Abstract
OBJECTIVE
The relationship between acute pancreatitis and incident diabetes is unclear. We assessed whether a resolved single event of acute pancreatitis in childhood was associated with incident diabetes in adulthood.
RESEARCH DESIGN AND METHODS
A nationwide, population-based study of 1,802,110 Israeli adolescents (mean age 17.4 years [range 16–20]) who were examined before compulsory military service between 1979 and 2008 and whose data were linked to the Israeli National Diabetes Registry (INDR). Resolved pancreatitis was defined as a history of a single event of acute pancreatitis with normal pancreatic function at enrollment. Logistic regression analysis was applied.
RESULTS
Incident diabetes developed in 4.6% of subjects with resolved pancreatitis (13 of 281; none of these cases were identified as type 1 diabetes) and 2.5% among the unexposed group (44,463 of 1,801,716). Resolved acute pancreatitis was associated with incident diabetes with an odds ratio (OR) of 2.23 (95% CI 1.25–3.98) with adjustment for age, sex, and birth year. Findings persisted after further adjustments for baseline BMI and sociodemographic confounders (OR 2.10 [95% CI 1.15–3.84]). Childhood pancreatitis was associated with a diagnosis of diabetes at a younger age, with 92% of diabetes case subjects diagnosed before 40 years of age compared with 47% in the unexposed group (P = 0.002). The association accentuated when the study sample was limited to individuals of unimpaired health or normal BMI at baseline.
CONCLUSIONS
A history of acute pancreatitis in childhood with normal pancreatic function in late adolescence is a risk factor for incident type 2 diabetes, especially at young adulthood.
Introduction
Diabetes occurs when the pancreas is unable to secrete sufficient insulin to maintain normal glycemia due to autoimmune inflammation, nonautoimmune inflammation, infiltration, neoplasia, resection, or unknown causes (1). Whereas chronic pancreatitis was linked to a higher risk for diabetes (2,3), acute pancreatitis may be accompanied by transient hyperglycemia (4,5), but resolves in most patients without additional recognized sequelae (6). Nevertheless, a sequela of β-cell damage that is accompanied with an increased risk for diabetes later in life may be underappreciated. This possibility is becoming of growing clinical importance given a fivefold increase in the cases of acute pancreatitis diagnosed during childhood and adolescence in the past two decades, approaching the incidence in adults, that had been primarily attributed to greater awareness among clinicians (7,8).
In contrast to the adult population (9), acute pancreatitis during childhood or adolescence is usually not associated with the metabolic syndrome, but primarily attributed to obstructive bile disease, medications, or idiopathic etiologies (10–12). In 2018, a National Institutes of Health–funded prospective study of pediatric acute recurrent and chronic pancreatitis was initiated to characterize disease risk factors, natural history, and outcomes, including risk for incident diabetes (13). To our knowledge, there is no equivalent study conducted on nonrecurrent acute pancreatitis, and it is unknown whether history of apparently resolved pancreatitis increases the risk for diabetes later in life. The long-term risk of diabetes among patients with childhood resolved pancreatitis that had not progressed to diabetes in childhood is unclear and is of importance given the key role of gradual deterioration in β-cell mass and function in the pathophysiology of diabetes (14). In this study, we analyzed the association between history of clinically resolved acute pancreatitis and future diabetes risk in a nationwide cohort of 1.8 million adolescents.
Research Design and Methods
Study Population
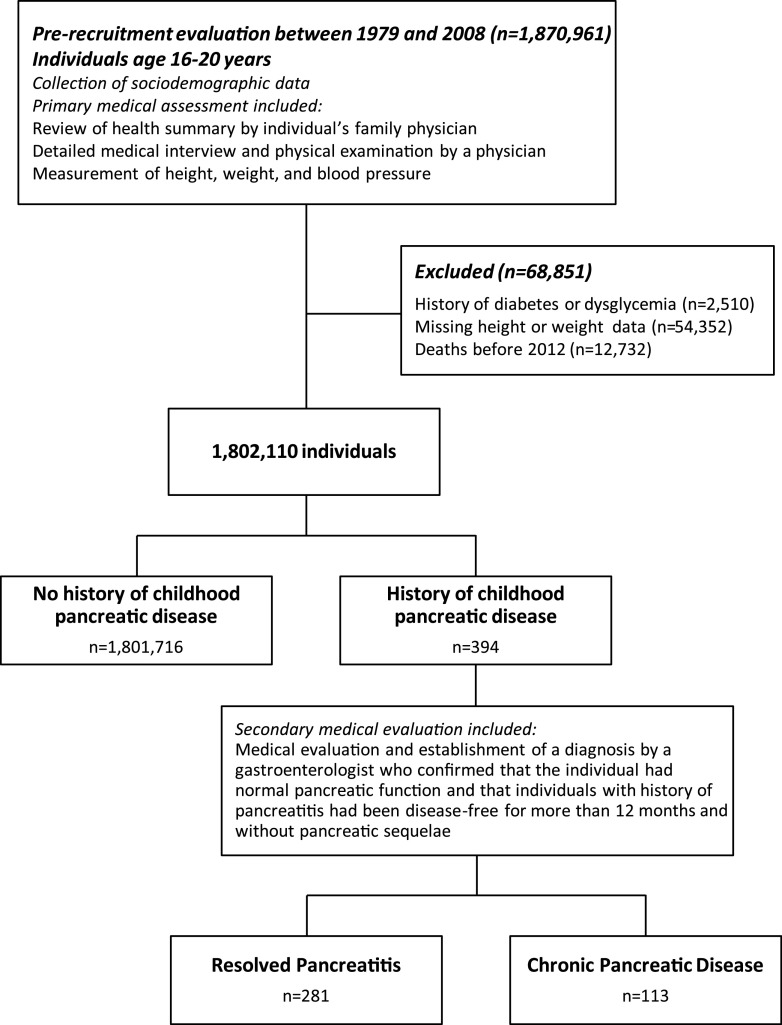
Included in the study were all potential Israeli adolescent recruits who underwent a medical examination to assess their fitness for military service 1 year before conscription. The assessment process and designation of individuals to study groups are presented in Fig. 1. The study sample included all Israeli adolescents who were evaluated at 16–20 years of age between 1 January 1979 and 31 December 2008 (n = 1,870,961). Excluded from the analysis were persons with a history of diabetes or dysglycemia, those who died before establishment of the Israel National Diabetes Registry (INDR) in 2012 (as diabetes was not reported in those who died prior to its establishment), and those with missing height or weight data given the critical role attributed to obesity in the development of diabetes among young adults (15). The final study sample included 1,802,110 individuals (59% men). The Institutional Review Board of the Israel Defense Forces Medical Corps approved this study and waived the requirement for written informed consent.
Figure 1.
Examination process and study sample buildup.
Clinical Assessment and Diagnosis of Childhood Resolved Pancreatitis
The medical assessment performed by a physician as part of the military enrollment process included a detailed interview, review of medical history and records, and physical examination. If this assessment revealed abnormal findings or a positive medical history, the examinee would have been referred to appropriate tests or consultation by a board-certified specialist to determine his or her medical fitness.
The diagnosis of resolved acute pancreatitis was made by a board-certified gastroenterologist and included cases with a history of a single event of acute pancreatitis that occurred at least 1 year prior to study enrollment and had normal pancreatic function without any evidence for chronic pancreatic sequelae. Notably, all individuals who were classified as having a single event of acute pancreatitis had no episodes of recurrent pancreatitis during their mandatory military service (2-year and 3-year period for women and men, respectively), thereby providing a period of at least 3 years that was used to ascertain classification of no more than a single event of acute pancreatitis. Information regarding the age at pancreatic episode, laboratory test results, and diagnostic imaging findings assessed by gastroenterologists were unavailable to us. Conscripts without a history of childhood pancreatic disease were assigned to the unexposed group. Conscripts with two or more episodes of acute pancreatitis, chronic pancreatitis, or history of subtotal or partial pancreatectomy were collectively assigned as having chronic pancreatic disease (n = 113). These cases were excluded from the primary analysis and analyzed separately.
INDR
The primary outcome of the study was incident diabetes of any type documented in the INDR. The INDR database is a national registry managed by the Israel Center for Disease Control in the Ministry of Health. Since 2012, all HMOs that provide health care services to all residents are requested by law to report annually on incident and prevalent cases of diabetes. Diabetes was defined in cases which met one or more of the following criteria in the previous year: 1) glycated hemoglobin (HbA1c) ≥6.5% (48 mmol/mol); 2) serum glucose concentrations of ≥200 mg/dL (11.1 mmol/L) in two tests performed at an interval of at least 1 month apart; and 3) three purchases or more of glucose-lowering medications. The sensitivity of the INDR is 95%, and the specificity is 93%. The latter increases to >98% for cases that were reported in 2 separate years. While data regarding the existence of islet autoantibodies were unavailable, the INDR includes data regarding the class of glucose-lowering drugs, which were used to define the type of diabetes. Diabetes was classified as type 1 diabetes in case subjects who were actively treated with short-acting insulin and met at least one of the following criteria: 1) short-acting insulin purchase within 1 year from diabetes diagnosis; and 2) insulin purchase without any prior history of oral glucose-lowering medication purchase. The remaining case subjects were classified as having type 2 diabetes. If clinical data regarding glucose-lowering medications were missing, patients in the case group were classified as having diabetes of uncertain type. Case subjects with gestational diabetes mellitus are not reported to the INDR.
The INDR database contains information regarding date of diagnosis for patients diagnosed after 1 January 2000. Date of diabetes diagnosis was available for 74.4% of case subjects. Their baseline characteristics were comparable to those without data regarding the time of diabetes diagnosis (Supplementary Table 1). Medical data at enrollment were linked to the INDR using civilian identification number. Follow-up extended from initial medical assessment until 31 December 2016.
Study Variables
Age at examination and year of birth were treated as continuous variables. Education was divided into three groups: <10, 11, or 12 years of formal schooling. Residential socioeconomic status, based on locality of residence at the time of examination, was coded on a 1 to 10 scale (16) and grouped into low (1–4), medium (5–7), and high (8–10) categories. Cognitive performance level was assessed by general intelligence score, which was strongly associated with diabetes risk in a subpopulation of this cohort (17,18) and grouped into low (less than or equal to −1 SD), medium (between −1 SD and +1 SD), and high (greater than or equal to +1 SD) (17). Country of origin was classified by the examinee’s father’s country of birth or by the grandfather’s country of birth if the father was born in Israel (19). BMI was calculated based on measured height and weight (weight in kilograms divided by height in meters squared). The classification to BMI categories of the U.S. Centers for Disease Control and Prevention was applied as it was previously validated and selected by the Israel Ministry of Health as the routine reference for anthropometric data in children (20). BMI was classified into three categories: underweight (<5th percentile), normal BMI (≥5th percentile to <85th percentile), and overweight and obese (≥85th percentile).
Statistical Analysis
Categorical variables were compared by χ2 or Fisher exact tests. A t test was used to test differences in continuous variables. All tests used were two-tailed, and P < 0.05 was considered statistically significant. We applied logistic regression analysis to determine the odds ratio (OR) for diabetes in the group with resolved pancreatitis compared with the unexposed population. In the prespecified minimally adjusted model, we adjusted for sex, birth year, and age at examination. The fully adjusted multivariable model additionally included BMI, cognitive performance level, education level, socioeconomic status, and country of origin. We calculated the E-value, which is the minimum strength of association that an unmeasured confounder would need to have with both the exposure and outcome to fully account for the association between a history of resolved pancreatitis and incident diabetes (21,22).
Several sensitivity analyses were conducted: 1) to minimize misclassification in diabetes diagnosis, a stricter definition of diabetes was used, in which diabetes diagnosis was valid only if diabetes criteria were fulfilled on 2 separate calendar years; 2) to minimize confounding by coexisting morbidities, the study sample was restricted to individuals with unimpaired health status (defined as lack of chronically prescribed medical treatment and absence of a history of any chronic disease or a history of a major operation) (23,24); 3) to better control for adolescence obesity, analysis was limited to individuals with normal BMI at enrollment; and 4) to assess the robustness of the adjusted OR of the entire cohort, E-value was calculated (21,22). 5) Cox proportional hazards models were used to determine time to diabetes diagnosis and to estimate the hazard ratio for developing diabetes in the group with resolved pancreatitis, compared with the unexposed group. For this analysis, follow-up extended from initial medical assessment to diabetes diagnosis, death, or 31 December 2016, whichever came first. Individuals who developed diabetes without a recorded date of diagnosis (n = 11,515) were excluded. 6) The study outcome was set as diabetes diagnosis prior to 40 years of age, the mean age of diagnosis in the cohort, as well as to other age cutoffs. 7) To account for the increasing rates of childhood pancreatitis in recent years, we defined an unexposed group in which the proportions of enrollment year were similar to that in the exposed group.
The association between examinees with chronic pancreatic disease and incident diabetes risk was determined. All statistical analyses were conducted with SPSS software, version 25.0 (IBM).
Results
Baseline demographic and clinical characteristics of 1,801,997 examinees are presented in Table 1. Sociodemographic characteristics were similar at initial medical assessment, albeit the proportion of men was higher in the resolved pancreatitis group. Individuals with a history of resolved pancreatitis were more overweight and obese at adolescence compared with unexposed (18.9% vs. 12.6%; P = 0.008), but mean BMI was statistically comparable between groups in both men and women (P = 0.224 and P = 0.092, respectively). Rates of unimpaired health status at baseline were also comparable between the study groups (78% and 81%, respectively; P = 0.71).
Table 1.
Baseline characteristics of the study population
| History of childhood resolved pancreatitis | No history of childhood pancreatic disease | P value | |
|---|---|---|---|
| Number | 281 | 1,801,716 | |
| Age, years | 17.5 ± 0.6 | 17.4 ± 0.4 | <0.001 |
| Male, number (%) | 195 (69.4) | 1,043,796 (57.9) | <0.001 |
| Female, number (%) | 86 (30.6) | 757,920 (42.1) | |
| Male height, cm | 174.7 ± 6.6 | 174.0 ± 6.8 | 0.143 |
| Female height, cm | 162.6 ± 6.6 | 162.3 ± 6.1 | 0.593 |
| Male BMI, kg/m2 | 21.9 ± 3.7 | 21.6 ± 3.4 | 0.224 |
| Female BMI, kg/m2 | 22.4 ± 4.3 | 21.7 ± 3.4 | 0.092 |
| BMI categories, number (%)† | 0.002 | ||
| Underweight | 24 (8.5) | 123,829 (6.9) | |
| Normal weight | 204 (72.6) | 1,451,498 (80.6) | |
| Overweight and obese | 53 (18.9) | 226,389 (12.6) | |
| Completed high school education, % | 87 | 83 | 0.071 |
| Residential socioeconomic status, % | 0.533 | ||
| Low | 23 | 26 | |
| High | 23 | 22 | |
| Cognitive performance level, % | 0.115 | ||
| Low | 15 | 16 | |
| Medium | 67 | 71 | |
| High | 18 | 14 | |
| Country of origin, % | <0.001 | ||
| Israel | 7 | 7 | |
| Former USSR | 28 | 16 | |
| Asia | 23 | 24 | |
| Africa | 19 | 23 | |
| Europe | 23 | 28 | |
| Ethiopia | 1 | 1 | |
| Period of enrollment, number (%) | 0.001 | ||
| 1979–1988 | 50 (17.8) | 452,032 (25.1) | |
| 1989–1998 | 94 (33.5) | 653,227 (36.3) | |
| 1999–2008 | 137 (48.8) | 696,457 (38.7) | |
| Unimpaired health, %‡ | 78 | 81 | 0.71 |
| Duration of follow-up, years§ | 20.0 ± 7.8 | 21.6 ± 8.2 | 0.001 |
| Age at end of follow-up, years§ | 37.5 ± 7.9 | 38.9 ± 8.3 | 0.004 |
Data are means ± SD unless otherwise indicated. USSR, Union of Soviet Socialist Republics.
BMI categories classified according to percentiles: underweight (<5th), normal BMI (≥5th to <85th), and overweight and obese (≥85th). BMI percentiles determined by the U.S. Centers for Disease Control and Prevention sex- and age-specific BMI range.
Unimpaired health was defined as lack of chronically prescribed medical treatment and absence of a history of any major disease or a history of a major operation.
Follow-up extended from initial medical assessment until 31 December 2016.
Among 1,802,110 individuals, a total of 281 (0.016%) had a history of childhood-resolved acute pancreatitis. There were 13 (4.63%) and 44,463 (2.47%) case subjects with incident diabetes among those with resolved pancreatitis and the unexposed population, respectively. A history of childhood pancreatitis was associated with an OR for diabetes of 2.23 (95% CI 1.25–3.98) after adjustment for sex, birth year, and age (Fig. 2). This finding was consistent after further adjustment for baseline BMI, education level, cognitive performance, socioeconomic status, and country of origin (adjusted OR 2.10 [95% CI 1.15–3.84]; P = 0.016). Results persisted when a stricter definition of diabetes was used (adjusted OR 2.22 [95% CI 1.19–4,16]; P = 0.013) or when the analysis was limited to individuals with unimpaired health to minimize confounding by coexisting illness (adjusted OR 2.27 [95% CI 1.17–4.40]). The risk for incident diabetes with a history of resolved pancreatitis was accentuated when only individuals with normal BMI were included (adjusted OR 3.09 [95% CI 1.57–6.08]) (Fig. 2). Of note, the observed adjusted OR between a history of resolved pancreatitis and incident diabetes of the entire cohort could be explained away by an unmeasured confounder that was associated with the latter both by an OR of 3.62 each, above and beyond the measured confounders, but weaker confounding could not do so; the CI could be moved to include the null by an unmeasured confounder that was associated with both a history of resolved pancreatitis and incident diabetes by an OR of at least 1.57 each, whereas weaker confounding could not do so.
Figure 2.
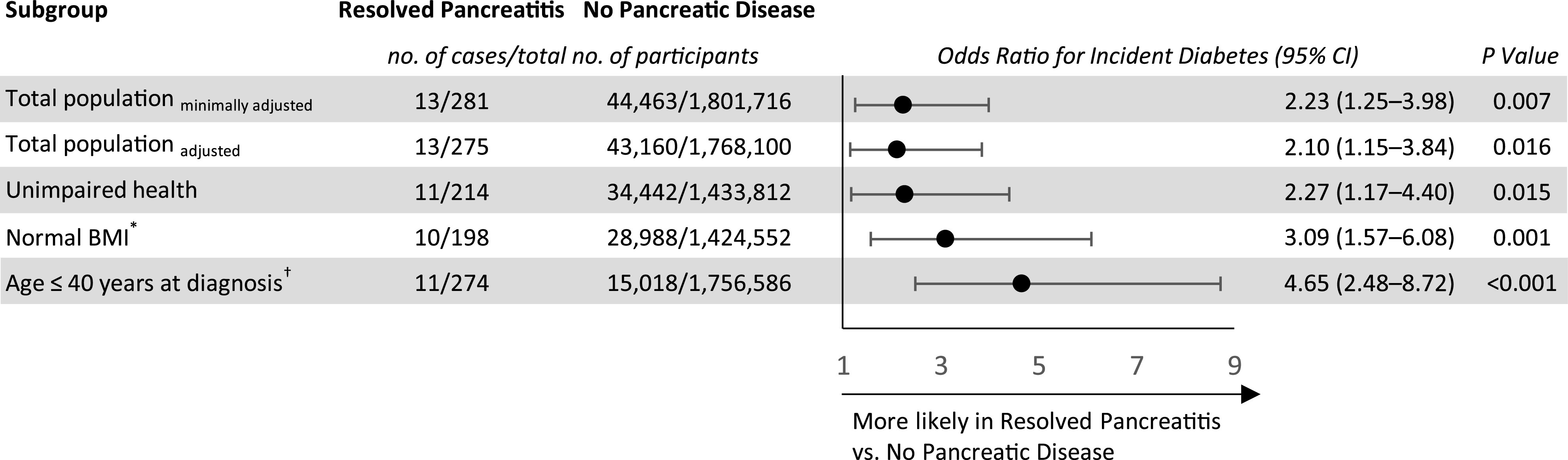
The association between history of childhood resolved pancreatitis and diabetes risk at young adulthood. Logistic regression analysis to determine the OR for diabetes in the group with resolved pancreatitis compared with the unexposed population. Prespecified minimally adjusted model includes sex, birth year, and age at examination. Fully adjusted multivariable model additionally includes BMI, cognitive performance level, education level, socioeconomic status, and country of origin. Fully adjusted model was applied for unimpaired health, normal BMI, and age ≤40 years at diagnosis analyses presented. *Applying both restrictions of unimpaired health and normal BMI at baseline yielded an adjusted OR of 3.41 (95% CI 1.66–7.01). †Included were individuals with information on age of diagnosis. To account for those excluded because they had missing data on age of diagnosis, we show their characteristics in a separate analysis (Supplementary Table 1).
Time-to-diabetes incidence was shorter in the resolved pancreatitis group compared with the unexposed group (mean ± SD, 18.7 ± 5.0 vs. 23.2 ± 7.2 years; P = 0.029) (Supplementary Table 2). Cox proportional hazard models yielded similar results to the logistic regression models with an adjusted hazard ratio of 2.36 (95% CI 1.34–4.16; P = 0.003) (Supplementary Table 2 and Supplementary Fig. 1).
Diabetes among individuals with a history of childhood pancreatitis was diagnosed at a younger age compared with diabetes incidence in the unexposed group (mean age ± SD, 36.4 ± 4.8 vs. 40.7 ± 7.2 years; P = 0.037). Notably, 92% of case patients with diabetes among individuals with a history of childhood pancreatitis occurred before 40 years of age, compared with 47% in the unexposed group (P = 0.002). Accordingly, the point estimate was accentuated when incident diabetes by 40 years of age was the outcome (adjusted OR 4.65 [95% CI 2.48–8.72]) (Fig. 2 and Supplementary Table 3). The difference in the age of diabetes diagnosis persisted in a subanalysis in which the examinees in the unexposed group enrolled in the study in years with similar proportions to that of the exposed group (mean age ± SD, 36.4 ± 4.8 vs. 39.9 ± 7.5 years; P = 0.029). Of note, with the latter unexposed group, point estimates were similar with an adjusted OR of 2.08 (95% CI 1.14–3.81; P = 0.017). None of the case subjects with diabetes in the group with resolved pancreatitis were classified as having type 1 diabetes.
We detected 113 individuals (0.006% of the entire cohort) with a history of chronic pancreatic disease, of whom 9 (8%) were diagnosed with diabetes during the follow-up period. A history of chronic pancreatic disease was associated with a minimally adjusted OR for diabetes of 6.19 (95% CI 2.93–13.08) with a minimal OR attenuation in the fully adjusted model (adjusted OR 5.67 [95% CI 2.62–12.25]) (Supplementary Table 4).
Conclusions
In this nationwide population-based study, a history of childhood resolved acute pancreatitis without clinical or laboratory evidence of compromised endocrine or exocrine pancreatic functions in adolescence, doubled the risk for incident diabetes, and was associated with an earlier age of diabetes diagnosis independent of BMI and other confounding factors. An analysis limited to those with unimpaired health and normal BMI at late adolescence strengthened this association.
The etiologies of acute pancreatitis in the pediatric age group are diverse compared with adults and may include obstructive causes, medications, idiopathic, systemic diseases, trauma, and viral, metabolic, and genetic causes, in descending order of frequency (10–12). Although genetic predisposition and metabolic factors may underlie recurrent acute pancreatitis as well as chronic pancreatitis, they are relatively rare in children who suffer from a single event of acute pancreatitis (12). Our classification of resolved acute pancreatitis was strict in limiting the history to no more than a single event and assuring a minimum disease-free period of at least 3 years, making genetic factors or hypertriglyceridemia as less likely etiologies in our cohort. While pancreatitis was coded without a definitive etiology, most individuals who developed diabetes did not have a chronic comorbidity or chronically prescribed medical treatment at enrollment, consistent with an idiopathic nature.
In adults, the relationship between acute pancreatitis and diabetes is proposed to be bidirectional (9). The main studies that evaluated acute pancreatitis as a cause for diabetes suggested a high prevalence of diabetes (16–54%) among patients after acute pancreatitis (25–28) and reported a relative high prevalence of a history of acute pancreatitis (1.1%) among patients with adult-onset diabetes (29). Similar to our findings, two population-based studies from Taiwan National Health Insurance Research Database reported hazard ratios of 2.54 and 2.15 for incident diabetes following acute pancreatitis compared with matched control subjects without such a diagnosis (30,31). In children and adolescents, there is scarce evidence linking a history of acute pancreatitis with incident diabetes. Raman et al. (4) reported that 4.5% of 176 children and adolescents with acute pancreatitis developed diabetes during hospitalization and defined diabetes as at least a single measurement of glucose >200 mg/dL combined with a prescription of insulin at hospital discharge. Notably, most patients had significant comorbidities, and the long-term diabetes risk was not assessed.
There are several potential mechanisms that can account for the association between resolved acute pancreatitis and an increased risk for diabetes later in life. It has been shown that a reduction in β-cell mass may be associated with a higher risk for diabetes (32). Thus, it could be that inflammation of the exocrine pancreas in pancreatitis collaterally damages β-cells of the endocrine islets, resulting in a reduced islet mass. Clinical resolution of acute pancreatitis as judged by standard clinical measures may not necessarily reflect absence of some residual damage to β-cell mass, especially in the first years after injury. Rather, this may manifest later in life with the coexistence of other diabetes risk factors. In that respect, we found that the average age of diabetes diagnosis was younger by 4 years among those with a history of acute pancreatitis even when adolescent BMI was carefully controlled. This may suggest a lower capacity to overcome the individual’s underlying degree of insulin resistance and that a shorter duration is sufficient to induce β-cell failure and hyperglycemia, supporting the hypothesis that diabetes in young adulthood may be more strongly dependent on β-cell failure than in those who develop type 2 diabetes later in life (15). The occurrence of β-cell damage during somatic growth might also reduce replication capacity of β-cells and the number of existing islets, as the contribution of new islet formation to the overall increase in β-cell mass from birth to adolescence was estimated to be only 12% (33). Unavailability of the precise age of pancreatitis diagnosis and its severity is one of the study limitations, as the rate of expansion in β-cell mass is suggested to be higher in early childhood compared with adolescence (33). However, the higher risk estimates for incident diabetes among those with chronic pancreatic injury is in line with a dose-dependent effect of such a mechanism. In that respect, asymptomatic undiagnosed chronic pancreatitis may be an underrecognized cause of diabetes (2). Elucidating the mechanism underlying increased diabetes risk following resolved acute pancreatitis is limited by lack of data on β-cell reserve and insulin resistance. As data on the presence of autoantibodies were unavailable to us, we cannot completely exclude the possibility of misclassification of type 1 diabetes (34). Nevertheless, none of our case subjects with diabetes with a history of resolved pancreatitis had a concurrent autoimmune disease at medical evaluation or were prescribed a short-acting insulin despite their relatively young age at diabetes diagnosis.
Our study has several limitations. First, as we did not have information about the etiology and the clinical severity of pancreatitis and on recurrence after military service, it is possible that the increased risk was mediated by recurrent attacks, which might have occurred later in adulthood. However, it is suggested that ∼65–85% of children with a single event of pancreatitis do not suffer from recurrent episodes (10,11) and that among those with acute recurrent pancreatitis, the median progression time to chronic pancreatitis is ∼1 year (interquartile range 1.5 months to 2.7 years) (35). When taking into consideration that for classification we had data available on additional incident attacks of pancreatitis during the time interval between prerecruitment medical assessment and military discharge, the possibility that recurrent pancreatitis solely mediated the association is unlikely. Second, lifestyle data at enrollment and during follow-up were unavailable to us such as smoking, alcohol consumption, or physical activity. Of note, accounting for sociodemographic background, which was shown to strongly correlate with lifestyle habits in our cohort (36), minimally changed the point estimates. Third, data regarding the age of diabetes diagnosis were missing for 26.6% of the cohort. However, the baseline characteristics of those who developed diabetes and for whom the age at diabetes diagnosis was available were similar to those without this data item, and similar point estimates were computed when diabetes diagnosis was limited only to those with a date of diagnosis. Fourth, we lacked longitudinal data on diabetes severity in our study groups, and our observations are based on a relatively small number of incident case subjects with diabetes among those with a history of resolved pancreatitis. Yet, results withstood an extensive sensitivity analyses and were minimally affected by adjustment for obesity and sociodemographic variables, and it would be required that a strong unknown confounder be present to account for the association. Finally, although our study design aimed to reduce previous exposure to dysglycemia, it lacked a structured screening for glucose levels. Hence, we cannot exclude the possibility that patients with undiagnosed dysglycemia were included in our study and that the onset of diabetes may have occurred years before (37). The strengths of our study include systematic collection of clinical data throughout three decades, using linkage between two nationally representative databases, strict definition of resolved acute pancreatitis, and strict control for health and obesity status at baseline.
In conclusion, a clinically resolved single event of acute pancreatitis is associated with an increased risk for diabetes at young adulthood, independent of adolescent obesity or sociodemographic factors, and is associated with a younger age of disease diagnosis. These patients therefore may require a tighter follow-up and aggressive control of other diabetes risk factors. Future studies are required to confirm these results in other populations and test the role of early therapeutic intervention at the prediabetes stage to prevent or delay diabetes onset.
Supplementary Material
Article Information
Acknowledgments
The authors thank the members of the Steering Committee of the INDR for the support in facilitating the linkage between the two databases described above. The authors also thank the administrative staff of the Department of Military Medicine at Hebrew University for the technical support throughout this study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.D.B. conceived and designed the study, statistically analyzed and interpreted the data, and drafted and revised the manuscript. A.B. designed the study, statistically analyzed and interpreted the data, and drafted and revised the manuscript. I.Z., M.L., T.S., and D.T. acquired data. T.C.-Y., O.M., O.P.-H., R.G.K., I.R., A.A., and H.C.G. contributed to the discussion and critically revised the manuscript. E.D. performed statistical analysis. A.S. acquired data, contributed to the discussion, and critically revised the manuscript. A.T. designed the study, contributed to the discussion, and critically revised the manuscript. G.T. designed and supervised the study, statistically analyzed and interpreted the data, and drafted and revised the manuscript. G.T. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 55th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 16–20 September 2019.
Footnotes
C.D.B. and A.B. equally contributed to this work.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1562/-/DC1.
References
- 1.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes--2019. Diabetes Care 2019;42(Suppl. 1):S13–S28 [DOI] [PubMed] [Google Scholar]
- 2.Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev 2012;28:338–342 [DOI] [PubMed] [Google Scholar]
- 3.Andersen DK, Korc M, Petersen GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes 2017;66:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman VS, Loar RW, Renukuntla VS, et al. Hyperglycemia and diabetes mellitus in children with pancreatitis. J Pediatr 2011;158:612–616.e1 [DOI] [PubMed] [Google Scholar]
- 5.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974;139:69–81 [PubMed] [Google Scholar]
- 6.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med 2016;375:1972–1981 [DOI] [PubMed] [Google Scholar]
- 7.Morinville VD, Barmada MM, Lowe ME. Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas 2010;39:5–8 [DOI] [PubMed] [Google Scholar]
- 8.Poddar U, Yachha SK, Borkar V, Srivastava A, Kumar S. A report of 320 cases of childhood pancreatitis: increasing incidence, etiologic categorization, dynamics, severity assessment, and outcome. Pancreas 2017;46:110–115 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes--2019. Diabetes Care 2019;42(Suppl. 1):S34–S45 [DOI] [PubMed] [Google Scholar]
- 10.Pohl JF, Uc A. Paediatric pancreatitis. Curr Opin Gastroenterol 2015;31:380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai HX, Lowe ME, Husain SZ. What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr 2011;52:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uc A, Husain SZ. Pancreatitis in children. Gastroenterology 2019;156:1969–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uc A, Perito ER, Pohl JF, et al.; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) . International Study Group of Pediatric Pancreatitis: In Search for a CuRE cohort study: design and rationale for INSPPIRE 2 from the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47:1222–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol 2018;6:69–80 [DOI] [PubMed] [Google Scholar]
- 16.Israel Central Bureau of Statistics Characterization and classification of local authorities by the socio-economic level of the population 2006 [Internet], 2009. Available from https://www.cbs.gov.il/en/publications/Pages/2006/Characterization-and-Classification-of-Local-Authorities-by-the-Socio-Economic%20-Level-of-the-Population-2006.aspx. Accessed 13 September 2019
- 17.Twig G, Gluzman I, Tirosh A, et al. Cognitive function and the risk for diabetes among young men. Diabetes Care 2014;37:2982–2988 [DOI] [PubMed] [Google Scholar]
- 18.Cukierman-Yaffe T, Kasher-Meron M, Fruchter E, et al. Cognitive performance at late adolescence and the risk for impaired fasting glucose among young adults. J Clin Endocrinol Metab 2015;100:4409–4416 [DOI] [PubMed] [Google Scholar]
- 19.Twig G, Afek A, Shamiss A, et al. Adolescence BMI and trends in adulthood mortality: a study of 2.16 million adolescents. J Clin Endocrinol Metab 2014;99:2095–2103 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein A, Haelyon U, Krolik E, Sack J. Comparison of body weight and height of Israeli schoolchildren with the Tanner and Centers for Disease Control and Prevention growth charts. Pediatrics 2001;108:E108. [DOI] [PubMed] [Google Scholar]
- 21.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med 2017;167:268–274 [DOI] [PubMed] [Google Scholar]
- 22.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019;321:602–603 [DOI] [PubMed] [Google Scholar]
- 23.Twig G, Yaniv G, Levine H, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med 2016;374:2430–2440 [DOI] [PubMed] [Google Scholar]
- 24.Twig G, Tirosh A, Leiba A, et al. BMI at age 17 years and diabetes mortality in midlife: a nationwide cohort of 2.3 million adolescents. Diabetes Care 2016;39:1996–2003 [DOI] [PubMed] [Google Scholar]
- 25.Das SLM, Singh PP, Phillips ARJ, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2014;63:818–831 [DOI] [PubMed] [Google Scholar]
- 26.Appelros S, Lindgren S, Borgström A. Short and long term outcome of severe acute pancreatitis. Eur J Surg 2001;167:281–286 [DOI] [PubMed] [Google Scholar]
- 27.Tu J, Zhang J, Ke L, et al. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol 2017;17:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doepel M, Eriksson J, Halme L, Kumpulainen T, Höckerstedt K. Good long-term results in patients surviving severe acute pancreatitis. Br J Surg 1993;80:1583–1586 [DOI] [PubMed] [Google Scholar]
- 29.Woodmansey C, McGovern AP, McCullough KA, et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care 2017;40:1486–1493 [DOI] [PubMed] [Google Scholar]
- 30.Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of diabetes mellitus after first-attack acute pancreatitis: a national population-based study. Am J Gastroenterol 2015;110:1698–1706 [DOI] [PubMed] [Google Scholar]
- 31.Lee YK, Huang MY, Hsu CY, Su YC. Bidirectional relationship between diabetes and acute pancreatitis: a population-based cohort study in Taiwan. Medicine (Baltimore) 2016;95:e2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir GC, Bonner-Weir S. Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 2004;53(Suppl. 3):S16–S21 [DOI] [PubMed] [Google Scholar]
- 33.Meier JJ, Butler AE, Saisho Y, et al. β-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018;6:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Ooi CY, Werlin S, et al. Risk factors associated with pediatric acute recurrent and chronic pancreatitis: lessons from INSPPIRE. JAMA Pediatr 2016;170:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kark JD, Laor A. Cigarette smoking and educational level among young Israelis upon release from military service in 1988--a public health challenge. Isr J Med Sci 1992;28:33–37 [PubMed] [Google Scholar]
- 37.Porta M, Curletto G, Cipullo D, et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care 2014;37:1668–1674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.