Abstract
Secukinumab was the first fully human anti-interleukin-17a monoclonal antibody and successfully treated moderate-severe psoriasis. These new, targeted, medications are becoming more ubiquitous, but long-term side effects are not fully known. Post-market surveillance is crucial to identify delayed adverse events, analogous to the paradoxical development of pustular psoriasis in a subset of patients treated with the anti-tumor necrosis factor-alpha class drugs. Dyshidrotic eczema and pompholyx are rare variants of dermatitis characterized by vesicles or bullae on the palms, soles and sides of the fingers. The etiology of dyshidrotic eczema is not always known, but medications have been implicated in a minority of patients. Herein, we present two cases of dyshidrotic eczema developing in patients on secukinumab for psoriasis. Extended follow-up and larger numbers of patients are needed to fully understand the potential association between secukinumab and dyshidrotic eczema.
Keywords: Secukinumab, IL-17, psoriasis, biologics, dyshidrotic eczema
Introduction
Psoriasis is a common skin disorder, affecting 0.1%–3% of the population. It usually manifests on the extensor surfaces of the limbs, ears, and torso; however, any skin surface can be affected. There are numerous variants of psoriasis and chronic plaque psoriasis is the most common. The development of monoclonal antibody therapy (i.e. biologics) has revolutionized how moderate to severe psoriasis is treated. These new agents are expensive, but provide the most effective, longest lasting results, even in recalcitrant cases. These newer agents have a more favourable side effect profile compared to other available treatments of psoriasis. Long-term repercussions are not fully known.
Numerous factors have been identified that precipitate and exacerbate psoriasis. Temperature, humidity, alcohol, stress, infection (e.g. post-streptococcal guttate psoriasis), trauma (Koebnerization), and medications are known precipitants and exacerbators of psoriasis. Some implicated drugs include beta-blockers, anti-malarial drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), tetracyclines and TNFα inhibitors which can precipitate and exacerbate psoriasis and psoriasiform eruptions.1–3
Secukinumab is a fully human recombinant IgG1κ monoclonal antibody that is a specific antagonist of interleukin-17a (IL-17a), encoded by the IL17A gene. IL-17a is a pro-inflammatory cytokine secreted by activated T-cells, specifically Th17 helper T-cells, which are now regarded as the main immune cell involved in the pathogenesis of psoriasis. Secukinumab received USFDA approval in January 2015, for the treatment of severe psoriasis. Subsequently, it was also approved for psoriatic arthritis and ankylosing spondylitis. In clinical trials for psoriasis, secukinumab was shown to effectively achieve disease control with a favourable side effect profile and less immunosuppression, compared to other systemic therapies such as corticosteroids, apremilast, acitretin, methotrexate, cyclosporine and TNFα-inhibitors. As it is a newer therapy, long-term data and post-market adverse event reporting is limited and ongoing. Here, we report two cases of patients developing dyshidrotic eczema (DE) after using secukinumab. This has not previously been published, but the FDA has currently reported 12 cases of DE in patients taking secukinumab.4
Case report
A 52-year-old female with severe guttate psoriasis since October, 2016, and no history of eczema, was treated with secukinumab after failing topical corticosteroid and Vitamin D analogs. She did not take any other medications. She started secukinumab on June 16, 2017, resulting in rapid clearance of her psoriasis. Eight months after starting secukinumab, she developed widespread eczema and a dyshidrotic, vesiculobullous eruption mainly on the palms (Figure 1). She had no exposure to other relevant medications, and did not change occupation or personal skin care products prior to the eruption. Secukinumab was stopped and she started cyclosporine and guselkumab, which target the calcineurin pathway and the IL-23 pathway, respectively. Her symptoms resolved over the ensuing weeks and she did not have recurrence of her DE and her psoriasis remains stable.
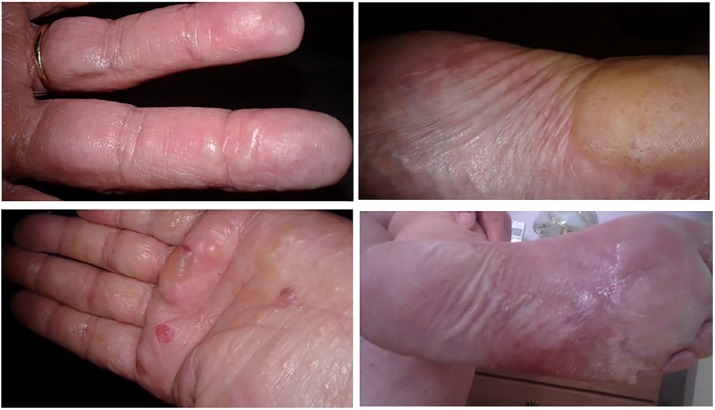
Figure 1.
Patient 1, dyshidrotic vesicles, bullae and erosions on the palms, pads and sides of the fingers, and more macerated, weepy plaques and erosions on the soles of the feet.
The second patient is a 69-year-old female with palmoplantar psoriasis and inflammatory arthritis diagnosed 4 years ago. She was recalcitrant to topical corticosteroids, could not tolerate methotrexate (i.e. transaminitis), and could only tolerate soriatane every other day due to transaminitis and steatosis. She received secukinumab and developed a vesiculobullous and pompholyx-like eruption 7 weeks after starting secukinumab (Figure 2). The drug was stopped and she was started on infliximab and Otezla, which cleared her lesions over the ensuing weeks to months. Her psoriasis remains stable and in remission. Written informed consent for clinical photographs used for publication was provided by both patients.
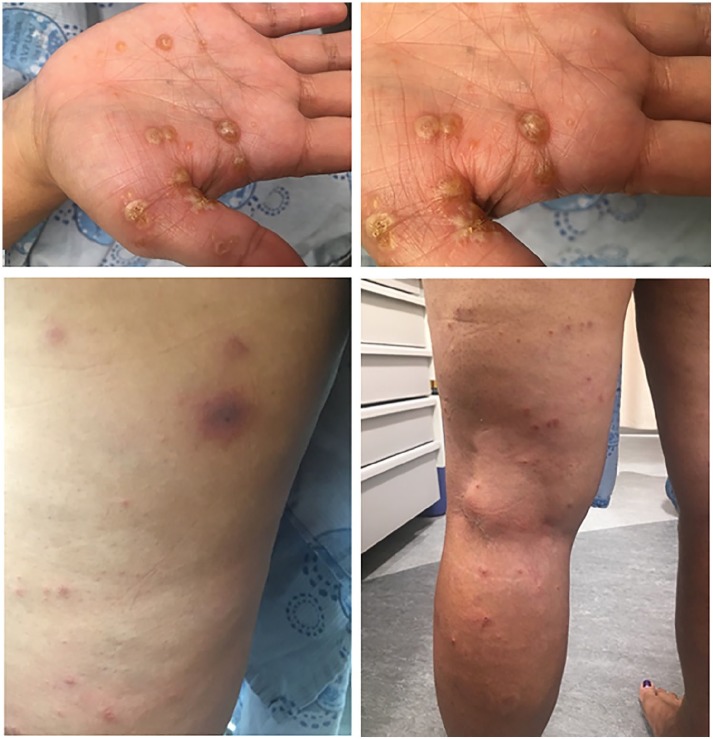
Figure 2.
Patient 2, dyshidrotic vesiculobullous eruption on the palms, pads and sides of the fingers (Top panels). She also had eczematous papules and more ecchymotic-purpuric, erythematous to violaceous plaques on the lower extremities (bottom panels).
Discussion
The Th17 helper T-cell is an integral effector in the pathogenesis of psoriasis as well as other autoimmune conditions. Thl7 T-cells act via IL-17a, which is an endogenously produced cytokine that has been shown to be elevated in psoriasis and other auto-immune conditions. Elevated levels of IL-17a are also found in psoriatic plaques. Secukinumab is a human immunoglobulin-G1κ monoclonal antibody that selectively binds to the IL-17a cytokine and inhibits its interaction with the IL-17 receptor.2,5,6 IL-17a is a naturally occurring cytokine involved in normal inflammatory and immune responses. Through this pathway, secukinumab inhibits the release of pro-inflammatory cytokines and chemokines.2,5,6 Treatment with secukinumab may reduce epidermal neutrophils and IL-17a levels in psoriatic plaques.5
The most common side effects of secukinumab, include nasopharyngitis, diarrhea, and upper respiratory tract infection (URTI). Cutaneous side effects include injection site reaction, oral herpes reactivation, and less frequently urticaria. During clinical trials the use of secukinumab was found to result in flares of Crohn’s disease in some patients with the condition. There have been 12 reported cases of DE while on secukinumab, but none that were identified during clinical trials. All documented cases occurred between 1 and 12 months after starting secukinumab.2,3,4
DE is a clinical variant of eczema that manifests as a symmetric, palmoplantar, vesiculobullous eruption, usually in additions to the typical eczematous plaques seen in atopic dermatitis. This form of eczema has many causes including allergic contact dermatitis, pre-existing atopic dermatitis, tinea, bacterial infections, and medications such as IVIg and mycophenolate mofetil.7
The pathogenesis of DE is not well understood. It is a chronic, relapsing, spongiotic dermatosis belonging to the spectrum of eczema.5,6 Langerhans cells have been shown to be increased in number and several T-cell lineages. In dyshidrotic skin, CD3, CD8, CD45, anti-human fibrinogen anti-myeloperoxidase (MPO), anti human fibrinogen, and anti-human IgE antibodies in lesions were increased in lesions. These findings suggest that DE is a Th2 mediated process.10
It is possible that the blockade of the Th17 immune response with secukinumab causes shunting to other T-cell pathways, including those implicated in other disease states, such as DE. The Th2 immune pathway, therefore, may play a role in the pathogenesis of dyshidrotic and atopic eczema. Another example of this phenomenon is the exacerbation of Crohn’s disease in patients commencing secukinumab. Crohn’s disease favours the Th1 immune pathway and blockade of Th17 lineage may result in shunting toward a Th1 phenotype, exacerbating Crohn’s disease. For paradoxical psoriasiform exanthems and pustular psoriasis with TNF-alpha inhibitors, there were increased levels of interferon-alpha (IFN-alpha) and a number of plasmacytoid dendritic cells in affected skin, an association that has been shown to be related to the pathogensis of psoriasis.8,9
Secukinumab is a newer treatment for psoriasis and not all adverse reactions are known. As more patients are prescribed this medication, for longer durations, other adverse reactions can be better characterized and managed.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Written and verbal informed consent for patient information and photos was provided by both patients for this publication.
References
- 1. Rallis E, Korfitis C, Stavropoulou E, et al. Onset of palmoplantar pustular psoriasis while on adalimumab for psoriatic arthritis: a ‘class effect’ of TNF-α antagonists or simply an anti-psoriatic treatment adverse reaction? J Dermatolog Treat 2010; 21(1): 3–5. [DOI] [PubMed] [Google Scholar]
- 2. Ryoo JY, Yang HJ, Ji E, et al. Meta-analysis of the efficacy and safety of secukinumab for the treatment of plaque psoriasis. Ann Pharmacother 2016; 50(5): 341–351. [DOI] [PubMed] [Google Scholar]
- 3. van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol 2016; 75(1): 83–98. [DOI] [PubMed] [Google Scholar]
- 4. EHealthMe. Cosentyx and Dyshidrotic eczema – from FDA reports. https://www.ehealthme.com/ds/cosentyx/dyshidrotic-eczema/ (accessed 4 April 2018).
- 5. Novartis. Cosentyx (secukinumab) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2016, https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/cosentyx.pdf [Google Scholar]
- 6. Sanford M, McKeage K. Secukinumab: first global approval. Drugs 2015; 75(3): 329–338. [DOI] [PubMed] [Google Scholar]
- 7. Houck G, Saeed S, Stevens GL, et al. Eczema and the spongiotic dermatoses: a histologic and pathogenic update. Semin Cutan Med Surg 2004; 23(1): 39–45. [DOI] [PubMed] [Google Scholar]
- 8. Park JJ, Lee SC. A case of tumor Necrosis factor-α inhibitors-induced pustular psoriasis. Ann Dermatol 2010; 22(2): 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Gannes GC, Ghoreishi M, Pope J, et al. Psoriasis and pustular dermatitis triggered by TNF-α inhibitors in patients with rheumatologic conditions. Arch Dermatol 2007; 143: 223–231. [DOI] [PubMed] [Google Scholar]
- 10. Abreu-Velez AM, Pinto FJ, Jr, Howard MS. Dyshidrotic eczema: relevance to the immune response in situ. N Am J Med Sci 2009; 1(3): 117–120. [PMC free article] [PubMed] [Google Scholar]