Abstract
Thrombotic thrombocytopenic purpura (TTP) is usually a fatal disease caused by a deficiency of the metalloproteinase, ADAMTS13, often due to autoimmunity. This leads to the development of pathogenic multimers of von Willebrand factor (vWF), causing an inappropriate interaction of platelets and vWF. This results in a thrombotic microangiopathy, which is treated with therapeutic plasma exchange and immune suppression. Although this treatment has reduced the mortality of TTP to only about 20%, there have been no recent significant advances in the treatment of TTP. Recently, a novel agent has been approved for use in TTP. Caplacizumab, which binds to the A1 domain of vWF, prevents the adhesion of platelets to vWF. It is a first in-class ‘nanobody’, that in clinical trials has shown marked efficacy in treating TTP and its complications. This review will discuss the development and implications of caplacizumab in the treatment of TTP.
Keywords: ADAMTS13, caplacizumab, nanobody, TTP, vWF
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare (two cases per million people per year)1 thrombotic microangiopathy characterized by microangiopathic hemolytic anemia and severe thrombocytopenia. TTP is usually caused by the pathologic immune mediated production of anti-ADAMTS13 autoantibodies that neutralize or induce the clearance of the ADAMTS13 protein. ADAMTS13 is a disintegrin and metalloproteinase present in plasma that cleaves von Willebrand factor (vWF) multimers. Without the proteolytic activity of ADAMTS13, the uncleaved ultra-large vWF multimers accumulate and induce excessive platelet binding and activation leading to the formation of disseminated microthrombosis. Often these microthrombi are large enough to induce tissue ischemia, and result in platelet consumption and microangiopathic hemolytic anemia, leading to the classic clinical presentation. TTP can present in isolation as a primary disease or as a secondary presentation associated with other autoimmune diseases (systemic lupus erythematosus), malignancy, infection, pregnancy or with drug exposures. In the United States, TTP is most common in the fourth decade of life, in women (2–3:1), and in blacks (seven times higher incidence).2,3 Despite multiple treatment modalities, long term morbidity and mortality associated with each presentation of TTP remains high with recent mortality rates as high as 20% with each episode.4 Most commonly, patient’s acute presentations are complicated by cerebral vascular events, acute coronary events, acute renal injury, and bowel ischemia.5–7 Long-term complications of TTP remain despite improvements in treatment. Patients experiencing at least one episode of TTP are more likely to have arterial hypertension, have unresolved neurocognitive deficits, suffer from depression, and are more likely to die at an early age than matched controls.6
TTP is a life-threatening disease requiring urgent diagnosis and treatment, often in intensive care units. The mainstay of treatment involves therapeutic plasma exchange (TPE).8 Previous studies have shown that TPE should be started as soon as possible (ideally within 4–8 h of presentation) when TTP is suspected.8,9 TPE replenishes ADAMTS13 activity and removes the immunologic inhibitor improving survival rates from <20% pretreatment era to >80%.8
Despite these gains in the treatment of TTP, up to 20% of patients may remain refractory from initial therapy. In addition, the clinical course for patients with TTP is often characterized by multiple relapses, with 30–40% of patients suffering at least one relapse requiring re-initiation of treatment.5,7 Relapses are characterized by a severe deficiency of ADAMTS13 and the persistence or recurrence of anti-ADAMTS13 antibodies. At each episode of relapse, the patient is at risk for significant morbidity and mortality including the acute complications of TTP and the resultant end-organ ischemia as well as the complications of prolonged in-patient treatment.
Current therapies include TPE and immunomodulation, replenishment of functional ADAMTS13 enzyme, and control of the underlying autoimmune disease, but these do not directly address the pathologic formation of microvascular thrombi. Caplacizumab, a ‘nanobody’ (described in the following) that inhibits the vWF-platelet glycoprotein-Ib interaction, blocks the adhesion of platelets to vWF multimers preventing the formation of the pathological microthrombi, and thus preventing end-organ ischemic damage. This novel treatment may actually be the key to improving the prognosis associated with TTP.
Development of caplacizumab
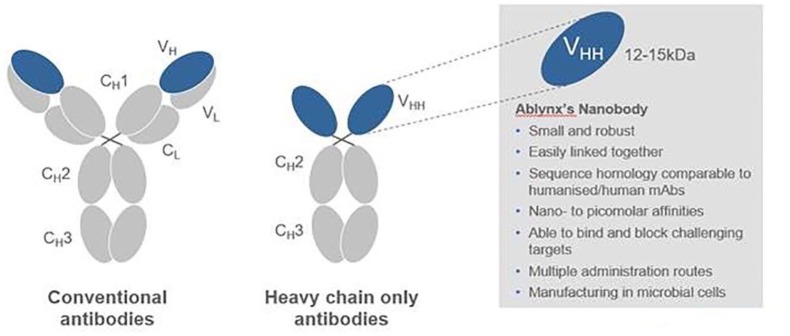
The decades-long process that eventually brought caplacizumab forth as a treatment for TTP, like many discoveries in science, started as a serendipitous finding. As the story goes, students in a biology class were assigned the task of isolating antibodies from human blood in the late 1980s.10 Because of a number reasons, these students deferred performing this experiment with human blood and instead, used camel blood. The results showed that camel blood contained a heavy-chain only antibody [sometimes referred to as camel single-domain antibody], compared with the typical immunoglobulin composed of a heavy- and light-chain (Figure 1). This was followed by several years of research that confirmed this finding and detailed the antibody repertoire of the Camelidae species, showing that heavy-chain only immunoglobulins were present in old- and new-world camelids.11 Despite the lack of a light-chain, these antibodies had an ‘extensive antigen-binding repertoire’ and it was hypothesized that this could ‘. . . be an invaluable asset in the development. . . for diagnostics, therapeutic and biochemical purposes.’11
Figure 1.
Comparison of conventional versus camel derived antibodies.
Following the identification of these heavy-chain only camel antibodies, their potential therapeutic role in a number of conditions were explored. Although a more complete list can be found elsewhere,12 some of these uses included:
inhibiting enzymes, such as erythrocyte carbonic anhydrase and porcine pancreatic alpha-amylase;13,14
targeting ∝-lactamases in bacteria, in order to overcome antibiotic resistance;15
preventing the formation of amyloid fibrils;16
binding to TNFα in a mouse model of rheumatoid arthritis;17
neutralizing scorpion venom;18
targeting tumors in scid mice.19
In the last-mentioned study,19 the variable regions of these heavy chains (denoted as VHH) was combined with another VHH into a homodimer. In addition, since these VHH domains were the smallest known fully functional structures derived from immunoglobulins, they were coined as a ‘nanobody’,20 as noted above. Despite the wide array of potential uses of a nanobody, be it as a single chain or as a dimer, the main focus of research became the inhibition of the vWF A1 domain interaction with the platelet glycoprotein-Ib receptor.
In particular, use of the dimerized version of the anti-vWF nanobody, initially known as ALX-0081 (Figure 2), was first explored in a cardiovascular setting.21 Using a 3-alanine linker, the bivalent ALX-0081 bound tighter to the A1 domain of vWF and in an ex vivo model simulating high-flow conditions, platelet adhesion was prevented. Furthermore, in a baboon model, a lower rate of bleeding was observed when compared with traditional anti-platelet agents, such as abciximab and clopidogrel.21 A follow-up proof of principle study on the platelets from patients electively undergoing percutaneous coronary intervention showed that ALX-0081 was able to completely prevent platelet adhesion to collagen.22 A substudy noted that this inhibition of the vWF–platelet interaction led to an improvement in endothelial function, as measured by endothelial pulse amplitude tonometry and the presence of endothelial microparticles.23
Figure 2.
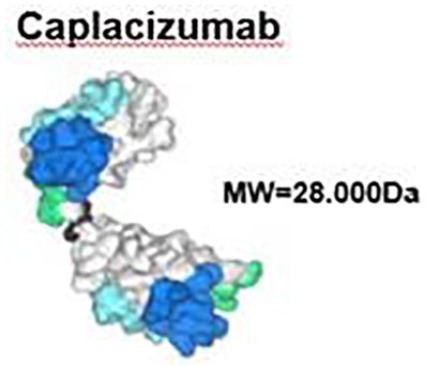
Structure of caplacizumab.
While the preliminary data for the use of ALX-0081 in blocking vWF–platelet adhesion in the cardiovascular setting appeared promising, attention was shifted towards blocking this interaction in TTP. As noted above, this deadly condition is characterized in part by an inappropriate binding of platelets to vWF, and as such, this was a natural extension of ALX-0081’s activity. Using a baboon model of TTP, in which the infusion of an anti-ADAMTS13 antibody created the phenotype of TTP, the efficacy and safety of ALX-0081 was examined.24 ALX-0081 was administered either prophylactically or after laboratory findings of TTP were present and it was noted that the presence of ALX-0081 was effective in preventing and treating the effects of TTP. Reassuringly, even though there was a complete inhibition of vWF activity noted, an increase in hemorrhagic complications was not observed.
Given these findings, a phase II trial in humans with TTP was in order.
TITAN trial
The use of ALX-0081, now termed caplacizumab, for treating acquired TTP was evaluated in the phase II TITAN study.25 This study was a single-blind, parallel design, randomized, placebo-controlled study at 56 sites worldwide conducted from October 2010 to January 2014. The study population included 75 patients experiencing an acute episode of acquired TTP with a platelet count of less than 100,000 per cubic millimeter, requiring plasma exchange, and without active bleeding. Patients were randomized in a 1:1 ratio to the study drug or placebo. Patients experiencing either their initial episode or recurrent episode of TTP were included.
Patients in both arms received standard-of-care treatment for acquired TTP including daily plasma exchange and immunosuppressive therapy. Approximately, 90% of patients received steroids in both arms; 5.6% of patients in the treatment group and 23.1% of patients in the placebo arm received rituximab. Patients in the treatment arm received an IV loading dose of 10 mg of caplacizumab or placebo prior to the start of the first plasma exchange following enrollment in the study. Study drug or placebo was then administered subcutaneously daily throughout the treatment period, within 30 min of the end of each plasma exchange. Study subjects continued treatment for 30 days after the last plasma exchange for a maximum duration of 90 days and could go home with the drug.
The primary end point was the time to confirmed normalization of platelet count to 150,000 per cubic millimeter, with a confirmation platelet count at 48 h and a lactate dehydrogenase (LDH) that was no more than twice the upper limit of normal range. Secondary end points included exacerbations (defined as recurrent thrombocytopenia within 30 days after the end of daily plasma exchange requiring re-initiation of daily exchange), relapse (a recurrent TTP event more than 30 days from the end of daily plasma exchange), complete remission status, duration and volume of plasma exchange, mortality, and safety. A number of exploratory end points including time to resolution of creatinine elevation, time to resolution of troponin (I or T) elevation, and time to resolution of elevated LDH were also examined. Pharmacokinetic data and pharmacodynamics data of the study drug were also collected.
A total of 75 patients underwent randomization (69 of whom enrolled before the initiation of plasma exchange). Patients receiving the study drug saw a 39% reduction in median time to response (resolution of thrombocytopenia) with an event ratio of 2.20 [95% confidence interval (CI), 1.28–3.87, p = 0.005]. Three patients in the caplacizumab group experienced exacerbations as compared with 11 patients in the placebo group. After cessation of the study drug, eight patients in the caplacizumab group had a relapse during the 1-month follow-up period (most occurring within 10 days of drug cessation) as compared with zero patients in the placebo group. Complete remission after the initial course of daily plasma exchanged occurred more frequently in the caplacizumab group 81% versus 46% in the placebo group. Two deaths occurred during the trial, both in the placebo group (one from severe refractory TTP and one from cerebral hemorrhage.)
As could be expected, in both groups, exacerbations correlated with continued low levels of ADAMTS13 activity. In all patients experiencing relapse within 10 days of stopping the study drug, ADAMTS13 levels <10% were seen shortly before stopping treatment. Patients experiencing complete remission were more likely to have an increase of ADAMTS13 activity greater than 10% during the treatment period.
A post hoc analysis also showed that the mean number of days of plasma exchange and the mean volume of plasma utilized were lower in the caplacizumab group during daily plasma exchange (5.9 versus 7.9 days, and 19.9 versus 28.3 l) and during the overall treatment period, including exacerbations (7.7 versus 11.7 days and 25.8 versus 41.8 l).
Also in post hoc analysis, indicators of organ damage including creatinine elevation, troponin I or T, and elevated LDH showed a trend toward improvement in the mean time to resolution of these markers in the caplacizumab group when compared with placebo.
Caplacizumab rapidly inhibited vWF as indicated by suppression of vWF-ristocetin cofactor activity to a mean of less than 20% by day one and throughout the treatment period. These values returned to baseline within 1 week following the end of treatment. Drug-induced antidrug antibodies were detected in three patients in the caplacizumab group (9%), but pharmacokinetic and pharmacodynamic profiles were not affected indicating that there was no neutralizing activity.
Headache and epistaxis were the most common adverse events. Serious adverse events were reported in 37% of patients in the caplacizumab group and 32% in the placebo group. The number of bleeding-related adverse events was higher in the caplacizumab group than in the placebo group, however, only three events were considered severe (i.e. interrupting daily activities, substantial affecting clinical status, or requiring intensive therapeutic intervention).
The authors stated that the use of caplacizumab results in a more rapid resolution of TTP episodes as indicated by faster platelet-count normalization. They suggest that caplacizumab immediately inhibits the pathophysiological mediator of microthrombosis leading to improvement in organ-damage markers as well as the numbers of exacerbations. Given that caplacizumab prevents platelet-aggregation and microthrombus formation faster than conventional therapy, the authors suggest that this treatment could potentially prevent short- and long-term end-organ injury due to ischemia. They also argue that the difference seen in relapse rates between the groups is likely an effect of caplacizumab delaying an exacerbation until after the period of drug administration. Given that patients in the relapse group had ADAMTS13 activity persistently <10% the authors suggest that ADAMTS13 activity level could potentially guide decisions on the duration of caplacizumab treatment in addition to guiding immunosuppressive treatment.
An additional post hoc analysis of follow-up data from the TITAN trial showed improvement in the frequency of major embolic and thrombotic events as well as death in the caplacizumab group as compared with the placebo group. In the 30 days following initial treatment with caplacizumab or placebo, 11.4% of patients in the caplacizumab group experienced a major thromboembolic event, experienced an exacerbation of TTP or died compared with 43.2% of placebo-treated patients. No patients in the caplacizumab group died.26
HERCULES trial
Given the positive results of the TITAN trial, the phase III HERCULES trial eventually followed.27 From November 2015 to April 2017, 145 patients were enrolled in the randomized, double-blind, placebo control trial at 92 sites world-wide. Patients were randomly assigned to receive caplacizumab or placebo in addition to the standard-of-care treatment for TTP. Standard-of-care treatment again included daily plasma exchange until at least 2 days after platelet count normalization, steroids, and other immunosuppressive therapy in accordance to clinical practice at each site. In total, 43% of patients received rituximab during the study period. Other immune-modulating therapies used in the trial included mycophenolate mofetil, hydroxychloroquine, bortezomib, cyclophosphamide, cyclosporine, IVIG, and splenectomy. Randomization was stratified according to neurologic involvement. The treatment regimen was identical to that of the TITAN trial except for allowance of extending therapy for an additional 28 days past the initial 30 days as guided by risk factors for TTP recurrence. In the event of recurrence, patients were switched to open-label caplacizumab and daily plasma exchange was re-initiated. Patients were followed for an additional 28 days after the treatment period and any recurrences during this period were managed with standard-of-care therapy without re-initiation of the study drug.
Again, the primary outcome was time to normalization of the platelet count. Notable secondary outcomes included a composite of TTP-related death, recurrence of TTP, or major thromboembolic event during the treatment period, recurrence of TTP at any time during the trial, refractory TTP, and time to normalization of three organ-damage markers (LDH, serum creatinine, troponin).
Demographic and baseline disease characteristics in the two groups were similar except for the percentage of patients with initial as compared with recurrent TTP episodes, with more patients presenting with their initial presentation in the caplacizumab group. The diagnosis of TTP was confirmed by baseline ADAMTS13 activity <10% in 85% of patients and in all but seven patients, the diagnosis was confirmed by history or an ADAMTS13 activity level <10% at some other point during the trial.
Median time to normalization of the platelet count was significantly shorter in the caplacizumab group with a rate ratio of 1.55 (95% confidence interval 1.09–2.19; p = 0.01). Patients who received caplacizumab had a 74% lower incidence (12% in the caplacizumab group versus 49% in the placebo group) of the composite outcome of TTP-related death, recurrence of TTP, or major thromboembolic event during the treatment period. Patients in the caplacizumab group also had a 67% lower incidence of recurrence (12% of patients in the caplacizumab group and 38% in placebo group) and there were no patients with refractory disease in the treatment group. Normalization of the three organ damage markers again occurred somewhat sooner in patients who received caplacizumab.
Patients in the caplacizumab group required 38% fewer days of plasma exchange, received 41% less plasma volume, had a 65% shorter stay in the intensive care unit, and 31% shorter duration of hospitalization.
Immunogenicity again did not play a role in either clinical efficacy or in laboratory measures of inhibitor of vWF. There were significantly more bleeding events in the caplacizumab group, but these were largely mild or moderate in severity and commonly mucocutaneous bleeding. There were four deaths during the trial, and all were TTP related (one in the treatment group, three in the control group). No deaths occurred while on treatment with caplacizumab.
The HERCULES trail again showed improvement in TTP therapy with caplacizumab, by illustrating faster resolution of thrombocytopenia, decreased health care resource utilization, and improvement in TTP-related death, recurrence, and major thrombotic events. There were also fewer relapses in the HERCULES trial compared with the TITAN trial validating the theory that continued treatment with caplacizumab and modulation of immunotherapy in the setting of continued low ADAMTS13 activity levels was beneficial.
Discussion
Caplacizumab clearly improves important patient centered outcomes such as- thromboembolic event rate, death rate, relapse rate, hospitalization/ICU time, days of TPE, and time to normalization of markers of end-organ ischemic damage. It was recently approved by the FDA for acquired TTP in February 201928 after receiving fast track designation. Since that time, providers have been considering how to incorporate caplacizumab into their standard treatment algorithms for TTP. It is estimated that up to 20% of patients die from each episode of TTP despite current available treatments, with most deaths occurring within 30 days of diagnosis. It is important to note that caplacizumab has not been studied in congenital TTP populations and it is unclear what if any role it has in treating these patients.
As demonstrated in the TITAN and HERCULES trials caplacizumab decreases morbidity and mortality of an already morbid disease. It prevents the interaction between vWF and platelets yielding a direct and timely intervention to block the formation of microthrombi. The combination of caplacizumab with TPE protects patients with acquired TTP from the end-organ. Damage associated with platelet consumption and microvascular thrombosis while immunosuppression reaches full effect. This protection could alter the course of the disease, potentially preventing long-term morbidity and mortality. As also illustrated in the trials, caplacizumab may also prevent recurrence in a significant number of patients. Long-term results from the POST-HERCULES trial are still pending but they hope to provide more information regarding the long-term safety and efficacy of caplacizumab, the repeated use of caplacizumab, and to further characterize the long-term impact of TTP.
Despite the clear advantages of treatment with caplacizumab, this therapy does not address the underlying pathophysiology of TTP; as it does not address the ADAMTS13 inhibitor or its production/elimination. Caplacizumab simply blocks the downstream effects of platelet consumption and microthrombus production. Treatment of the underlying auto-antibody production would still be an essential part of TTP treatment with or without caplacizumab therapy. Treatment strategies usually involve steroids and in refractory cases other immunosuppressant medications.
The humanized anti-CD20 monoclonal antibody, rituximab, was originally used in TTP patients with refractory disease or in those with early exacerbations. Rituximab acts through B-cell depletion, suppressing the production of the anti-ADAMTS13 antibody and increasing ADAMTS13 activity. In several retrospective studies, most patients (about 90%) treated with weekly rituximab after suboptimal response to standard therapy were able to achieve remission in generally less than 4 weeks.29–32 In two prospective trials of patients with recurrent or refractory disease >90% remission rates were seen in patients treated with rituximab and no relapses were observed during the first year.33,34
The up-front use of rituximab in the initial acute setting is more controversial. In 2011, a phase II trial demonstrated that front line rituximab plus TPE resulted in a shorter hospitalization and fewer relapses than compared with historical controls.35 Fewer and later relapses were also observed in rituximab treated patients in two of the largest TTP patient data registries.31,34 It is currently unclear if all patients benefit from rituximab36 use upfront as many patients respond well to TPE and steroids alone.
Other immune modulatory agents have been reportedly used in the relapse or refractory setting including vincristine,37–39 cyclosporine,40–44 and cyclophosphamide.36,45–48 With a greater understanding of the pathophysiology of TTP, other agents are being studied as potential therapies. N-acetylcysteine, which has been shown to reduce large vWF multimers and inhibit vWF-dependent platelet aggregation has been used in refractory cases and pilot studies are currently underway to assess its effectiveness.49 Bortezomib, a proteasome inhibitor targeting plasma cell depletion50,51 and recombinant ADAMTS13 are also being evaluated.52
The major barrier of caplacizumab use has been cost of the drug. Current pricing of caplacizumab at the time of publication was more than US$8000 per dose with treatment regimens recommending daily dosing for 30 days following the last plasma exchange with the possibility of extended treatment pending the recovery of ADAMTS13 activity. Given this high cost, hospitals have been hesitant to incorporate the drug into their standard formulary and providers have been cautious with implementing a standard use policy. Many patients will completely recover from the first episode of TTP with no complications or relapses with TPE and steroids alone.3,7,8 Given this, there is appropriate concern that up front universal utilization of caplacizumab could lead to over treatment in a significant number of patients as well as contribute to the already significant inflation of health care costs, similarly to the up-front use of rituximab.
In addition, given its limited availability (likely limited to tertiary care centers) and the delay in treatment required for insurance approval, clinical practice stands to differ significantly from the phase II/III protocol in that patients will most likely not receive the first IV loading dose within the first 24 h of illness presentation or prior to the first TPE. Whether this change in dosing administration limits the potential benefits of the medication is not currently known, and results from a ‘real-world’ cohort would ultimately demonstrate caplacizumab’s practical usage.
Interestingly, a recent correspondence in the New England Journal of Medicine was published that recounted the treatment of a Jehovah’s witness with TTP who refused transfusion of plasma.53 A 63-year-old woman presented with spontaneous purpura, mild anemia, thrombocytopenia, elevated LDH, and <5% ADAMTS13 enzyme activity. She was treated initially with high dose steroids, rituximab, and anti-hemophilic factor VIII. On days 4–10 of her hospital stay she developed worsening confusion and multiple episodes of transient right facial droop and aphasia. She was initiated on caplacizumab on hospital day 11 and on day 14 her platelet count had normalized. She suffered only mild epistaxis as a side effect from caplacizumab. Her neurologic symptoms resolved, and she was discharged home to complete 30 days of caplacizumab treatment. At the time of follow up, she remained well and continued to have normal ADAMTS13 activity. This interesting case poses the possible future treatment of TTP without TPE, especially in patients with a contraindication to this therapy. Caplacizumab could prevent the negative pathophysiologic effects of microthrombus formation, while immune modulatory therapy controls the ADAMTS13 inhibitor. This methodology could also avoid the complications of plasma exchange including complications of central lines, risk of infusion reactions and in severe cases anaphylaxis to plasma, and life-threatening hypocalcemia.3
Ultimately, the goal remains to improve outcomes for patients with TTP and to avoid relapse and long-term complications. New therapies, such as caplacizumab will play a key, though not clearly defined, role in treating this complex and morbid disease. Treatment algorithms will continue to be refined, as currently it is not clear which patient is more likely to relapse, which will not respond to standard treatment algorithms, and which is at greatest risk for recurrence: ultimately requiring more aggressive and expensive treatment methodologies.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Ara Metjian has received research funding and honoraria from: Ablynx/Sanofi, the makers of caplacizumab; Genentech, the makers of rituximab; Shire, the makers of rADAMTS13. Ashley Hanlon has no conflicts of interest to declare.
ORCID iD: Ara Metjian  https://orcid.org/0000-0001-9890-8639
https://orcid.org/0000-0001-9890-8639
Contributor Information
Ashley Hanlon, Duke University, Durham, NC, USA.
Ara Metjian, Duke University, Box #3422, 100 Trent Drive, Durham, NC 27708-0187, USA.
References
- 1. Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol 2016; 3: e237–e245. [DOI] [PubMed] [Google Scholar]
- 2. Scully M, Cataland S, Coppo P. et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost 2017; 15: 312–322. [DOI] [PubMed] [Google Scholar]
- 3. Page EE, Kremer Hovinga JA, Terrell DR, et al. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv 2017; 1: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood 2017; 129: 2836–2846. [DOI] [PubMed] [Google Scholar]
- 5. Dervenoulas J, Tsirigotis P, Bollas G, et al. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS): treatment outcome, relapses, prognostic factors. A single-center experience of 48 cases. Ann Hematol 2000; 79: 66–72. [DOI] [PubMed] [Google Scholar]
- 6. Deford CC, Reese JA, Schwartz LH, et al. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood 2013; 122: 2023–2029; quiz 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kremer Hovinga JA, Vesely SK, Terrell DR, et al. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood 2010; 115: 1500–1511; quiz 662. [DOI] [PubMed] [Google Scholar]
- 8. Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis study group. N Engl J Med 1991; 325: 393–397. [DOI] [PubMed] [Google Scholar]
- 9. Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158: 323–335. [DOI] [PubMed] [Google Scholar]
- 10. Gross M. One reason to get the hump. 2000. https://www.theguardian.com/science/2000/sep/14/technology1
- 11. Hamers-Casterman C, Atarhouch T, Muyldermans S, et al. Naturally occurring antibodies devoid of light chains. Nature 1993; 363: 446–448. [DOI] [PubMed] [Google Scholar]
- 12. Van Bockstaele F, Holz JB, Revets H. The development of nanobodies for therapeutic applications. Curr Opin Investig Drugs 2009; 10: 1212–1224. [PubMed] [Google Scholar]
- 13. Lauwereys M, Arbabi Ghahroudi M, Desmyter A, et al. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J 1998; 17: 3512–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desmyter A, Spinelli S, Payan F, et al. Three camelid VHH domains in complex with porcine pancreatic alpha-amylase. Inhibition and versatility of binding topology. J Biol Chem 2002; 277: 23645–23650. [DOI] [PubMed] [Google Scholar]
- 15. Conrath KE, Lauwereys M, Galleni M, et al. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrob Agents Chemother 2001; 45: 2807–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumoulin M, Last AM, Desmyter A, et al. A camelid antibody fragment inhibits the formation of amyloid fibrils by human lysozyme. Nature 2003; 424: 783–788. [DOI] [PubMed] [Google Scholar]
- 17. Coppieters K, Dreier T, Silence K, et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum 2006; 54: 1856–1866. [DOI] [PubMed] [Google Scholar]
- 18. Hmila I, Abdallah RB, Saerens D, et al. VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI’. Mol Immunol 2008; 45: 3847–3856. [DOI] [PubMed] [Google Scholar]
- 19. Cortez-Retamozo V, Lauwereys M, Hassanzadeh Gh G, et al. Efficient tumor targeting by single-domain antibody fragments of camels. Int J Cancer 2002; 98: 456–462. [DOI] [PubMed] [Google Scholar]
- 20. Cortez-Retamozo V, Backmann N, Senter PD, et al. Efficient cancer therapy with a Nanobody-based conjugate. Cancer Res. 2004; 64: 2853–2857. [DOI] [PubMed] [Google Scholar]
- 21. Ulrichts H, Silence K, Schoolmeester A, et al. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood 2011; 118: 757–765. [DOI] [PubMed] [Google Scholar]
- 22. van Loon JE, de Jaegere PP, Ulrichts H, et al. The in vitro effect of the new antithrombotic drug candidate ALX-0081 on blood samples of patients undergoing percutaneous coronary intervention. Thromb Haemost 2011; 106: 165–171. [DOI] [PubMed] [Google Scholar]
- 23. Muller O, Bartunek J, Hamilos M, et al. von Willebrand factor inhibition improves endothelial function in patients with stable angina. J Cardiovasc Transl Res 2013; 6: 364–370. [DOI] [PubMed] [Google Scholar]
- 24. Callewaert F, Roodt J, Ulrichts H, et al. Evaluation of efficacy and safety of the anti-VWF Nanobody ALX-0681 in a preclinical baboon model of acquired thrombotic thrombocytopenic purpura. Blood 2012; 120: 3603–3610. [DOI] [PubMed] [Google Scholar]
- 25. Peyvandi F, Callewaert F. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2016; 374: 2497–2498. [DOI] [PubMed] [Google Scholar]
- 26. Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost 2017; 15: 1448–1452. [DOI] [PubMed] [Google Scholar]
- 27. Scully M, Cataland SR, Peyvandi F, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2019; 380: 335–346. [DOI] [PubMed] [Google Scholar]
- 28. U. S. Food & Drug Administration. FDA approved caplacizumab-yhdp, 2019, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approved-caplacizumab-yhdp
- 29. Jasti S, Coyle T, Gentile T, et al. Rituximab as an adjunct to plasma exchange in TTP: a report of 12 cases and review of literature. J Clin Apher 2008; 23: 151–156. [DOI] [PubMed] [Google Scholar]
- 30. Ling HT, Field JJ, Blinder MA. Sustained response with rituximab in patients with thrombotic thrombocytopenic purpura: a report of 13 cases and review of the literature. Am J Hematol 2009; 84: 418–421. [DOI] [PubMed] [Google Scholar]
- 31. Page EE, Kremer Hovinga JA, Terrell DR, et al. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood 2016; 127: 3092–3094. [DOI] [PubMed] [Google Scholar]
- 32. de la Rubia J, Moscardo F, Gomez MJ, et al. Efficacy and safety of rituximab in adult patients with idiopathic relapsing or refractory thrombotic thrombocytopenic purpura: results of a Spanish multicenter study. Transfus Apher Sci 2010; 43: 299–303. [DOI] [PubMed] [Google Scholar]
- 33. Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol 2007; 136: 451–461. [DOI] [PubMed] [Google Scholar]
- 34. Froissart A, Buffet M, Veyradier A, et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French thrombotic microangiopathies reference center. Crit Care Med 2012; 40: 104–111. [DOI] [PubMed] [Google Scholar]
- 35. Scully M, McDonald V, Cavenagh J, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood 2011; 118: 1746–1753. [DOI] [PubMed] [Google Scholar]
- 36. Hertzberg MS, Koutts J. Oral cyclophosphamide for refractory or relapsing thrombotic thrombocytopenic purpura (TTP). Aust N Z J Med 1997; 27: 439. [DOI] [PubMed] [Google Scholar]
- 37. Ferrara F, Copia C, Annunziata M, et al. Vincristine as salvage treatment for refractory thrombotic thrombocytopenic purpura. Ann Hematol 1999; 78: 521–523. [DOI] [PubMed] [Google Scholar]
- 38. Bobbio-Pallavicini E, Porta C, Centurioni R, et al. Vincristine sulfate for the treatment of thrombotic thrombocytopenic purpura refractory to plasma-exchange. The Italian cooperative group for TTP. Eur J Haematol 1994; 52: 222–226. [DOI] [PubMed] [Google Scholar]
- 39. Mazzei C, Pepkowitz S, Klapper E, et al. Treatment of thrombotic thrombocytopenic purpura: a role for early vincristine administration. J Clin Apher 1998; 13: 20–22. [DOI] [PubMed] [Google Scholar]
- 40. Cataland SR, Jin M, Lin S, et al. Cyclosporin and plasma exchange in thrombotic thrombocytopenic purpura: long-term follow-up with serial analysis of ADAMTS13 activity. Br J Haematol 2007; 139: 486–493. [DOI] [PubMed] [Google Scholar]
- 41. Jhaveri KD, Scheuer A, Cohen J, et al. Treatment of refractory thrombotic thrombocytopenic purpura using multimodality therapy including splenectomy and cyclosporine. Transfus Apher Sci 2009; 41: 19–22. [DOI] [PubMed] [Google Scholar]
- 42. Pasquale D, Vidhya R, DaSilva K, et al. Chronic relapsing thrombotic thrombocytopenic purpura: role of therapy with cyclosporine. Am J Hematol 1998; 57: 57–61. [DOI] [PubMed] [Google Scholar]
- 43. Bachman WR, Brennan JK. Refractory thrombotic thrombocytopenic purpura treated with cyclosporine. Am J Hematol 1996; 51: 93–94. [DOI] [PubMed] [Google Scholar]
- 44. Kierdorf H, Maurin N, Heintz B. Cyclosporine for thrombotic thrombocytopenic purpura. Ann Intern Med 1993; 118: 987–988. [DOI] [PubMed] [Google Scholar]
- 45. Zheng X, Pallera AM, Goodnough LT, et al. Remission of chronic thrombotic thrombocytopenic purpura after treatment with cyclophosphamide and rituximab. Ann Intern Med 2003; 138: 105–108. [DOI] [PubMed] [Google Scholar]
- 46. Stein GY, Zeidman A, Fradin Z, et al. Treatment of resistant thrombotic thrombocytopenic purpura with rituximab and cyclophosphamide. Int J Hematol 2004; 80: 94–96. [DOI] [PubMed] [Google Scholar]
- 47. Beloncle F, Buffet M, Coindre JP, et al. Splenectomy and/or cyclophosphamide as salvage therapies in thrombotic thrombocytopenic purpura: the French TMA reference center experience. Transfusion 2012; 52: 2436–2444. [DOI] [PubMed] [Google Scholar]
- 48. Zappasodi P, Corso A, Castagnola C, et al. A successful combination of plasma exchange and intravenous cyclophosphamide in a patient with a refractory thrombotic thrombocytopenic purpura. Eur J Haematol 1999; 63: 278–279. [DOI] [PubMed] [Google Scholar]
- 49. Rottenstreich A, Hochberg-Klein S, Rund D, et al. The role of N-acetylcysteine in the treatment of thrombotic thrombocytopenic purpura. J Thromb Thrombolysis 2016; 41: 678–683. [DOI] [PubMed] [Google Scholar]
- 50. Shortt J, Oh DH, Opat SS. ADAMTS13 antibody depletion by bortezomib in thrombotic thrombocytopenic purpura. N Engl J Med 2013; 368: 90–92. [DOI] [PubMed] [Google Scholar]
- 51. Eskazan AE. Bortezomib therapy in patients with relapsed/refractory acquired thrombotic thrombocytopenic purpura. Ann Hematol 2016; 95: 1751–1756. [DOI] [PubMed] [Google Scholar]
- 52. Scully M, Knobl P, Kentouche K, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood 2017; 130: 2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chander DP, Loch MM, Cataland SR, et al. Caplacizumab therapy without plasma exchange for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2019; 381: 92–94. [DOI] [PubMed] [Google Scholar]