Abstract
From February 2015 to October 2017, among 20 men who have sex with men with Mycoplasma genitalium-associated nongonococcal urethritis, 15% had macrolide resistance and S83I ParC mutations. Azithromycin followed by moxifloxacin cleared Mycoplasma genitalium in 2 of 2 with and 11 of 13 without S83I mutations. Dual failures were cleared after doxycycline. S83I mutations were not associated with moxifloxacin failure.
Mycoplasma genitalium (MG) is associated with nongonococcal urethritis (NGU) in men,1 several reproductive tract sequelae in women,2 and risk for human immunodeficiency virus (HIV) infection.3 Although MG prevalencevaries by geographic region and risk group, men who have sex with men (MSM) are among those at highest risk.4 Traditionally, azithromycin (AZM) has been recommended as first-line therapy for MG-associated NGU (MG-NGU), and moxifloxacin (MOX) is recommended when AZM treatment fails. However, the high prevalence of macrolide resistance (range, 7–100%)5,6 and increasing reports of fluoroquinolone (range, 2–47%)7,8 and dual drug (range, 1–33%)9,10 resistance have generated substantial concern over our continuing ability to treat MG infections.
Molecular tools to detect resistance-associated mutations at the time of diagnostic testing now permit resistance-guided therapy, which has yielded promising results.11 However, this is currently limited to detection of macrolide resistance. The greater variety of mutations in the quinolone resistance determining region of the parC and gyrA genes and uncertainty over which are most relevant has, thus far, prevented implementation of this approach for fluoroquinolones. To address this, we evaluated the role of resistance-associated mutations in treatment outcomes among MSM with MG-NGU enrolled in a cohort study.
MATERIALS AND METHODS
From February 2015 to October 2017, we recruited people with NGU attending the Public Health-Seattle and King County STD Clinic who were age 16 years or older, assigned male sex at birth, and had no female sex partners in the past year. Patients were ineligible if they had no sex in the past 60 days, antibiotics in the past 30 days, urethral contact to Neisseria gonorrhoeae (GC), no contact information, or no freezer at home for storing urine specimens for microbiota analyses (not reported here).
Enrolled participants attended 5 in-clinic visits at 3-week intervals, at which the clinician collected a urethral swab specimen for Gram staining and first-void urine. We defined NGU as greater than or equal to 5 polymorphonuclear leukocytes (PMNs) per high-power field (HPF) plus either urethral symptoms or visible urethral discharge on examination in the absence of GC. NGU was treated with AZM (1 g). Participants reported sociobehavioral data on computerassisted self-interviews and daily antibiotics, urethral symptoms, and sexual activity on weekly Web-based diaries.
Prior to implementing routine MG testing in April 2016, we stored specimens at −80°C and tested them retrospectively for MG. Upon receiving retrospective MG test results, we asked participants with positive results to undergo an additional post-study test and provided MOX (400 mg daily, 10–14 days)12 if MG-positive. Starting in April 2016, we performed MG tests within 10 days of each visit. Participants still received AZM for NGU at enrollment, but those with MG were recalled and provided MOX.
We tested urine for GC and Chlamydia trachomatis using the Aptima Combo 2 assay and MG using the Aptima assay with analyte-specific reagents (Hologic, Marlborough, Massachusetts). We performed DNA extraction from MG-positive specimens in Aptima transport medium using a MagNA Pure 96 Instrument (Roche, Pleasanton, California) with large volume (1 mL) universal pathogen extraction protocol and elution in 50 μL. We estimated organism load using real-time quantitative polymerase chain reaction with a limit of detection of less than 5 genome equivalents (geq) per reaction.13,14 Macrolide resistance mutations (MRM) and ParC mutations were detected with PyroMark Q96 sequencing14 and conventional Sanger sequencing,15 respectively. GyrA mutations were not sought because they have never been associated with MOX treatment failure without the simultaneous presence of ParC mutations.16
We compared treatment outcomes by pretreatment resistance mutation profile using Fisher’s exact tests. We defined microbiologic cure as an MG-negative test after treatment. We defined clinical cure as either (1) less than 5 PMNs/HPF, or (2) neither symptoms nor visible discharge after treatment. We used linear regression with robust standard errors to estimate the association between pretreatment resistance mutation profile and log10 organism load. We compared treatment outcomes by pretreatment organism load using Wilcoxon rank-sum tests.
The University of Washington Human Subjects Division approved all study procedures. Participants provided written informed consent. We compensated participants US $360 through a tiered schedule. We used Stata 13 (StataCorp, College Station, TX), 2-sided tests, and alpha = 0.05.
RESULTS
Twenty (22%) of 92 participants had MG-NGU at enrollment. Among these, 12 (60%) were non-Hispanic white, median age was 30.5 years (interquartile range, 26.5–42.5), and all selfidentified as cisgender men. Two (10%) were living with HIV. At enrollment (i.e., pretreatment), 18 men (90%) had MRM, and 3 (15%) had the S83I ParC fluoroquinolone resistance-associated mutation (Table 1). Five men (25%) had other ParC mutations not previously associated with fluoroquinolone resistance All with ParC mutations also had MRM.
TABLE 1.
Prevalence of Resistance-associated Mutations in Pretreatment Specimens and Summary of Treatment Outcomes Among MSM with MG-NGU
| MG Cleared After: | |||||
|---|---|---|---|---|---|
| Mutation Profile | N = 20, n (Column %) | AZM, n (Row %) | AZM + MOX, n (Row %) | AZM + MOX + DOX, n (Row %) | Unknown, n (Row %) |
| None | 2 (10) | 1 (50) | — | 1 (50) | — |
| MRM only | |||||
| A2058G | 5 (25) | 1 (20) | 3 (60) | — | 1 (20) |
| A2059G | 5 (25) | 3 (60) | 1 (20) | 1 (20) | |
| MRM and nonresistance ParC mutation | |||||
| A2058G and E72D | 1 (5) | — | 1 (100) | — | — |
| A2059G and P62S | 2 (10) | — | 2 (100) | — | — |
| A2059G and P62S/silent C234T | 1 (5) | — | 1 (100) | — | — |
| A2058G and S83N | 1 (5) | — | 1 (100) | — | — |
| MRM and S83I ParC mutation | |||||
| A2058G and S83I | 1 (5) | — | 1 (100) | — | — |
| A2058T and S83I | 1 (5) | — | 1 (100) | — | — |
| A2059G and S83I | 1 (5) | — | — | — | 1 (100) |
DOX, doxycycline.
Treatment Outcomes
AZM Alone
In the absence of routine MG testing, 5 men (25%) were only managed syndromically (participants 1–5, Figure 1). Median time from specimen collection to receipt of MG test results was 162 days (interquartile range, 118.5–174.25). Pretreatment organism loads were low (range, not detectable to 3643 geq/5 μL template). None received MOX during follow-up. Although clinical cure occurred in 4 men (80%), MG was eradicated in only 2 (40%), 1 (participant 2) with and 1 (participant 1) without MRM. In 3 men (participants 3–5), MG was persistently detected at each study visit, or for 88 to 90 days after enrollment. Two men (participants 3 and 4) returned for a post-study test; both had MG, but treatment outcomes were not available. All 3 men with persistent infections had MRM; 1 (participant 5) also had the S83I mutation. Despite not meeting our NGU definition, each had either symptoms or 5 PMNs/HPF or greater at several subsequent visits.
Figure 1.
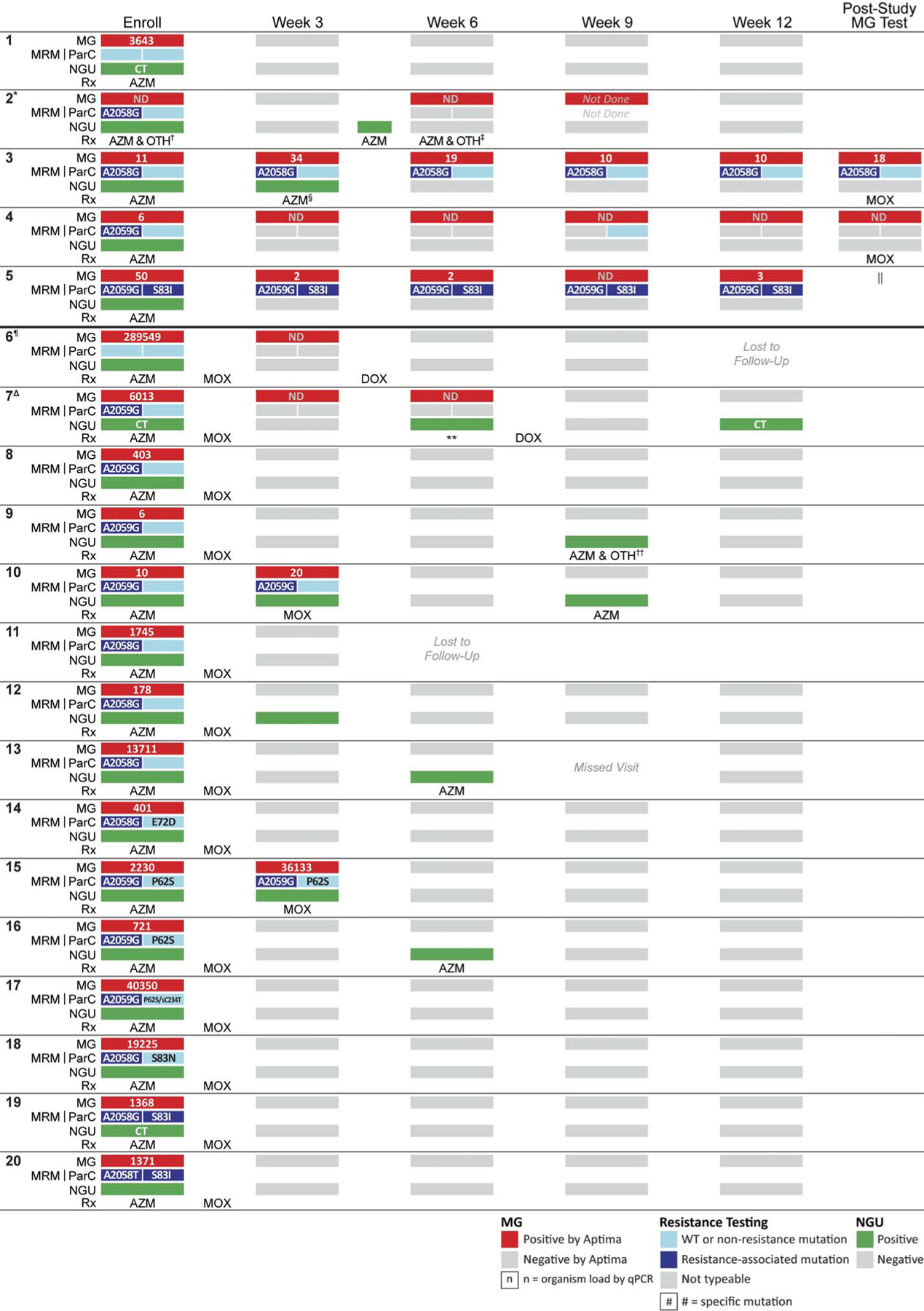
Clinical presentation and management, detection of mutations, and organism load among MSM with MG-NGU. CT, Chlamydia trachomatis; DOX, doxycycline; ND, non-detectable; OTH, other antibiotic therapy; qPCR, quantitative polymerase chain reaction; Rx, antibiotic therapy; sC234T, silent C234T. *Reported condomless insertive anal sex and insertive oral sex (presumed condomless) between enroll and week 6 visits. †Ceftriaxone, provided with AZM for proctitis. ‡Ceftriaxone, provided with AZM for rectal gonorrhea. §Retreated with AZM because reexposed to untreated sex partner. ||Patient did not respond to our attempts to recall him to the clinic for a post-study MG test. ¶Reported condomless insertive anal sex and insertive oral sex (presumed condomless) between enroll and week 3 visits. ΔReported no urethral sexual exposures between enroll and week 6 visits. **No antibiotics per MD while we waited to see the next MG test result and discussed what third-line therapy to use. ††Nitrofurantoin for UTI, plus AZM for NGU.
AZM Followed by MOX
MG test results were available during follow-up for 15 men (participants 6–20). They received initial presumptive AZM followed by MOX after their MG-positive test. Pretreatment organism loads ranged from 6 to 289,549 geq/5 μL template. Clinical and microbiologic cure occurred in 12 men (80%). All 12 had MRM; 2 (participants 19 and 20) also had the S83I mutation. One man with MRM but no ParC mutations (participant 12) experienced persistent NGU at week 3 despite eradication of MG. This resolved by week 6 without additional therapy.
Two men (13%) were potential dual treatment failures (participants 6 and 7). Although participant 7 had MRM, neither had ParC mutations. Participant 6 reported insertive oral sex and condomless insertive anal sex between enrollment and week 3 and, thus, could have been reinfected. However, participant 7 did not report any urethral sexual exposures between enrollment and week 6. Both received doxycycline (DOX) (100 mg twice daily, 7 days), after which MG was not detected.
Organism Load and Resistance-associated Mutations
Among men with no resistance-associated mutations, MRM only, and both MRM and S83I, pretreatment organism load ranged from 3,643 to 289,54aa9 geq/5 μL; not detectable to 40,350 geq/5 μL; and 50 to 1,371 geq/5 μL template, respectively. Men with MRM alone had 0.01-fold lower median pretreatment organism load than the 2 men with no resistance-associated mutations (95% confidence interval, 0.0002–0.55; P = 0.03); men with MRM and S83I also had 0.01-fold lower median organism load (95% confidence interval, 0.0002–0.83; P = 0.04). Nevertheless, there was wide variation, and some men with MRM had higher organism loads than men without MRM. Treatment failure after AZM alone was not associated with pretreatment organism load. However, pretreatment organism load was somewhat higher among the 2 men with treatment failure after sequential AZM and MOX than among those with microbiologic cure (median, 147,781 vs. 1368 geq/5 μL template; P = 0.09).
DISCUSSION
Among MSM with MG-NGU in Seattle, 90% had MRM, and 15% had both MRM and the S83I ParC mutation. The efficacy of AZM was low, and treatment failures correlated with detection of MRM. In contrast, AZM followed by MOX had relatively high efficacy, but there was no association between the S83I mutation and MOX failure. DOX was effective in 2 cases of possible dual AZM and MOX failure.
The 90% prevalence of MRM in our study is even higher than prior studies of MSM from North America17,18 and Europe10,19 and similar to observations from Australia.20 Although the prevalence of the S83I mutation that we observed was also similar to other reports,21 the lack of association with MOX failure was surprising, given prior associations with treatment failure22–25 and high minimum inhibitory concentrations.26,27 However, 4 other MG cases with the S83I mutation have been cured with fluoroquinolones.8,24 These treatment successes despite the S83I mutation highlight the potential role of other factors, including decreased fitness, organism load,28 and interactions with other ParC or GyrA mutations.25,27 Potential detection of non-viable nucleic acids with the highly sensitive Aptima assay, the length of time between completion of treatment and retesting, and spontaneous clearance may also have contributed. The lower median pretreatment organism loads with MRM may indicate decreased fitness and have contributed to spontaneous clearance.
Given the low efficacy of DOX,29 we were surprised that it appeared to eradicate MG after suspected dual treatment failure. The persistently positive MG tests may reflect detection of nonviable nucleic acids rather than dual treatment failure. Alternatively, initial treatment with AZM and MOX may have reduced the organism load, similar to what has been observed after initial treatment with DOX under resistance-guided therapy.11 Pristinamycin,22 spectinomycin,30 and minocycline,25 which have been used successfully in some dual treatment failures, are not all widely available. DOX may be another alternative for suspected dual treatment failures. Nonetheless, new antimicrobials are urgently needed.29
Strengths of this study include the prospective design and frequent clinical evaluation. However, our sample size was small, and we could not estimate the frequency of “de novo” mutations after specific antibiotics. Specimens were not tested for GyrA mutations, and these may play a role.
MRM in MG are now nearly ubiquitous in some populations. Persistent asymptomatic infection may be common after ineffective therapy. Resistance-guided therapy based on MRM is currently effective11; however, more data are needed to identify appropriate predictors of fluoroquinolone treatment failure.
Acknowledgments:
The authors thank the study participants and the Public Health-Seattle and King County STD Clinic staff. They also gratefully acknowledge Sean Proll for assisting with the figures; Linda Cles, Cynthia Khoury, and Sabina Astete for performing MG testing; and Gitte Jensen and the staff at the Statens Serum Institute Virus and Microbiological Special Diagnostics Department for technical assistance with tests for MG organism load and resistance-associated mutations in MG. Finally, they thank Hologic for donation of test collection kits and reagents.
Conflicts of Interest and Sources of Funding: This study was funded by the National Institutes of Health (grant U19 AI113173). L.C.C. was funded by the National Institutes of Health (grant TL1 TR002318 trainee support). Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the University of Washington Institute of Translational Health Sciences and supported by the National Institutes of Health (grant UL1 TR002319). J.S.J. has received speaker’s fees from Hologic, Cepheid, and SpeeDx and serves on the scientific advisory board of Roche Molecular Systems, Abbott Molecular Inc., and Cepheid. The Statens Serum Institut has received remuneration for contract work from SpeeDx, Hologic, NYtor, Diagenode, Nabriva, and GlaxoSmithKline. P.A.T. has received remuneration for honoraria, reagents, test kits, and contract work from Hologic; consulting services and contract work from SpeeDx; and contract work from Abbott and Cepheid. M.R.G. has conducted studies unrelated to this work supported by grants from Hologic and GlaxoSmithKline. L.E.M. has received honoraria, reagents, and test kits for diagnostic assays from Hologic. All other authors declare that they have no conflict of interest.
Footnotes
A portion of this work was presented at the 2018 International Union Against Sexually Transmitted Infection World and European Conference, held June 27–30, 2018, in Dublin, Ireland.
REFERENCES
- 1.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61:418–426. [DOI] [PubMed] [Google Scholar]
- 3.Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 23:611–620. [DOI] [PubMed] [Google Scholar]
- 4.Baumann L, Cina M, Egli-Gany D, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmetaanalysis. Sex Transm Infect 2018; 94:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coorevits L, Traen A, Binge L, et al. Macrolide resistance in Mycoplasma genitalium from female sex workers in Belgium. J Glob Antimicrob 2018; 12:149–152. [DOI] [PubMed] [Google Scholar]
- 6.Gesink DC, Mulvad G, Montgomery-Andersen R, et al. Mycoplasma genitalium presence, resistance and epidemiology in Greenland. Int J Circumpolar Health 2012; 71:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernesky MA, Jang D, Martin I, et al. Mycoplasma genitalium antibiotic resistance-mediating mutations in Canadian women with or withaout Chlamydia trachomatis infection. Sex Transm Dis 2017; 44: 433–435. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi M, Ito S, Yasuda M, et al. Remarkable increase in fluoroquinoloneresistant Mycoplasma genitalium in Japan. J Antimicrob Chemother 2014; 69:2376–2382. [DOI] [PubMed] [Google Scholar]
- 9.Hadad R, Golparian D, Lagos AC, et al. Macrolide and fluoroquinolone resistance in Mycoplasma genitalium in two Swedish counties, 2011–2015. APMIS 2018; 126:123–127. [DOI] [PubMed] [Google Scholar]
- 10.Mulligan V, Lynagh Y, Clarke S, et al. Prevalence, macrolide resistance, fluoroquinolone resistance in Mycoplasma genitalium in men who have sex with men attending an sexually transmitted disease clinic in Dublin, Ireland in 2017–2018. Sex Transm Dis 2019; 46:e35–e37. [DOI] [PubMed] [Google Scholar]
- 11.Read TRH, Fairley CK, Murray GL, et al. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis 2018; 68:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2015. Atlanta, GA, 2015. [Google Scholar]
- 13.Jensen JS. Protocol for the detection of Mycoplasma genitalium by PCR from clinical specimens and subsequent detection of macrolide resistance-mediating mutations in region V of the 23S rRNA gene. Methods Mol Biol 2012; 903:129–139. [DOI] [PubMed] [Google Scholar]
- 14.Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: a Danish nationwide retrospective survey. Clin Infect Dis 2014; 59:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deguchi T, Maeda S, Tamaki M, et al. Analysis of the gyrA and parC genes of Mycoplasma genitalium detected in first-pass urine of men with non-gonococcal urethritis before and after fluoroquinolone treatment. J Antimicrob Chemother 2001; 48:742–744. [DOI] [PubMed] [Google Scholar]
- 16.Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 2017; 14:139–152. [DOI] [PubMed] [Google Scholar]
- 17.Dionne-Odom J, Geisler WM, Aaron KJ, et al. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in Alabama. Clin Infect Dis 2018; 66:796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gratrix J, Plitt S, Turnbull L, et al. Prevalence and antibiotic resistance of Mycoplasma genitalium among STI clinic attendees in Western Canada: a cross-sectional analysis. BMJ Open 2017; 7:e016300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbera MJ, Fernandez-Huerta M, Jensen JS, et al. Mycoplasma genitalium macrolide and fluoroquinolone resistance: prevalence and risk factors among a 2013–2014 cohort of patients in Barcelona Spain. Sex Transm Dis 2017; 44:457–462. [DOI] [PubMed] [Google Scholar]
- 20.Couldwell DL, Jalocon D, Power M, et al. Mycoplasma genitalium: high prevalence of resistance to macrolides and frequent anorectal infection in men who have sex with men in western Sydney. Sex Transm Infect 2018; 94:406–410. [DOI] [PubMed] [Google Scholar]
- 21.Xiao L, Waites KB, Van Der Pol B, et al. Mycoplasma genitalium infections with macrolide and fluoroquinolone resistance-associated mutations in heterosexual African American couples in Alabama. Sex Transm Dis 2018; 46:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 2015; 60:1228–1236. [DOI] [PubMed] [Google Scholar]
- 23.Couldwell DL, Tagg KA, Jeoffreys NJ, et al. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS 2013; 24: 822–828. [DOI] [PubMed] [Google Scholar]
- 24.Murray GL, Bradshaw CS, Bissessor M, et al. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis 2017; 23:809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deguchi T, Ito S, Yasuda M, et al. Emergence of Mycoplasma genitalium with clinically significant fluoroquinolone resistance conferred by amino acid changes both in GyrA and ParC in Japan. J Infect Chemother 2017; 23:648–650. [DOI] [PubMed] [Google Scholar]
- 26.Fookes MC, Hadfield J, Harris S, et al. Mycoplasma genitalium: whole genome sequence analysis, recombination and population structure. BMC Genomics 2017; 18:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamasuna R, Le PT, Kutsuna S, et al. Mutations in ParC and GyrA of moxifloxacin-resistant and susceptible Mycoplasma genitalium strains. PLoS One 2018; 13:e0198355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker J, Fairley CK, Bradshaw CS, et al. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin Infect Dis 2013; 56: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 29.Jensen JS, Bradshaw C. Management of Mycoplasma genitalium infections—can we hit a moving target? BMC Infect Dis 2015; 15:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falk L, Jensen JS. Successful outcome of macrolide-resistant Mycoplasma genitalium urethritis after spectinomycin treatment: a case report. J Antimicrob Chemother 2017; 72:624–625. [DOI] [PubMed] [Google Scholar]


