Abstract
Background
Vitamin D is important for brain function and linear growth. Vitamin D deficiency during pregnancy has been linked with impaired neurodevelopment during early childhood. However, there is limited evidence from population-based studies on the long-term impact of vitamin D deficiency on cognitive development and linear growth. The objective of the current analysis is to examine whether vitamin D deficiency during infancy and early childhood is associated with cognitive development and linear growth measured in school age.
Methods
This is a follow-up study of a placebo-controlled trial among 1000 North Indian children 6–30 months of age. We measured growth and neurodevelopment in 791 of these children when they were 6–9 years old. Neurodevelopment was measured using the Wechsler Intelligence Scale for Children, 4th edition INDIA, the Crichton Verbal Scale, NEPSY-II subtests, and the BRIEF 2. We categorized vitamin D concentrations during infancy and early childhood according to the US Institute of Medicine’s recommendations; serum 25(OH)D < 12 ng/ml as deficient; 12–20 ng/ml as inadequate; > 20 ng/ml as sufficient. In multivariable regression models, adjusting for relevant confounders, we estimated the association between vitamin D status, growth and neurodevelopmental outcomes.
Results
Among the 791 children, baseline vitamin D status was available for 716. Of these, 45.8% were vitamin D deficient, 32.7% were inadequate, and 21.5% were sufficient. Vitamin D status was not associated with any of the cognitive outcomes or linear growth [Adjusted β coefficient for height for age z-score between deficient and sufficient children was − 0.06 (95% CI − 0.24 to 0.11)] at follow up.
Conclusion
Our findings do not support the notion that poor vitamin D status in early childhood is an important limitation for cognitive development and linear growth.
Trial Registration
The trial was first registered at www.clinicaltrials.gov as NCT00717730 in July, 2008, and at CTRI/2010/091/001090 in August, 2010 and then as CTRI/2016/11/007494 in November 2016.
Keywords: Vitamin D, Wechsler intelligence scale for children, 4th edition INDIA, Crichton verbal scale, A developmental neuropsychological assessment II, The behavior rating inventory of executive function 2, Linear growth, School age
Background
Vitamin D deficiency is one of the most common micronutrient deficiencies worldwide [1]. In the Indian subcontinent, the prevalence of vitamin D deficiency is estimated to be from 50 to 90% using an internationally accepted reference value [2]. Vitamin D acts by binding to the nuclear vitamin D receptors (VDR), which are widely distributed throughout the human brain in most neurons and some glial cells [3, 4]. Animal studies have shown that vitamin D deficiency during pregnancy causes extreme alterations in the brain at birth [5, 6]. This provides a biological plausibility for a link between vitamin D status and neurodevelopment.
There is evidence from observational studies on the association between vitamin D status during pregnancy or cord blood vitamin D at birth with cognitive, language, and behavioral development in different periods of childhood [7–12]. Previously, we have shown that vitamin D status was not associated with neurodevelopment as measured by a brief screening tool, the Ages and Stages Questionnaire 3rd edition (ASQ-3) during early childhood [13]. The consequences of vitamin D deficiency in early life on neurodevelopmental may not become evident until later in childhood. Furthermore, the predictive ability of early neurodevelopmental assessments is poor, and cognitive assessments in school-aged children have shown to be stable over time [14, 15]. We measured vitamin D status in 1000 young North Indian children and conducted a comprehensive assessment of cognitive performance and growth approximately 6 years later [16, 17]. This study gave us a unique opportunity to explore the extent to which vitamin D deficiency during early childhood is associated with impaired cognitive development and linear growth at school age.
Methods
Study design and participants
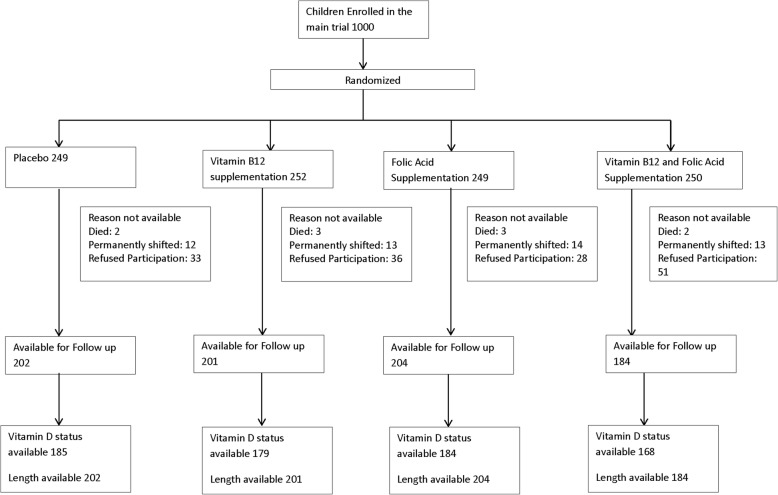
We followed up children who previously participated in a randomized double-blind placebo-controlled trial (n = 1000) on the effect of two recommended daily allowances (RDA) of vitamin B12 and/or folic acid daily for 6 months in Delhi, North India [18]. The main outcome of the study was incidence of infections. In September 2016, we approached all these children, and were able to get in contact with 798 of whom 791 consented to participate in the follow-up study. (Fig. 1) All families were initially contacted by phone to be invited to participate in the study. A physical visit was made to the family’s address if no contact could be made. We requested the families who had moved out of the study area to come to the study clinic for 1 day. On the day of the assessment, consent was taken from the children’s caregiver for the participation in the study and we gathered information on socio-economic situation of the family such as parental education and occupation and various household assets.
Fig. 1.
Participant Flow
Assessment
Cognition
We assessed cognitive development with the Wechsler Intelligence Scale for Children, 4th edition INDIA, the Crichton Verbal Scale, NEPSY-II subtests and the BRIEF 2.
The Wechsler Intelligence Scale for Children 4th edition (India) (WISC-IVINDIA) is an assessment tool of intellectual ability in children validated for the Indian population with Indian norms [19]. We assessed seven subtests (listed in the parenthesis) that were summed up to three index scores; the Perceptual Reasoning (Block design, Picture concept, Matrix reasoning), Processing Speed (Symbol search, Letter-number sequences) and Working Memory (Digit span, Coding).
The Crichton Vocabulary Scales (CVS) assess verbal skills in children 4 to 18 years through knowledge of words [20]. The CVS has been translated to Hindi and provides a standard score with Indian norms [21].
NEPSY-II is a neuropsychological test battery for children aged 3 to 16 years with American norms [22]. Seven age-appropriate subtests were administered; Inhibition and Design Fluency, Word Generation, Visuo-motor Precision and Manual Motor Sequences, Affect Recognition and Geometric Puzzles. No modifications and cultural adjustments were necessary to perform the tests in this setting.
The Behavior Rating Inventory of Executive Function 2nd edition (BRIEF 2) is a parent report questionnaire for children 5 to 18 years assessing executive functions in everyday life with American norms [23]. The scale comprises three clinical indexes; the Behavior, Emotion and Regulation Index, and an overall Global Executive Composite. The questionnaire was translated and validated to Hindi in close collaboration with the developers (PARiConnect).
Growth
Trained field supervisors measured weight and height at follow up. Weight was measured to the nearest 50 g using a Digitron scales. Height was measured with Seca 213 to the nearest 0.1 cm. Inter- and intra-observer standardization exercises for weight and height assessments were conducted before study initiation for outcome ascertainment team; these are repeated every 3 months.
Analytical procedures
At baseline, 3 ml blood was collected into an evacuated tube containing EDTA (BD, Franklin Lakes, NJ, USA) from all children. Plasma was separated from the whole blood by centrifugation at room temperature (450 x g × 10 min), transferred into storage vials and stored at − 20 °C until analysis. Plasma concentration of vitamin-D was measured by quantitative electro-chemiluminescence binding assay (Roche Diagnostics, Mannheim, Germany) at the Department of Biochemistry, Christian Medical College, Vellore, India [24]. Plasma homocysteine (tHcy) was analyzed using commercial kits (Abbott Park, IL, USA) [25]. Plasma concentrations of vitamin B12 and folate were determined by microbiological assays using a chloramphenicol-resistant strain of Lactobacillus casei and colistin sulfate-resistant strain of Lactobacillus leichmannii, respectivel y[26, 27]. Plasma soluble transferrin receptor (sTfR) was analyzed using an immunoturbidimetric assay [28].
Statistical analysis
Proportions, means (SD) or medians (IQR) were calculated for categorical and continuous variables by vitamin-D status at baseline. We categorized vitamin D status according to the US Institute of Medicine’s recommendations; serum 25(OH)D < 12 ng/ml as ‘deficient’; 12–20 ng/ml as ‘inadequate’; > 20 ng/ml as ‘sufficient’ [29]. For the cognitive outcomes, we calculated a combined WISC-IVINDIA and CVS z-score based on converted z-scores for the three index scores in the WISC-IVINDIA and the total CVS score. We also calculated a combined NEPSY-II z-score based on converted z-scores in the seven subtests. For the BRIEF 2, we used the overall Global Executive Composite score in the analyses. Children’s height-for-age z-score (HAZ) at follow up was calculated based on WHO growth standards [30]. The wealth of an individual was determined by a wealth index created using principal component analysis based on assets owned by the household [31]. Using the score from the principal component analysis, the population was divided into five equal wealth quintiles i.e. poorest, very poor, poor, less poor and least poor.
We used multivariable linear regression to investigate the association between vitamin D status at baseline and the cognitive outcomes and HAZ score at follow up. We used generalized linear model (GLM) with the gaussian distribution family, and identity link function to calculate beta-coefficients for the cognitive outcomes and the HAZ scores. We used GLM with the poisson distribution family, and log link to calculate the relative risk (RR) for stunting [32].
We used a method of purposeful selection of covariates to identify variables for the multivariable models [33, 34]. We included in the multivariable models the variables that changed the beta coefficient or relative risk of the outcome variables by 20% from the univariable models. We present the adjusted models, including variables that were identified in the process. The candidate variables for these models were age and sex of the child, maternal and paternal years of schooling, paternal occupation, wealth quintiles at follow up and baseline log (base e) cobalamin, folate, and total homocysteine concentration and the intervention groups.
Statistical analyses were performed using STATA version 15 (Stata Corporation, College Station, TX). We used generalized additive models in the statistical software R version 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria) to explore nonlinear associations between the plasma vitamin D concentration at baseline and the combined WISC-IVINDIA and CVS z-score, the combined NEPSY-II z-score and the Global BRIEF-2 score at follow up after adjustment for potential confounders [35].
Results
Of the 1000 children in the main study, 791 children consented to participate in the follow-up study. Fig. 1 shows the flow of the participants. The demographic information and nutritional status of the children at baseline and follow up are presented in Table 1. Baseline vitamin D status was available for 716 children who consented. Of these, 328 (45.8%) were deficient, 234 (32.7%) were inadequate, and 154 (21.5%) sufficient [29]. The mean (SD) and median (IQR) of vitamin D concentration at baseline were 14.6 (8.6) ng/ml and 12.8 (8.3–18.7) ng/ml, respectively.
Table 1.
Demographic information and nutritional status of the 716 North Indian children at baseline (6 to 30 months) and follow up (6 to 9 years)
| Vitamin D sufficient (n = 154) |
Vitamin D inadequate (n = 234) |
Vitamin D deficient (n = 328) |
|
|---|---|---|---|
| Child characteristics at baseline (6 to 30 months) | |||
| Boys, n (%) | 85 (55.2) | 125 (53.4) | 161 (49.1) |
| Ever breastfed, n (%) | 154 (100) | 232 (99.1) | 323 (98.5) |
| Growth Z scores, mean (sd) | |||
| Weight-for-height (WHZ) | −0.9 (0.9) | − 0.9 (0.9) | − 0.8 (0.9) |
| Height-for-age (HAZ) | −1.7 (1.1) | − 1.6 (1.1) | − 1.6 (1.2) |
| Weight-for-age (WAZ) | − 1.6 (0.9) | −1.5 (1) | − 1.5 (1) |
| Wasted (<−2 WHZ) | 18 (11.7) | 28 (12) | 34 (10.4) |
| Stunted (<−2 HAZ) | 59 (38.3) | 86 (36.7) | 128 (39) |
| Underweight (<−2 WAZ) | 47 (30.5) | 77 (32.9) | 105 (32) |
| Biomarkers: | |||
| Cobalamin, mean (sd) pmol/L | 312.7 (186.7) | 332.6 (215.3) | 305.9 (175.2) |
| Cobalamin < 200 pmol/L, n (%) | 47 (30.5) | 72 (30.8) | 106 (32.3) |
| Folate, mean (sd) nmol/L | 16.7 (14.6) | 16.1 (13.7) | 14.9 (14.4) |
| Folate < 7.5 nmol/L, n (%) | 48 (31.2) | 69 (29.5) | 115 (35.1) |
| Homocysteine, mean (sd) μmol/L | 14.4 (8.8) | 13.3 (6.7) | 13.7 (7.3) |
| Homocysteine > 10 μmol/L, n (%) | 104 (68.4) | 144 (62.1) | 204 (62.4) |
| Soluble transferrin receptor, mean (sd) nmol/L | 4.3 (2.8) | 4.4 (3.3) | 4.7 (3.1) |
| Soluble transferrin receptor> 4.7 nmol/L, n (%) | 40 (26) | 76 (32.5) | 111 (33.8) |
| Child characteristics at follow up (6 to 9 years) | |||
| Age follow up (yrs) mean (SD) | 7.9 (0.6) | 7.8 (0.6) | 7.9 (0.6) |
| Schooling | |||
| No School, n (%) | 3 (1.9) | 6 (2.6) | 4 (1.2) |
| Hindi medium, n (%) | 61 (39.6) | 88 (37.6) | 129 (39.3) |
| English medium, n (%) | 90 (58.4) | 140 (59.8) | 195 (59.4) |
| Family characteristics at follow up | |||
| Mothers years of schooling | |||
| No schooling, n (%) | 54 (35.5) | 65 (28) | 76 (23.3) |
| Primary (1–5 years), n (%) | 23 (15.1) | 18 (7.8) | 49 (15) |
| Middle (6–12 years), n (%) | 65 (42.8) | 123 (53) | 153 (46.9) |
| Higher (> 12 years), n (%) | 10 (6.6) | 26 (11.2) | 48 (14.7) |
| Fathers occupation | |||
| Government job or private services, n (%) | 88 (57.1) | 135 (57.9) | 165 (50.8) |
| Self-employed, n (%) | 31 (20.1) | 56 (24) | 95 (29.2) |
| Daily wager/farming, n (%) | 29 (18.8) | 31 (13.3) | 52 (16) |
| No job/other, n (%) | 6 (3.9) | 11 (4.7) | 13 (4) |
| Wealth Quintile | |||
| Poorest, n (%) | 39 (25.3) | 47 (20.1) | 60 (18.3) |
| Very Poor, n (%) | 36 (23.4) | 38 (16.2) | 65 (19.8) |
| Poor, n (%) | 31 (20.1) | 57 (24.4) | 60 (18.3) |
| Less Poor, n (%) | 32 (20.8) | 43 (18.4) | 69 (21) |
| Least Poor, n (%) | 16 (10.4) | 49 (20.9) | 74 (22.6) |
The estimates from both univariable and multivariable analyses comparing the combined WISC-IVINDIA and CVS z-score, the combined NEPSY-II z-score and the Global BRIEF-2 score between vitamin D inadequate, deficient, and vitamin D sufficient children are shown in Table 2. There were no significant differences between the vitamin D sufficient children, inadequate and deficient children on any of the cognitive outcomes.
Table 2.
The association between baseline Vitamin D status and cognitive scores at follow up in North Indian children 6 to 9 years
| WISC-IVINDIA and CVS z-score | NEPSY z-score | Global BRIEF score | ||||
|---|---|---|---|---|---|---|
| Unadjusted β coefficient (95% CI) |
Adjusted β coefficient (95% CI)a |
Unadjusted β coefficient (95% CI) |
Adjusted β coefficient (95% CI)a |
Unadjusted β coefficient (95% CI) |
Adjusted β coefficient (95% CI)a |
|
| Vitamin D sufficient | Reference | Reference | Reference | |||
| Vitamin D inadequate | 0.03 (−0.17 to 0.23) | −0.12 (−0.30 to 0.05) | −0.01 (−0.21 to 0.19) | −0.15 (−0.33 to 0.04) | 0.21 (−2.10 to 2.48) | 0.87 (−1.40 to 3.14) |
| Vitamin D deficient | 0.02 (− 0.16 to 0.22) | − 0.13 (− 0.29 to 0.04) | −0.02 (− 0.21 to 0.17) | −0.16 (− 0.34 to 0.02) | −1.10 (−3.21 to 1.05) | −0.38 (− 2.53 to 1.77) |
aadjusted for log folate, log soluble transferrin receptor and log homocysteine level at baseline, and the wealth index, paternal occupational status and maternal education at follow-up and intervention group
Table 3 shows the association between baseline vitamin D status and linear growth at follow up. Of the children, 15.8, 12.4 and 17.5% were stunted in the vitamin D deficient, inadequate and sufficient group, respectively. Vitamin D status was not associated with the HAZ score or the proportion of children stunted at follow up.
Table 3.
The association between baseline Vitamin D status and linear growth at follow up in North Indian children 6 to 9 years
| HAZ scores at follow up | Stunted at follow up | |||
|---|---|---|---|---|
| Unadjusted β coefficient (95% CI) |
Adjusted β coefficient (95% CI)* |
Unadjusted RR (95% CI) |
Adjusted RR (95% CI)a |
|
| Vitamin D sufficient | Reference | Reference | ||
| Vitamin D inadequate | 0.06 (−0.13 to 0.26) | −0.07 (− 0.26 to 0.12) | 0.70 (0.42 to 1.20) | 0.87 (0.50 to 1.50) |
| Vitamin D deficient |
0.08 (−0.10 to 0.27) |
−0.06 (− 0.24 to 0.11) | 0.90 (0.57 to 1.44) | 1.14 (0.70 to 1.86) |
aadjusted for log folate, log soluble transferrin receptor and log homocysteine level, at baseline and the wealth index, paternal occupational status and maternal education at follow-up and intervention group
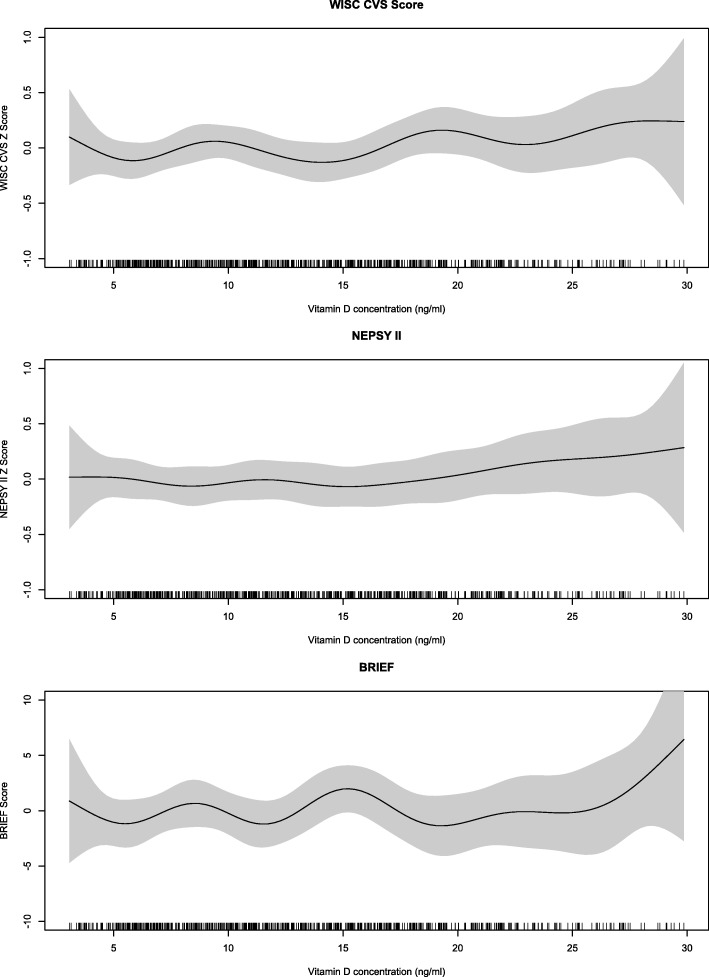
The association between vitamin D concentration at baseline and the cognitive outcomes at follow up are depicted in Fig. 2. The GAMs did not reveal any non-linear associations between vitamin D level at baseline and the combined WISC-IVINDIA and CVS z-score and the Global BRIEF-2 score at follow up.
Fig. 2.
Association between baseline vitamin D level and the combined WISC IVINDIA and Crichton Vocabulary Scale (CVS) z-score, the combined NEPSY II z scores and the BRIEF P Global Executive composite score at follow up in North Indian children 6 to 9 years. The graph was constructed using generalized additive models in R, the solid line depicts the association of vitamin D level at baseline and Global BRIEF score at follow up. The shaded area spans the 95% confidence interval of this association
Discussion
We did not find any evidence for a link between early vitamin D status and long-term cognition and linear growth.
To our knowledge, this is the first study that has examined the relationship between vitamin D status in early childhood and cognitive development at school age. The findings from this study are in accordance with findings from the same cohort when neurodevelopment was measured in early childhood [13]. The findings are also similar to those from a cohort study in India, where vitamin D status in early childhood was not associated with the gross motor functioning among school aged children [36]. Studies that have examined the association between cord blood vitamin D concentrations and neurodevelopment measured in early and middle childhood have shown mixed results [37, 38]. Furthermore, studies that have examined the associations between vitamin D deficiency during pregnancy and neurodevelopment during early and middle childhood have also shown inconsistent results [7–12, 39]. Three studies found an association between pregnancy vitamin D status and neurodevelopment outcomes in children before 4 years of age [8, 11, 38], while one study found marginal associations with language scores at 10 years of age [10]. The inconsistencies are likely due to differences in the populations, the timing of vitamin D assessment during pregnancy, the use of different cut-offs for vitamin D deficiency, age of the child at developmental assessments, and the way potential confounders were handled. The large sample size, the broad range of cognitive assessments, many with Indian norms, and the timing of the assessments at school age provide strengths to our findings that early vitamin D status most likely is not associated with cognitive function on long term.
We found no association between vitamin D status at baseline and linear growth at follow up. There are similar findings described in pre-school children in Nepal and in uninfected HIV exposed infants in Africa [40, 41]. In contrast, low-birth-weight (1.8 to 2.5 kg) infants in India, who received 1 RDA of vitamin D supplementation showed significantly higher length and weight at 6 months of age compared to those who received placebo [42]. Vitamin D helps the growth plate cells to be more sensitive to growth hormone action which plays an important role in linear growth at school age [43]. Vitamin D also maintains bone health to ensure normal calcium and phosphate levels in the blood [44]. Thus, our findings of no association between vitamin D deficiency and linear growth may be unexpected. A probable explanation could be that the children have to be severely deficient in vitamin D before it has consequences for their bone growth. There may be deficiencies of other growth-limiting macro and micronutrients such as calcium, zinc, vitamin B12 that account for the variance of growth between these study children. Furthermore, lower proportion of animal source protein in food, may also contribute to poor growth in this population [45]. The role of vitamin D might thus be negligible in the light of other growth limiting factors in this population.
Dietary sources of vitamin D are limited mainly to oily fish, eggs, and fortified foods [46]. As most people in the northern part of India are vegetarians, the predominant dietary source of vitamin D among the study children would be milk. Milk is rarely fortified with vitamin D in India and the vitamin D content of unfortified milk is very low (2 IU/100 mL). Prevalence of lactose intolerance also contributes to vitamin D deficiency in this setting [47]. Currently, there is no national program of vitamin D supplementation for infants and children, but the Indian Academy of Pediatrics guidelines recommend daily vitamin D supplementation in doses of 400 IU up to 1 year of age and 600 IU from 1 to 18 years of age [48].
The main strength of the study is that we measured vitamin D status in a large sample of children during what is considered a critical window for the brain development, and measured the cognitive outcomes during a period where valid and stable estimates can be obtained. The study includes high quality and comprehensive assessment of cognitive development, with the use of validated tests with Indian norms. We were able to include 80% of the children from the primary cohort after more than 5 years with no significant differences between the children who were included in the follow-up and not. An immunoassay method was used to assess vitamin D concentration. The immunoassay can underestimate serum 25(OH) D2 concentration compared to liquid chromatography-tandem mass spectrometry (LC-MS/MS) [49].
Conclusion
The results from the current study do not support that vitamin D status in early childhood is of importance for long-term growth and cognition.
Acknowledgements
We acknowledge the group of study psychologists and the coordinator who performed the cognitive assessments with the children and their caregivers; Farah Abbasi, Heena Chaudhary, Raqib Ali, Sugandhi Nagpal, Vaishali Panwar and Shruti Bisht. We acknowledge the input from Ratnasamy Selvakumar, Department of Biochemistry from Christian Medical College, Vellore, India for biochemical analysis. The Society for Applied Studies acknowledges the core support provided by the Department of Maternal, Newborn, Child and Adolescent Health, World Health Organisation, Geneva (WHO Collaborating Centre IND-158) and the Centre for Intervention Science in Maternal and Child Health (RCN Project No. 223269), Centre for International Health, University of Bergen (Norway). We also acknowledge Centre for Intervention Science in Maternal and Child Health (CISMAC; project number 223269), which is funded by the Research Council of Norway through its Centres of Excellence scheme and the University of Bergen (UiB), Norway.
Abbreviations
- BRIEF
Behavior rating inventory of executive function
- CI
Confidence interval
- CVS
Crichton Verbal Scale
- NEPSY
A developmental neuro psychological assessment
- RCT
Randomized controlled trial
- RDA
Recommended daily allowance
- SD
Standard deviation
- tHcy
Total Homocysteine
- WISC-IV INDIA
Wechsler intelligence scale for children, 4th edition INDIA
Authors’ contributions
RC, TS, ST, IK designed the research with contributions from all authors. RC and TS did the statistical analysis with support from ST, IK, MH, and NB. RC wrote the first draft. All authors contributed to the critical interpretation and writing of the paper and saw and approved the final version.
Funding
The study was funded by the Thrasher Research Fund (grant no 02827) and the Research Council of Norway (grant no. 172226 and 234495).
Availability of data and materials
Request for data pertaining to the current analysis may be sent to Dr. Sunita Taneja (Email id: sunita.taneja@sas.org.in).
Ethics approval and consent to participate
The follow-up study was approved from the ethics committee of Society for Applied Studies (India) and Norwegian Regional Committee for Medical and Health Research Ethics (REK VEST). The consent form for the main trial also sought permission from parents to store participating children’s blood specimen for use in future research. All parents consented for the same.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ranadip Chowdhury, Email: ranadip.chowdhury@sas.org.in.
Sunita Taneja, Email: sunita.taneja@sas.org.in.
Ingrid Kvestad, Email: inkv@norceresearch.no.
Mari Hysing, Email: Mari.Hysing@uib.no.
Nita Bhandari, Email: nita.bhandari@sas.org.in.
Tor A. Strand, Email: tors@me.com
References
- 1.Holick MF. Vitamin D: extraskeletal health. Rheum Dis Clin N Am. 2012;38:141–160. doi: 10.1016/j.rdc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Kamboj P, Dwivedi S, Toteja GS. Prevalence of hypovitaminosis D in India & way forward. IJMR. 2018;48(5):548–556. doi: 10.4103/ijmr.IJMR_1807_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3) Endocrinol Metab Clin N Am. 2010;39(2):255–269. doi: 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesby JP, Eyles DW, Burne TH, McGrath JJ. The effects of vitamin D on brain development and adult brain function. Mol Cell Endocrinol. 2011;347(1–2):121–127. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.O’Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne TH. Vitamin-D status during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology. 2007;32:227–234. doi: 10.1016/j.psyneuen.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin-D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 7.Strom M, Halldorsson TI, Hansen S, Granström C, Maslova E, Petersen SB, Cohen AS, Olsen SF. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann Nutr Metab. 2014;64:254–261. doi: 10.1159/000365030. [DOI] [PubMed] [Google Scholar]
- 8.Morales E, Guxens M, Llop S, Rodríguez-Bernal CL, Tardón A, Riaño I, Ibarluzea J, Lertxundi N, Espada M, Rodriguez A. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 2012;130:e913–e920. doi: 10.1542/peds.2011-3289. [DOI] [PubMed] [Google Scholar]
- 9.Keim SA, Bodnar LM, Klebanoff MA. Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol. 2014;28:434–444. doi: 10.1111/ppe.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehouse AJ, Holt BJ, Serralha M. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129:485–493. doi: 10.1542/peds.2011-2644. [DOI] [PubMed] [Google Scholar]
- 11.Hanieh S, Ha TT, Simpson JA, Thuy TT, Khuong NC, Thoang DD, Tran TD, Tuan T, Fisher J, Biggs BA. Maternal vitamin D status and infant outcomes in rural Vietnam: a prospective cohort study. PLoS One. 2014;9:e99005. doi: 10.1371/journal.pone.0099005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwer-Brolsma EM, Vrijkotte TG, Feskens EJ. Maternal vitamin D concentrations are associated with faster childhood reaction time and response speed, but not with motor fluency and flexibility, at the age of 5–6 years: the Amsterdam born children and their development (ABCD) study. Br J Nutr. 2018;120(3):345–352. doi: 10.1017/S0007114518001319. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury R, Taneja S, Bhandari N, Kvestad I, Strand TA. Bhan MK. Vitamin-D status and neurodevelopment and growth in young north Indian children: a secondary data analysis. Nutr J, 2017; 16: 59 doi: 10.1186/s12937-017-0285-y. [DOI] [PMC free article] [PubMed]
- 14.Månsson Johanna, Stjernqvist Karin, Serenius Fredrik, Ådén Ulrika, Källén Karin. Agreement Between Bayley-III Measurements and WISC-IV Measurements in Typically Developing Children. Journal of Psychoeducational Assessment. 2018;37(5):603–616. doi: 10.1177/0734282918781431. [DOI] [Google Scholar]
- 15.Schneider W, Niklas F, Schmiedeler S. Intellectual development from early childhood to early adulthood: The impact of early IQ differences on stability and change over time. Learn Individ Differ 2014;32:156–162. doi: https://doi.org/10.1016/j.lindif.2014.02.001.
- 16.Winje BA, Kvestad I, Krishnamachari S, Manji K, Taneja S, Bellinger DC, et al. Does early vitamin B<sub>12</sub> supplementation improve neurodevelopment and cognitive function in childhood and into school age: a study protocol for extended follow-ups from randomised controlled trials in India and Tanzania. BMJ Open. 2018;8(2):e018962. doi: 10.1136/bmjopen-2017-018962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury R, Taneja S, Bhandari N, Sinha B, Upadhyay RP, Bhan M, Strand TA. Vitamin-D deficiency predicts infections in young north Indian children: a secondary data analysis. PLoS One. 2017;12(3):e0170509. doi: 10.1371/journal.pone.0170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taneja S, Strand TA, Kumar T, Mahesh M, Mohan S, Manger MS. Folic acid and vitamin B-12 supplementation and common infections in 6-30-mo-old children in India: a randomized placebo-controlled trial. Am J Clin Nutr. 2013;98(3):731–737. doi: 10.3945/ajcn.113.059592. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. WISC-IV India. Wechsler intelligence scale for children - fourth (India edition) New Delhi: Pearson; 2016. [Google Scholar]
- 20.Raven J, Rust J, Squire A. Manual for Coroured progressive matrices and Crichton vocabulary scale. NCS Pearson Inc: UK; 2008. [Google Scholar]
- 21.Raven J, Rust J, Squire A. Raven’s Coloured Progressive Matrices and Crichton Vocabulary Scales (Hindi Edition). Chennai, India: Pearson Clinical & Talent Assessment; 2015.
- 22.Brooks Brian L., Sherman Elisabeth M. S., Strauss Esther. NEPSY-II: A Developmental Neuropsychological Assessment, Second Edition. Child Neuropsychology. 2009;16(1):80–101. doi: 10.1080/09297040903146966. [DOI] [Google Scholar]
- 23.Gioia Gerard A., Isquith Peter K., Roth Robert M. Encyclopedia of Clinical Neuropsychology. Cham: Springer International Publishing; 2018. Behavior Rating Inventory for Executive Function; pp. 532–538. [Google Scholar]
- 24.Cobas E411 Vitamin-D Total Reagent Insert (06268668001V1). Roche Diagnostics Web site. Available online: http://www.cobas.com/home/product/clinical-and-immunochemistry-testing/elecsys-vitamin-d-total-assay.html Accessed 17 April 2019.
- 25.Shipchandler MT, Moore EG. Rapid, fully automated measurement of plasma homocyst (e) ine with the Abbott IMx analyzer. Clin Chem. 1995;41(7):991e4. doi: 10.1093/clinchem/41.7.991. [DOI] [PubMed] [Google Scholar]
- 26.O'broin S, Kelleher B. Microbiological assay on microtitre plates of folate in serum and red cells. J Clin Pathol. 1992;45(4):344e7. doi: 10.1136/jcp.45.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelleher BP, Walshe KG, Scott JM, O'Broin SD. Microbiological assay for vitamin B12 with use of a colistin-sulfate-resistant organism. Clin Chem. 1987;33(1):52e4. doi: 10.1093/clinchem/33.1.52. [DOI] [PubMed] [Google Scholar]
- 28.Cotton F, Thiry P, Boeynaems J. Measurement of soluble transferrin receptor by immunoturbidimetry and immunonephelometry. Clin Biochem. 2000;33(4):263e7. doi: 10.1016/S0009-9120(00)00071-0. [DOI] [PubMed] [Google Scholar]
- 29.Spiro A., Buttriss J. L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutrition Bulletin. 2014;39(4):322–350. doi: 10.1111/nbu.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr 2006; 95(S450): 76–85 doi: https://doi.org/10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed]
- 31.International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-4), 2015-16. Mumbai: International Institute for Population Sciences (IIPS) and Macro International; 2015.
- 32.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 33.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol and Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. New York: Wiley; 2013. [Google Scholar]
- 35.Wood SN. Modelling and smoothing parameter estimation with multiple quadratic penalties. J Royal Stat Soc. 2000;62:413–428 doi: https://doi.org/10.1111/1467-9868.00240.
- 36.Filteau S, Rehman AM, Yousafzi A, Chugh R, Kaur M, Sachdev HPS, Trilok-Kumar G. Association of vitamin D status, bone health and anthropometry, with gross motor development and performance of school-aged Indian children who were born at term low birth weight. BMJ Open. 2016;6:e009268. doi: 10.1136/bmjopen-2015-009268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu P, Tong SL, Hao JH, Tao RX, Huang K, Hu WB, Zhou QF, Jiang XM, Tao FB. Cord blood vitamin D and neurocognitive development are nonlinearly related in toddlers. J Nutr. 2015;145(6):1232–1238. doi: 10.3945/jn.114.208801. [DOI] [PubMed] [Google Scholar]
- 38.Gould JF, Anderson AJ, Yelland LN, Smithers LG, Skeaff CM, Zhou SJ, Gibson RA, Makrides M. Association of cord blood vitamin-D with early childhood growth and neurodevelopment. J Paediatr Child Health. 2017;53(1):75–83. doi: 10.1111/jpc.13308. [DOI] [PubMed] [Google Scholar]
- 39.Darling AL, Rayman MP, Steer CD, Golding J, Lanham-New SA, Bath SC. Association between maternal vitamin D status in pregnancy and neurodevelopmental outcomesin childhood: results from the Avon longitudinal study of parents and children (ALSPAC) Br J Nutr. 2017;117(12):1682–1692. doi: 10.1017/S0007114517001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avagyan D, Neupane SP, Gundersen TE, Madar AA. Vitamin D status in preschool children in rural Nepal. Public Health Nutr. 2016;19(3):470–476. doi: 10.1017/S136898001500083X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudfeld CR, Duggan C, Abound S, Kupka R, Manji KP, Kisenge R, Fawzi WW. Vitamin D status is associated with mortality, morbidity, and growth failure among a prospective cohort of HIV-infected and HIV-exposed Tanzanian infants. J Nutr. 2015;145(1):121–127. doi: 10.3945/jn.114.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar GT, Sachdev HS, Chellani H, Rehman AM, Singh V, Arora H, Filteau S. Effect of weekly vitamin-D ements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ. 2011;342:d2975. doi: 10.1136/bmj.d2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esposito S, Leonardi A, Lanciotti L, Cofini M, Muzi G, Penta L. Vitamin D and growth hormone in children: a review of the current scientific knowledge. J Transl Med. 2019;17(1):87. doi: 10.1186/s12967-019-1840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eaton JC, Rothpletz-Puglia P, Dreker MR, Iannotti L, Lutter C, Kaganda J, Rayco-Solon P. Effectiveness of provision of animal-source foods for supporting optimal growth and development in children 6 to 59 months of age. Cochrane Database of Systematic Reviews. 2019(2):CD012818. 10.1002/14651858.CD012818.pub2. [DOI] [PMC free article] [PubMed]
- 46.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi. India. 2002;87(2):111–113. doi: 10.1136/adc.87.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritu G, Gupta A. Vitamin-D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6:729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khadilkar A, Khadilkar V, Chinnappa J, Rathi N, Khadgawat R, Balasubramanian S, et al. Prevention and treatment of vitamin D and calcium deficiency in children and adolescents: Indian academy of pediatrics (IAP) guidelines. Indian Pediatr. 2017;54:567–573. doi: 10.1007/s13312-017-1070-x. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Zeng Q, Yuan J, Xie Z. Performance evaluation of two immunoassays for 25-hydroxyvitamin D. J Clin Biochem Nutr. 2016;58(3):186–192. doi: 10.3164/jcbn.15-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Request for data pertaining to the current analysis may be sent to Dr. Sunita Taneja (Email id: sunita.taneja@sas.org.in).