Abstract
Rationale
Vascular permeability is a hallmark of acute respiratory distress syndrome (ARDS) and ventilator-induced lung injury pathobiology; however, the mechanisms underlying this vascular dysregulation remain unclear, thereby impairing the development of desperately needed effective therapeutics. We have shown that sphingosine-1-phosphate (S1P) and 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720) analogues are useful tools for exploring vascular barrier regulation mechanisms.
Objective
To experimentally define the effects of FTY720 regioisomers on lung endothelial cell barrier regulation.
Methods
Specific barrier-regulatory receptor and kinase inhibitors were utilized to probe signaling mechanisms involved in FTY720 regioisomer-mediated human lung endothelial cell barrier responses (trans-endothelial electrical resistance, TER). Docking simulations with the S1P1 receptor were performed to further evaluate FTY720 regioisomer signaling.
Results
FTY720 regioisomers produced potent endothelial cell barrier disruption reflected by declines in TER alterations. Pharmacologic inhibition of Gi-coupled S1P receptors (S1P1, S1P2, S1P3) failed to alter FTY720 regioisomer-mediated barrier disruption; findings that were corroborated by docking simulations demonstrating FTY720 regiosomers were repelled from S1P1 docking, in contrast to strong S1P1 binding elicited by S1P. Inhibition of either the barrier-disrupting PAR-1 receptor, the VEGF receptor, Rho-kinase, MAPK, NFkB, or PI3K failed to alter FTY720 regioisomer-induced endothelial cell barrier disruption. While FTY720 regioisomers significantly increased protein phosphatase 2 (PP2A) activity, PP2A inhibitors failed to alter FTY720 regioisomer-induced endothelial cell barrier disruption.
Conclusions
Together, these results imply a vexing model of pulmonary vascular barrier dysregulation in response to FTY720-related compounds and highlight the need for further insights into mechanisms of vascular integrity required to promote the development of novel therapeutic tools to prevent or reverse the pulmonary vascular leak central to ARDS outcomes.
Keywords: FTY720, sphingosine 1-phosphate, regioisomer, endothelial, permeability, acute respiratory distress syndrome
Introduction
Sustained vascular barrier leak is a critical contributor to the morbidity and mortality observed in acute inflammatory diseases, such as acute respiratory distress syndrome (ARDS) and sepsis. For critically ill patients experiencing respiratory failure as a result of ARDS, an inflammatory lung syndrome with high mortality rate of 30–40%,1–3 reversal of the diminished pulmonary vascular barrier integrity is an important clinical goal. Increases in lung vascular leakage, inflammatory cell influx, and inflammatory cytokine expression are all hallmarks of ARDS pathology4,5; however, mechanisms underlying ARDS are still unclear and effective therapeutics targeting the vasculature are still needed.
Disruption of lung vascular endothelial cell (EC) monolayer integrity leads to respiratory failure due to flooding of interstitial and alveolar compartments with fluid, protein, and inflammatory cells.6 Effective therapeutic agents to prevent or reverse inflammation-mediated vascular barrier leak are lacking.7 We previously demonstrated the potent barrier-enhancing properties of the endogenous phospholipid sphingosine 1-phosphate (S1P), the related pharmaceutical agent 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720), and several novel synthetic analogs of FTY720 including (S)-FTY720-phosphonate (or TySIPonate)8–11 in models of inflammatory lung injury. S1P, a sphingolipid produced by multiple cell types, initiates a series of downstream effects through the ligation of the Gi-coupled S1P1 receptor (S1P1), culminating in enhancement of the EC cortical actin ring, improved cell–cell and cell–matrix interactions, and increased barrier function in vitro.10,12,13 Subsequently, the S1P1 receptor has also been proven to be protective in in vivo murine models of acute lung injury.14 The pharmaceutical agent FTY720, a structural analog of S1P, potently enhances lung EC barrier function via Gi-coupled receptor signaling.9,15 Phosphonate and enephosphonate analogs of FTY720, such as TySIPonate, demonstrate similar but not identical barrier regulatory properties to S1P and FTY720.8 Oxazolo-oxazole derivatives of FTY720 reduce EC permeability induced by LPS or TNFα in vitro.16 Moreover, S1P, FTY720, and TySIPonate attenuate lipopolysaccharide (LPS)-induced preclinical lung injury.8,17,18 More recent studies19 examined additional FTY720 analogs, where TER and labeled dextran studies demonstrate that (R)-methoxy-, fluoro-, and β-glucuronide FTY720 analogs display in vitro barrier-enhancing properties comparable or superior to FTY720 and S1P due to S1P1-dependent receptor ligation. Thus, S1P, FTY720, and various analogs (such as TySIPonate) represent a novel class of agents that are potential therapeutic options for addressing the increased vascular permeability observed in inflammatory lung diseases such as ARDS.
However, both S1P and FTY720 exhibit specific characteristics that imply limited therapeutic utility in ARDS patients. The endogenous ligand S1P exhibits a limited therapeutic window with higher concentrations (>5 µM) increasing in vitro lung EC permeability,8 and intratracheal S1P administration, producing pulmonary edema in vivo via ligation of the abundant S1P3 receptor on epithelium. This results in disruption of the epithelial barrier20 and produces contraction of human airway smooth muscle cells,21 increased airway hyper-responsiveness in mice,22 and cardiac toxicity via S1P3 activation in the heart.23,24 While FTY720 is an FDA-approved therapy for multiple sclerosis due to its effectiveness as an immunosuppressant via down-regulated S1P1 signaling,25,26 this immunosuppressive effect may be harmful in critically ill patients with sepsis or other infectious disease processes. In addition, multiple studies have recently demonstrated detrimental effects on vascular permeability of higher concentrations and prolonged exposure to FTY720.27 Higher concentrations of FTY720 produce tissue edema28 and exacerbate both ventilator-induced lung injury29 and bleomycin-induced lung injury11,27 in preclinical models. The barrier-disruptive effects of high concentrations of FTY720 are likely mediated through down-regulation of endothelial S1P1 expression and subsequent increased permeability in the absence of S1P1 ligation and signaling.11,28 TySIPonate, unlike FTY720, does not down-regulate S1P1 expression and, therefore, remains highly protective in preclinical models of bleomycin-induced lung injury.11
Another concern when considering this class of sphingolipids as potential therapeutic agents in the critically ill is that subtle changes in the chemical structure produce dramatically paradoxical effects. In contrast to vascular protective effects of (R)-methoxy-FTY720, the (S)-methoxy-FTY720 enantiomer disrupts lung EC barrier integrity in association with actin stress fiber formation and robust intracellular Ca++ release but independent of myosin light chain or ERK phosphorylation.19 More importantly, the (S)- and (R)-FTY720 regioisomers or positional isomer analogs,8 analogs of FTY720 with the same molecular formula but with altered functional group positions, exhibit exactly opposing effects on EC barrier regulation. At modest concentrations, FTY720 is EC barrier-enhancing, whereas the (S)- and (R)-FTY720 regioisomers potently disrupt EC barrier integrity as monitored by TER and flux of labeled dextran.8
In the current study, we sought to enhance understanding of the mechanistic basis for (S)- and (R)-FTY720 regioisomer-mediated vascular permeability and characterize the mechanisms by which this novel class of agents modulate EC permeability.
Methods
Synthesis of FTY70 regioisomers
Regioisomers of FTY720 (S enantiomer or 3S; R enantiomer or 3R) were synthesized as previously described.30
Reagents
S1P was purchased from Sigma-Aldrich (St. Louis, MO), and FTY720 was generously provided by Novartis (Basel, Switzerland) or purchased from AbMole BioScience (Kowloon, Hong Kong, China) for phosphatase assays. S1P1 inverse agonist SB649146 was generously provided by Glaxo Smith Kline (King of Prussia, PA). NFkB, okadaic acid, S1P2, and S1P3 inhibitors were purchased from Cayman Chemicals (Ann Arbor, MI). MAPK, PI3K, PKC, Rho-kinase, and VEGFR2 inhibitors, in addition to pertussis toxin (PTX), were purchased from EMD Millipore Corporation (Billerica, MA). PAR-1 neutralizing antibody and the Calyculin A inhibitor were purchased from Santa Cruz Biotechnology (Dallas, TX). PP2A catalytic subunit (PP2Ac, Cayman Chemical), malachite green oxalate salt (Sigma-Aldrich, St. Louis, MO), and threonine-phosphopeptide (K-R-pT-I-R-R, pT, New England Peptide LLC, Gardner, MA) were obtained for cell-free assays. All other reagents were obtained from Sigma-Aldrich, unless otherwise noted.
Cell culture
Human pulmonary artery endothelial cells (HPAEC or ECs) were obtained from Lonza (Walkersville, MD) and were cultured as described previously12 in the manufacturer’s recommended endothelial growth medium-2 (EGM-2). Cells were grown at 37℃ in a 5% CO2 incubator, and passages 6 to 9 were used for experiments. Media was changed one day before experimentation.
Trans-endothelial electrical resistance
Lung EC were grown to confluence in polycarbonate wells containing evaporated gold microelectrodes, and trans-endothelial electrical resistance (TER) monolayer measurements were performed using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as described previously in detail.10 TER values from each microelectrode were pooled as discrete time points and plotted versus time as the mean ± S.E.M.
Cell-free PP2A assay
Recombinant PP2A catalytic subunit was incubated 30 min at 4℃ in 4-nitrophenyl-phosphate (pNPP) buffer (50 mM Tris-HCl, pH 7.0, 0.1 mM CaCl2) plus 5 μM of agonist. Reactions proceeded 10 min at 30℃ with intermittent shaking in the presence of pT substrate described above. Reactions were stopped by placing on ice, with aliquots evaluated by malachite green assay relative to freshly prepared phosphate standards and read at 630 nm (Multiskan Spectrum plate reader, Thermo Scientific, Pittsburgh, PA) as detailed in Lek et al.31 PP2A activity is expressed as pmol phosphate/min/µg protein normalized to the methanol vehicle as baseline.
Statistical analysis
For all experiments (n = 3 or more), values are shown as the mean ± SEM, and data were analyzed using standard Student’s t test or two-way ANOVA. Significance in all cases was defined at p < 0.05.
S1P1 receptor docking simulations
The Molecular Operating Environment® (MOE®) software was used32 to perform ∼300 simulations. Single crystal X-ray data of the S1P1 receptor (PDB file code 3V2Y in MOE®) provided coordinates for the S1P1 receptor co-crystallized with the S1P1 antagonist W146 at 2.8 Å resolution.33 With W146 being localized within the active site of S1P1, the antagonist bound in 3V2Y was substituted with the substrates to be simulated, and the latter were placed into the active site of S1P1 without overlapping any receptor components. The placement phase used in the docking experiments was the default “Triangle Matcher” method with London dG scoring. The refinement method was set to the Induced Fit receptor model for a more realistic ligand–receptor interaction with GBVI/WSA dG scoring. Each experiment ran 100 ligand poses pre-set within the active site of S1P1, and the 25 energetically most favorable poses were saved. After running all simulations, the results were sorted in ascending order of the energy values of each conformer, and the lowest energy ligand–receptor pose (tightest binding) of each substrate was chosen, displayed in the receptor’s active site, and evaluated for its structural and conformational features. The molecular surface of the receptor’s active site was visualized for structural and steric analysis.
Results
Evaluation of G protein-coupled S1P receptor involvement in (S)- and (R)-FTY720 regioisomer barrier disruption
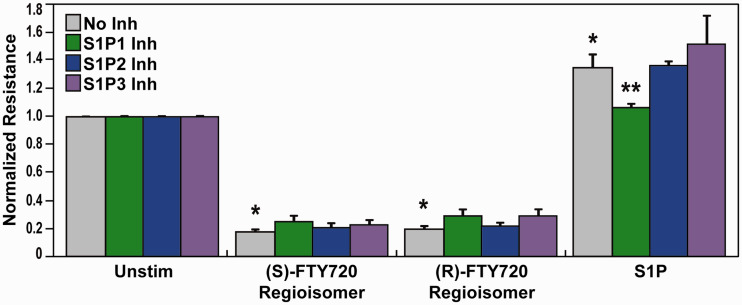
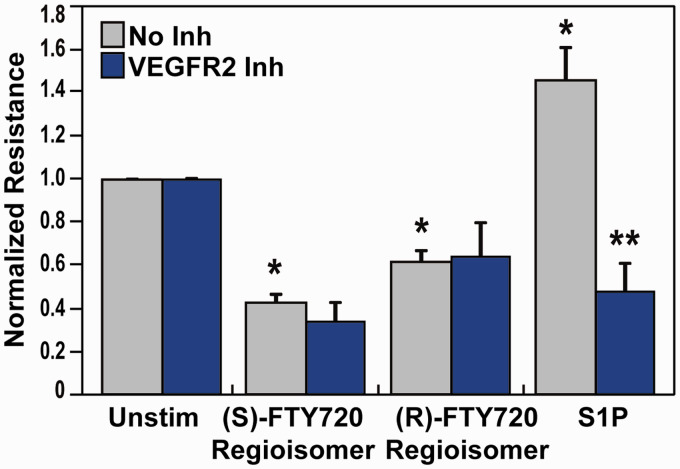
The (S)- and (R)-FTY720 regioisomers produced potent and dose-dependent barrier disruption in lung ECs as determined by TER, a highly sensitive in vitro measure of permeability.8 Since S1P receptors are critical modulators of barrier regulation by S1P and other related compounds,34,35 we explored the role of S1P receptors in barrier disruption by the (S)- and (R)-FTY720 regioisomers. Pretreatment of lung ECs with SB649146, an inverse agonist of the S1P1 receptor,36 attenuated S1P-induced barrier enhancement (Fig. 1) but did not ameliorate FTY720 regioisomer-induced barrier disruption as monitored by TER (Fig. 1). Pretreatment with either JTE-013, a selective S1P2 receptor antagonist,37 or BML-241, a selective S1P3 receptor antagonist,38 also did not significantly block TER permeability induced by the EC barrier disruptive (S)- and (R)-FTY720 regioisomers (Fig. 1).
Fig. 1.
Effect of S1P receptor inhibition on (S)- and (R)-FTY720 regioisomer-induced barrier disruption. HPAEC were plated on gold microelectrodes for TER measurements as described in the Methods section. Bar graphs depict pooled TER data from HPAEC pre-treated for 1 h with no inhibitor (grey), 10 µM SB649146 (an inverse agonist of the S1P1 receptor, green), JTE-013 (a selective S1P2 receptor antagonist, blue), or BML-241 (a selective S1P3 receptor antagonist, purple), then stimulated with (S)- FTY720 regioisomer (10 µM), (R)-FTY720 regioisomer (10 µM), or S1P (1 µM) as indicated. The data are expressed as change in TER, compared to normalized unstimulated or inhibitor only controls, at 6 h ((S)- and (R)-FTY720 regioisomers) or 10 min (S1P) after agonist stimulation (±SEM). Normalized resistance values over 1 indicate EC barrier enhancement. Normalized resistance values under 1 indicate EC barrier disruption. n = 3-4 independent experiments per condition; *p < 0.01 agonist alone versus unstimulated cells; p < 0.05 agonist with inhibitor pretreatment versus agonist alone.
S1P: sphingosine-1-phosphate; FTY720: 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol.
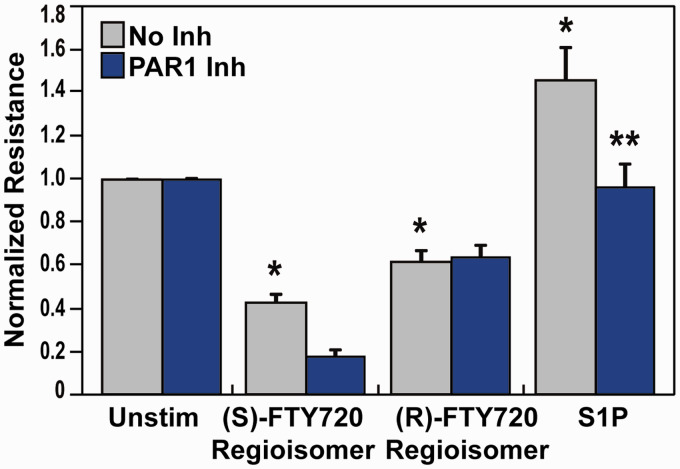
We and others have shown that multiple receptors, such as PAR-1 and APC, are linked to S1P signaling.39–42 Activated protein C (APC) regulates EC barrier integrity via endothelial protein C receptor (EPCR). We have previously demonstrated that APC binding to EPCR cleaves the PAR-1 receptor, thereby activating PAR-1 with subsequent transactivation of S1P1.39–42 Exploration of the role of PAR-1 receptors in barrier disruption by the (S)- and (R)-FTY720 regioisomers revealed that a PAR-1 blocking antibody (Fig. 2) attenuated S1P-induced barrier enhancement, due to PAR1-S1P1 transactivation,39–42 but failed to alter FTY720 regioisomer-induced barrier disruption.
Fig. 2.
Effect of PAR-1 receptor inhibition on (S)- and (R)-FTY720 regioisomer-induced barrier disruption. HPAEC were plated on gold microelectrodes for TER measurements as described in the Methods section. Bar graphs depict pooled TER data from HPAEC pre-treated for 1 h with no inhibitor (grey) or PAR-1 blocking antibody (20 µg/ml, blue), then stimulated with (S)- FTY720 regioisomer (10 µM), (R)-FTY720 regioisomer (10 µM), or S1P (1 µM) as indicated. Data are expressed as change in TER, compared to normalized unstimulated or inhibitor only controls, at 6 h ((S)- and (R)-FTY720 regioisomers) or 10 min (S1P) after agonist stimulation (±S.E.M.). Normalized resistance values over 1 indicate EC barrier enhancement. Normalized resistance values under 1 indicate EC barrier disruption. n = 3 independent experiments per condition; *p < 0.01 agonist alone versus unstimulated cells; p < 0.05 agonist with inhibitor pretreatment versus agonist alone.
S1P: sphingosine-1-phosphate; FTY720: 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol.
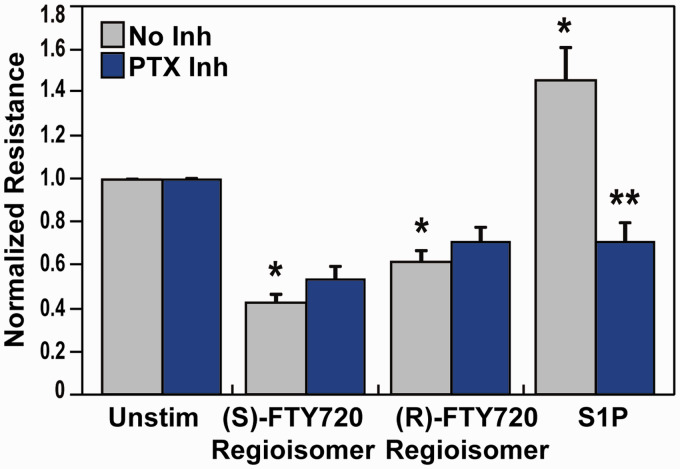
Similarly, preincubation with the G protein-coupled receptor inhibitor PTX produced prominent attenuation S1P-induced barrier enhancement as we previously reported10 but failed to ameliorate TER permeability induced by the FTY720 regioisomers (Fig. 3). These data demonstrate the absence of typical S1P1 or related sphingolipid receptors (S1P1, S1P2, S1P3, PAR-1, G protein-coupled receptors) involvement in FTY720 regioisomer-mediate barrier signaling, indicating subtle FTY720 structural changes significantly alter binding in ECs.
Fig. 3.
Effect of G protein inhibition on (S)- and (R)-FTY720 regioisomer-induced barrier disruption. HPAEC were plated on gold microelectrodes for TER measurements as described in the Methods section. Bar graphs depict pooled TER data from HPAEC pre-treated for 4 h with no inhibitor (grey) or pertussis toxin (PTX, 100 ng/ml, blue), then stimulated with (S)- FTY720 regioisomer (10 µM), (R)-FTY720 regioisomer (10 µM), or S1P (1 µM) as indicated. The data are expressed as change in TER, compared to normalized unstimulated or inhibitor only controls, at 6 h ((S)- and (R)-FTY720 regioisomers) or 10 min (S1P) after agonist stimulation (±S.E.M.). Normalized resistance values over 1 indicate barrier enhancement. Normalized resistance values under 1 indicate barrier disruption. n = 5 independent experiments per condition; *p < 0.01 agonist alone versus unstimulated cells; p < 0.01 agonist with inhibitor pretreatment versus agonist alone.
S1P: sphingosine-1-phosphate; FTY720: 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol.
FTY720 regioisomer docking simulations with S1P1
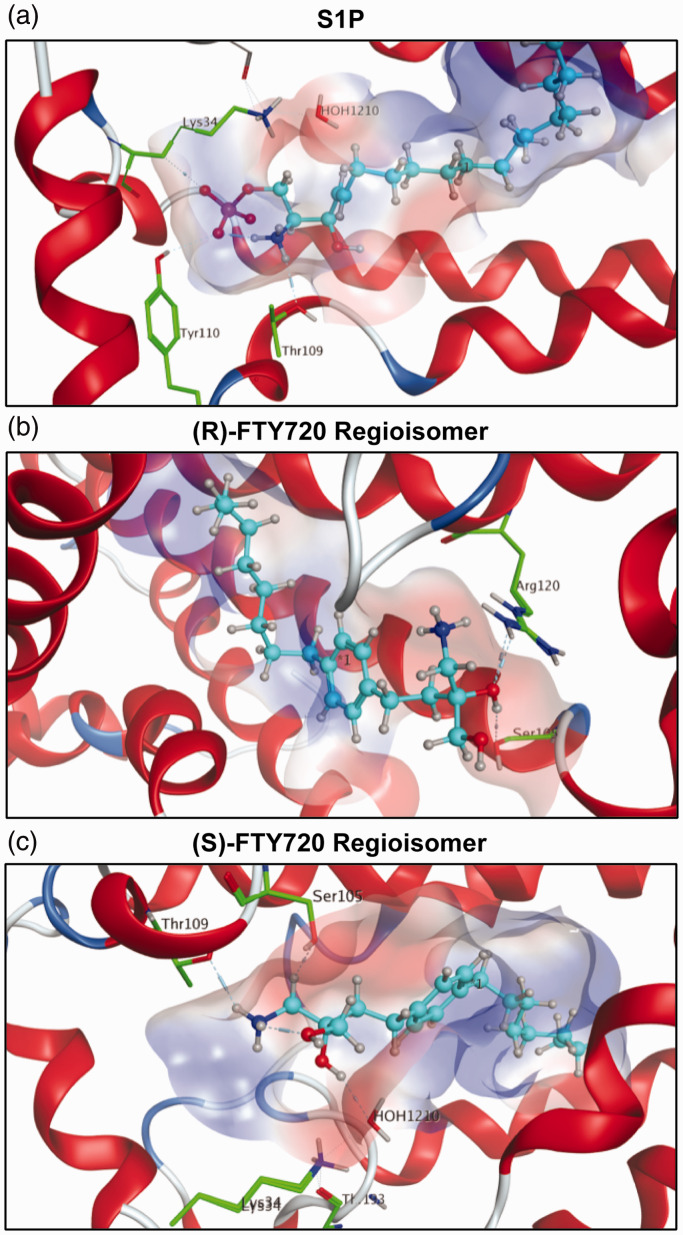
The binding behavior of endogenous S1P and synthetic S1P analogues was examined utilizing molecular dynamic docking simulations. As S1P is the endogenous substrate for S1P1, high binding affinity with Ki values from 7.9 to 9.4, this was defined as the standard to which the synthetic analogues and their optimized conformations in the active site of S1P1 were compared43 with a calculated potential energy of (–351 kcal/mol). The phosphate of the head group is decisive in fixing S1P within the receptor involving residues Lys34 and Tyr110. Additionally, residue Thr109 further stabilizes the head group of S1P within the active site’s cavity of S1P1 (Fig. 4a). The corresponding Ki values for the unphosphorylated (S)- and (R)-FTY720 regioisomers (–1–2 kcal/mol) indicate very weak affinity for S1P1 in the absence of phosphorylation. The binding energies of both regioisomers were similar in value to each other: −1.92 kcal/mol for the (R)-FTY720 regioisomer and −0.81 kcal/mol for the (S)-FTY720 regioisomer, suggesting extremely weak binding affinity, if any, for the unphosphorylated regioisomers to S1P1. The 3D visualization of the (S)- and (R)-FTY720 regioisomer simulations displays few intermolecular interactions with the receptor’s active site. A “bent” alkyl chain of (R)-FTY720 regioisomer suggests high steric strain contributing to the poor affinity of unphosphorylated (R)-FTY720 regioisomer to S1P1 (Fig. 4b). In the case of (S)-FTY720 regioisomer, only the protonated amine of the substrate exhibits a hydrogen bond to residue Thr109 (Fig. 4c), again supporting the low score achieved when docked in the active site of S1P1. Both simple potential energy analysis and 3D visualization again elucidate that the presence of the negatively-charged phosphate moiety is critical for efficient binding.
Fig. 4.
S1P1 docking simulations with S1P and (S)- and (R)-FTY720 regioisomers. In all visualizations, the substrate is highlighted in light blue. The polarity of the active site’s cavity is indicated by colors: red regions define hydrophilicity, blue areas mark lipophilicity. Receptor residues engaging in interactions with the substrate are highlighted in green with emphasis of respective hydrogen bonds. (a) S1P in its most stable conformer within the active site of S1P1 with a calculated relative energy of −351.72 kcal/mol. The phosphate moiety of S1P is largely responsible for the strong binding, as one of the negatively charged oxygens of the phosphoester acts as a hydrogen bond acceptor for residue Tyr110, and one forms a hydrogen bond with residue Lys34. The protonated amino group of S1P donates to another hydrogen bond with Thr109 and forms an intramolecular hydrogen bond with the phosphate oxygen that binds to Tyr110. The calculations suggest that residues Lys34, Thr109, and Tyr110 lock the hydrophilic phosphate head group of S1P into position, while the hydrophobic tail of S1P is held between the seven lipophilic transmembrane helices of S1P1, well isolated from polar media. The hydroxyl group at the C3 position fails to display interactions with the active site of S1P1. (b) The unphosphorylated FTY720 regioisomer 3R is depicted in the active site of S1P1 in its energetically most favorable conformer, its energy being calculated as −1.92 kcal/mol, indicating no favorable S1P1 binding. Aside from the substrate’s C3 hydroxyl group forming hydrogen bonds with residues Ser105 and Arg120, the docking simulation did not show any other interactions. (c) The unphosphorylated FTY720 regioisomer 3S is depicted in its calculated energetically most stable conformation in the active site of S1P1 with a relative energetic value of −0.81 kcal/mol, which also suggests poor binding to S1P1 in comparison to S1P. The docking simulation of unphosphorylated 3S to S1P1 shows hydrogen binding of the protonated amine of 3S to residue Thr109. In the calculations, the hydroxyl group at C3 further coordinates to a water molecule which in turn hydrogen bonds to residue Lys34. No direct interaction of 3S with any residues except for Thr109 could be observed in the calculations, explaining the poor affinity of the unphosphorylated substrate to S1P1.
S1P: sphingosine-1-phosphate; FTY720: 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol.
Effect of inhibitors of barrier-regulatory pathways on (S)- and (R)-FTY720 regioisomer-mediated barrier disruption
A well-known modulator of EC barrier permeability is the VEGF/VEGFR2 receptor pathway,44–47 which has been linked to the S1P1 receptor via crosstalk.48,49 We next explored the potential link between VEGF receptors and EC barrier disruption evoked by (S)- and (R)-FTY720 regioisomers. Preincubation of lung EC with a VEGFR2 inhibitor (Fig. 5) resulted in attenuation of S1P-induced barrier enhancement (likely due to VEGFR2-S1P1 crossactivation)48,49 but did not ameliorate (S)- or (R)-FTY720 regioisomer-induced barrier disruption.
Fig. 5.
Effect of VEGFR2 receptor inhibition on (S)- and (R)-FTY720 regioisomer-induced barrier disruption. HPAEC were plated on gold microelectrodes for TER measurements as described in the Methods section. Bar graphs depict pooled TER data from HPAEC pre-treated for 1 h with no inhibitor (grey) or a VEGFR2 kinase IV inhibitor (1 µM, blue), then stimulated with (S)- FTY720 regioisomer (10 µM), (R)-FTY720 regioisomer (10 µM), or S1P (1 µM) as indicated. The data are expressed as change in TER, compared to normalized unstimulated or inhibitor only controls, at 6 h ((S)- and (R)-FTY720 regioisomers) or 10 min (S1P) after agonist stimulation (±S.E.M.). Normalized resistance values over 1 indicate barrier enhancement. Normalized resistance values under 1 indicate barrier disruption. n = 3 independent experiments per condition; *p < 0.01 agonist alone versus unstimulated cells; p < 0.05 agonist with inhibitor pretreatment versus agonist alone.
S1P: sphingosine-1-phosphate; FTY720: 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol.
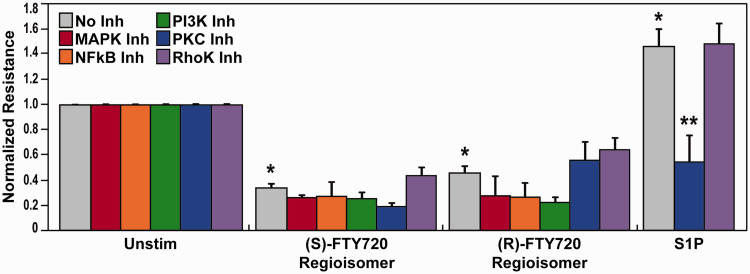
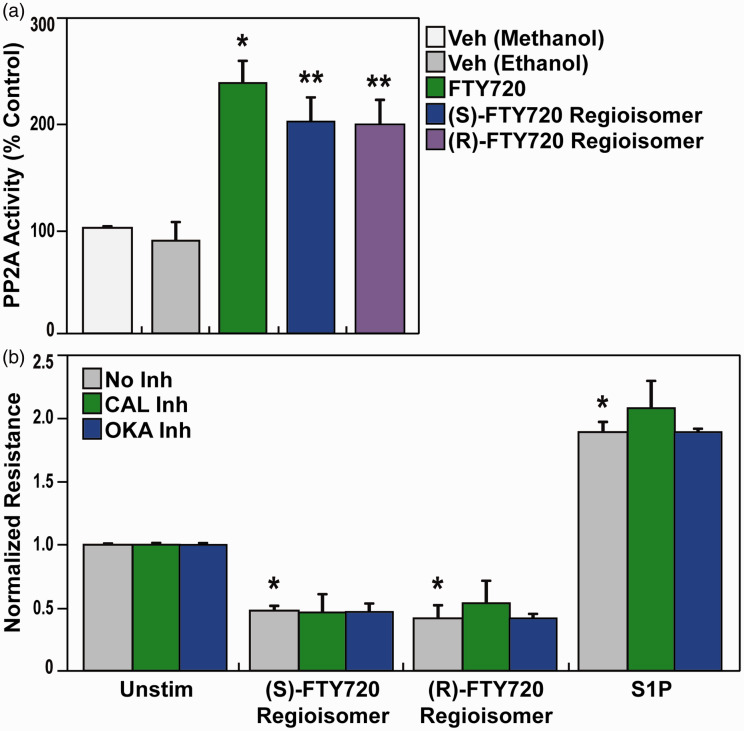
Pretreatment with various inhibitors of EC barrier-regulatory signaling pathways such as MAPK, NFkB, PI3K, PKC, or Rho-kinase (Fig. 6) failed to attenuate (S)- and (R)-FTY720 regioisomers-evoked permeability. Based on reports of FTY720 effects on phosphatase 2A activity,50–52 we next evaluated (S)- and (R)-FTY720 regioisomer-induced PP2A activity responses. Both FTY720 regioisomers were observed to stimulate PP2A activity (Fig. 7a); however, neither treatment with calyculin A (PP1/2A-C inhibitor) or okadaic acid (PP2A inhibitor) (Fig. 7b) affected the rapid (S)- and (R)-FTY720 regioisomers-induced barrier disruption in lung ECs as monitored by TER. Thus, these studies, designed to mechanistically examine FTY720 regioisomer-mediated barrier disruption, failed to resolve the mechanism of FTY720 regioisomer-induced loss of EC barrier integrity.
Fig. 6.
Involvement of various EC barrier-regulatory signaling pathways in (S)- and (R)-FTY720 regioisomer-induced barrier disruption. HPAEC were plated on gold microelectrodes for TER measurements as described in the Methods section. Bar graphs depict pooled TER data from HPAEC pre-treated for 1 h with no inhibitor (grey), PD98059 (MAPK inhibitor, (25 µM, red), CAY10512 (NFkB inhibitor, 4 µM, orange), LY294002 (PI3K inhibitor, 25 µM, green), Gö 6983 (PKC α, β, γ, δ, ζ inhibitor, 1 µM, blue), or Y-27632 (Rho-kinase inhibitor, 10 µM, purple), then stimulated with (S)- FTY720 regioisomer (10 µM), (R)-FTY720 regioisomer (10 µM), or S1P (1 µM) as indicated. The data are expressed as change in TER, compared to normalized unstimulated or inhibitor only controls, at 6 h ((S)- and (R)-FTY720 regioisomers) or 10 min (S1P) after agonist stimulation (±SEM). Normalized resistance values over 1 indicate EC barrier enhancement. Normalized resistance values under 1 indicate EC barrier disruption. n = 3–5 independent experiments per condition; *p < 0.01 agonist alone versus unstimulated cells; p < 0.01 agonist with inhibitor pretreatment versus agonist alone.
S1P: sphingosine-1-phosphate; FTY720: 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol.
Fig. 7.
Effect of (S)- and (R)-FTY720 regioisomers on PP2A activity and induced barrier disruption. (a) Bar graphs depict pooled PP2A activity data quantified as the amount of free PO4 cleaved from pT substrate relative to a standard curve for methanol vehicle (white), ethanol vehicle (grey), FTY720 (5 µM) (green), (S)-FTY720 regioisomer (5 µM) (blue), or (R)-FTY720 regioisomer (5 µM) (purple) (±S.D.). n = 3 independent experiments per condition; *p < 0.05 agonist versus ethanol vehicle alone, p < 0.05 agonist versus methanol vehicle alone. (b) HPAEC were plated on gold microelectrodes for TER measurements as described in the Methods section. Bar graphs depict pooled TER data from HPAEC pre-treated for 1 h with no inhibitor (grey), CAL inhibitor (calyculin A, PP1/2A-C inhibitor, 2 nM, green), or OKA inhibitor (okadaic acid, PP2A inhibitor, 50 nM, blue), then stimulated with (S)-FTY720 regioisomer (10 µM), (R)-FTY720 regioisomer (10 µM), or S1P (1 µM) as indicated. The data are expressed as change in TER, compared to normalized unstimulated or inhibitor only controls, at 4 h ((S)- and (R)-FTY720 regioisomers) or 10 min (S1P) after agonist stimulation (±S.E.M.). Normalized resistance values over 1 indicate EC barrier enhancement. Normalized resistance values under 1 indicate EC barrier disruption. n = 3 independent experiments per condition; *p < 0.01 agonist alone versus unstimulated cells.
S1P: sphingosine-1-phosphate; FTY720: 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol.
Discussion
We have attempted to further elucidate the role of FTY720 regioisomers on lung injury, specifically on in vitro pulmonary vascular barrier function and related signaling. Pulmonary vascular leak is a vital therapeutic target in acute inflammatory diseases such as ARDS, a condition afflicting an estimated 400,000 people annually with morbidity rates exceeding 30%.2 To date, there are no effective treatments that target the underlying pulmonary vascular leak that characterizes this syndrome.7 In prior studies, we identified S1P10,17,18 and the related pharmacologic agent, FTY720,9,17,53 as potent pulmonary vascular barrier-enhancing agents both in vitro and in vivo. However, as a therapy, S1P suffers from the potential to produce adverse effects, including cardiac toxicity, pulmonary edema at higher doses, and airway hyperresponsiveness.8,20,22–24 Similarly, FTY720 has the potential to induce bradycardia, immunosuppression, and increased vascular leak at higher doses,8,24–26,54 with preclinical models confirming detrimental effects on vascular permeability at higher concentrations.11,27–29
Multiple groups have synthesized derivatives of FTY720 in order to identify possible antiangiogenic agents55 that exhibit pro-apoptotic effects,56 S1P receptor affinity, and lymphopenia induction.16,24,57–62 In contrast, we have chosen to focus on their value as potential barrier-regulatory therapeutic agents.8,11,19,63 Given the potential therapeutic limitations of S1P and FTY720 in patients with ARDS, this study looked to further characterize FTY720 regioisomers for insight into vascular leak and to subsequently better understand the mechanisms by which novel class of agents can be created to better modulate permeability.
Unlike S1P and FTY720, the (S)- and (R)-FTY720 regioisomers produce potent and dose-dependent barrier disruption in lung ECs8 via poorly understood atypical signaling cascades that do not result in actin stress fiber formation, MLC phosphorylation, ERK phosphorylation, or intracellular calcium release.8 Our studies demonstrate that FTY720 regioisomer-mediated permeability in lung ECs is not altered by attenuation of Gi-coupled receptors, S1P1, S1P2, or S1P3 (Figs 1 and 3), findings supported by S1P1 docking simulations (Fig. 4). Inhibition of the PAR-1 receptor (Fig. 2) or the VEGF receptor (Fig. 5) also did not alter FTY720 regioisomer-induced barrier disruption. Key cytoskeletal regulatory signaling pathways involved in lung EC barrier disruption were not activated during FTY720 regioisomer permeability including Rho-kinase, MAPK, NFkB, or PI3K pathways (Fig. 6). While pretreatment with PP2A inhibitors (Fig. 7b) did not affect rapid FTY720 regioisomers permeability in lung ECs, the (S)- and (R)-FTY720 regioisomers significantly enhanced PP2A activity (Fig. 7a) as previously reported for Parkinson’s disease models using MN9D dopaminergic cells.64–66
In summary, our results demonstrate that subtle FTY720 structural changes significantly alter EC barrier-regulatory properties, similar to our previous reports.19 Despite structural similarity to the parent FTY720 compound, the unphosphorylated (S)- and (R)-FTY720 regioisomers increase EC vascular permeability via an unknown rapid, non-S1P receptor-mediated mechanism that does not involve classic cytoskeletal regulators but whose rapidity suggests a receptor-mediated effect. Given the evolutionary association, sphingolipid receptors, cannabinoid, and other G protein-coupled receptors may be considered as novel (S)- and (R)-FTY720 regioisomer receptors and studies to explore these possibilities are underway. In addition to ligating a potentially novel class of receptors, FTY720 regioisomers are highly hydrophobic, indicating that the highly hydrophobic tail may penetrate the cell membrane without a requirement for receptor binding, to induce rapid increases in EC permeability. Our results with PP2A activity are consistent with this possibility, although we failed to identify evidence of PP2A involvement or cytoskeletal signaling. Thus, our mechanistic insights into FTY720-mediated barrier disruptive mechanisms remain limited. Minor structural alterations in S1P/FTY720 should be evaluated for their impact on vascular permeability regulation while designing novel agents to treat inflammatory disorders such as ARDS.
Highlights
There is an unmet medical need for novel therapies in ARDS
This study examines the effects of FTY720 regioisomers on vascular permeability
FTY720 regioisomers do not utilize S1P receptor signaling pathways to alter vascular permeability
These mechanistic insights may assist in the development of novel ARDS therapeutic strategies.
Acknowledgments
We would like to dedicate this research article to the late Dr. Robert Bittman. Dr. Bittman was a dear friend and exceptional lipid biochemist without whose insight, enthusiasm, and friendship this research would have never been completed.
Conflict of interest
Joe G.N. Garcia, MD is the founder, CEO, and majority shareholder of Aqualung Therapeutics, Corp., which does not have any relevant conflicts of interest. All other authors have no relevant conflicts of interest.
Funding
This work was supported by NIH grants P01-HL58064 and HL126609 (JGNG); Doyle/Hoy/Perez Research, El Paso Community Foundation and Paso Del Norte Health Foundation (RGP), and access to MOE® was supported by NINDS grant RO1NS091238 (RP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ORCID iD
Joe G. N. Garcia https://orcid.org/0000-0002-6934-0420
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–1349. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998; 157: 294–323. [DOI] [PubMed] [Google Scholar]
- 5.Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med 1993; 21: 131–143. [DOI] [PubMed] [Google Scholar]
- 6.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 2001; 91: 1487–1500. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007; 369: 1553–1564. [DOI] [PubMed] [Google Scholar]
- 8.Camp SM, Bittman R, Chiang ET, et al. Synthetic analogs of FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] differentially regulate pulmonary vascular permeability in vivo and in vitro. J Pharmacol Exp Ther 2009; 331: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudek SM, Camp SM, Chiang ET, et al. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 2007; 19: 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia JG, Liu F, Verin AD, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Investig 2001; 108: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Sammani S, Moreno-Vinasco L, et al. FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury. Crit Care Med 2014; 42: e189–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudek SM, Jacobson JR, Chiang ET, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 2004; 279: 24692–24700. [DOI] [PubMed] [Google Scholar]
- 13.Shikata Y, Birukov KG, Garcia JG. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol 2003; 94: 1193–1203. [DOI] [PubMed] [Google Scholar]
- 14.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol 2010; 43: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Chiang ET, Simmons JT, et al. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. Eur Respir J 2011; 38: 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imeri F, Fallegger D, Zivkovic A, et al. Novel oxazolo-oxazole derivatives of FTY720 reduce endothelial cell permeability, immune cell chemotaxis and symptoms of experimental autoimmune encephalomyelitis in mice. Neuropharmacology 2014; 85: 314–327. [DOI] [PubMed] [Google Scholar]
- 17.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004; 169: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 18.McVerry BJ, Peng X, Hassoun PM, et al. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 2004; 170: 987–993. [DOI] [PubMed] [Google Scholar]
- 19.Camp SM, Chiang ET, Sun C, et al. Pulmonary endothelial cell barrier enhancement by novel FTY720 analogs: methoxy-FTY720, fluoro-FTY720, and beta-glucuronide-FTY720. Chem Phys Lipids 2015; 191: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gon Y, Wood MR, Kiosses WB, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA 2005; 102: 9270–9275. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Rosenfeldt HM, Amrani Y, Watterson KR, et al. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J 2003; 17: 1789–1799. [DOI] [PubMed] [Google Scholar]
- 22.Roviezzo F, Di Lorenzo A, Bucci M, et al. Sphingosine-1-phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am J Respir Cell Mol Biol 2007; 36: 757–762. [DOI] [PubMed] [Google Scholar]
- 23.Hale JJ, Doherty G, Toth L, et al. Selecting against S1P3 enhances the acute cardiovascular tolerability of 3-(N-benzyl)aminopropylphosphonic acid S1P receptor agonists. Bioorg Med Chemi Lett 2004; 14: 3501–3505. [DOI] [PubMed] [Google Scholar]
- 24.Forrest M, Sun SY, Hajdu R, et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther 2004; 309: 758–768. [DOI] [PubMed] [Google Scholar]
- 25.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006; 355: 1124–1140. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med 2012; 366: 339–347. [DOI] [PubMed] [Google Scholar]
- 27.Shea BS, Brooks SF, Fontaine BA, et al. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol 2010; 43: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oo ML, Chang SH, Thangada S, et al. Engagement of S1P-degradative mechanisms leads to vascular leak in mice. J Clin Investig 2011; 121: 2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller HC, Hocke AC, Hellwig K, et al. The sphingosine-1 phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulm Pharmacol Ther 2011; 24: 377–385. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Sun C, Valentine WJ, et al. Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J Org Chem 2009; 74: 3192–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lek S, Vargas-Medrano J, Villanueva E, et al. Recombinant alpha- beta- and gamma-synucleins stimulate protein phosphatase 2A catalytic subunit activity in cell free assays. J Vis Exp 2017; 126: e55361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Website CCGO, www.chemcomp.com/Products.htm (accessed 18 March 2019).
- 33.Hanson MA, Roth CB, Jo E, et al. Crystal structure of a lipid G protein-coupled receptor. Science 2012; 335: 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, et al. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 2009; 78: 743–768. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 2009; 77: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters CM, Long J, Gorshkova I, et al. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. FASEB J 2006; 20: 509–511. [DOI] [PubMed] [Google Scholar]
- 37.Osada M, Yatomi Y, Ohmori T, et al. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 2002; 299: 483–487. [DOI] [PubMed] [Google Scholar]
- 38.Koide Y, Hasegawa T, Takahashi A, et al. Development of novel EDG3 antagonists using a 3D database search and their structure-activity relationships. J Med Chem 2002; 45: 4629–4638. [DOI] [PubMed] [Google Scholar]
- 39.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 2005; 105: 3178–3184. [DOI] [PubMed] [Google Scholar]
- 40.Finigan JH, Dudek SM, Singleton PA, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem 2005; 280: 17286–17293. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien LA, Richardson MA, Mehrbod SF, et al. Activated protein C decreases tumor necrosis factor related apoptosis-inducing ligand by an EPCR-independent mechanism involving Egr-1/Erk-1/2 activation. Arterioscler Thromb Vasc Biol 2007; 27: 2634–2641. [DOI] [PubMed] [Google Scholar]
- 42.van der Poll T, Levi M. Crosstalk between inflammation and coagulation: the lessons of sepsis. Curr Vasc Pharmacol 2012; 10: 632–638. [DOI] [PubMed] [Google Scholar]
- 43.Deng Q, Clemas JA, Chrebet G, et al. Identification of Leu276 of the S1P1 receptor and Phe263 of the S1P3 receptor in interaction with receptor specific agonists by molecular modeling, site-directed mutagenesis, and affinity studies. Mol Pharmacol 2007; 71: 724–735. [DOI] [PubMed] [Google Scholar]
- 44.Becker PM, Verin AD, Booth MA, et al. Differential regulation of diverse physiological responses to VEGF in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 2001; 281: L1500–L1511. [DOI] [PubMed] [Google Scholar]
- 45.Jin F, Hagemann N, Sun L, et al. High-density lipoprotein (HDL) promotes angiogenesis via S1P3-dependent VEGFR2 activation. Angiogenesis 2018; 21: 381–394. [DOI] [PubMed] [Google Scholar]
- 46.Medford AR, Ibrahim NB, Millar AB. Vascular endothelial growth factor receptor and coreceptor expression in human acute respiratory distress syndrome. J Crit Care 2009; 24: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu Y, Camp SM, Sun X, et al. Sp1-mediated nonmuscle myosin light chain kinase expression and enhanced activity in vascular endothelial growth factor-induced vascular permeability. Pulm Circ 2015; 5: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaengel K, Niaudet C, Hagikura K, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 2012; 23: 587–599. [DOI] [PubMed] [Google Scholar]
- 49.Ryu JM, Baek YB, Shin MS, et al. Sphingosine-1-phosphate-induced Flk-1 transactivation stimulates mouse embryonic stem cell proliferation through S1P1/S1P3-dependent beta-arrestin/c-Src pathways. Stem Cell Res 2014; 12: 69–85. [DOI] [PubMed] [Google Scholar]
- 50.Cristobal I, Madoz-Gurpide J, Manso R, et al. Potential anti-tumor effects of FTY720 associated with PP2A activation: a brief review. Curr Med Res Opin 2016; 32: 1137–1141. [DOI] [PubMed] [Google Scholar]
- 51.Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol 2013; 14: e229–e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrestha J, Ki SH, Shin SM, et al. Synthesis of novel FTY720 analogs with anticancer activity through PP2A activation. Molecules 2018; 23: 2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez T, Estrada-Hernandez T, Paik JH, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem 2003; 278: 47281–47290. [DOI] [PubMed] [Google Scholar]
- 54.Brown BA, Kantesaria PP, McDevitt LM. Fingolimod: a novel immunosuppressant for multiple sclerosis. Ann Pharmacother 2007; 41: 1660–1668. [DOI] [PubMed] [Google Scholar]
- 55.Nakayama S, Uto Y, Tanimoto K, et al. TX-2152: a conformationally rigid and electron-rich diyne analogue of FTY720 with in vivo antiangiogenic activity. Bioorg Med Chem 2008; 16: 7705–7714. [DOI] [PubMed] [Google Scholar]
- 56.Don AS, Martinez-Lamenca C, Webb WR, et al. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J Biol Chem 2007; 282: 15833–15842. [DOI] [PubMed] [Google Scholar]
- 57.Zhu R, Snyder AH, Kharel Y, et al. Asymmetric synthesis of conformationally constrained fingolimod analogues – discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist. J Med Chem 2007; 50: 6428–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanessian S, Charron G, Billich A, et al. Constrained azacyclic analogues of the immunomodulatory agent FTY720 as molecular probes for sphingosine 1-phosphate receptors. Bioorg Med Chem Lett 2007; 17: 491–494. [DOI] [PubMed] [Google Scholar]
- 59.Foss FW, Jr, Clemens JJ, Davis MD, et al. Synthesis, stability, and implications of phosphothioate agonists of sphingosine-1-phosphate receptors. Bioorg Med Chem Lett 2005; 15: 4470–4474. [DOI] [PubMed] [Google Scholar]
- 60.Clemens JJ, Davis MD, Lynch KR, et al. Synthesis of 4(5)-phenylimidazole-based analogues of sphingosine-1-phosphate and FTY720: discovery of potent S1P1 receptor agonists. Bioorg Med Chem Lett 2005; 15: 3568–3572. [DOI] [PubMed] [Google Scholar]
- 61.Hale JJ, Neway W, Mills SG, et al. Potent S1P receptor agonists replicate the pharmacologic actions of the novel immune modulator FTY720. Bioorg Med Chem Lett 2004; 14: 3351–3355. [DOI] [PubMed] [Google Scholar]
- 62.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science (New York, NY) 2002; 296: 346–349. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Bittman R, Garcia JG, et al. Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate. Microvasc Res 2015; 99: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lou H, Montoya SE, Alerte TN, et al. Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J Biol Chem 2010; 285: 17648–17661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng X, Tehranian R, Dietrich P, et al. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 2005; 118: 3523–3530. [DOI] [PubMed] [Google Scholar]
- 66.Vargas-Medrano J, Krishnamachari S, Villanueva E, et al. Novel FTY720-based compounds stimulate neurotrophin expression and phosphatase activity in dopaminergic cells. ACS Med Chem Lett 2014; 5: 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]