, INSIG2, and ADRA2A. As we can see in Figure 2 , complex pathways are involved in the modulation of energy intake and energy expenditure, that is orexigenic and anorexigenic mechanisms. NPY/AgRP neurons and POMC neurons play a fundamental role in the downstream of the pathway of LEP, CNR1, and HTR2C. NPY is an orexigenic mediator whereas MC4R exerts anorexigenic effects. HTR2C mediates the effects of multiple genes, such as LEP, CNR1, and BDNF. Dopamine regulates appetite mainly through brain reward circuits. Leptin is an upstream molecular bridging the peripheral tissue and central nervous system. Secreted from fat cells, leptin acts on the hypothalamus through the blood-brain barrier, and ultimately suppresses appetite by triggering multiple signaling cascades like NPY, MC4R, CNR1. In peripheral tissues, multiple hormones or peptides affect lipid metabolism, such as leptin, neuropeptide Y, melanocortin, endocannabinoids, insulin, and norepinephrine, etc. Inhibition of lipolysis and stimulation of lipogenesis lead to hyperlipemia and obesity.
Figure 2.
Gene-gene interaction among genes associated with atypical antipsychotic-induced lipid disturbances. The central and peripheral pathways are separated by brain-blood barrier, with leptin through it. Neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons and pro-opiomelanocortin (POMC) neurons play a fundamental role in the downstream of the pathway of LEP, CNR1, and HTR2C. NPY is an orexigenic mediator whereas melanocortin-4 receptor (MC4R) exert anorexigenic effects. HTR2C mediates the effects of multiple genes, such as LEP, CNR1, and BDNF. Dopamine regulate appetite mainly through brain reward circuits. As a bridge between the central and peripheral ways, leptin inhibits food intake by triggering multiple signaling cascades like NPY, MC4R, CNR1. In peripheral tissues, multiple hormones or peptides modulating lipid metabolism, such as leptin, neuropeptide Y, melanocortin, endocannabinoids, insulin, and norepinephrine etc. Inhibition of lipolysis and stimulation of lipogenesis lead to hyperlipemia and obesity. The solid and dotted lines indicate upregulation and downregulation respectively. ↑: activated by AAPDs; ↓: inhibited by AAPDs; yellow arrows indicate compensatory effects rather than direct effects; NE, norepinephrine.
Conclusion
Pharmacogenomics helps to find possible genetic polymorphisms related to lipid disturbances induced by atypical antipsychotic drugs and the variation among different patients. Nevertheless, inconsistency and limitations have impeded the progress in this field. As mentioned above, discrepancies often occur between different studies on the same SNP. Several reasons are to be noted. A small sample size may reduce the statistical power; and the representativeness of the sample can be weakened if the frequency of a certain base is low in a small sample. Ethnic, gender, and age differences should be taken into account. Evidence has shown worse lipid metabolic dysfunction in female schizophrenia patients due to antipsychotics (Castellani et al., 2019). Sex differences were also found in gene expression associated with antipsychotic induced weight gain (Sainz et al., 2019). Younger age has been reported to be a risk of greater antipsychotic-related weight gain (Maayan and Correll, 2010; Greil et al., 2013). The degree of the metabolic side effects varies from antipsychotics and doses. Evidence suggested a dose-dependent effect between serum levels and metabolic side effects of clozapine and olanzapine although the relationship between daily dose and metabolic disturbances is not clear. (Simon et al., 2009). First episode drug-naive patients are more sensitive to the AAPDs than chronic ones, which may cause the distinct exposure to the adverse drug reaction. Long-term treatment and short-term treatment might be different in the effects of weight gain or metabolic syndrome. To observe the alteration of BMI, a long enough study duration makes it easier to get a significant result. For instance, LEP -2548A/G polymorphism showed nonsignificant association with short-term (6-week and 3-month) weight change but was associated with 9-month antipsychotic-induced weight gain (Templeman et al., 2005). Meanwhile, although metabolic syndrome was verified to be related to weight gain for patients under the treatment of clozapine (Bai et al., 2011), different indicators (BMI or blood lipid levels, etc.) and diverse definitions can lead to inconsistency. Significant end points vary from body weight (or BMI) increase ≥7% of baseline to change of metabolic parameters or presence of metabolic syndrome. Hyperlipidemia (hypertriglyceridemia or hypercholesterolemia) may be the outcome of weight gain or a direct effect of antipsychotics. A rodent experiment found that only olanzapine significantly induced weight increase in rats, but both olanzapine and clozapine elevated blood lipid levels after 9-week treatment at clinic equivalent doses (Liu et al., 2017). Therefore, different outcome variables of lipid disturbances may be analyzed separately. As for animal studies, we should pay more attention to the different regions (the striatum, the hypothalamus, etc.) and species differences when we compare the various conclusions from a bulk of published work. For example, anatomical studies and physiological experiments have suggested significant interspecies differences in the distribution of the cannabinoid 1 receptor both in central and peripheral nervous system (Howlett et al., 2002). Furthermore, since some genes participate in the metabolic side effects as well as the pharmaceutical effect, the functions of the AAPDs can be complex to analyze.
Genetic correlation study consists of single nucleotide polymorphisms, haplotype analysis, gene-gene interaction, genome wide association study (GWAS), etc. Several susceptibility gene loci have been reported but the exact mechanism hasn’t been illuminated. Additionally, correlations do not imply causations. Hence, in vitro and in vivo studies are needed to explore the specific effects of AAPDs on these related genes and gene-gene interactions. Further functional analyses are also required to verify which are the functional polymorphisms and the specific functional consequences of these SNPs. Moreover, both obesity and schizophrenia are polygenic diseases, so we can speculate that the metabolic side effects of atypical antipsychotics cannot be monogenic. Research strategies of monogenic diseases are inapplicable to find out the complex causations currently. Given the limitations of such research, more efficient methods are in great need. The International Schizophrenia Consortium proposed a polygenic risk score (PRS) test for schizophrenia (International Schizophrenia C. et al., 2009). Now schizophrenia polygenic risk score has been reported to be a potential predictor of antipsychotic efficacy in patients with first-episode psychosis (Jian-Ping Zhang et al., 2018). In addition, the hypothesis of an omnigenic model proposed by Boyle et al. provide us with a new perspective to understand gene effects—core genes and peripheral genes. (Boyle et al., 2017).
Future Perspective
Although the pharmacological mechanisms and pharmacogenomics of atypical antipsychotic drugs seem to be hard to figure out, research on weight gain and dyslipidemia induced by atypical antipsychotics is of great significance. On one hand, combining genetic markers and relevant clinical indicators, a pharmacogenetic model can be built to predict the risk of lipid disturbances for an individual patient using atypical antipsychotics. Consequently, it will allow clinicians to select appropriate medication with less metabolic side effects and enough efficacy for every patient and promote individualized treatment. A multigene risk-model has showed promising results of predicting antipsychotic-induced weight gain (Tiwari et al., 2014). Moreover, the combinatorial model of genetic and clinical data could help to identify patients at high risk for early weight gain (Vandenberghe et al., 2016). On the other hand, the more we know about the mechanism of the metabolic side effects of atypical antipsychotics, the better we can tackle with this troublesome adverse drug reaction. More specifically, maybe we can develop novel molecules with high receptor selectivity or new drug targets. And toxicities could be designed out by counter-screening approaches combined with medicinal chemistry methods when discovering selectively non-selective drugs representing highly effective treatments (Roth et al., 2004). Besides, combination with lipid-lowering medication might help to attenuate weight gain or dyslipidemia. Metformin is a good try. In a double-blind and placebo-controlled study, metformin addition showed great efficacy, safety, and good adherence in preventing olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients (Wu et al., 2008). In addition, recently a preclinical study indicates that the selective protein kinase Cβ (PKCβ) inhibitor, ruboxistaurin (LY-333531) prevent long-term clozapine-induced weight gain through the inhibition of the lipid droplet-selective autophagy process (Rimessi et al., 2017). In summary, further studies focusing on the prediction model and drug combination are needed. Decreasing the adverse drug reaction will help improve the compliance of patients, ensure the therapeutic effect, and promote the life quality of them.
Author Contributions
NL wrote the manuscript and designed the figures and the table. TC and XW participated in the survey of the literatures and organization of the table. MT and DX contributed to manuscript reviewing and revisions. HC conceived the idea, supervised the whole work, and critically revised the paper.
Funding
This work was supported by the National Natural Science Foundation of China (81401113) and the Natural Science Foundation of Hunan Province (2017JJ3444).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AAPD, atypical antipsychotic drug; 5-HT, 5-hydroxytryptamine/serotonin; DRD2, dopamine receptor D2; LEP, leptin; NPY, neuropeptide Y; MC4R, the melanocortin 4 receptor; BDNF, the brain-derived neurotrophic factor; CNR1, the cannabinoid 1 receptor; INSIG, insulin-induced gene; ADRA2A, adrenergic α-2A receptors; SNP, single nucleotide polymorphism; BMI, body mass index; HDL, high-density lipoprotein; AgRP, agouti-related protein; CART, amphetamine-regulated transcript; POMC, pro-opiomelanocortin.
References
- Achiron A., Gurevich M. (2006). Peripheral blood gene expression signature mirrors central nervous system disease: the model of multiple sclerosis. Autoimmun. Rev. 5 (8), 517–522. 10.1016/j.autrev.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Alladi C. G., Mohan A., Shewade D. G., Rajkumar R. P., Adithan S., Subramanian K. (2017). Risperidone-Induced adverse drug reactions and role of DRD2 (-141 C Ins/Del) and 5HTR2C (-759 C > T) genetic polymorphisms in patients with schizophrenia. J. Pharmacol. Pharmacother. 8 (1), 28–32. 10.4103/jpp.JPP_197_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D. B., Mentore J. L., Heo M., Chandler L. P., Cappelleri J. C., Infante M. C., et al. (1999). Antipsychotic-induced weight gain: a comprehensive research synthesis. Am. J. Psychiatry 156 (11), 1686–1696. 10.1176/ajp.156.11.1686 [DOI] [PubMed] [Google Scholar]
- Angelucci F., Mathe A. A., Aloe L. (2000). Brain-derived neurotrophic factor and tyrosine kinase receptor TrkB in rat brain are significantly altered after haloperidol and risperidone administration. J. Neurosci. Res. 60 (6), 783–794. [DOI] [PubMed] [Google Scholar]
- Arora S., Anubhuti (2006). Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides 40 (6), 375–401. 10.1016/j.npep.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Bai O., Chlan-Fourney J., Bowen R., Keegan D., Li X. M. (2003). Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J. Neurosci. Res. 71 (1), 127–131. 10.1002/jnr.10440 [DOI] [PubMed] [Google Scholar]
- Bai Y. M., Lin C. C., Chen J. Y., Chen T. T., Su T. P., Chou P. (2011). Association of weight gain and metabolic syndrome in patients taking clozapine: an 8-year cohort study. J. Clin. Psychiatry 72 (6), 751–756. 10.4088/JCP.09m05402yel [DOI] [PubMed] [Google Scholar]
- Bath K. G., Lee F. S. (2006). Variant BDNF (Val66Met) impact on brain structure and function. Cogn. Affect. Behav. Neurosci. 6 (1), 79–85. 10.3758/CABN.6.1.79 [DOI] [PubMed] [Google Scholar]
- Beckers S., Zegers D., de Freitas F., Mertens I. L., Van Gaal L. F., Van Hul W. (2011). Association study of MC4R with complex obesity and replication of the rs17782313 association signal. Mol. Genet. Metab. 103 (1), 71–75. 10.1016/j.ymgme.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Bensaid M., Gary-Bobo M., Esclangon A., Maffrand J. P., Le Fur G., Oury-Donat F., et al. (2003). The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol. Pharmacol. 63 (4), 908–914. 10.1097/00007890-199904150-00827 [DOI] [PubMed] [Google Scholar]
- Bluher M., Engeli S., Kloting N., Berndt J., Fasshauer M., Batkai S., et al. (2006). Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55 (11), 3053–3060. 10.2337/db06-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Thanos P. K., Gold M. S. (2014). Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 5, 919. 10.3389/fpsyg.2014.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhaus D. W., Weinhardt K. K., Taylor M., DeSouza A., McNeeley P. M., Szczepanski K., et al. (1997). RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology 36 (4-5), 621–629. 10.1016/S0028-3908(97)00049-X [DOI] [PubMed] [Google Scholar]
- Boyle E. A., Li Y. I., Pritchard J. K. (2017). An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell 169 (7), 1177–1186. 10.1016/j.cell.2017.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R. L., Mansfield J. P., Maratos-Flier E. (2005). Neuropeptides, including neuropeptide Y and melanocortins, mediate lipolysis in murine adipocytes. Obes. Res. 13 (4), 653–661. 10.1038/oby.2005.73 [DOI] [PubMed] [Google Scholar]
- Brandl E. J., Frydrychowicz C., Tiwari A. K., Lett T. A., Kitzrow W., Buttner S., et al. (2012). Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain. Prog. Neuropsychopharmacol. Biol. Psychiatry 38 (2), 134–141. 10.1016/j.pnpbp.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Cai H. L., Tan Q. Y., Jiang P., Dang R. L., Xue Y., Tang M. M., et al. (2015). A potential mechanism underlying atypical antipsychotics-induced lipid disturbances. Translational Psychiatry 5, e661. 10.1038/tp.2015.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani L. N., Costa-Dookhan K. A., McIntyre W. B., Wright D. C., Flowers S. A., Hahn M. K., et al. (2019). Preclinical and clinical sex differences in antipsychotic-induced metabolic disturbances: a narrative review of adiposity and glucose metabolism. J. Psychiatr. Brain Sci. 4, e190013. 10.20900/jpbs.20190013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. S., Yang Y. K., Yeh T. L., Lee I. H., Yao W. J., Chiu N. T., et al. (2008). Correlation between body mass index and striatal dopamine transporter availability in healthy volunteers–a SPECT study. Neuroimage 40 (1), 275–279. 10.1016/j.neuroimage.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Chen C. C., Hsu L. W., Huang K. T., Goto S., Chen C. L., Nakano T. (2017). Overexpression of Insig-2 inhibits atypical antipsychotic-induced adipogenic differentiation and lipid biosynthesis in adipose-derived stem cells. Sci. Rep. 7 (1), 10901. 10.1038/s41598-017-11323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. C., Liao D. L., Hsiung C. A., Chen C. Y., Liao Y. C., Chen C. H. (2008). Chronic treatment with aripiprazole induces differential gene expression in the rat frontal cortex. Int. J. Neuropsychopharmacol. 11 (2), 207–216. 10.1017/s1461145707008048 [DOI] [PubMed] [Google Scholar]
- Chlan-Fourney J., Ashe P., Nylen K., Juorio A. V., Li X. M. (2002). Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res. 954 (1), 11–20. 10.1016/S0006-8993(02)03215-8 [DOI] [PubMed] [Google Scholar]
- Chowdhury N. I., Tiwari A. K., Souza R. P., Zai C. C., Shaikh S. A., Chen S., et al. (2013). Genetic association study between antipsychotic-induced weight gain and the melanocortin-4 receptor gene. Pharmacogenomics J. 13 (3), 272–279. 10.1038/tpj.2011.66 [DOI] [PubMed] [Google Scholar]
- Clifton P. G., Lee M. D., Dourish C. T. (2000). Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacol. (Berl) 152 (3), 256–267. 10.1007/s002130000504 [DOI] [PubMed] [Google Scholar]
- Cordeira J. W., Frank L., Sena-Esteves M., Pothos E. N., Rios M. (2010). Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. 30 (7), 2533–2541. 10.1523/JNEUROSCI.5768-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts A. A., Izzo A. A. (2004). The gastrointestinal pharmacology of cannabinoids: an update. Curr. Opin. Pharmacol. 4 (6), 572–579. 10.1016/j.coph.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Czerwensky F., Leucht S., Steimer W. (2013. a). Association of the common MC4R rs17782313 polymorphism with antipsychotic-related weight gain. J. Clin. Psychopharmacol. 33 (1), 74–79. 10.1097/JCP.0b013e31827772db [DOI] [PubMed] [Google Scholar]
- Czerwensky F., Leucht S., Steimer W. (2013. b). MC4R rs489693: a clinical risk factor for second generation antipsychotic-related weight gain? Int. J. Neuropsychopharmacol. 16 (9), 2103–2109. 10.1017/S1461145713000849 [DOI] [PubMed] [Google Scholar]
- de Kuijper G., Mulder H., Evenhuis H., Visser F., Hoekstra P. J. (2013). Effects of controlled discontinuation of long-term used antipsychotics on weight and metabolic parameters in individuals with intellectual disability. J. Clin. Psychopharmacol. 33 (4), 520–524. 10.1097/JCP.0b013e3182905d6a [DOI] [PubMed] [Google Scholar]
- de Leon J., Correa J. C., Ruaño G., Windemuth A., Arranz M. J., Diaz F. J. (2008). Exploring genetic variations that may be associated with the direct effects of some antipsychotics on lipid levels. Schizophrenia Res. 98 (1), 40–46. 10.1016/j.schres.2007.10.003 [DOI] [PubMed] [Google Scholar]
- De Luca V., Souza R. P., Viggiano E., Sickert L., Teo C., Zai C., et al. (2011). Genetic interactions in the adrenergic system genes: analysis of antipsychotic-induced weight gain. Hum. Psychopharmacol. 26 (6), 386–391. 10.1002/hup.1219 [DOI] [PubMed] [Google Scholar]
- Delis F., Rosko L., Shroff A., Leonard K. E., Thanos P. K. (2017). Oral haloperidol or olanzapine intake produces distinct and region-specific increase in cannabinoid receptor levels that is prevented by high fat diet. Prog. Neuropsychopharmacol. Biol. Psychiatry 79 (Pt B), 268–280. 10.1016/j.pnpbp.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Matias I. (2005). Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 8 (5), 585–589. 10.1038/nn1457 [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Goparaju S. K., Wang L., Liu J., Batkai S., Jarai Z., et al. (2001). Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410 (6830), 822–825. 10.1038/35071088 [DOI] [PubMed] [Google Scholar]
- Duan J., Wainwright M. S., Comeron J. M., Saitou N., Sanders A. R., Gelernter J., et al. (2003). Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 12 (3), 205–216. 10.1093/hmg/ddg055 [DOI] [PubMed] [Google Scholar]
- Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., et al. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112 (2), 257–269. 10.1016/S0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- Elias C. F., Aschkenasi C., Lee C., Kelly J., Ahima R. S., Bjorbæk C., et al. (1999). Leptin differentially regulates NPY and pomc neurons projecting to the lateral hypothalamic area. Neuron 23 (4), 775–786. 10.1016/S0896-6273(01)80035-0 [DOI] [PubMed] [Google Scholar]
- Emsley R. (2018). Antipsychotic maintenance treatment in schizophrenia and the importance of preventing relapse. World Psychiatry: Off. J. World Psychiatr. Association (WPA) 17 (2), 168–169. 10.1002/wps.20521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhoury M. (2017). Role of the endocannabinoid system in the pathophysiology of schizophrenia. Mol. Neurobiol. 54 (1), 768–778. 10.1007/s12035-016-9697-5 [DOI] [PubMed] [Google Scholar]
- Fang H., Zhen Y. F., Liu X. Y., Xu G., Soares J. C., Zhao J., et al. (2016). Association of the BDNF Val66Met polymorphism with BMI in chronic schizophrenic patients and healthy controls. Int. Clin. Psychopharmacol. 31 (6), 353–357. 10.1097/YIC.0000000000000142 [DOI] [PubMed] [Google Scholar]
- Faton S., Tassin J.-P., Duranton F., Bagnol D., Lajoix A.-D. (2018). 5-HT2C receptors in the ventral tegmental area, but not in the arcuate nucleus, mediate the hypophagic and hypolocomotor effects of the selective 5-HT2C receptor agonist AR231630 in rats. Behav. Brain Res. 347, 234–241. 10.1016/j.bbr.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Favalli G., Li J., Belmonte-de-Abreu P., Wong A. H., Daskalakis Z. J. (2012). The role of BDNF in the pathophysiology and treatment of schizophrenia. J. Psychiatr. Res. 46 (1), 1–11. 10.1016/j.jpsychires.2011.09.022 [DOI] [PubMed] [Google Scholar]
- Ferno J., Raeder M. B., Vik-Mo A. O., Skrede S., Glambek M., Tronstad K. J., et al. (2005). Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 5 (5), 298–304. 10.1038/sj.tpj.6500323 [DOI] [PubMed] [Google Scholar]
- Fonseka T. M., Tiwari A. K., Goncalves V. F., Lieberman J. A., Meltzer H. Y., Goldstein B. I., et al. (2015). The role of genetic variation across IL-1beta, IL-2, IL-6, and BDNF in antipsychotic-induced weight gain. World J. Biol. Psychiatry 16 (1), 45–56. 10.3109/15622975.2014.984631 [DOI] [PubMed] [Google Scholar]
- Giegling I., Balzarro B., Porcelli S., Schafer M., Hartmann A. M., Friedl M., et al. (2013). Influence of ANKK1 and DRD2 polymorphisms in response to haloperidol. Eur. Arch. Psychiatry Clin. Neurosci. 263 (1), 65–74. 10.1007/s00406-012-0348-1 [DOI] [PubMed] [Google Scholar]
- Gregoor J. G., van der Weide J., Loovers H. M., van Megen H. J., Egberts T. C., Heerdink E. R. (2010). Association between LEP and LEPR gene polymorphisms and dyslipidemia in patients using atypical antipsychotic medication. Psychiatr. Genet. 20 (6), 311–316. 10.1097/YPG.0b013e32833b6378 [DOI] [PubMed] [Google Scholar]
- Gregoor J. G., van der Weide J., Loovers H. M., van Megen H. J., Egberts T. C., Heerdink E. R. (2011). Polymorphisms of the LEP, LEPR and HTR2C gene: obesity and BMI change in patients using antipsychotic medication in a naturalistic setting. Pharmacogenomics 12 (6), 919–923. 10.2217/pgs.11.40 [DOI] [PubMed] [Google Scholar]
- Greil W., Haberle A., Schuhmann T., Grohmann R., Baumann P. (2013). Age and adverse drug reactions from psychopharmacological treatment: data from the AMSP drug surveillance programme in Switzerland. Swiss. Med. Wkly 143, w13772. 10.4414/smw.2013.13772 [DOI] [PubMed] [Google Scholar]
- Hamdani N., Tabeze J. P., Ramoz N., Ades J., Hamon M., Sarfati Y., et al. (2008). The CNR1 gene as a pharmacogenetic factor for antipsychotics rather than a susceptibility gene for schizophrenia. Eur. Neuropsychopharmacol. 18 (1), 34–40. 10.1016/j.euroneuro.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Hamdi A., Porter J., Prasad C. (1992). Decreased striatal D2 dopamine receptors in obese Zucker rats: changes during aging. Brain Res. 589 (2), 338–340. 10.1016/0006-8993(92)91296-Q [DOI] [PubMed] [Google Scholar]
- Hirvonen M. M., Laakso A., Nagren K., Rinne J. O., Pohjalainen T., Hietala J. (2009). C957T polymorphism of dopamine D2 receptor gene affects striatal DRD2 in vivo availability by changing the receptor affinity. Synapse 63 (10), 907–912. 10.1002/syn.20672 [DOI] [PubMed] [Google Scholar]
- Hong C. J., Liou Y. J., Bai Y. M., Chen T. T., Wang Y. C., Tsai S. J. (2010). Dopamine receptor D2 gene is associated with weight gain in schizophrenic patients under long-term atypical antipsychotic treatment. Pharmacogenet. Genomics 20 (6), 359–366. 10.1097/FPC.0b013e3283397d06 [DOI] [PubMed] [Google Scholar]
- Howlett A. C., Barth F., Bonner T. I., Cabral G., Casellas P., Devane W. A., et al. (2002). International union of pharmacology. XXVII. classification of cannabinoid receptors. Pharmacol. Rev. 54 (2), 161–202. 10.1124/pr.54.2.161 [DOI] [PubMed] [Google Scholar]
- International Schizophrenia C. Purcell S. M., Wray N. R., Stone J. L., Visscher P. M., O’Donovan M. C., et al. (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460 (7256), 748–752. 10.1038/nature08185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa G., Wilson C., Terry A. V., Jr., Pillai A. (2010). An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol. Dis. 39 (3), 327–333. 10.1016/j.nbd.2010.04.017 [DOI] [PubMed] [Google Scholar]
- Jbilo O., Ravinet-Trillou C., Arnone M., Buisson I., Bribes E., Peleraux A., et al. (2005). The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 19 (11), 1567–1569. 10.1096/fj.04-3177fje [DOI] [PubMed] [Google Scholar]
- Jindal R. D., Pillai A. K., Mahadik S. P., Eklund K., Montrose D. M., Keshavan M. S. (2010). Decreased BDNF in patients with antipsychotic naive first episode schizophrenia. Schizophr. Res. 119 (1-3), 47–51. 10.1016/j.schres.2009.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. G., Lee H. J., Park Y. M., Choi J. E., Han C., Kim Y. K., et al. (2008). Possible association between the -2548A/G polymorphism of the leptin gene and olanzapine-induced weight gain. Prog. Neuropsychopharmacol. Biol. Psychiatry 32 (1), 160–163. 10.1016/j.pnpbp.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Kirk S. L., Cahir M., Reynolds G. P. (2006). Clozapine, but not haloperidol, increases neuropeptide Y neuronal expression in the rat hypothalamus. J. Psychopharmacol. 20 (4), 577–579. 10.1177/0269881106061199 [DOI] [PubMed] [Google Scholar]
- Kuzman M. R., Medved V., Bozina N., Hotujac L., Sain I., Bilusic H. (2008). The influence of 5-HT(2C) and MDR1 genetic polymorphisms on antipsychotic-induced weight gain in female schizophrenic patients. Psychiatry Res. 160 (3), 308–315. 10.1016/j.psychres.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Kuzman M. R., Medved V., Bozina N., Grubisin J., Jovanovic N., Sertic J. (2011). Association study of MDR1 and 5-HT2C genetic polymorphisms and antipsychotic-induced metabolic disturbances in female patients with schizophrenia. Pharmacogenomics J. 11 (1), 35–44. 10.1038/tpj.2010.7 [DOI] [PubMed] [Google Scholar]
- Lam D. D., Przydzial M. J., Ridley S. H., Yeo G. S., Rochford J. J., O’Rahilly S., et al. (2008). Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149 (3), 1323–1328. 10.1210/en.2007-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. D., Garfield A. S., Marston O. J., Shaw J., Heisler L. K. (2010). Brain serotonin system in the coordination of food intake and body weight. Pharmacol. Biochem. Behav. 97 (1), 84–91. 10.1016/j.pbb.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Lazzari P., Serra V., Marcello S., Pira M., Mastinu A. (2017). Metabolic side effects induced by olanzapine treatment are neutralized by CB1 receptor antagonist compounds co-administration in female rats. Eur. Neuropsychopharmacol. 27 (7), 667–678. 10.1016/j.euroneuro.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Le Hellard S., Theisen F. M., Haberhausen M., Raeder M. B., Ferno J., Gebhardt S., et al. (2009). Association between the insulin-induced gene 2 (INSIG2) and weight gain in a German sample of antipsychotic-treated schizophrenic patients: perturbation of SREBP-controlled lipogenesis in drug-related metabolic adverse effects? Mol. Psychiatry 14 (3), 308–317. 10.1038/sj.mp.4002133 [DOI] [PubMed] [Google Scholar]
- Lencz T., Robinson D. G., Napolitano B., Sevy S., Kane J. M., Goldman D., et al. (2010). DRD2 promoter region variation predicts antipsychotic-induced weight gain in first episode schizophrenia. Pharmacogenet. Genomics 20 (9), 569–572. 10.1097/FPC.0b013e32833ca24b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett T. A. P., Wallace T. J. M., Chowdhury N. I., Tiwari A. K., Kennedy J. L., Müller D. J. (2011). Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol. Psychiatry 17, 242. 10.1038/mp.2011.109 [DOI] [PubMed] [Google Scholar]
- Liou Y. J., Bai Y. M., Lin E., Chen J. Y., Chen T. T., Hong C. J., et al. (2012). Gene-gene interactions of the INSIG1 and INSIG2 in metabolic syndrome in schizophrenic patients treated with atypical antipsychotics. Pharmacogenomics J. 12 (1), 54–61. 10.1038/tpj.2010.74 [DOI] [PubMed] [Google Scholar]
- Lipska B. K., Khaing Z. Z., Weickert C. S., Weinberger D. R. (2001). BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur. J. Neurosci. 14 (1), 135–144. 10.1046/j.1460-9568.2001.01633.x [DOI] [PubMed] [Google Scholar]
- Liu X., Wu Z., Lian J., Hu C.-H., Huang X.-F., Deng C. (2017). Time-dependent changes and potential mechanisms of glucose-lipid metabolic disorders associated with chronic clozapine or olanzapine treatment in rats. Sci. Rep. 7 (1), 2762. 10.1038/s41598-017-02884-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochman J., Balcar V. J., Stastny F., Sery O. (2013). Preliminary evidence for association between schizophrenia and polymorphisms in the regulatory regions of the ADRA2A, DRD3 and SNAP-25 Genes. Psychiatry Res. 205 (1-2), 7–12. 10.1016/j.psychres.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Lord C. C., Wyler S. C., Wan R., Castorena C. M., Ahmed N., Mathew D., et al. (2017). The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J. Clin. Invest. 127 (9), 3408–3412. 10.1172/jci93362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L., Correll C. U. (2010). Management of antipsychotic-related weight gain. Expert Rev. Neurother. 10 (7), 1175–1200. 10.1586/ern.10.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A. K., Correll C. U., Chowdhury N. I., Muller D. J., Gregersen P. K., Lee A. T., et al. (2012). Association between common variants near the melanocortin 4 receptor gene and severe antipsychotic drug-induced weight gain. Arch. Gen. Psychiatry 69 (9), 904–912. 10.1001/archgenpsychiatry.2012.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. A., Aguado M., Fruhbeck G. (2000). Interactions between leptin and NPY affecting lipid mobilization in adipose tissue. J. Physiol. Biochem. 56 (1), 1–8. 10.1007/bf03179770 [DOI] [PubMed] [Google Scholar]
- McGrath J., Saha S., Chant D., Welham J. (2008). Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 30, 67–76. 10.1093/epirev/mxn001 [DOI] [PubMed] [Google Scholar]
- McPherson R., Gauthier A. (2004). Molecular regulation of SREBP function: the Insig-SCAP connection and isoform-specific modulation of lipid synthesis. Biochem. Cell Biol. 82 (1), 201–211. 10.1139/o03-090 [DOI] [PubMed] [Google Scholar]
- Mehrpouya-Bahrami P., Chitrala K. N., Ganewatta M. S., Tang C., Murphy E. A., Enos R. T., et al. (2017). Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 7 (1), 15645. 10.1038/s41598-017-15154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal-Zubiaga J., Melser S., Benard G., Ramos A., Reguero L., Arrabal S., et al. (2016). Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 7, 476. 10.3389/fphys.2016.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. J., Vancampfort D., Sweers K., van Winkel R., Yu W., De Hert M. (2013). Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull 39 (2), 306–318. 10.1093/schbul/sbr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P., Milano W., Petrella C., Canestrelli B., Maj M. (2010). Endocannabinoid Pro129Thr FAAH functional polymorphism but not 1359G/A CNR1 polymorphism is associated with antipsychotic-induced weight gain. J. Clin. Psychopharmacol. 30 (4), 441–445. 10.1097/JCP.0b013e3181e742c5 [DOI] [PubMed] [Google Scholar]
- Mottillo S., Filion K. B., Genest J., Joseph L., Pilote L., Poirier P., et al. (2010). The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll C.ardiol. 56 (14), 1113–1132. 10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- Mulder H., Franke B., van der-Beek van der A. A., Arends J., Wilmink F. W., Scheffer H., et al. (2007). The association between HTR2C gene polymorphisms and the metabolic syndrome in patients with schizophrenia. J. Clin. Psychopharmacol. 27 (4), 338–343. 10.1097/JCP.0b013e3180a76dc0 [DOI] [PubMed] [Google Scholar]
- Muller D. J., Zai C. C., Sicard M., Remington E., Souza R. P., Tiwari A. K., et al. (2012). Systematic analysis of dopamine receptor genes (DRD1-DRD5) in antipsychotic-induced weight gain. Pharmacogenomics J. 12 (2), 156–164. 10.1038/tpj.2010.65 [DOI] [PubMed] [Google Scholar]
- Nakata M., Yada T. (2008). Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet beta-cells via CB1 receptors. Regul Pept. 145 (1-3), 49–53. 10.1016/j.regpep.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Nakazi M., Bauer U., Nickel T., Kathmann M., Schlicker E. (2000). Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 361 (1), 19–24. 10.1007/s002109900147 [DOI] [PubMed] [Google Scholar]
- Nielsen M. O., Rostrup E., Wulff S., Glenthoj B., Ebdrup B. H. (2016). Striatal reward activity and antipsychotic-associated weight change in patients with schizophrenia undergoing initial treatment. JAMA Psychiatry 73 (2), 121–128. 10.1001/jamapsychiatry.2015.2582 [DOI] [PubMed] [Google Scholar]
- Nogueiras R., Wiedmer P., Perez-Tilve D., Veyrat-Durebex C., Keogh J. M., Sutton G. M., et al. (2007). The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest. 117 (11), 3475–3488. 10.1172/jci31743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opgen-Rhein C., Brandl E. J., Muller D. J., Neuhaus A. H., Tiwari A. K., Sander T., et al. (2010). Association of HTR2C, but not LEP or INSIG2, genes with antipsychotic-induced weight gain in a German sample. Pharmacogenomics 11 (6), 773–780. 10.2217/pgs.10.50 [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Batkai S., et al. (2005). Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 115 (5), 1298–1305. 10.1172/JCI23057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panariello F., Polsinelli G., Borlido C., Monda M., De Luca V. (2012). The role of leptin in antipsychotic-induced weight gain: genetic and non-genetic factors. J. Obes. 2012, 572848. 10.1155/2012/572848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. M., Chung Y. C., Lee S. H., Lee K. J., Kim H., Byun Y. C., et al. (2006). Weight gain associated with the alpha2a-adrenergic receptor -1,291 C/G polymorphism and olanzapine treatment. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 141B (4), 394–397. 10.1002/ajmg.b.30311 [DOI] [PubMed] [Google Scholar]
- Park S. W., Phuong V. T., Lee C. H., Lee J. G., Seo M. K., Cho H. Y., et al. (2011. a). Effects of antipsychotic drugs on BDNF, GSK-3beta, and beta-catenin expression in rats subjected to immobilization stress. Neurosci. Res. 71 (4), 335–340. 10.1016/j.neures.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Park Y. M., Choi J. E., Kang S. G., Koo S. H., Kim L., Geum D., et al. (2011. b). Cannabinoid type 1 receptor gene polymorphisms are not associated with olanzapine-induced weight gain. Hum. Psychopharmacol. 26 (4-5), 332–337. 10.1002/hup.1210 [DOI] [PubMed] [Google Scholar]
- Pelleymounter M. A., Cullen M. J., Wellman C. L. (1995). Characteristics of BDNF-induced weight loss. Exp. Neurol. 131 (2), 229–238. 10.1016/0014-4886(95)90045-4 [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer F. X., Aronne L. J., Heshmati H. M., Devin J., Rosenstock J., Group R.I.-N.A.S. (2006). Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295 (7), 761–775. 10.1001/jama.295.7.761 [DOI] [PubMed] [Google Scholar]
- Potvin S., Zhornitsky S., Stip E. (2015). Antipsychotic-induced changes in blood levels of leptin in schizophrenia: a meta-analysis. Can J. Psychiatry 60 (3 Suppl 2), S26–S34. [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. P., McGowan O. O. (2017). Mechanisms underlying metabolic disturbances associated with psychosis and antipsychotic drug treatment. J. Psychopharmacol. 31 (11), 1430–1436. 10.1177/0269881117722987 [DOI] [PubMed] [Google Scholar]
- Reynolds G. P., Zhang Z. J., Zhang X. B. (2002). Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet 359 (9323), 2086–2087. 10.1016/S0140-6736(02)08913-4 [DOI] [PubMed] [Google Scholar]
- Rimessi A., Pavan C., Ioannidi E., Nigro F., Morganti C., Brugnoli A., et al. (2017). Protein Kinase C β: a new target therapy to prevent the long-term atypical antipsychotic-induced weight gain. Neuropsychopharmacology 42, 1491. 10.1038/npp.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringen P. A., Engh J. A., Birkenaes A. B., Dieset I., Andreassen O. A. (2014). Increased mortality in schizophrenia due to cardiovascular disease – a non-systematic review of epidemiology, possible causes, and interventions. Front. Psychiatry 5 (137). 10.3389/fpsyt.2014.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risselada A. J., Vehof J., Bruggeman R., Wilffert B., Cohen D., Al Hadithy A. F., et al. (2010). Association between the 1291-C/G polymorphism in the adrenergic alpha-2a receptor and the metabolic syndrome. J. Clin. Psychopharmacol. 30 (6), 667–671. 10.1097/JCP.0b013e3181fbfac4 [DOI] [PubMed] [Google Scholar]
- Rojczyk E., Pałasz A., Wiaderkiewicz R. (2015). Effect of short and long-term treatment with antipsychotics on orexigenic/anorexigenic neuropeptides expression in the rat hypothalamus. Neuropeptides 51, 31–42. 10.1016/j.npep.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Rosas-Vargas H., Martinez-Ezquerro J. D., Bienvenu T. (2011). Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch. Med. Res. 42 (6), 482–494. 10.1016/j.arcmed.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Roth B. L., Sheffler D. J., Kroeze W. K. (2004). Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 3 (4), 353–359. 10.1038/nrd1346 [DOI] [PubMed] [Google Scholar]
- Ruohonen S., Valve L., Tuomainen K., Ailanen L., Roytta M., Manz G., et al. (2018). Increased energy expenditure, lipolysis, and hyperinsulinemia confer resistance to central obesity and type 2 diabetes in mice lacking alpha2A-adrenoceptors. Neuroendocrinology 107 (4), 324–339. 10.1159/000492387 [DOI] [PubMed] [Google Scholar]
- Russo P., Strazzullo P., Cappuccio F. P., Tregouet D. A., Lauria F., Loguercio M., et al. (2007). Genetic variations at the endocannabinoid type 1 receptor gene (CNR1) are associated with obesity phenotypes in men. J. Clin. Endocrinol. Metab. 92 (6), 2382–2386. 10.1210/jc.2006-2523 [DOI] [PubMed] [Google Scholar]
- Ryu S., Cho E. Y., Park T., Oh S., Jang W.-S., Kim S.-K., et al. (2007). –759 C/T polymorphism of 5-HT2C receptor gene and early phase weight gain associated with antipsychotic drug treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 31 (3), 673–677. 10.1016/j.pnpbp.2006.12.021 [DOI] [PubMed] [Google Scholar]
- Sáinz N., Barrenetxe J., Moreno-Aliaga M. J., Martínez J. A. (2015). Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism 64 (1), 35–46. 10.1016/j.metabol.2014.10.015 [DOI] [PubMed] [Google Scholar]
- Sainz J., Prieto C., Crespo-Facorro B. (2019). Sex differences in gene expression related to antipsychotic induced weight gain. PLoS One 14 (4), e0215477. 10.1371/journal.pone.0215477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher A., Husum H., Holst B., Egerod K. L., Mellerup E. (2010). Risperidone treatment increases CB1 receptor binding in rat brain. Neuroendocrinology 91 (2), 155–168. 10.1159/000245220 [DOI] [PubMed] [Google Scholar]
- Shen J., Ge W., Zhang J., Zhu H. J., Fang Y. (2014). Leptin -2548g/a gene polymorphism in association with antipsychotic-induced weight gain: a meta-analysis study. Psychiatr. Danub 26 (2), 145–151. [PubMed] [Google Scholar]
- Shen W. J., Yao T., Kong X., Williams K. W., Liu T. (2017). Melanocortin neurons: Multiple routes to regulation of metabolism. Biochim Biophys Acta Mol. Basis Dis. 1863 (10 Pt A), 2477–2485. 10.1016/j.bbadis.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard M. N., Zai C. C., Tiwari A. K., Souza R. P., Meltzer H. Y., Lieberman J. A., et al. (2010). Polymorphisms of the HTR2C gene and antipsychotic-induced weight gain: an update and meta-analysis. Pharmacogenomics 11 (11), 1561–1571. 10.2217/pgs.10.123 [DOI] [PubMed] [Google Scholar]
- Sickert L., Muller D. J., Tiwari A. K., Shaikh S., Zai C., De Souza R., et al. (2009). Association of the alpha 2A adrenergic receptor -1291C/G polymorphism and antipsychotic-induced weight gain in European-Americans. Pharmacogenomics 10 (7), 1169–1176. 10.2217/pgs.09.43 [DOI] [PubMed] [Google Scholar]
- Simon V., van Winkel R., De Hert M. (2009). Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J. Clin. Psychiatry 70 (7), 1041–1050. 10.4088/jcp.08r04392 [DOI] [PubMed] [Google Scholar]
- Skledar M., Nikolac M., Dodig-Curkovic K., Curkovic M., Borovecki F., Pivac N. (2012). Association between brain-derived neurotrophic factor Val66Met and obesity in children and adolescents. Prog. Neuropsychopharmacol. Biol. Psychiatry 36 (1), 136–140. 10.1016/j.pnpbp.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Srisai D., Gillum M. P., Panaro B. L., Zhang X. M., Kotchabhakdi N., Shulman G. I., et al. (2011). Characterization of the hyperphagic response to dietary fat in the MC4R knockout mouse. Endocrinology 152 (3), 890–902. 10.1210/en.2010-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott L. H., Sun L. M., Akana S. F., Strack A. M., Lowenstein D. H., Dallman M. F., et al. (1995). Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374 (6522), 542–546. 10.1038/374542a0 [DOI] [PubMed] [Google Scholar]
- Templeman L. A., Reynolds G. P., Arranz B., San L. (2005). Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet. Genomics 15 (4), 195–200. 10.1097/01213011-200504000-00002 [DOI] [PubMed] [Google Scholar]
- Tiwari A. K., Zai C. C., Likhodi O., Lisker A., Singh D., Souza R. P., et al. (2010. a). A common polymorphism in the cannabinoid receptor 1 (CNR1) gene is associated with antipsychotic-induced weight gain in Schizophrenia. Neuropsychopharmacology 35 (6), 1315–1324. 10.1038/npp.2009.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A. K., Zai C. C., Meltzer H. Y., Lieberman J. A., Muller D. J., Kennedy J. L. (2010. b). Association study of polymorphisms in insulin induced gene 2 (INSIG2) with antipsychotic-induced weight gain in European and African-American schizophrenia patients. Hum. Psychopharmacol. 25 (3), 253–259. 10.1002/hup.1111 [DOI] [PubMed] [Google Scholar]
- Tiwari A. K., Brandl E. J., Weber C., Likhodi O., Zai C. C., Hahn M. K., et al. (2013). Association of a functional polymorphism in neuropeptide Y with antipsychotic-induced weight gain in schizophrenia patients. J. Clin. Psychopharmacol. 33 (1), 11–17. 10.1097/JCP.0b013e31827d145a [DOI] [PubMed] [Google Scholar]
- Tiwari A. K., Brandl E. J., Chowdhury N. I., Zai C. C., Lieberman J. A., Meltzer H. Y., et al. (2014). A Genetic risk-model for antipsychotic induced weight gain. Biol. Psychiatry 75 (9), 73S–73S. [Google Scholar]
- Tsai A., Liou Y. J., Hong C. J., Wu C. L., Tsai S. J., Bai Y. M. (2011). Association study of brain-derived neurotrophic factor gene polymorphisms and body weight change in schizophrenic patients under long-term atypical antipsychotic treatment. Neuromol. Med. 13 (4), 328–333. 10.1007/s12017-011-8159-5 [DOI] [PubMed] [Google Scholar]
- Tybura P., Trzesniowska-Drukala B., Bienkowski P., Beszlej A., Frydecka D., Mierzejewski P., et al. (2014). Pharmacogenetics of adverse events in schizophrenia treatment: comparison study of ziprasidone, olanzapine and perazine. Psychiatry Res. 219 (2), 261–267. 10.1016/j.psychres.2014.05.039 [DOI] [PubMed] [Google Scholar]
- Upadhyay N., Patel A., Chan W., Aparasu R. R., Ochoa-Perez M., Sherer J. T., et al. (2019). Reversibility of psychotropic medication induced weight gain among children and adolescents with bipolar disorders. Psychiatry Res. 276, 151–159. 10.1016/j.psychres.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Vähätalo L. H., Ruohonen S. T., Mäkelä S., Ailanen L., Penttinen A. M., Stormi T., et al. (2015). Role of the endocannabinoid system in obesity induced by neuropeptide Y overexpression in noradrenergic neurons. Nutr. Diabetes 5 (4), e151–e151. 10.1038/nutd.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahatalo L. H., Ruohonen S. T., Makela S., Kovalainen M., Huotari A., Makela K. A., et al. (2015). Neuropeptide Y in the noradrenergic neurones induces obesity and inhibits sympathetic tone in mice. Acta Physiol. (Oxf) 213 (4), 902–919. 10.1111/apha.12436 [DOI] [PubMed] [Google Scholar]
- Van Gaal L. F., Rissanen A. M., Scheen A. J., Ziegler O., Rössner S. (2005). Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365 (9468), 1389–1397. 10.1016/S0140-6736(05)66374-X [DOI] [PubMed] [Google Scholar]
- Vandenberghe F., Saigi-Morgui N., Delacretaz A., Quteineh L., Crettol S., Ansermot N., et al. (2016). Prediction of early weight gain during psychotropic treatment using a combinatorial model with clinical and genetic markers. Pharmacogenet. Genomics 26 (12), 547–557. 10.1097/FPC.0000000000000249 [DOI] [PubMed] [Google Scholar]
- Voigt J.-P., Fink H. (2015). Serotonin controlling feeding and satiety. Behav. Brain Res. 277, 14–31. 10.1016/j.bbr.2014.08.065 [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Wang G.-J., Baler R. D. (2011). Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 15 (1), 37–46. 10.1016/j.tics.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg C., Langhans W., Hrupka B. J. (2003). Evidence for a role of the 5-HT2C receptor in central lipopolysaccharide-, interleukin-1 beta-, and leptin-induced anorexia. Pharmacol. Biochem. Behav. 74 (4), 1025–1031. 10.1016/S0091-3057(03)00030-3 [DOI] [PubMed] [Google Scholar]
- Wang Y. C., Bai Y. M., Chen J. Y., Lin C. C., Lai I. C., Liou Y. J. (2005). Polymorphism of the adrenergic receptor alpha 2a -1291C > G genetic variation and clozapine-induced weight gain. J. Neural Transm. (Vienna) 112 (11), 1463–1468. 10.1007/s00702-005-0291-7 [DOI] [PubMed] [Google Scholar]
- Wang C., Bomberg E., Levine A., Billington C., Kotz C. M. (2007). Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am. J. Physiol. Regul Integr. Comp. Physiol. 293 (3), R1037–R1045. 10.1152/ajpregu.00125.2007 [DOI] [PubMed] [Google Scholar]
- Weston-Green K., Huang X. F., Deng C. (2012). Alterations to Melanocortinergic, GABAergic and Cannabinoid Neurotransmission Associated with Olanzapine-Induced Weight Gain. Plos One 7 (3), 12. 10.1371/journal.pone.0033548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierucka-Rybak M., Wolak M., Juszczak M., Drobnik J., Bojanowska E. (2016). The inhibitory effect of combination treatment with leptin and cannabinoid Cb1 receptor agonist on food intake and body weight gain is mediated by serotonin 1b and 2c receptors. J. Physiol. Pharmacol. 67 (3), 457–463. [PubMed] [Google Scholar]
- Wiley J. L., Kendler S. H., Burston J. J., Howard D. R., Selley D. E., Sim-Selley L. J. (2008). Antipsychotic-induced alterations in CB1 receptor-mediated G-protein signaling and in vivo pharmacology in rats. Neuropharmacology 55 (7), 1183–1190. 10.1016/j.neuropharm.2008.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. R., Zhao J. P., Guo X. F., He Y. Q., Fang M. S., Guo W. B., et al. (2008). Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am. J. Psychiatry 165 (3), 352–358. 10.1176/appi.ajp.2007.07010079 [DOI] [PubMed] [Google Scholar]
- Xiu M. H., Hui L., Dang Y. F., Hou T. D., Zhang C. X., Zheng Y. L., et al. (2009). Decreased serum BDNF levels in chronic institutionalized schizophrenia on long-term treatment with typical and atypical antipsychotics. Prog. Neuropsychopharmacol. Biol. Psychiatry 33 (8), 1508–1512. 10.1016/j.pnpbp.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Xu B., Goulding E. H., Zang K., Cepoi D., Cone R. D., Jones K. R., et al. (2003). Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6 (7), 736–742. 10.1038/nn1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D., Brown M. S., Goldstein J. L. (2002). Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 99 (20), 12753–12758. 10.1073/pnas.162488899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevtushenko O. O., Cooper S. J., O’Neill R., Doherty J. K., Woodside J. V., Reynolds G. P. (2008). Influence of 5-HT2C receptor and leptin gene polymorphisms, smoking and drug treatment on metabolic disturbances in patients with schizophrenia. Br. J. Psychiatry 192 (6), 424–428. 10.1192/bjp.bp.107.041723 [DOI] [PubMed] [Google Scholar]
- Yu W., De Hert M., Moons T., Claes S. J., Correll C. U., van Winkel R. (2013). CNR1 gene and risk of the metabolic syndrome in patients with schizophrenia. J. Clin. Psychopharmacol. 33 (2), 186–192. 10.1097/JCP.0b013e318283925e [DOI] [PubMed] [Google Scholar]
- Zai G. C., Zai C. C., Chowdhury N. I., Tiwari A. K., Souza R. P., Lieberman J. A., et al. (2012). The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog. Neuropsychopharmacol. Biol. Psychiatry 39 (1), 96–101. 10.1016/j.pnpbp.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Zai C. C., Tiwari A. K., Zai G. C., Maes M. S., Kennedy J. L. (2018). New findings in pharmacogenetics of schizophrenia. Curr. Opin. Psychiatry 31 (3), 200–212. 10.1097/YCO.0000000000000417 [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Tan Y. L., Zhou D. F., Cao L. Y., Wu G. Y., Xu Q., et al. (2007). Serum BDNF levels and weight gain in schizophrenic patients on long-term treatment with antipsychotics. J. Psychiatr. Res. 41 (12), 997–1004. 10.1016/j.jpsychires.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Zhou D. F., Wu G. Y., Cao L. Y., Tan Y. L., Haile C. N., et al. (2008). BDNF levels and genotype are associated with antipsychotic-induced weight gain in patients with chronic schizophrenia. Neuropsychopharmacology 33 (9), 2200–2205. 10.1038/sj.npp.1301619 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen M., Wu Z., Chen J., Yu S., Fang Y., et al. (2013). Association study of Val66Met polymorphism in brain-derived neurotrophic factor gene with clozapine-induced metabolic syndrome: preliminary results. PLoS One 8 (8), e72652. 10.1371/journal.pone.0072652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. P., Lencz T., Zhang R. X., Nitta M., Maayan L., John M., et al. (2016). Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis. Schizophr. Bull 42 (6), 1418–1437. 10.1093/schbul/sbw058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Jian-Ping, Robinson Delbert, Yu Jin, Gallego Juan, Fleischhacker W. Wolfgang, . Kahn Rene S, et al. (2018). Schizophrenia polygenic risk score as a predictor of antipsychotic efficacy in first-episode psychosis. Am. J. Psychiatry 0 (0), appi.ajp.2018.17121363. 10.1176/appi.ajp.2018.17121363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ren H., Wang Q., Deng W., Yue W., Yan H., et al. (2019). Testing the role of genetic variation of the MC4R gene in Chinese population in antipsychotic-induced metabolic disturbance. Sci. Chin. Life Sci. 62 (4), 535–543. 10.1007/s11427-018-9489-x [DOI] [PubMed] [Google Scholar]