Abstract
Objective
Sarcoidosis is an idiopathic inflammatory disorder that is difficult to treat. There is accumulating evidence that constitutive activation of Janus kinase–signal transducer and activator of transcription (JAK‐STAT) signaling occurs in sarcoidosis and represents a target for treatment. Here we report the efficacy of tofacitinib, a Janus kinase (JAK) inhibitor, in a single patient with multiorgan sarcoidosis.
Methods
A patient with long‐standing multiorgan sarcoidosis who was unresponsive to other commonly used therapies, including methotrexate, prednisone, and tumor necrosis factor α blockade, was treated with tofacitinib.
Results
Tofacitinib treatment resulted in clinical remission of cutaneous sarcoidosis lesions and resolution of positron emission tomography avid lesions in internal organs after 6 months. An evaluation of lesional tissue and blood before and during treatment showed resolution of granulomatous inflammation and normalization of disease biomarkers.
Conclusion
This case illustrates the promise of JAK inhibition as a strategy to treat recalcitrant sarcoidosis and suggests that further study of JAK inhibitors in sarcoidosis is needed.
Introduction
Sarcoidosis is an inflammatory disorder characterized by granulomatous inflammation in affected tissue and can involve nearly any organ. Sarcoidosis can be difficult to treat. Glucocorticoids, which have many adverse effects, are a mainstay of treatment. We and others have shown that Janus kinase–signal transducer and activator of transcription (JAK‐STAT) signaling is constitutively active in sarcoidosis 1, 2, 3 as well as granuloma annulare, another granulomatous disease 3. We hypothesize that JAK‐STAT activation in sarcoidosis is a result of increased production of cytokines, such as interferon‐γ (IFN‐γ) and interleukin 6 (IL‐6), by T cells and macrophages, respectively 3.
We recently reported remission of refractory cutaneous sarcoidosis during treatment with tofacitinib, a Janus kinase 1 (JAK1) and JAK3 inhibitor, in three consecutive patients 2, 3. In these patients, we showed that tofacitinib resulted in histologic resolution of granulomatous inflammation in the skin as well as normalization of JAK‐STAT signaling in the skin and blood 2, 3. In six cases of thoracic sarcoidosis, four from lung and two from lymph node biopsies, we showed not only a similar pattern of phosphorylated‐STAT1 (p‐STAT1) and p‐STAT3 activation but also elevated levels of p‐STAT1 and p‐STAT3 as in cutaneous sarcoidosis. We were unable, however, to evaluate in our patients treated with tofacitinib whether internal organ sarcoidosis was also impacted by treatment; thus, it is not known if internal organ sarcoidosis is similarly responsive to tofacitinib. In this report, we evaluate the efficacy of tofacitinib in a single patient with long‐standing multiorgan sarcoidosis recalcitrant to several therapies, including methotrexate, infliximab, and prednisone. The effect of tofacitinib on internal organ sarcoidosis activity was evaluated with serial positron emission tomography–computed tomography (PET‐CT) imaging, and disease activity in the skin and blood was evaluated with serial cellular and molecular analyses before and during treatment.
Methods
A 60‐year‐old woman with a 21‐year history of severe sarcoidosis involving the lungs, lymph nodes, bone, and skin was treated with tofacitinib. The diagnosis of sarcoidosis was established by multiple biopsies showing noncaseating granulomas. The patient's prior and recent treatments are summarized in Figure 1A. A skin biopsy and plasma were collected, and whole‐body 18F‐flurordeoxyglucose PET‐CT was obtained. These studies were repeated after 6 months of tofacitinib therapy to assess the clinical and molecular response.
Figure 1.
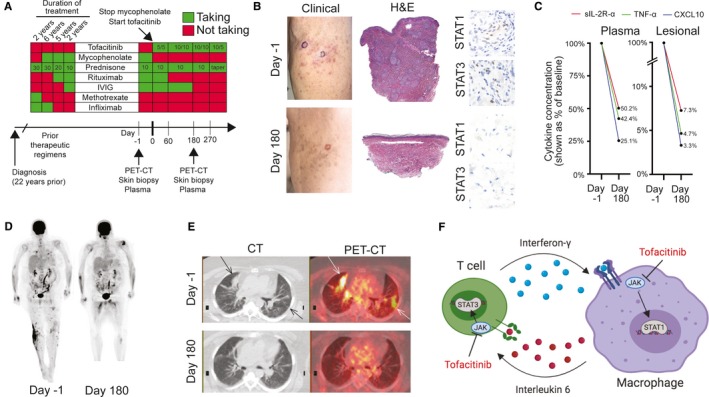
Response of sarcoidosis to tofacitinib. A, Overview of treatments. Doses for the medications were as follows: tofacitinib (daily dose (milligrams) in the morning/daily dose (milligrams) in the evening), mycophenolate sodium at 720 mg twice daily, prednisone (shown as a daily dose in milligrams), rituximab (1000 mg weekly for 2 weeks, repeated every 5‐6 months), intravenous immunoglobulin (IVIG) (1000 mg divided over 2 days, repeated monthly), methotrexate (12.5 mg/wk), infliximab (5 mg/kg every 4‐8 weeks). PET‐CT, positron emission tomography–computed tomography. B, Left panels: clinical photograph of cutaneous involvement by sarcoidosis before treatment (day −1) and after 6 months (day 180) of tofacitinib treatment. Circled areas show where biopsies were performed. In the day 180 photograph, only postinflammatory pigmentation is present clinically, and there was no palpable component to the lesions. Middle panels: hematoxylin and eosin (H&E)–stained skin biopsies showing representative images before treatment (day −1) and after 6 months (day 180) of tofacitinib treatment. Right panels: immunohistochemistry showing phosphorylated‐STAT1 (p‐STAT1) and p‐STAT3 staining before treatment (day −1, upper 2 panels) and after 6 months (day 180, lower 2 panels) of tofacitinib treatment. Activated Janus kinase–signal transducer and activator of transcription (JAK‐STAT) signaling is indicated by nuclear staining. C, Levels of soluble interleukin 2 receptor α (sIL‐2R‐α), tumor necrosis factor α (TNF‐α), and C‐X‐C motif chemokine ligand 10 (CXCL10) in plasma (left panel) and cutaneous tissue interstitial fluid (right panel, labeled “lesional”) before treatment (day −1) and after 6 months (day 180) of tofacitinib treatment. D, Whole‐body 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) before treatment (day −1) and after 6 months (day 180) of tofacitinib treatment. E, Axial view of the lungs showing computed tomography (CT) (left panels) and composite PET‐CT (right panels) before treatment (day −1) and after 6 months (day 180) of tofacitinib treatment. Arrows show areas of disease activity present at baseline. F, Tofacitinib can inhibit both Janus kinase 1 (JAK1) and JAK2 activation of STAT1 induced by interferon‐γ as well as JAK1 and JAK2 activation of STAT3 induced by interleukin 6 and, in doing so, disrupts cytokine signaling loops important for granuloma formation and maintenance. Other JAK‐STAT‐dependent cytokines may also be important.
Plasma was collected after performing density gradient centrifugation on blood collected in EDTA‐coated tubes. Skin biopsies were divided in half. One portion was placed in 10% buffered formalin for histology and immunohistochemistry. The other half was placed in phosphate buffered saline on ice and immediately minced with a sterile scalpel and incubated at 37°C for 1 hour to allow diffusion of soluble factors present in the tissue (tissue interstitial fluid [TIF] preparation). TIF and plasma were analyzed using both a 65‐Plex cytokine/chemokine and 14‐Plex soluble cytokine receptor quantification assay (Eve Technologies) (see 1). Immunohistochemistry was performed with p‐STAT1 (Tyr701) and p‐STAT3 (Tyr705) antibodies (Cell Signaling Technologies).
Immunohistochemistry was performed using standard methods. The p‐STAT1 antibody recognizes STAT1 only when it is phosphorylated at Tyr701 (Cell Signaling Technologies). The p‐STAT3 antibody recognize STAT3 only when it is phosphorylated at Tyr705 (Cell Signaling Technologies).
For PET‐CT imaging, the patient followed a high‐fat/low‐carbohydrate diet and a prolonged fast of greater than 12 hours, as is the current clinical protocol for sarcoidosis studies 4. The patient was injected with a standard dose of fluorine‐18‐fluorodeoxyglucose, and imaging from the vertex of the skull to the toes was performed with low‐dose computed tomography and positron emission tomography (PET).
Results
At the time of our initial evaluation, the patient's sarcoidosis was not controlled with a combination of prednisone, mycophenolate sodium, rituximab, and intravenous immunoglobulin (IVIG), which she had been taking for 7 years (Figure 1A). Because her disease had previously progressed on medications, including methotrexate and infliximab, her prior and current physicians had used alternative agents, such as rituximab and IVIG, commonly used for nerve involvement.
A cutaneous examination revealed numerous subcutaneous nodules and plaques. A skin biopsy showed granulomatous inflammation consistent with sarcoidosis (Figure 1B), and immunohistochemistry showed the presence of p‐STAT1 and p‐STAT3 staining (Figure 1B) consistent with activation of JAK‐STAT signaling, as seen in our prior work 2, 3. PET‐CT showed intense focal uptake in the lungs, lymph nodes, vertebrae and iliac crest, and subcutaneous tissues consistent with sarcoidosis. It is notable that the above findings were present during treatment with a significantly immunosuppressive regimen (Figure 1D).
Mycophenolate was discontinued, and tofacitinib, 5 mg twice daily, was initiated. After 1 month, there was improvement in the skin lesions, but after 2 months (when she would have been due for rituximab), she noted worsening arthralgias, and so the tofacitinib was increased to 10 mg twice daily. After 4 months of treatment, there was clinical resolution of cutaneous disease. After 6 months, a biopsy from previously lesional skin revealed histologic resolution of granulomas and reduction in STAT1 and STAT3 activation (Figure 1B). A measurement of cytokine and soluble protein levels in lesional skin tissue showed a reduction in sarcoidosis biomarkers, including soluble interleukin 2 receptor, tumor necrosis factor α, and C‐X‐C motif chemokine ligand 10 (Figure 1C). A measurement of cytokine levels in the plasma mirrored the reduction in levels of these sarcoidosis biomarkers observed in the skin on tofacitinib (Figure 1C). Changes in the levels of other markers observed during treatment can be found in 1.
In addition to the skin and plasma analyses, PET‐CT was repeated after 6 months of tofacitinib treatment and showed complete resolution of prior sarcoidosis involvement of subcutaneous tissues and internal organs, including lungs, lymph nodes, and bone (Figure 1D and E). Focal PET avidity was noted in the skeletal muscle of the tongue, proximal thighs, and left rotator cuff and was felt to be nonspecific. PET‐CT was again performed 3 months later and showed resolution of these nonspecific areas of PET avidity as well as continued remission of sarcoidosis at prior sites of involvement.
The patient remains on tofacitinib, IVIG has been discontinued, and prednisone is being tapered. The dose of tofacitinib has been decreased to 10 mg in the morning and 5 mg at night with stable improvement (Figure 1A). The patient has tolerated tofacitinib well, with only a urinary tract infection that resolved with antibiotic treatment.
Discussion
These data demonstrate remission of recalcitrant cutaneous and internal organ sarcoidosis with tofacitinib. Histologic resolution of granulomatous inflammation and a marked decrease in sarcoidosis biomarkers in both blood and tissue were observed. In sarcoidosis, dysregulated cytokines, such as IFN‐γ and IL‐6, which help drive granuloma formation, signal via the JAK‐STAT pathway. Inhibition of this pathway with a JAK inhibitor disrupts these cytokine signals between T cells and macrophages (Figure 1F). Improvement in concomitant sarcoidosis has also been reported in two patients with myelodysplastic disorders treated with ruxolitinib, a JAK1 and JAK2 inhibitor, and in a third patient with sarcoidosis‐like systemic granulomatosis also treated with ruxolitinib 5, 6, 7.
There is significant emerging evidence that JAK inhibitors are targeted treatment for sarcoidosis, an often‐morbid disease for which there is a paucity of safe, reliably effective therapies. Prospective studies of JAK inhibitors in sarcoidosis are underway (ClinicalTrials.gov identifiers NCT03910543 and NCT03793439).
Author Contributions
All authors drafted the article, revised it critically for important intellectual content, and approved the final version to be published.
Supporting information
Acknowledgments
We appreciate the assistance of the Yale Dermatopathology Laboratory, especially E. Hast and D. Mekael. We appreciate the participation of the patient in this work. Figure 1F was created using BioRender and published with permission (Dr. Damsky).
Dr. Damsky's work was supported by the Dermatology Foundation. Dr. King's work was supported by a gift from the Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
1William Damsky, MD, PhD, Bryan D. Young, MD, PhD, Edward J. Miller, MD, PhD, J. Antonio Obando, MD, Brett King, MD, PhD: Yale School of Medicine, New Haven, Connecticut; 2Brett Sloan, MD: University of Connecticut School of Medicine, Farmington.
Dr. Damsky has received research funding from Pfizer, the manufacturer of tofacitinib; however, this was not used to support this work, which was initiated before the research funding. Dr. Damsky has also served as a consultant for Eli Lily and Company. Dr. Miller has received grants from Braco and Eidos and has served as a consultant for General Electric, Eidos, Alnylam, and Pfizer, outside the submitted work. Dr. King is an investigator for Concert Pharmaceuticals Inc, Eli Lilly and Company, and Pfizer Inc; he has served as a consultant to and/or has served on advisory boards for Aclaris Therapeutics, Arena Pharmaceuticals, Concert Pharmaceuticals Inc, Dermavant Sciences, Eli Lilly and Company, and Pfizer Inc; he is on speakers bureaus for Pfizer Inc, Regeneron, and Sanofi Genzyme. No other disclosures relevant to this article were reported.
Contributor Information
William Damsky, Email: william.damsky@yale.edu.
Brett King, Email: brett.king@yale.edu.
References
- 1. Zhou T, Zhang W, Sweiss NJ, Chen ES, Moller DR, Knox KS, et al. Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PLoS One 2012;7:e44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Damsky W, Thakral D, Emeagwali N, Galan A, King B. Tofacitinib treatment and molecular analysis of cutaneous sarcoidosis. N Engl J Med 2018;379:2540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damsky W, Thakral D, McGeary MK, Leventhal J, Galan A, King B. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol 2019. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol 2014;21:925–39. [DOI] [PubMed] [Google Scholar]
- 5. Rotenberg C, Besnard V, Brillet PY, Giraudier S, Nunes H, Valeyre D. Dramatic response of refractory sarcoidosis under ruxolitinib in a patient with associated JAK2‐mutated polycythemia. Eur Respir J 2018;52:1801482. [DOI] [PubMed] [Google Scholar]
- 6. Wei JJ, Kallenback LR, Kreider M, Leung TH, Rosenbach M. Resolution of cutaneous sarcoidosis after Janus kinase inhibitor therapy for concomitant polycythemia vera. JAAD Case Rep 2019;5:360–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levraut M, Martis N, Viau P, Suarez F, Queyrel V. Refractory sarcoidosis‐like systemic granulomatosis responding to ruxolitinib. Ann Rheum Dis 2019;78:1606–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


