Abstract
BACKGROUND.
To design better antimicrobial stewardship programs, detailed data on the primary drivers and patterns of antibiotic use are needed.
OBJECTIVE.
To characterize the indications for antibiotic therapy, agents used, duration, combinations, and microbiological justification in 6 acute-care US facilities with varied location, size, and type of antimicrobial stewardship programs.
DESIGN, PARTICIPANTS, AND SETTING.
Retrospective medical chart review was performed on a random cross-sectional sample of 1,200 adult inpatients, hospitalized (>24 hrs) in 6 hospitals, and receiving at least 1 antibiotic dose on 4 index dates chosen at equal intervals through a 1-year study period (October 1, 2009-September 30, 2010).
METHODS.
Infectious disease specialists recorded patient demographic characteristics, comorbidities, microbiological and radiological testing, and agents used, dose, duration, and indication for antibiotic prescriptions.
RESULTS.
On the index dates 4,119 (60.5%) of 6,812 inpatients were receiving antibiotics. The random sample of 1,200 case patients was receiving 2,527 antibiotics (average: 2.1 per patient); 540 (21.4%) were prophylactic and 1,987 (78.6%) were therapeutic, of which 372 (18.7%) were pathogen-directed at start. Of the 1,615 empirical starts, 382 (23.7%) were subsequently pathogen-directed and 1,231 (76.2%) remained empirical. Use was primarily for respiratory (27.6% of prescriptions) followed by gastrointestinal (13.1%) infections. Fluoroquinolones, vancomycin, and antipseudomonal penicillins together accounted for 47.1% of therapy-days.
CONCLUSIONS.
Use of broad-spectrum empirical therapy was prevalent in 6 US acute care facilities and in most instances was not subsequently pathogen directed. Fluoroquinolones, vancomycin, and antipseudomonal penicillins were the most frequently used antibiotics, particularly for respiratory indications.
Antibiotics are among the most commonly prescribed drugs in hospitalized patients. Antibiotic use is an important driver of antimicrobial resistance and a growing cause of morbidity and mortality worldwide.1 The association between antibiotic use and resistance emphasizes the importance of antimicrobial stewardship programs (ASPs), which are designed to optimize clinical outcomes while minimizing unintended consequences of antimicrobial use, such as toxicity, the selection of pathogenic organisms, increased cost, and the emergence of resistance.2,3 ASPs can improve antibiotic use and decrease adverse events.4 Although the benefits of ASPs are widely recognized, ASP infrastructures vary widely in US acute care facilities and detailed data on the determinants of antibiotic prescribing are needed to design better ASPs.4,5
Large-scale benchmarking and point prevalence surveys of US and European hospitals have reported that 19%−59% of adult inpatients receive antibiotic therapy,6–10 chiefly for respiratory infections, with broad-spectrum beta-lactam, vancomycin, and fluoroquinolone antibiotics constituting the majority of use.11–14 Approximately 22% to 49% of broad-spectrum antibiotic use has been found to be redundant and inappropriate,15–17 with more than half of treatments usually lacking microbiological documentation of infection.13,18,19
US studies published in the past decade are largely confined to large teaching hospitals9 and focus on specific populations,20–22 indications, and antibiotic classes.23 Recent studies that have examined the prevalence of antibiotic use have not looked further at the determinants of antibiotic prescriptions24 and are limited by a cross-sectional design10 that does not permit an assessment at different times in the year when prevalence of infection may vary. An expanded understanding of the epidemiology of antibiotic prescribing could improve the quality of inpatient antibiotic use and inform the implementation of ASPs. We aimed to characterize the indications for the start of antibiotic therapy, agents used, duration, combinations, and microbiological justification in 6 acute care facilities that vary with respect to location, size, type, and presence of ASPs.
METHODS
Setting
We conducted a retrospective medical chart review study in a pragmatic sample of 6 US hospitals: 4 community and 2 university-affiliated hospitals. Details of the facilities and their ASPs have been described in an earlier article.25 Three of the 6 sites had formal ASPs. All sites had pharmacy and therapeutics committees and restricted formularies with facility-specific criteria for dispensing certain antimicrobials.
Study Design
Medical charts were randomly selected from a population of all inpatient admissions in the 12-month period from October 1, 2009, through September 30, 2010. Details of this study design have been published.25 In brief, 4 dates (November 20, 2009; February 10, 2010; May 20, 2010; August 10, 2010) were chosen at equal intervals through the study period and patient medical charts were randomly selected from a pharmacy-sourced database, with an active antibiotic prescription serving as the enrollment trigger. For each index date and site, abstractors enrolled approximately 50 nonduplicate, adult (>18 years of age) inpatients admitted for at least 24 hours to nonpsychiatric wards.
Data Collection
Trained infectious disease physician reviewers used standardized electronic data entry forms on the Research Electronic Data Capture software26 to record patient demographic characteristics, drug name, dose, duration, indication, and documentation source for up to 8 antibiotics started and/or coadministered in the index window, defined as 72 hours before and 14 days after the index date. Reviewers were asked to make a clinical judgment about duration of antibiotics on the basis of all the information available in the record when documentation for starting or stopping antibiotics was conflicting. The International Classification of Diseases, Ninth Revision, codes for up to 5 admission diagnoses were extracted and used to calculate the Charlson Comorbidity Index.27 Patients were considered immune-compromised if they had human immunodeficiency virus with CD4 count less than 200 cells/mL, bone marrow or solid organ transplant, or chemotherapy for cancer. The Sabadell modification of the McCabe-Jackson score was used to assign prognosis at discharge and included 4 subjective categories: good prognosis, poor long-term prognosis (>6 months) with unlimited intensive care unit readmission, poor short-term prognosis (<6 months) with debatable intensive care unit readmission, and death expected during hospitalization with intensive care unit readmission not recommended.28 The culture isolate source, hospital day of specimen collection, and culture result were recorded for all microbiological tests in the index window. Human subjects approval was obtained from institutional review boards at all facilities before data collection. Data were analyzed using Stata, version 11 (StataCorp).
Definitions
Antibiotic prescription included any antibiotic dispensed (including a single-dose administration) by the hospital pharmacy. Antibiotic use was measured by days of therapy (DOTs), equal to the total duration of an order measured in hospital days, over the number of patient-days, measured as the total length of stay in days, multiplied by 100. Single-dose use was counted as 1 DOT as previously.25 Duration of antibiotic therapy include duration of therapy after hospital discharge unless an antibiotic was discontinued before discharge and changed to an oral formulation that would be started after discharge.
Total antibiotic use in the hospitals can be measured using defined daily doses (DDD) per 100 patient-days. DDDs reflect the average maintenance dose for an antibiotic’s major indication over the number of patient-days, measured as the total length of stay in days, multiplied by 100. We quantified antibacterial use by using DOT rather than DDD29 because DDDs do not reflect the dosages used in clinical practice for many antimicrobials in the United States and the total rate of measured antibacterial use.30,31
A regimen was considered empirical throughout if it was never pathogen directed—that is, there was no evidence in the medical chart to suggest that the physicians changed antimicrobial therapy on the basis of etiology (physician notes, culture and sensitivity data, Gram stains, or rapid tests).
Prophylactic indication included all prescription orders for pre- or postoperative surgical prophylaxis or any other administration with the purpose of preventing infection or managing a chronic condition, including neutropenia and cystic fibrosis. Therapeutic indication included all antibiotic prescription orders that had a presumed or documented nonprophylactic indication. Therapeutic prescriptions were divided into pathogen-directed from start—when the bacterial etiology of the infection was known before the start of therapy—and empirical therapy, which included prescriptions started without identification of a pathogen.
RESULTS
Prevalence of Antibiotic Use
Across the 6 hospitals and during the study period, there were 97,850 in-patient admissions with a total duration of hospitalization of 592,840 patient-days and a mean length of stay (calculated as the ratio of reported patient-days to inpatient admissions) of 6.06 days. Several of the facilities were unable to provide reliable aggregate data in either of the commonly used measures (DDDs or DOTs) and thus total antibiotic use was reported only when there was complete information. Total antibiotic use for facilities that provided data was 86.3 DDD per 100 patient-days and 46.6 antibiotic prescriptions per 100 patient-days (Table 1). There were 6,812 patients hospitalized on the 4 review dates, of whom 4,119 (60.4%) had an active antibiotic order. Of these, reviewers enrolled 1,200 randomly selected nonduplicate charts of nonpsychiatric, nonpediatric admissions.
TABLE 1.
Prevalence of Antibiotic Use per Hospital
| Variable | Hospital A | Hospital B | Hospital C | Hospital D | Hospital E | Hospital F | Total |
|---|---|---|---|---|---|---|---|
| Facility type | Community-private | Teaching | Teaching | Community-private | Community-public | Community-private | … |
| Study period antibiotic use in DDD/100 PDa | NA | 81.8 | NA | 95.8 | NA | 89.7 | 86.3 |
| Study period antibiotic use in prescriptions/100 PDa,b | NA | 59.5 | 51.8 | NA | 12.3 | 52.2 | 46.6 |
| Inpatients on ABT, no. (%)c | 489 (47.6%) | 1,056 (48.5%) | 1,448 (78.3%) | 365 (46.6%) | 241 (60.9%) | 519 (65.9%) | 4,119 (60.4%) |
| LOS of analyzed cases, median (IQR), d | 8 (5–14) | 11 (5.5–26) | 21 (9–48) | 8 (5–12) | 7(4–14) | 8 (5–15) | 9 (5–18) |
| Duration of antimicrobial therapy, median (IQR), d | 4 (2–7) | 4 (2–7.5) | 7(3–13) | 3 (1–5) | 5 (3–7) | 4 (2–8) | 4.5 (3–7) |
NOTE. ABT, antibiotic therapy; DDD, defined daily dose; IQR, interquartile range; LOS, length of stay; NA, not available; PD, patient days.
Data from hospital B based on inpatient pharmacy orders summarized by antibiotic stewardship program coordinator; hospitals D and F report total patient ordering data. Three facilities (A, C, E) were unable to provide reliable aggregate data in DDD/PD because of duplicate or inaccurate entries in pharmacy order databases. Total includes only hospitals with complete information.
Inpatient admissions include acute and emergency department visits for all facilities. Data for hospitals A-C is for 2010 only (A and C sourced from state’s Office of Statewide Health Planning and Development database, B from hospital administrative report).
Two facilities (A and D) were unable to provide reliable data. In hospitals B and F, the number of prescriptions includes dose changes and adjustments as separate events. Total includes only hospitals with complete information.
Characteristics of Patients Receiving Antibiotics
Table 2 displays characteristics of the patients included in the chart review and analysis. Study patients had a mean age of 61.9 years and a mean length of stay of 14.7 days. Of these patients, 508 (42.3%) had 1 or more comorbid conditions (defined on the basis of the Charlson Comorbidity Index), 536 (44.7%) had poor prognosis (on the basis of the McCabe-Jackson score), 150 (12.5%) were severely immunocompromised, 300 (25.0%) had received antibiotics during the 30 days prior to the study day, and 366 (30.5%) were critically ill (hospitalized in the intensive care unit).
TABLE 2.
Characteristics of 1,200 Enrolled Patients
| Variable | Value | |
|---|---|---|
| Age, mean (SD), y | 61.9 | 18 |
| ICU admission | 366 | (30.5) |
| LOS, mean (SD), d | 14.7 | 22.9 |
| History of antibiotic allergies on admission | 311 | (25.9) |
| Beta-lactam allergy | 220 | (18.3) |
| Received antibiotics within previous 30 d | 300 | (25.0) |
| Severely immune-compromised | 150 | (12.5) |
| Chemotherapy | 82 | (6.8) |
| Solid organ transplant | 31 | (2.6) |
| AIDSa | 9 | (0.7) |
| Bone marrow transplant | 8 | (0.7) |
| Otherb | 33 | (2.7) |
| Charlson Comorbidity Indexc | ||
| No comorbidities (0) | 692 | (57.7) |
| Mild (1–5) | 224 | (18.7) |
| Severe (>5) | 284 | (23.7) |
| McCabe-Jackson scored | ||
| Good prognosis | 664 | (55.3) |
| Poor long-term prognosis (>6 mo) | 355 | (29.6) |
| Poor short-term prognosis (<6 mo) | 123 | (10.3) |
| Death expected during hospitalization | 58 | (4.8) |
NOTE. Data are no. (%) of patients unless otherwise indicated. ICU, intensive care unit; LOS, length of stay; PD, patient-days.
AIDS defined as human immunodeficiency virus with CD4 count <200 cells/mL.
Other conditions deemed as severe immunosuppression: 12 cases of blood cancers or other chronic or acute oncohematological conditions, 9 cases of cystic fibrosis, 2 cases of long-term steroid use for autoimmune disorders.
The Charlson Comorbidity Index is a weighted measure of the presence of 22 chronic conditions.27 Up to 5 International Classification of Diseases, Ninth Revision, codes per patient were recorded with chief complaints and infectious syndromes given a priority; The index was calculated during analysis using the user-written CHARLSON add-on module in Stata, version 11.
Assigned patients into 1 of 4 categories based on judgment of how clinical information on admission predicted likelihood of survival and recovery. We used a modified version of the severity of illness score validated in Fernandez et al.28
Patient-Level Indications for Antibiotic Use
Of the 1,200 study patients, 282 (23.5%) received antibiotics only for prophylaxis and 804 (67.0%) only for therapeutic indications, whereas 114 (9.5%) received antibiotics for both prophylaxis and therapeutic indications (Table 3). Of the 918 patients (76.5%) who received antibiotics for therapeutic indications, antibiotics were pathogen directed at start in 120 patients (13.1%) and antibiotics were started empirically in 798 patients (86.9%). Among 798 patients who were started on empirical antibiotics, in 216 patients (27.1%) they were subsequently pathogen directed and in 582 patients (72.9%) antibiotics remained empirical. Of the 918 patients receiving therapeutic antibiotics, 365 (39.8%) received antibiotics for respiratory tract infections, 200 (21.8%) for gastrointestinal infections, 178 (19.4%) for bloodstream infections, 171 (18.6%) for urinary tract infections, and 171 (18.6%) for skin and soft-tissue infections (Table 3).
TABLE 3.
Patient- and Prescription-Level Indications and Suspected Primary Infection Site for Antibiotic Use
| Course indication | Patients (N = 1,200) | Prescriptions (N = 2,527) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | DOT | % | |
| THERAPYa | 918 | (76.5) | 1,987 | (78.6) | 14,353 | 84.30 |
| PROPHYLAXISb | 395 | (32.9) | 540 | (21.4) | 2,673 | 15.70 |
| Respiratory tract | 365 | (39.8) | 697 | (27.6) | 4,660 | 32.47 |
| CAP | 200 | (21.7) | 429 | (21.6) | 2,763 | 19.25 |
| HCAP | 86 | (9.4) | 142 | (7.2) | 944 | 6.58 |
| VAP | 28 | (3.0) | 60 | (3.0) | 472 | 3.29 |
| Other RTI | 26 | (2.8) | 38 | (1.9) | 377 | 2.63 |
| COPD exacerbation | 25 | (2.7) | 28 | (1.4) | 104 | 0.72 |
| Gastrointestinal (GI) | 200 | (21.8) | 332 | (13.1) | 2,345 | 16.34 |
| GI/intra-abdominal (not C. difficile) | 136 | (14.8) | 267 | (13.4) | 1,803 | 12.56 |
| C. difficile | 64 | (7.0) | 72 | (3.6) | 542 | 3.78 |
| Bloodstreamc | 178 | (19.4) | 260 | (10.3) | 2,941 | 20.49 |
| Bacteremia/sepsis (not device-associated) | 144 | (15.7) | 290 | (14.6) | 2,195 | 15.29 |
| Central line-associated BSI | 34 | (3.7) | 50 | (2.5) | 387 | 2.70 |
| Cardiovascular infection | 17 | (1.8) | 30 | (1.5) | 359 | 2.50 |
| Urinary tract | 171 | (18.6) | 243 | (9.6) | 996 | 6.94 |
| UTI (not device-associated) | 154 | (16.8) | 233 | (11.7) | 867 | 6.04 |
| Catheter-associated UTI | 17 | (1.8) | 29 | (1.5) | 129 | 0.90 |
| Skin and soft-tissue | 171 | (18.6) | 298 | (11.8) | 1,940 | 13.52 |
| Skin/soft-tissue infection | 107 | (11.6) | 214 | (10.8) | 1,174 | 8.18 |
| Bone infection | 32 | (3.5) | 59 | (3.0) | 504 | 3.51 |
| Wound/SSI | 32 | (3.5) | 49 | (2.5) | 262 | 1.83 |
| Other | 105 | (11.4) | 158 | (6.3) | 1,471 | 10.25 |
| Other/unknown | 36 | (3.9) | 69 | (3.5) | 439 | 3.06 |
| Fever/leukocytosis | 27 | (2.9) | 42 | (2.1) | 464 | 3.23 |
| CNS infection | 20 | (2.2) | 43 | (2.2) | 396 | 2.76 |
| Other (nonbacteriological) | 15 | (1.6) | 17 | (0.9) | 162 | 1.13 |
| Unclear/not specified | 7 | (0.8) | 9 | (0.5) | 10 | 0.07 |
NOTE. Patients may have received a prescription for more than 1 indication, and prescriptions may be associated with more than 1 indication. BSI, bloodstream infection; CAP, community-associated pneumonia; C. difficile, Clostridium difficile; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; DOT, days of therapy; HCAP, healthcare-associated pneumonia; PCP, Pneumocystis pneumonia; RTI, respiratory tract infection; SSI, surgical site infection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Percentages out of 918 patients receiving therapy and 2,527 prescriptions.
Prophylaxis included all prescription orders for the prevention of infection pre- or postoperatively, or for the management of a chronic condition, including in patients with neutropenia, cystic fibrosis, and Pneumocystis carinii pneumonia.
Bloodstream infection is defined as patients with an organism grown in blood cultures or sepsis syndrome without any organism grown in blood cultures.
Microbiological Cultures
Of the 918 patients receiving antibiotics for therapeutic indications, diagnostic cultures were not obtained in 98 patients (10.7%) (Table 4). Of 820 patients with cultures obtained, 649 (79.1%) had a specimen for culture obtained before or on the same day of therapy start. Blood and urine were the most common specimens for cultures (79.0% and 61.5%, respectively). The most common pathogen isolated was Staphylococcus aureus (18.8%), followed by coagulase-negative staphylococci (17.8%) and Escherichia coli (13.9%). Infections were polymicrobial in 29.5% of cases (Table 4).
TABLE 4.
Summary of Microbiological Culture Results for 918 Patients Receiving Therapy
| Variable | No. (%) | |
|---|---|---|
| Number of cultures | ||
| 0 | 98 | (10.7) |
| 1–5 | 616 | (74) |
| >5 | 141 | (15.3) |
| Specimen source (if ≥1 culture) | 820 | (100) |
| Blood | 648 | (79.0) |
| Urine | 504 | (61.5) |
| Sputum | 215 | (26.2) |
| Other | 354 | (43.2) |
| Culture timing | ||
| Prior to or on first day of therapy | 649 | (79.1) |
| After start of therapy | 171 | (20.9) |
| Organism isolated | ||
| Total | 404 | (49.3) |
| Staphylococcus aureus | 76 | (18.8) |
| Coagulase-negative staphylococci | 72 | (17.8) |
| Escherichia coli | 56 | (13.9) |
| Enterococcus spp. | 47 | (11.6) |
| Pseudomonas aeruginosa | 42 | (10.4) |
| Klebsiella pneumoniae | 33 | (8.2) |
| Clostridium difficile | 24 | (5.9) |
| Enterobacter spp. | 14 | (3.5) |
| Acinetobacter baumannii | 8 | (2.0) |
| Polymicrobial | 119 | (29.5) |
| Other organism | 140 | (34.6) |
Of 820 patients with cultures obtained, 729 (88.9%) were treated with empirical antibiotics; among these 729 patients, an organism was identified in 216 (29.6%), whose treatments were subsequently pathogen directed. Most patients who had their therapy adjusted had a positive culture from tissue, wound, or sputum samples (online Table 1). In 69 out of 582 (12%) patients, cultures were never ordered and were not used to direct empirically-started therapy.
Prescription-Level Indications for Antibiotic Use
Since patients may have received a prescription for more than 1 indication and antibiotic prescriptions may have been associated with more than 1 indication, we also determined the indications for antibiotic use at the prescription level. Of 2,527 antibiotic prescriptions during the study period, 540 (21.4%) were used for prophylaxis and 1,987 (78.6%) were used for therapeutic indication; of these, 372 (18.7%) were pathogen-directed at start and 1,615 (81.3%) were started as empirical; of which, 382 (23.7%) were subsequently pathogen-directed, and 1,233 (76.3%) remained empirical. Use was primarily for respiratory (27.6% of prescriptions and 32.5% of therapy days) followed by gastrointestinal (13.1% and 16.34%), skin and soft-tissue (11.8% and 10.5%), bloodstream (10.3% and 20.5%), and urinary tract (9.6% and 6.5%) infections (Table 3).
Drug-Specific Prescribing Patterns
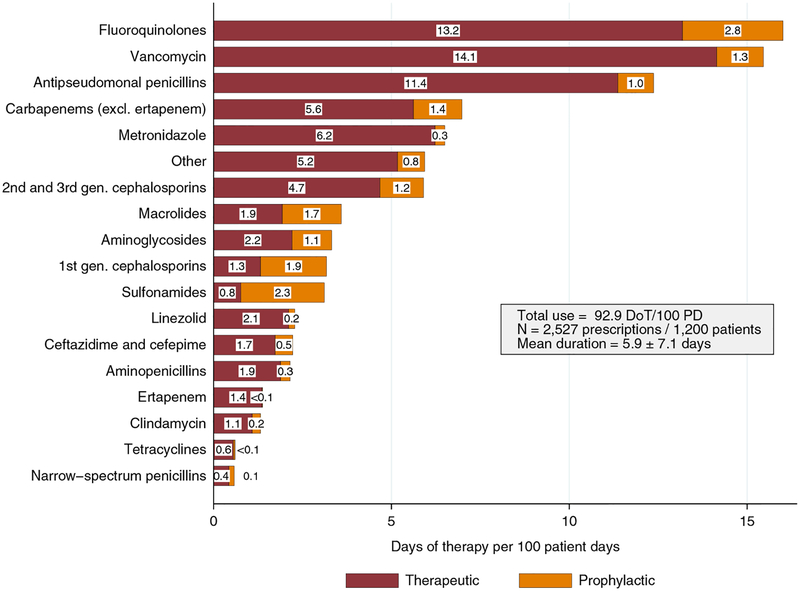
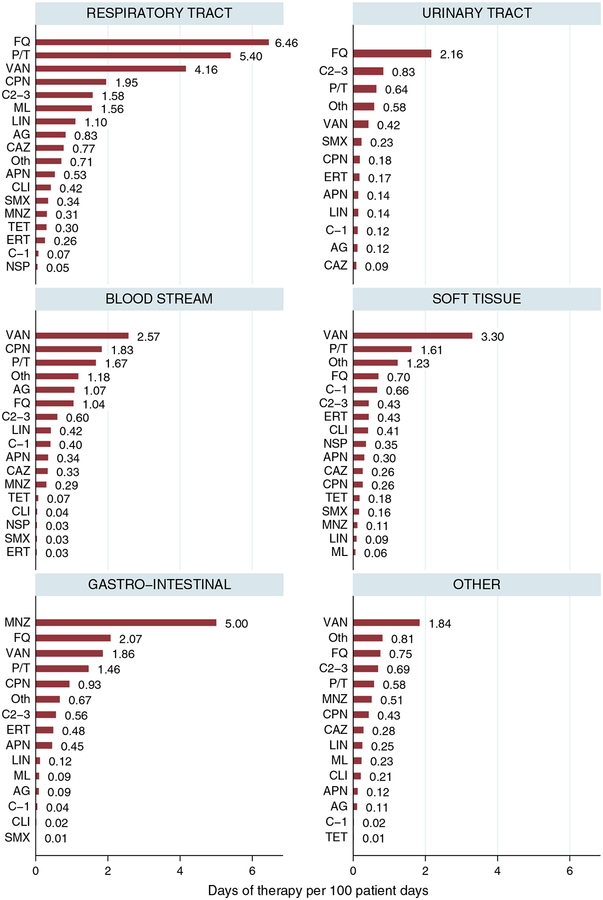
Broad- and extended-spectrum regimens accounted for most antibiotic use in our study (Table 5). Fluoroquinolones, vancomycin, and antipseudomonal penicillins were the most frequently prescribed antibiotics, together accounting for 47.1% of therapy days (Table 5) and 43.8% of DOT per 100 patient-days (Figure 1). Fluoroquinolone monotherapy was the most common prescription (17.2%), followed by vancomycin (16.6%) and piperacillin/tazobactam (13.3%). Fluoroquinolones were the most commonly prescribed antibiotics for respiratory and urinary tract infections (6.46 and 2.16 DOT per 100 patient-days, respectively) (Figure 2). Among respiratory tract infections, for community-acquired pneumonia, the most common empirically started antibiotics were fluoroquinolones (31.5%), antipseudomonal penicillins (17%), and macrolides (12.8%). However, for healthcare-associated pneumonia, the most common empirically started antibiotics were antipseudomonal penicillins (29.6%), fluoroquinolones (27.5%), and vancomycin (19.7%).
TABLE 5.
Days of Therapy (DOT), by Antibiotic Class
| Antibiotic class | DOT, % |
|---|---|
| Fluoroquinolones | 17.2% |
| Vancomycin | 16.6% |
| Piperacillin/tazobactam | 13.3% |
| Carbapenems (excluding ertapenem) | 7.5% |
| Metronidazole | 7% |
| Othersa | 6% |
| Cephalosporins (2nd- and 3rd-gen.) | 6% |
| Macrolides | 4% |
| Aminoglycosides | 4% |
| Cephalosporins (1st-gen.) | 3% |
| Sulfonamides | 3% |
| Linezolid | 2% |
| Ceftazidime and cefepime | 2% |
| Aminopenicillins | 2% |
| Ertapenem | 1% |
| Clindamycin | 1% |
| Tetracyclines | 1% |
| Narrow spectrum penicillins | <1% |
Others includes daptomycin, tigecycline, colistin, and nitrofurantoin.
FIGURE 1.
Therapeutic vs prophylactic antibiotic use rates, expressed in days of therapy (DOT) per 100 patient-days (PD). Boxes inside bars show the number of DOT per 100 PD corresponding to therapeutic (black) and prophylactic prescriptions (gray). “Other” includes daptomycin, tigecycline, colistin, and nitrofurantoin.
FIGURE 2.
Therapeutic antibiotic use by infection site, expressed in days of therapy per 100 patient-days. Bars show the number of days of therapy per 100 patient-days (N = 2,527 prescriptions, 1,200 patients). AG, aminoglycosides; APN, aminopenicillins; C1–3, first- to third-generation cephalosporins, CAZ, ceftazidime and cefepime; CPN, carbapenems (excluding ertapenem); ERT, ertapenem; FQ, fluoroquinolones; LIN, linezolid; ML, macrolides; MNZ, metronidazole; NSP, narrow-spectrum penicillins; P/T, antipseudomonal penicillins; SMX, sulfonamides; TET, tetracyclines; VAN, vancomycin; Oth, other (includes daptomycin, tigecycline, nitrofurantoin, and colistin).
Figure 1 shows therapeutic versus prophylactic antibiotic use rates (expressed in DOT per 100 patient-days) for each antibiotic class. Antibiotics were mainly used for therapeutic indication except sulfonamides and first-generation cephalosporins, which were mainly used for prophylactic purpose. Online Figure 1 demonstrates the empirical versus pathogen-directed antibiotic use rates (expressed in DOT per 100 patient-days) per category of antibiotics. Tetracyclines, clindamycin, and antipseudomonal penicillins were used 32.9-, 15.4-, and 7.5-fold more often as empirical treatment throughout hospitalization compared with pathogen-directed therapy at start, respectively. Narrow-spectrum penicillins and aminoglycosides were used 12.5- and 2.0-fold more often than pathogen-directed therapy throughout hospitalization compared with empirical treatment at start, respectively.
Of the 1,200 study patients, antibiotics were administered in combination in 361 patients (30.1%). Vancomycin in combination with antipseudomonal penicillin accounted for more than 15% of empirical antibiotic combinations followed by metronidazole plus fluoroquinolone (9.7%) and vancomycin plus third-generation cephalosporins (5.5%) (online Table 2).
DISCUSSION
Our study can inform efforts to develop nationwide stewardship strategies that are effective in altering inpatient prescribing behaviors and address the problem of overprescribing antimicrobials. Approximately 60% of hospitalized patients received at least 1 antibiotic, and 77% of these were for therapeutic use. In patients with therapeutic antibiotic use, 87% were started empirically and in 73% of these patients, antibiotics remained empirical throughout hospitalization. Respiratory tract infections accounted for the most antibiotic prescriptions (27.6% of antibiotic prescriptions, 32.5% of DOT). Fluoroquinolones, vancomycin, and antipseudomonal penicillins were the most frequently prescribed antibiotics, together accounting for 47.1% of therapy days. We observed that approximately 11% of patients receiving antibiotics had no cultures ordered and in 21% of patients, cultures were obtained only after the initiation of antibiotics. In addition, we found that in 12% of patients who were initially treated with empirical antimicrobial therapy, the therapy remained empirical throughout hospitalization despite identification of a specific organism based on culture.
A recent study by Fridkin et al,24 which used an administrative database of 323 hospitals, found that approximately 56% of patients received antibiotics during their hospitalization. Similar to our findings, 16% of inpatients treated with antibiotics for urinary tract infections had no urine culture ordered and about 9% of patients receiving intravenous vancomycin had no diagnostic culture obtained. Magill et al10 observed that respiratory tract infection was the most common indication for antibiotic use, and use of broad-spectrum antimicrobial drugs such as piperacillin-tazobactam and vancomycin was common among randomly selected patients in 183 acute care hospitals. Although our study included only 6 hospitals in contrast to the larger Fridkin and Magill studies, our use of infectious disease physicians as medical chart reviewers allowed us to include data elements to more specifically determine whether therapy was pathogen directed and the timing of such directed therapy.
We found that empirical broad-spectrum antibiotic use was common for therapeutic indications, and in most cases treatment remained empirical throughout. Fluoroquinolones, vancomycin, and antipseudomonal penicillins were the most frequently prescribed antibiotics. Most of their use was for respiratory infections and was empirical throughout without subsequent change to pathogen-directed therapy despite identification of an organism based on culture in approximately a quarter of cases. Thus, ASPs targeted at appropriate use of fluoroquinolones, vancomycin, and antipseudomonal penicillins for respiratory indications hold the greatest potential to improve antibiotic use.
Antibiotics are commonly administered to patients cared for in US hospitals and are among the most frequent causes of adverse drug events among hospitalized US patients, and complications, such as increasing antibiotic resistance and Clostridium difficile infection, can be severe and even deadly.32 However, studies have shown that antibiotics are prescribed incorrectly in up to 50% of cases.2 One study reported that 30% of antibiotics received by hospitalized adult patients outside of critical care were unnecessary.17 Evidence is accumulating that interventions to optimize inpatient antibiotic prescribing can improve patient outcomes.33 The importance of ASPs is being increasingly recognized.34,35 Determining whether an antibiotic prescription is prophylactic, pathogen directed, or empirical requires detailed information that is obtained only through medical chart reviews. Our study provides an updated and expanded understanding of microbiological documentation of antibiotic use, which is critical to ongoing efforts to improve ASPs and the quality of inpatient antibiotic use.
The finding that respiratory infections were the most common indication for antibiotic prescriptions highlights the potential role of newer diagnostic molecular and serologic tests and the emerging role of novel markers, such as procalcitonin, to guide initiation and duration of antibiotic treatment in patients with acute respiratory infections and to reduce antibiotic exposure across settings.36,37 Similarly, other newer diagnostic methods such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry have shown that time to effective antibiotic therapy can be decreased, significantly enhancing the ASP efforts.38 Further studies are needed to define the usage of inpatient-specific pathogen-directed antibiotics and whether these newer diagnostic methods may enhance antimicrobial stewardship efforts in combination with physician education, since we found that physicians may often not adjust antimicrobial therapy despite identification of a specific organism.
Although ASPs frequently restrict vancomycin and piperacillin/tazobactam use, fluoroquinolones are often overlooked.5 Indeed, these antibiotics have been associated with adverse ecological effects of antibiotic therapy, such as the selection of multidrug-resistant organisms.39 Even modest improvements in the appropriate use of antibiotics may have large benefits, as Fridkin et al24 illustrated through a simulation exercise that a 30% reduction in broad-spectrum antimicrobial use would lead to a 26% reduction in C. difficile infection. A monitoring system that involves antibiotic use measurement to inform quality improvement activities is urgently needed.24 Thus, fluoroquinolones may represent a more effective target for improvement of patient outcomes, even though they do not have a large impact on the pharmacy budget.5
There are several limitations. First, our results were based on a pragmatic sample of 6 hospitals (both community and teaching) and may not be generalizable to all US hospitals. However, results that were directly comparable with those obtained from a large and nationally representative study were found to be similar.10 Second, we obtained data from hospital billing records and variations among different institutions regarding the exact definitions used in data capture is a known limitation that may affect data interpretation in retrospective studies.40 Third, although a body of earlier research suggests a large share of prescribing is unnecessary, it was beyond the scope of our reviews to assess the appropriateness of empirical therapy and whether more optimal treatment alternatives existed. Additionally, the study design did not allow comparison between the participating hospitals with and without ASPs. Finally, although we followed a standardized approach in data collection, an inherent limitation to all multicenter medical chart review studies is variability in the quality of documentation across sites and cases.
We found that broad-spectrum antibiotic use is highly prevalent among hospitalized patients in the United States. Fluoroquinolones, vancomycin, and antipseudomonal penicillins are the most frequently used antibiotics, particularly for respiratory indications. In patients with therapeutic antibiotic use, most of them were empirically prescribed and remained so throughout even when a pathogen had been identified. Our study provides a foundation for future efforts among hospitals for developing nationwide stewardship strategies to alter behaviors on a large scale among prescribing physicians to address the worsening problem of overprescribing antimicrobials. Given the limited number of new antimicrobial agents in development, determining indications for the use of the currently available antibiotics is central to countering antimicrobial resistance.7
Supplementary Material
ACKNOWLEDGMENTS
We thank the University of Iowa Institute of Clinical and Translational Sciences CTSA (2 UL1 TR000442-06) for their support in the use of the Research Electronic Data Capture system.
Financial support. Centers for Disease Control, Division of Healthcare Quality Promotion (grant GS-10F-0163P to N.B., D.J.M., H.Y., B.J., S.A.W.), Science and Technology Directorate, Department of Homeland Security (contract HSHQDC-12-C-00058 to R.L.), Health Grand Challenges Program at Princeton University, Veterans Administration Health Service Research and Development (grant to D.J.M.), and Global Antibiotic Resistance Partnership project at Center for Disease Dynamics, Economics, & Policy funded by Bill & Melinda Gates Foundation (to S.G.).
Footnotes
Potential conflicts of interest. D.J.M. reports that he is a research consultant for Welch Allyn, Sanogiene, and 3M (not related to this work) and received travel expenses from Infectious Diseases Society of America, American Society for Microbiology, and Society for Healthcare Epidemiology of America for expenses to organize or present at national meetings. E.P. reports that he received an investigator-initiated grant from Cubist (not related to this work). E.S. reports that he was a member of 3M advisory board (not related to this work). All other authors report no conflicts of interest related to this article.
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of authors and might not reflect those of Centers for Disease Control and Prevention.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/ice.2015.226
REFERENCES
- 1.World Health Organization (WHO). WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. WHO website. http://www.who.int/mediacentre/news/releases/2014/amr-report/en/. Published April 30, 2014. Accessed November 5, 2014.
- 2.Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159–177. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky BA, Moran GJ, Napolitano LM, Vo L, Nicholson S, Kim M. A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis 2012;12:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johannsson B, Beekmann SE, Srinivasan A, Hersh AL, Laxminarayan R, Polgreen PM. Improving antimicrobial stewardship: the evolution of programmatic strategies and barriers. Infect Control Hosp Epidemiol 2011;32:367–374. [DOI] [PubMed] [Google Scholar]
- 5.Abbo L, Lo K, Sinkowitz-Cochran R, et al. Antimicrobial stewardship programs in Florida’s acute care facilities. Infect Control Hosp Epidemiol 2013;34:634–637. [DOI] [PubMed] [Google Scholar]
- 6.Ansari F, Erntell M, Goossens H, Davey P. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis 2009;49:1496–1504. [DOI] [PubMed] [Google Scholar]
- 7.MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infect Control Hosp Epidemiol 2008; 29:203–211. [DOI] [PubMed] [Google Scholar]
- 8.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011;53:1100–1110. [DOI] [PubMed] [Google Scholar]
- 10.Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014;312:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malacarne P, Rossi C, Bertolini G. Antibiotic usage in intensive care units: a pharmaco-epidemiological multicentre study. J Antimicrob Chemother 2004;54:221–224. [DOI] [PubMed] [Google Scholar]
- 12.Mettler J, Simcock M, Sendi P, et al. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: a prospective observational study. BMC Infect Dis 2007;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montravers P, Dupont H, Gauzit R, Veber B, Bedos JP, Lepape A. Strategies of initiation and streamlining of antibiotic therapy in 41 French intensive care units. Crit Care 2011;15:R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren MM, Gibb AP, Walsh TS. Antibiotic prescription practice in an intensive care unit using twice-weekly collection of screening specimens: a prospective audit in a large UK teaching hospital. J Hosp Infect 2005;59:90–95. [DOI] [PubMed] [Google Scholar]
- 15.Cusini A, Rampini SK, Bansal V, et al. Different patterns of inappropriate antimicrobial use in surgical and medical units at a tertiary care hospital in Switzerland: a prevalence survey. PLoS One 2010;5:e14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glowacki RC, Schwartz DN, Itokazu GS, Wisniewski MF, Kieszkowski P, Weinstein RA. Antibiotic combinations with redundant antimicrobial spectra: clinical epidemiology and pilot intervention of computer-assisted surveillance. Clin Infect Dis 2003;37:59–64. [DOI] [PubMed] [Google Scholar]
- 17.Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003;163:972–978. [DOI] [PubMed] [Google Scholar]
- 18.Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest 2006;129:1210–1218. [DOI] [PubMed] [Google Scholar]
- 19.Robert J, Pean Y, Varon E, et al. Point prevalence survey of antibiotic use in French hospitals in 2009. J Antimicrob Chemother 2012;67:1020–1026. [DOI] [PubMed] [Google Scholar]
- 20.Chun ED, Rodgers PE, Vitale CA, Collins CD, Malani PN. Antimicrobial use among patients receiving palliative care consultation. Am J Hosp Palliat Care 2010;27:261–265. [DOI] [PubMed] [Google Scholar]
- 21.Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children’s hospitals. Pediatrics 2010;126:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huttner B, Jones M, Huttner A, Rubin M, Samore MH. Antibiotic prescription practices for pneumonia, skin and soft tissue infections and urinary tract infections throughout the US Veterans Affairs system. J Antimicrob Chemother 2013;68:2393–2399. [DOI] [PubMed] [Google Scholar]
- 23.Zilberberg MD, Shorr AF, Micek ST, Mody SH, Kollef MH. Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: a single-center experience. Chest 2008;134:963–968. [DOI] [PubMed] [Google Scholar]
- 24.Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014;63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 25.Braykov NP, Morgan DJ, Schweizer ML, et al. Assessment of empirical antibiotic therapy optimisation in six hospitals: an observational cohort study. Lancet Infect Dis 2014;14:1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Sax FL. The therapeutic efficacy of critical care units from two perspectives: a traditional cohort approach vs a new case-control methodology. J Chronic Dis 1987;40:31–39. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez R, Baigorri F, Navarro G, Artigas A. A modified McCabe score for stratification of patients after intensive care unit discharge: the Sabadell score. Crit Care 2006;10:R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madaras-Kelly K. Optimizing antibiotic use in hospitals: the role of population-based antibiotic surveillance in limiting antibiotic resistance. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2003;23:1627–1633. [DOI] [PubMed] [Google Scholar]
- 30.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007;44:664–670. [DOI] [PubMed] [Google Scholar]
- 31.Dalton B, Sabuda D, Conly J. Trends in antimicrobial consumption may be affected by units of measure. Clin Infect Dis 2007;45:399–400. [DOI] [PubMed] [Google Scholar]
- 32.Weiss AJ, Elixhauser A. Characteristics of adverse drug events originating during the hospital stay, 2011: statistical brief #164. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Health Care Policy and Research, 2006. [PubMed] [Google Scholar]
- 33.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013;4:CD003543. [DOI] [PubMed] [Google Scholar]
- 34.Ohl CA, Luther VP. Antimicrobial stewardship for inpatient facilities. J Hosp Med 2011;6:S4–15. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan A, Fishman N. Antimicrobial stewardship 2012: science driving practice. Infect Control Hosp Epidemiol 2012;33:319–321. [DOI] [PubMed] [Google Scholar]
- 36.Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 2012;55:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuetz P, Briel M, Mueller B. Clinical outcomes associated with procalcitonin algorithms to guide antibiotic therapy in respiratory tract infections. JAMA 2013;309:717–718. [DOI] [PubMed] [Google Scholar]
- 38.Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013;57: 1237–1245. [DOI] [PubMed] [Google Scholar]
- 39.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 2004;38:S341–S345. [DOI] [PubMed] [Google Scholar]
- 40.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005;40:1620–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.