Abstract
Background
Acupoint application therapy (AAT) has been widely used to treat allergic inflammation induced by allergic rhinitis (AR). The therapeutic effect of acupoint application is obvious. But the underlying therapeutic mechanism is still indistinct. Nerve growth factor (NGF) expression showed a dramatic rise in nasal mucosa tissue after AR, and allergic inflammation also increased significantly. To demonstrate how AAT can improve allergic inflammation by down-regulating the expression of NGF, AR rat models were established by intraperitoneal injection of ovalbumin (OVA) and nasal drops in SD rats. The number of nasal rubbing, sneezing and the degree of runny nose were observed and the symptoms were scored by behavioral symptom scoring method within 3 min. The expression levels of NGF and its downstream key proteins, such as IL-4, IL-5, IL-13, IgE and IFN-γ were determined by q-PCR, Western blot analysis, ELISA and immunofluorescence staining. Furthermore, H&E staining and toluidine blue staining were used to observe the pathological structure of nasal mucosa and mast cells in nasal mucosa, and the ultrastructure of nasal mucosa was observed by electron microscopy.
Results
Our data demonstrated that acupoint application significantly reduced the score of behavioral symptoms, and decreased the expression levels of NGF and its downstream key proteins, including IL-4, IL-5, IL-13, IgE, as well as promoting the expression level of IFN-γ in nasal mucosa tissue in AR rats. Thus, the activation of IgE and viability of mast cells was inhibited.
Conclusion
Our findings suggest that AAT can attenuate allergic inflammation by inhibiting the expression of NGF and its downstream pathway.
Keywords: Acupoint application, Allergic rhinitis, Nerve growth factor, Allergic inflammation, IL-4, Mast cells
Introduction
An increased incidence of allergic rhinitis (AR) has been manifested worldwide in recent years [1, 2]. AR, a chronic inflammatory disease of nasal mucosa, is mediated by immunoglobulin E (IgE) in T helper (TH) 2 type [3, 4]. AR, which stimulates the body by allergen, promotes the transformation of TH reaction from TH1 reaction to TH2 reaction evidenced by inhibiting the expression level of IFN-gamma secreted by TH1 cells, and promoting the expression levels of IL-4, IL-5 and IL-13 secreted by TH2 cells [5]. These cytokines attract mast cells, eosinophils and basophils to inflammatory sites. When the allergen stimulates the collective again, it binds to IgE on the surface of mast cells and produces an allergic reaction [6–8]. The main symptoms of AR were runny nose, itching and sneezing, seriously affecting patients’ quality of life and work [9–11]. Neurotrophins play an immunoregulatory role [12, 13], and mast cells and eosinophils can produce neurotrophins. Nerve growth factor (NGF) is an immune related protein mainly by enhancing the immune response of TH2 [14]. NGF binds to its high affinity receptor TRKA [15], TRPV-1, activates neuronal ion channels and promotes the production of VIP1, which can enhance TH2 immune response [16].
As a unique external treatment method of Traditional Chinese Medicine, acupoint application method can prevent and treat diseases by applying traditional Chinese medicine into acupoints, such as pills, powder and ointment, and using the dual effects of drug percutaneous absorption and meridian effect [17]. Compared with western medicine, acupoint application therapy (AAT) has less side effects and better curative effect [18]. Nevertheless, the specific mechanism of AAT in inhibiting allergic inflammation is complex, which may be related to inhibition of TH2 immune response [19]. NGF can enhance TH2 immune response [16]. In view of the correlation between the two, AAT may inhibit allergic inflammation by inhibiting the expression of NGF and its downstream proteins, such as IL-4, IL-5 and IL-13. To identify this hypothesis, AR rat models were established to observe the changes of inflammation and the expression of NGF and its downstream proteins. H&E staining and toluidine blue staining were used to observe the pathological structure of nasal mucosa and mast cells in nasal mucosa, and the ultrastructure of nasal mucosa was observed by electron microscopy before and after the treatment in order to evaluate the therapeutic effect of AAT on AR.
Materials and methods
Experimental animals
All male Sprague-Dawley rats (180~200 g) were bought from Wenzhou Medical University (Wenzhou, China). All rats were housed in a temperature-controlled (20~22 °C) room on a 12 h light/dark cycle at 45 ± 5% humidity and were given standard laboratory diet. Approval for the study was obtained from the Institutional Animal Care and Use Committee of Wenzhou Medical University. All SD rats were randomly divided into four groups: control group, model group, Prescription NO.1 acupoint application group, Prescription NO.2 acupoint application group.
Ovalbumin (OVA) challenge protocol
Except the rats in control group, other SD rats were made into AR model. The rats in experimental groups were sensitized by intraperitoneal injection of 0.9% sodium chloride solution (1 mL) and OVA (0.3 mg) as antigen and aluminium hydroxide 30 mg as adjuvant once every other day for 8 times. Then they were sensitized by nasal drip containing 0.25% OVA and 0.9% sodium chloride solution once a day for 7 days. The control group was intraperitoneally injected with normal saline, nasal drip and atomization using the same method as above.
Drug preparation and location of acupoints
Based on the agreement parties of the rehabilitation center of the Second Affiliated Hospital of Wenzhou Medical University for more than 20 years, the winter disease and summer treatment ointment was prepared. The ingredients of ointment were white mustard seed, asarum, Angelica dahurica, and Corydalis at 1:1:2:2. A total of 100 items of fine powder were studied. The ointment was prepared with ginger juice or honey, and sealed for preservation. The ointment in Prescription NO.1 acupoint application group was mixed with ginger juice, and in Prescription NO.2 acupoint application group, the ointment was mixed with honey. The acupoints applied were Dazhui, Fengmen (Shuang), Feishu (Shuang), Pishu (Shuang). Point Location of Common Laboratory Animals was used as a Reference Basis for Location. Dazhui acupoint is located between the 7th cervical vertebra and the 1st thoracic vertebra in the middle part of the back. The throttle is located between the ribs of the second thoracic vertebra. Feishu is located in the intercostal space under the third thoracic vertebra. Pishu is located in the intercostal space of the 12th thoracic vertebra.
Behavioral tests
To observe the changes of nasal symptoms in SD rats, symptoms were observed and scored after sensitization (before AAT) and after 7, 14 and 28 days of the AAT. The specific method was to administrate 5 uL of 0.25% OVA and 0.9% sodium chloride solution nasal drip into each side. The number of nasal rubbing, sneezing and the degree of runny nose (within 3 min) were observed and the symptoms were scored. Specific scoring criteria are listed in Table 1 (Chinese Medical Association Council of Otolaryngology, Editorial Committee of Chinese Journal of Otorhinolaryngology. Chinese standard of diagnosis and curative effect evaluation criteria of allergic rhinitis [J]. Chin J Otorhinolaryngol, 1998,33 (3): 134–135). The allergic rhinitis model was considered successful when the total score was more than 5 points.
Table 1.
Scoring criteria for nasal symptoms
| Nasal symptoms | Scoring criteria | Score |
|---|---|---|
| Nasal itching | mild, rubbing the nose several times | 1 |
| severe, scratching the nose, face endless | 2 | |
| Continuous sneezing number | 1 ~ 3 | 1 |
| 4 ~ 10 | 2 | |
| more than 11 | 3 | |
| Runny nose | running to the front nostril | 1 |
| running over the front nostril | 2 | |
| running to all the face | 3 |
Acupoint application treatment
The application of acupoints was performed as mentioned above. Prescription No.1 was mixed with fresh Ginger Juice uniformly (1HG), and Prescription NO.2 was mixed with Honey uniformly (1HH). Rats in the control group (C group) were fed as usual. Rats in the model group (M group) were administrated 5 μl of OVA solution per nostril once a day. Rats in 1HG and 1HH groups were removed the hair on the back acupoints. In the 1HG group and 1HH group, isoflurane anesthesia was first used in rats, and then the ointment (0.5 cm × 0.5 cm) was applied at the acupoints for 1 h lasting for 28 days.
Western blotting
After removing nasal mucosa tissue, the protein was homogenized and then determined by BCA kit (Thermo Fisher Science, Inc., Waltham, MA, USA). Protein (10 μl) was separated by 12% SDS PAGE and transferred to PDVF membrane. It was sealed at room temperature for 2 h with 5% skimmed milk and incubated with corresponding primary and secondary antibodies. Finally, the film (Thermo Fisher Science, Inc., Waltham, MA, USA) was detected by ECL system and photographed.
Immunofluorescence staining
Nasal mucosa was fixed with 4% paraformaldehyde, and then dehydrated. After that, nasal mucosa was cut into sections (4 μm) following paraffin embedding. Slices were baked and dewaxed to water before incubated at room temperature with 3% hydrogen peroxide for 10 min. Subsequently, the slices were blocked in 10% goat serum and 0.3% Triton X-100 PBS solution for 1 h, followed by incubated overnight with corresponding antibodies (4 °C), and then IL-4 and IFN-gamma were double stained with mast cell markers. The primary antibodies and mast cell markers were rabbit anti-NGF (abcam, 1:100), rat anti-IL-4 (Santa, 1:200) and rabbit anti-Mast Cell Chymase (MCC, affinity, 1:100), rabbit anti-IFN-gamma (affinity, 1:100) and mouse anti-Mast Cell Tryptase (MCT, Santa, 1:200). The secondary antibody corresponds to the primary antibody. The nuclei were stained with DAPI. NGF, IL-4 and IFN-gamma positive cells in nasal mucosa sections were observed by fluorescence microscopy (FluoView FV1000, Olympus). Image Pro Plus 6 software (Media Cybernetics, Inc., Rockville, MD, USA) was used to analyze positive cells.
Quantitative real-time PCR (RT-qPCR)
Total RNA was extracted from nasal mucosa using Trizol (Takara, Tokyo, Japan) reagent. Genes were synthesized according to the instructions of the Bio-Rad kit. Then 1 μl of DNA was taken for polymerase chain reaction (PCR). GADPH was used to normalize based on CT value. All the primers were designed and synthesized by Shanghai Jierui Bioengineering Co., Ltd. The sequences of primers are shown in Table 2.
Table 2.
Primers for polymerase chain reaction
| Primers | Sequence (5′-3′) |
|---|---|
| NGF | Forward: TTTGAGACCAAGTGCCGAGC |
| Reverse: CACACACACGCAGGCTGTATCTAT | |
| IL-4 | Forward: CTTGCTGTCACCCTGTTCTGC |
| Reverse: GTGGTGTTCCTTGTTGCCGT | |
| IL-5 | Forward: AGACGATGAGGCTTCCTGTTC |
| Reverse: CTTCGCCACACTTCTCTTTTTG | |
| IL-13 | Forward: CCCTGACCAACATCTCCAGT |
| Reverse: AGGTCCACAGCTGAGATGTC | |
| IFN-γ | Forward: GAGGTGAACAACCCACAGATCCA |
| Reverse: CGACTCCTTTTCCGCTTCCTTAG | |
| IgE | Forward: CACTTCAAGGTTGCGGTCAA |
| Reverse: GCTCATAACACACAGGGCAG | |
| GADPH | Forward: GAGACAGCCGCATCTTCTTG |
| Reverse: TGACTGTGCCGTTGAACTTG |
Enzyme-linked immunosorbent assay (ELISA)
The levels of NGF, IL-4, IL-5, IL-13, IFN-gamma and IgE in serum of rats were determined by enzyme-linked immunosorbent assay (ELISA). At room temperature, samples and diluents were added to the pore, and biotin antibodies were added to the pore. After washing, HRP and chromogenic solution of biotin were added. Finally, the terminating solution is added. The absorbance (OD) of each pore was measured at the wavelength of 450 nm.
Hemotoxylin and eosin (H&E) staining and toluidine blue (TB) staining
H&E
Nasal mucosa was fixed with 4% paraformaldehyde, followed by dehydration and paraffin-embedding. After cut into sections (4 μm), the slices were baked before permeabilitization by xylene, dehydration by gradient alcohol, and dewaxing to water. Cells were stained by hematoxylin and eosin staining (Sigma Chemical Co.). Eosinophils were observed and counted. Double-blind and randomized analysis was performed.
TB
The pretreatment steps of the slices are as described above. The slices are stained with TB staining solution. Mast cells were observed and counted under a microscope. Double-blind and randomized analysis was performed.
Statistical analysis
The original data were processed by SPSS 22.0 statistical software. The experimental results were expressed by X (+s) mean (+standard deviation), and all experimental data were analyzed by Graphpadprism 6 statistical software. LSD test was used for the comparison of homogeneous variance and Dunnett’T3 test for the comparison of uneven variance. Statistic with P value < 0.05 was considered as statistically significant.
Results
Effect of AAT on ovalbumin-induced rhinitis symptoms
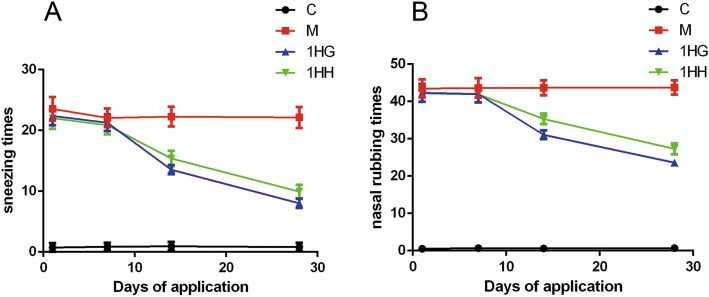
The number of sneezing, rubbing nose and the degree of runny nose were scored and recorded before and after 7, 14 and 28 days of AAT. The results showed that there were no obvious symptoms of rats in control group, and there was no significant difference in the scores of rats in each group on the 7th day after AAT (P > 0.05). After 14 and 28 days of application, the behavioral scores of both 1HG and 1HH groups were significantly lower than those of M group (P < 0.01). Compared with the 1HG group, the behavioral score of 1HH group was significantly higher (P < 0.05) (Tables 3 and 4) (Fig. 1).
Table 3.
The number of nose sneezes that occurred 10 min after OVA intranasal provocation in AR rats before and 7 days, 14 days and 28 days after acupoint application
| Group | n | 1st day | 7th day | 14th day | 28th day |
|---|---|---|---|---|---|
| C | 10 | 0.733 ± 0.704 | 0.867 ± 0.640 | 0.933 ± 0.704** | 0.800 ± 0.676** |
| M | 10 | 23.533 ± 1.960 | 22.067 ± 1.534 | 22.267 ± 1.624 | 22.133 ± 1.727 |
| 1HG | 10 | 22.400 ± 1.549 | 21.267 ± 1.387 | 13.533 ± 0.743** | 8.000 ± 0.756** |
| 1HH | 10 | 22.000 ± 1.773 | 20.867 ± 1.506 | 15.400 ± 1.298**# | 15.400 ± 1.298**# |
The values represent the mean ± S.E.M. Compared with M group, *, P < 0.05; **, P < 0.01; ***, P < 0.001; Compared with 1HG group, #, P < 0.05; ##, P < 0.01; ###, P < 0.001; 1HH Prescription NO.2 mixed with Honey, 1HG Prescription NO.1 mixed with fresh Ginger Juice, M group Model group, C group Control group, OVA Ovalbumin, AR Allergic rhinitis
Table 4.
The number of nose rubs that occurred 3 min after OVA intranasal provocation in AR rats before and 7 days, 14 days and 28 days after acupoint application
| Group | n | 1st day | 7th day | 14th day | 28th day |
|---|---|---|---|---|---|
| C | 10 | 0.533 ± 0.516 | 0.667 ± 0.488 | 0.600 ± 0.507** | 0.667 ± 0.617** |
| M | 10 | 43.467 ± 2.446 | 43.600 ± 2.613 | 43.667 ± 2.024 | 43.733 ± 1.907 |
| 1HG | 10 | 42.333 ± 2.380 | 42.000 ± 2.268 | 31.068 ± 1.163** | 23.600 ± 0.910** |
| 1HH | 10 | 42.200 ± 2.274 | 41.933 ± 2.219 | 35.333 ± 1.447**# | 27.333 ± 1.496**# |
The values represent the mean ± S.E.M. Compared with M group, *, P < 0.05; **, P < 0.01; ***, P < 0.001; Compared with 1HG group, #, P < 0.05; ##, P < 0.01; ###, P < 0.001; 1HH Prescription NO.2 mixed with Honey, 1HG Prescription NO.1 mixed with fresh Ginger Juice, M group Model group, C group Control group, OVA Ovalbumin, AR Allergic rhinitis
Fig. 1.
Effect of AAT on nasal symptoms in AR rats before and 7 days, 14 days and 28 days after acupoint application. Note: a The number of nose sneezes that occurred 10 min after OVA intranasal provocation; b The number of nose rubs that occurred 3 min after OVA intranasal provocation. The values represent the mean ± S.E.M (n = 10/group). Compared with M group, * P < 0.05, **P < 0.01,*** P < 0.001; compared with group 1HG, Significant differences # P < 0.05, ## P < 0.01, ### P < 0.001. AAT, Acupoint Application Therapy; AR, allergic rhinitis; OVA, ovalbumin; 1HH, prescription NO.2 mixed with Honey; 1HG, prescription NO.1 mixed with fresh Ginger Juice; M group, model group; C group, control group
Detection of NGF and its downstream IL-4, IL-5, IL-13 and IFN-gamma in nasal mucosa by western blotting
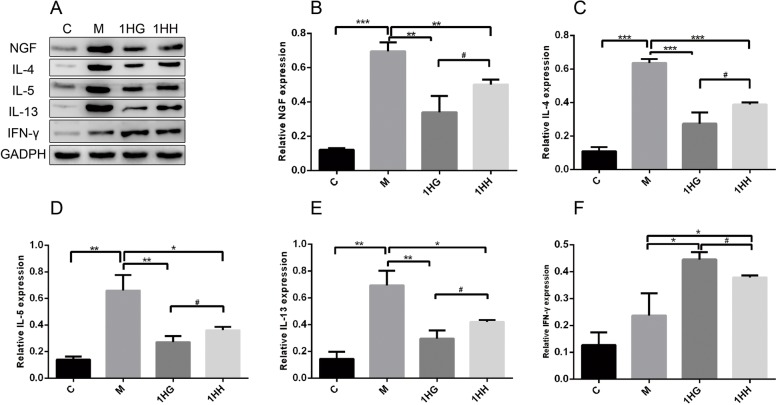
Compared with the control group, the expressions of NGF, IL-4, IL-5 and IL-13 in nasal mucosa of the model group increased significantly (P < 0.001, P < 0.01), while the expression of IFN-gamma increased slightly and failed to achieve any significant difference (P > 0.05). Compared with the model group, the expression of NGF, IL-4, IL-5 and IL-13 in nasal mucosa of the 1HG and 1HH groups decreased (P < 0.001, P < 0.01, P < 0.05), while the expression of IFN-gamma increased (P < 0.05). Compared with the 1HG group, the expression of NGF, IL-4, IL-5 and IL-13 in nasal mucosa of rats in 1HH group increased significantly (P < 0.05), while the expression of IFN-gamma decreased significantly (P < 0.05) (Fig. 2).
Fig. 2.
Effect of AAT on the expression of NGF, IL-4, IL-5, IL-13 and IFN-γ protein in nasal mucosa of AR rats. Note: a The protein level of NGF, IL-4, IL-5, IL-13 and IFN-γ was detected by western blot. b The protein level of NGF was detected by Western blot. c The protein level of IL-4 was detected by Western blot. d The protein level of IL-5 was detected by Western blot. e The protein level of IL-13 was detected by Western blot. f The protein level of IFN-γ was detected by Western blot. The values represent the mean ± S.E.M (n = 3/group). Compared with M group, *P < 0.05, **P < 0.01, ***P < 0.001; compared with 1HG group, Significant differences #P < 0.05, ## P < 0.01, ###P < 0.001; 1HH, prescription NO.2 mixed with Honey; 1HG, prescription NO.1 mixed with fresh Ginger Juice; M group, model group; C group, control group; AAT, Acupoint Application Therapy; AR, allergic rhinitis; NGF, nerve growth factor
Expression of cytokines and mast cells were detected by immunofluorescence technique
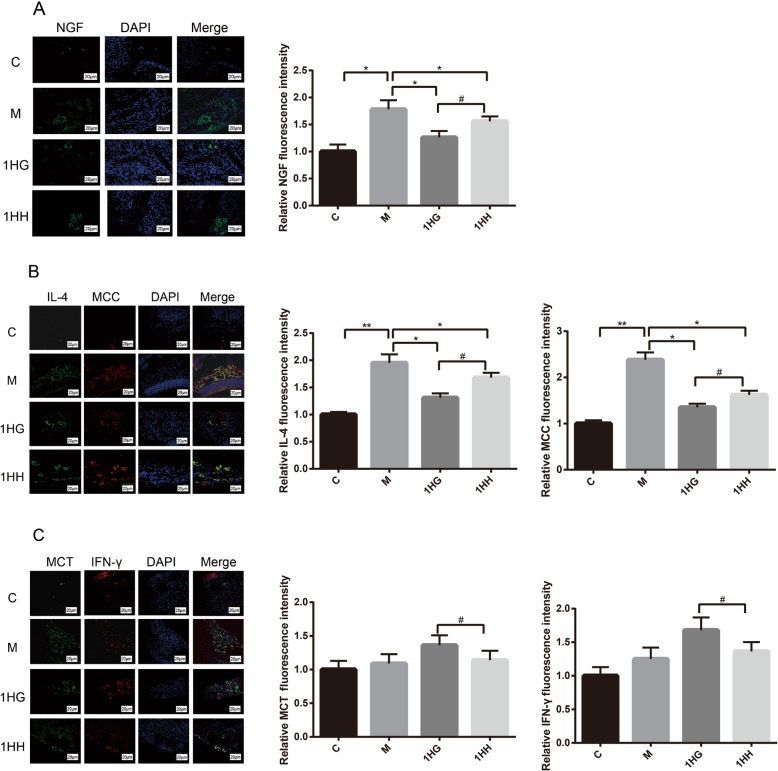
Compared with the control group, the positive spots of NGF and IL-4 protein in nasal mucosa of rats in model group increased significantly (P < 0.01, P < 0.05), but the positive spots of IFN-gamma protein did not increase significantly (P > 0.05). After 28 days of AAT, immunofluorescence results showed that the positive spots of NGF and IL-4 protein in nasal mucosa of rats in 1HG group and 1HH group significantly decreased compared with model group (P < 0.05). Compared with the 1HG group, the positive spots of NGF and IL-4 protein in nasal mucosa of 1HH group significantly increased (P < 0.05), while the positive spots of IFN-gamma protein significantly decreased (P < 0.05) (Fig. 3).
Fig. 3.
Effect of AAT on the expression of NGF、IL-4 and IFN-γ protein in nasal mucosa of AR rats. Note: a Effect of AAT on NGF protein expression in nasal mucosa with Olympus microscope. b The effects of Acupoint Application Therapy on mast cells localization of IL-4 with Olympus microscope. c The effects of Acupoint Application Therapy on mast cells localization of IFN-γ with Olympus microscope. The values represent the mean ± S.E.M (n = 3/group). Compared with M group, Significant differences *P < 0.05, **P < 0.01, ***P < 0.001; compared with 1HG group, Significant differences #P < 0.05, ##P < 0.01, ###P < 0.001; AAT, Acupoint Application Therapy; AR, allergic rhinitis; NGF, nerve growth factor; 1HH, prescription NO.2 mixed with Honey; 1HG, prescription NO.1 mixed with fresh Ginger Juice; M group, model group; C group, control group
Detection of cytokines and IgE gene expression in nasal mucosa by real-time fluorescence quantitative PCR
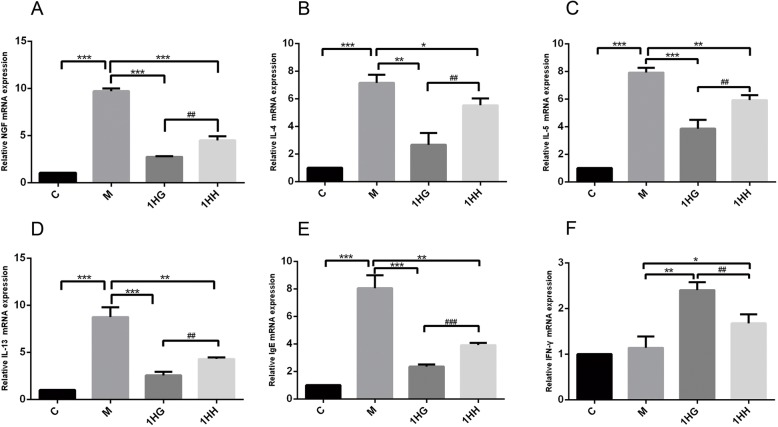
Compared with the control group, the expression of NGF, IL-4, IL-5, IL-13 and IgE mRNA in the model group increased (P < 0.001), while the expression of IFN-gamma mRNA increased slightly (P > 0.05). Compared with the model group, the expression of NGF, IL-4, IL-5, IL-13 and IgE mRNA in the nasal mucosa of rats in 1HG and 1HH groups decreased (P < 0.001, P < 0.01, P < 0.05), while the expression of IFN-gamma mRNA increased (P < 0.01, P < 0.05). Compared with the 1HG group, the expression of NGF, IL-4, IL-5, IL-13 and IgE mRNA in nasal mucosa of rats in 1HH group increased significantly (P < 0.001, P < 0.01), while the expression of IFN-gamma mRNA decreased significantly (P < 0.01) (Fig. 4).
Fig. 4.
Effect of AAT on the expression of NGF, IL-4, IL-5, IL-13, IgE and IFN-γ mRNA in nasal mucosa of AR rats. Note: a The mRNA level of NGF was detected by real-time qPCR. b The mRNA level of IL-4 was detected by real-time qPCR. c The mRNA level of IL-5 was detected by real-time qPCR. d The mRNA level of IL-13 was detected by real-time qPCR. e The mRNA level of IgE was detected by real-time qPCR. f The mRNA level of IFN-γ was detected by real-time qPCR. The values represent the mean ± S.E.M (n = 3/group). Compared with M group, Significant differences *P < 0.05, **P < 0.01, ***P < 0.001; compared with 1HG group, Significant differences #P < 0.05, ##P < 0.01, ###P < 0.001; AAT, Acupoint Application Therapy; AR, allergic rhinitis; NGF, nerve growth factor; qPCR, quantitative PCR; 1HH, prescription NO.2 mixed with Honey; 1HG, prescription NO.1 mixed with fresh Ginger Juice; M group, model group; C group, control group
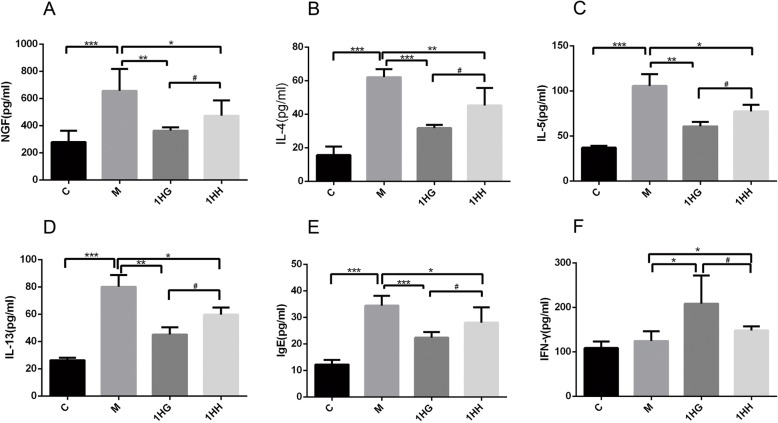
The serum levels of cytokines and IgE in AR rats were detected by ELISA
Compared with the control group, the serum levels of NGF, IL-4, IL-5, IL-13 and IgE in the model group increased (P < 0.001), and the expression of IFN-gamma increased slightly (P > 0.05). Compared with the model group, the serum levels of NGF, IL-4, IL-5, IL-13 and IgE in the 1HG group and 1HH group decreased (P < 0.001, P < 0.01, P < 0.05), while the expression of IFN-gamma increased (P < 0.05). Compared with the 1HG group, the serum levels of NGF, IL-4, IL-5, IL-13 and IgE in 1HH group were significantly increased (P < 0.05), while the expression of IFN-gamma was decreased significantly (P < 0.05) (Fig. 5).
Fig. 5.
Effect of AAT on the expression of NGF, IL-4, IL-5, IL-13, IgE and IFN-γ in serum of AR rats. Note: a The serum level of NGF was detected by enzyme-linked immunosorbent assay (ELISA). b The serum level of IL-4 was detected by ELISA. c The serum level of IL-5 was detected by ELISA. d The serum level of IL-13 was detected by ELISA. e The serum level of IgE was detected by ELISA. f The serum level of IFN-γ was detected by ELISA. The values represent the mean ± S.E.M (n = 3/group). Compared with M group, Significant differences *P < 0.05, **P < 0.01, ***P < 0.001; compared with 1HG group, Significant differences #P < 0.05, ##P < 0.01, ###P < 0.001; AAT, Acupoint Application Therapy; NGF, nerve growth factor; ELISA, enzyme-linked immuno sorbent assay; 1HH, prescription NO.2 mixed with Honey; 1HG, prescription NO.1 mixed with fresh Ginger Juice; M group, model group; C group, control group
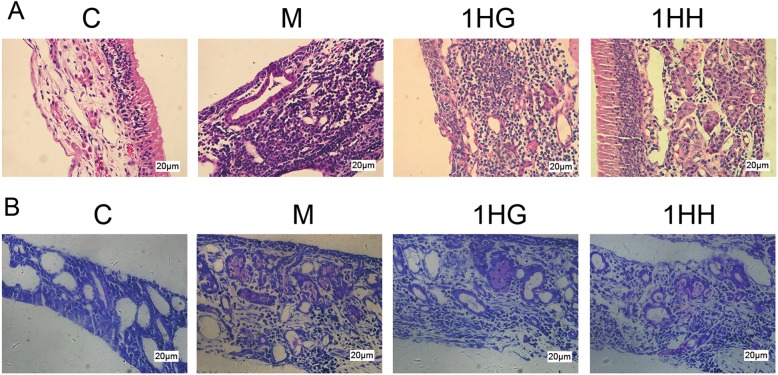
Pathological changes of nasal mucosa by H&E staining and the number and morphological changes of mast cells in nasal mucosa by TB staining
H&E staining
No obvious pathological changes were found in nasal mucosa of the control group. In model group, hyperemia, edema, gland hyperplasia, mucus secretion, tissue structure disorder and inflammatory cell infiltration appeared in nasal mucosa. After 28 days of AAT, the pathological degree of 1HG group and 1HH group were significantly reduced, and inflammatory cell infiltration was reduced. The pathological improvement degree in 1HG group was more obvious, and the number of inflammatory cells in nasal mucosa was the less.
TB staining
Mast cells were not found in the nasal mucosa of the control group, but a large number of purple-red mast cells were observed in the model group. After 28 days of treatment, the number of mast cells in both 1HH and 1HG groups decreased, especially in 1HG group (Fig. 6).
Fig. 6.
Effect of AAT on the proliferation of eosinophils and mast cells in nasal mucosa of AR rats
Note: a Nasal mucosa tissue was stained with H&E for eosinophils. Original magnification 400×, scale bar = 20 μm. b Nasal mucosa tissue was stained with TB for mast cells. Original magnification 400×, scale bar = 20 μm. The values represent the mean ± S.E.M (n = 3/group). Compared with M group, Significant differences *P < 0.05, **P < 0.01, ***P < 0.001; compared with 1HG group, Significant differences #P < 0.05, ##P < 0.01, ###P < 0.001; AAT, Acupoint Application Therapy; H&E,hematoxylin and eosin; TB, toluidine blue; 1HH, prescription NO.2 mixed with Honey; 1HG, prescription NO.1mixed with fresh Ginger Juice; M group, model group; C group, control group
Discussion
In this paper, we demonstrated that AAT can alleviate allergic inflammation in rats with AR, and inhibit IgE allergic reaction by inhibiting the activation of NGF and reducing the production of inflammatory factors (such as IL-4, IL-5, etc.). These results reveal the mechanism of AAT in the treatment of AR. A previous study reported that the occurrence of AR is closely related to the activation of NGF and neuroimmunity [20].
The common pathogenesis of AR is the imbalance of Th1/Th2 cell immunity. The main manifestation of AR is inflammatory disease with Th2 reaction [21]. Th2 cells mainly secrete cytokines such as IL-4, IL-5 and IL-13, which mainly stimulate humoral immune response. Among those cytokines, IL-4 most closely associated with AR [22, 23]. TH1 cells mainly secrete IFN-gamma and mediate cellular immune response. IFN-gamma closely related to AR [24]. The cytokines secreted by TH2 cells can promote the production of IgE (by B cells). IgE binds to high affinity receptor Fc receptor (Fc epsilon RI) (appeared in mast cells and basophilic granulocyte surface) [25], which promotes the degranulation of mast cells [26], and induces not only the release of histamine, leukotriene and other bioactive mediators, but a variety of pathological reactions (such as increased secretion of smooth muscle contraction and glandular mucus, dilatation of capillaries and small vessels, and increased permeability of blood vessels), leading to the occurrence of AR [27]. From the behavioral point of view, the symptoms of AR rats are consistent with the pathological reaction. After acupoint application, the symptoms of AR rats were alleviated slightly or significantly, indicating that acupoint application is effective for AR treatment.
NGF also participates in this immune response. NGF not only maintains the survival of neurons [28], but also plays an extensive role in immune, reproductive and hematopoietic systems [29–31], and directly participates in the immune response [32]. It has been reported that the cells involved in immune response, such as eosinophils, CD4 + T cells, some B cells and mast cells, can also produce and release NGF [33, 34]. There are a large number of sensory nerve fibers in nasal epithelial cells, most of which are markers of nociceptors, including trpv-1 and trpa-1 [35]. TH2 cytokines, such as IL-5, can activate lung noxious neurons and secrete VIP, thus stimulating lymphocyte to produce airway allergic reaction. These lymphocytes can secrete cytokines such as IL-5 and aggregate into effectors. Immune cells enter the disease state and further activate the sensory organs [36]. NGF promotes the proliferation and accumulation of immune cells through the aforementioned activation channels, and induces immune cells to release various bioactive factors (such as IL-4, IL-13, IL-5, IFN-gamma), which leads to the aggravation of allergic inflammation. In addition, immune cells such as eosinophils, mast cells and basophils can synthesize large amounts of NGF and further release [37], thus forming a positive feedback immune response, aggravating the progress of allergic rhinitis.
From the results of Western blot and PCR in this study, AAT can inhibit the mRNA and protein expression levels of NGF, IL-4, IL-5, IL-13, and promote the protein and mRNA expression of IFN-gamma. These results suggested that AAT can inhibit the formation of NGF in nasal mucosa and the positive feedback loop induced by NGF, while reduce the levels of IL-4 and NGF in nasal mucosa through nerve immune pathway of NGF/IL-4, and improve allergic inflammation in AR rats. In addition, our results suggest that IL-4 and IFN-gamma are co-expressed with mast cells. Compared with the control group, the co-expression of IL-4 and mast cell marker (chymase) [38] in nasal mucosa of AR rats increased. The up-regulation of chymase is related to the activation of mast cells. The results showed that mast cells were activated after AR. After acupoint application, the co-expression of IL-4 and chymase in nasal mucosa was lower than that in AR rats, and the co-expression of IFN-gamma and tryptase [39] was significantly higher than that in model group. Therefore, AAT can alleviate allergic inflammation in AR rats by inhibiting the expression of IL-4 in mast cells of nasal mucosa and increasing the expression of IFN-gamma in mast cells.
The powder is composed of four traditional Chinese medicine drugs, white mustard seed, asarum, Angelica dahurica and corydalis. White mustard seed has anti-inflammatory effect [40]. Angelica dahurica has anti-inflammatory effect by inhibiting mast cell degranulation and histamine release [41]. Asarum has anti-immune and anti-allergic effects. Compared with honey group, ginger group had stronger stimulation intensity, less volatilization of local water, and water content in skin increased sharply, which enhanced aseptic inflammation of local skin and made drugs more easily absorbed [42]. Therefore, compared with 1HH group, the symptoms in 1HG group were more obvious, which could inhibit the production of NGF, IL-4, IL-5, IL-13 and promote the production of IFN-gamma.
Conclusion
Acupoint application therapy can improve the allergic inflammation response of AR rats by reducing the expression of NGF, IL-4, IL-13, IL-5 and IgE in nasal mucosa of AR rats and enhancing the expression of IFN-gamma. Moreover, ginger has a stronger therapeutic effect than honey.
Acknowledgements
This research work was supported by the National Natural Science Foundation of China (No. 81873376), the Appropriate Technology Cultivation Project of Zhejiang Administration of Traditional Chinese Medicine (No.2014ZP005) and Basic Scientific Research Projects of Wenzhou Science and Technology Bureau (No.Y20180207,Y20160215).
Abbreviations
- AA
Acupoint application
- AR
Allergic rhinitis
- ELISA
Enzyme-linked ImmunoSorbent Assay
- HE
Hematoxylin-Eosinstaining
- IFN-gama
Interferon-gama
- IgE
Immunoglobulin E
- IL-13
Interleukin-13
- IL-4
Interleukin-4
- IL-5
Interleukin-5
- NGF
Nerve growth factor
- OVA
Ovalbumin
- PBS
Phosphate buffered saline
- PCR
Polymerase chain reaction
- PFA
Polymerisatum formaldehydum
- PVDF
Polyvinylidenedifluoride
- SD
Standard deviation
- SDS
Sodiumdodecyl sulfate
Authors’ contributions
WT, GY, XL, KZ and SJ conceived and designed the analysis. XC, QW, XY and RH collected the data. XC, QW, XY and RH contributed data or analysis tools. XC, QW, XY and RH performed the analysis. XC wrote the paper. All authors read and approved the manuscript.
Authors’ information
See information below the heading.
Funding
the National Natural Science Foundation of China (No. 81873376), the Appropriate Technology Cultivation Project of Zhejiang Administration of Traditional Chinese Medicine (No.2014ZP005) and Basic Scientific Research Projects of Wenzhou Science and Technology Bureau (No.Y20180207,Y20160215).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All procedures were approved by the Animal Care and Use Committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenzhan Tu and Xiaolong Chen contributed equally to this work.
Contributor Information
Kecheng Zhou, Email: zhou_kc@126.com.
Songhe Jiang, Email: jshwz@126.com.
References
- 1.Shin JH, Kim DH, Kim BY, et al. Anti-Interleukin-9 antibody increases the effect of allergen-specific immunotherapy in murine allergic rhinitis. Allergy Asthma Immunol Res. 2017;9:237–246. doi: 10.4168/aair.2017.9.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2–S8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 3.Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46:1139–1151. doi: 10.1111/cea.12780. [DOI] [PubMed] [Google Scholar]
- 4.Li LJ, Ma N, Zeng L, et al. Flagellin modulates IgE expression in B cells to initiate food allergy in mice. Am J Transl Res. 2016;8:2748–2757. [PMC free article] [PubMed] [Google Scholar]
- 5.Bosmans G, Shimizu Bassi G, Florens M, Gonzalez-Dominguez E, Matteoli G, Boeckxstaens GE. Cholinergic modulation of type 2 immune responses. Front Immunol. 2017;8:1873. doi: 10.3389/fimmu.2017.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng LE, Locksley RM. Allergic inflammation--innately homeostatic. Cold Spring Harb Perspect Biol. 2014;7:a016352. doi: 10.1101/cshperspect.a016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciprandi G, Marseglia GL, Castagnoli R, et al. From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol. 2015;11:1321–1333. doi: 10.1586/1744666X.2015.1086645. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Finn DF, Barlow JW, Walsh JJ. Mast cell stabilisers. Eur J Pharmacol. 2016;778:158–168. doi: 10.1016/j.ejphar.2015.05.071. [DOI] [PubMed] [Google Scholar]
- 9.Jaruvongvanich V, Mongkolpathumrat P, Chantaphakul H, Klaewsongkram J. Extranasal symptoms of allergic rhinitis are difficult to treat and affect quality of life. Allergol Int. 2016;65:199–203. doi: 10.1016/j.alit.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010;2:65–76. doi: 10.4168/aair.2010.2.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–1183. doi: 10.1067/mai.2003.1592. [DOI] [PubMed] [Google Scholar]
- 12.Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583–589. doi: 10.1016/j.jaci.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Raap U, Kapp A. Neuroimmunological findings in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2005;5:419–424. doi: 10.1097/01.all.0000183111.78558.4d. [DOI] [PubMed] [Google Scholar]
- 14.Raap U, Braunstahl GJ. The role of neurotrophins in the pathophysiology of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2010;10:8–13. doi: 10.1097/ACI.0b013e328334f5de. [DOI] [PubMed] [Google Scholar]
- 15.Miller FD, Kaplan DR. Neurobiology. TRK makes the retrograde. Science. 2002;295:1471–1473. doi: 10.1126/science.1069897. [DOI] [PubMed] [Google Scholar]
- 16.Noga O, Englmann C, Hanf G, Grutzkau A, Guhl S, Kunkel G. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin Exp Allergy. 2002;32:1348–1354. doi: 10.1046/j.1365-2745.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Yanhong LZ, Lei B, Zhixin Y, Shaowa L, Yongji L. A survey of treating allergic rhinitis with application of acupoint. J Clin Acupunct Moxibustion. 2010;26:68–70. [Google Scholar]
- 18.Xuan Lihua YB, Xu F, Shuyan Z, Zeyun M, Yonggang X, Xiang W, Xiaofeng M. Clinic research on preventing and treating bronchial asthma with different skin stimulus plaster in summer. China J Tradit Chin Med Pharm. 2012;27:1807–1810. [Google Scholar]
- 19.Lihua JYX. The action mechanism and clinical application of sticking points for allergic rhinitis. Jilin J Tradit Chin Med. 2015;35:84–87. doi: 10.1016/S0254-6272(15)30013-3. [DOI] [Google Scholar]
- 20.Chen PC, Hsieh MH, Kuo WS, Kao HF, Hsu CL, Wang JY. Water-soluble chitosan inhibits nerve growth factor and attenuates allergic inflammation in mite allergen-induced allergic rhinitis. J Allergy Clin Immunol. 2017;140:1146–1149.e1148. doi: 10.1016/j.jaci.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Malmhall C, Bossios A, Pullerits T, Lotvall J. Effects of pollen and nasal glucocorticoid on FOXP3+, GATA-3+ and T-bet+ cells in allergic rhinitis. Allergy. 2007;62:1007–1013. doi: 10.1111/j.1398-9995.2007.01420.x. [DOI] [PubMed] [Google Scholar]
- 22.Li K, Huang EP, Su J, et al. Therapeutic role for TSP-2 antibody in a murine asthma model. Int Arch Allergy Immunol. 2018;175:160–170. doi: 10.1159/000486313. [DOI] [PubMed] [Google Scholar]
- 23.Yu S, Han B, Liu S, et al. Derp1-modified dendritic cells attenuate allergic inflammation by regulating the development of T helper type1(Th1)/Th2 cells and regulatory T cells in a murine model of allergic rhinitis. Mol Immunol. 2017;90:172–181. doi: 10.1016/j.molimm.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Fouladi S, Masjedi M, Ghasemi R, Hakemi MG, Eskandari N. The in vitro impact of Glycyrrhizic acid on CD4+ T lymphocytes through OX40 receptor in the patients with allergic rhinitis. Inflammation. 2018;41:1690–1701. doi: 10.1007/s10753-018-0813-8. [DOI] [PubMed] [Google Scholar]
- 25.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelfand EW, Joetham A, Wang M, Takeda K, Schedel M. Spectrum of T-lymphocyte activities regulating allergic lung inflammation. Immunol Rev. 2017;278:63–86. doi: 10.1111/imr.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakli HA, Riley TD. Allergic rhinitis. Prim Care. 2016;43:465–475. doi: 10.1016/j.pop.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Tao Feng ZBaHZ The relationship between neurotrophic factors and respiratory allergic inflammation. J Southeast Univ (Medical Science Edition) 2007;26:322–325. [Google Scholar]
- 29.Park MJ, Kwak HJ, Lee HC, et al. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J Biol Chem. 2007;282:30485–30496. doi: 10.1074/jbc.M701081200. [DOI] [PubMed] [Google Scholar]
- 30.Shushen BWQ. Immunological effects of nerve growth factor. Int J Immunol. 1995;4:219–20.
- 31.Triaca V, Aloe L. Neuronal markers expression of NGF-primed bone marrow cells (BMCs) transplanted in the brain of 6-hydroxydopamine and ibotenic acid lesioned littermate mice. Neurosci Lett. 2005;384:82–86. doi: 10.1016/j.neulet.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 32.Frossard N, Freund V, Advenier C. Nerve growth factor and its receptors in asthma and inflammation. Eur J Pharmacol. 2004;500:453–465. doi: 10.1016/j.ejphar.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99:2214–2220. doi: 10.1182/blood.V99.6.2214. [DOI] [PubMed] [Google Scholar]
- 34.Raap U, Goltz C, Deneka N, et al. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol. 2005;115:1268–1275. doi: 10.1016/j.jaci.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talbot S, Abdulnour RE, Burkett PR, et al. Silencing Nociceptor neurons reduces allergic airway inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Zhihai ZS, Chenglong W, Weihong J. Detection of NGF mRNA expression in nasal mucosa of allergic rhinitis patients. Chin J Otorhinolaryngol-skull Base Surg. 2005;06:374–7.
- 38.Le DD, Schmit D, Heck S, et al. Increase of mast cell-nerve association and neuropeptide receptor expression on mast cells in perennial allergic rhinitis. Neuroimmunomodulation. 2016;23:261–270. doi: 10.1159/000453068. [DOI] [PubMed] [Google Scholar]
- 39.Baba S, Kondo K, Suzukawa M, Ohta K, Yamasoba T. Distribution, subtype population, and IgE positivity of mast cells in chronic rhinosinusitis with nasal polyps. Ann Allergy Asthma Immunol. 2017;119:120–128. doi: 10.1016/j.anai.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Yang R, Zhou Q, Wen C, et al. Mustard seed (Sinapis Alba Linn) attenuates imiquimod-induced psoriasiform inflammation of BALB/c mice. J Dermatol. 2013;40:543–552. doi: 10.1111/1346-8138.12119. [DOI] [PubMed] [Google Scholar]
- 41.Li D, Wu L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp Ther Med. 2017;14:874–880. doi: 10.3892/etm.2017.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jinfei Z, Qimiao H, Wenci C, Sisi L, Songhe J. Clinical study of Sanjiu herbal patch on acupoints with different intensity on persistent allergic rhinitis. J Emerg Tradit Chin Med. 2016;25:236–238+247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.