Abstract
Background:
There is some separate evidence that probiotic soy milk and Cuminum cyminum (C. cyminum) have positive effects on the prevention and reduction of diabetic complications. While the impact of probiotic soymilk mixed with herbal essential oils has not been investigated so far, the objective of this study is to examine the effects of probiotic soy milk using Lactobacillus plantarum A7 (KC 355240) added with essential oil of C. cyminum on diabetic rats.
Methods:
50 streptozotocin-nicotinamide (STZ-NA) induced diabetic Wistar rats were divided into five groups: Control group (C group), soy milk group (SM group), probiotic soy milk group (PSM group), soy milk containing essential oil of C. cyminum group (SMC group) and probiotic soy milk containing essential oil of C. cyminum group (PSMC group). The animals consumed these products (1 ml/day) for 30 days. The fasting blood glucose (FBS), the serum lipid levels, and body weight variation were analyzed in 10-day intervals.
Results:
FBS, total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) decreased significantly, whereas high-density lipoprotein cholesterol (HDL-C) increased in the PSMC group compared with that of other groups (P < 0.05). This product also led to weight gain (P < 0.05).
Conclusions:
A mixture of probiotic soy milk and herbal essential oil consumption could impose a positive effect on reducing FBS as well as serum lipid profile in STZ- NA diabetes-induced rat. Also, it results in an increase in their weight gain.
Keywords: Cuminum cyminum, diabetic, Lactobacillus plantarum A7, soy milk
Introduction
Diabetes is a prevalent chronic disease accompanied by long-term damage, dysfunction, and failure of different organs, particularly the eyes, kidneys, nerves, heart, and blood vessels.[1] An increasing aged population, urbanization, sedentary lifestyle and poor nutrition, as well as a rising level of obesity, are significant factors contributing to the rapid increase in the prevalence of diabetes all over the world, particularly among people in developing countries.[2] Moreover, diabetes imposes a substantial economic burden on the health care system in global communities.[3] Indeed, research on the control and prevention of diabetes is seriously in need.
Based on recent studies in the field, bioactive ingredients in soy milk can help protect against diabetes,[4] whereby soy milk consumption was associated with greater glycemic control, regulation of insulin and increased insulin sensitivity.[5] Soy milk consumption showed beneficial effects in reducing D-dimer (a fibrin degradation product) as one of the coagulation factors in diabetic patients with nephropathy was linked with soy milk consumption.[6]
Soy proteins, along with soy isoflavones, have been shown to have a potential role in reducing some atherogenic lipid and lipoprotein serum levels.[7] Since soy milk fermentation increases the bioavailability of its isoflavones, beneficial effects of the fermented product are higher than those of the unfermented product.[8] Fermentation is also deemed to be a likely factor affecting the ability of soy milk to regulate the effect on lipid metabolism gene expression.[9] In particular, fermented products with probiotic bacteria could alleviative the diabetic side effects, risk factors, and complications.[10] Probiotics are dietary supplements that, inadequate number, could modulate the activity of specific molecular pathways.[11]
Cuminum cyminum is an aromatic plant that is part of the Apiaceae family. Apart from its several usages in food products around the world, it has many applications in traditional herbal medicine to treat diseases including diabetes mellitus, dyspepsia and spasmodic gastrointestinal and inflammatory disorders.[12] Aqueous extract of C. cyminum has reduced risk factors associated with atherosclerosis, including high levels of cholesterol in plasma and had preventive effects on the body weight reduction in diabetic rats.[13] Also, methanolic extract of C. cyminum seed was capable of modulating blood glucose in diabetic rats.[14]
Although the positive effects of probiotic soy milk or C. cyminum in diabetes management were previously indicated, the impact of their combination has not been considered so far. The present study evaluates the effects of probiotic soy milk fermented by LA7 added with essential oil of C. cyminum on fasting blood glucose (FBS), serum lipid profiles and body weight in streptozotocin-nicotinamide-induced diabetic rats.
Methods
Soy milk samples
In the present study, four types of soy milk samples were prepared. Pasteurized plain soy milk was bought from Shir Soya Esfahan Company in Iran. The second type of soy milk samples was made by fermentation of the plain soy milk with Lactobacillus plantarum A7 (KC 355240, LA7) which was obtained from the food microbiology laboratory, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran. Overnight cultures were prepared using 1% inoculums in de-Man–Rogosa broth (MRS) (Merck-Germany) and aerobically incubated at 37°C for 24 h (Irankhodsaz, Iran). Active bacteria were achieved through two successive overnight cultures. A bacterial pellet was obtained by three-step centrifugation at 6000, 8000, 10000 rpm, each lasting for 15 min (Sequrita-B. Braun, Hamburg, Germany), followed by washing with normal saline. The bacterial sediment was suspended and diluted with a physiological serum to reach 0.5 optical density (OD) at 620 (λ) wave length determined by a spectrophotometer (Jenway-UK). Each flask containing soy milk (200 ml) was mixed with 1% (v/v) of the active culture, and the cultured samples underwent fermentation at 37°C in aerobic conditions. The third soy milk fortified with plant extract was made using the plain soy milk mixed with 0.02% (v/v) essential oil of C. cyminum (Barij Essence Pharmaceutical Company, Iran). Our previous study on the growth of LA7 in soy milk containing essential oil of C. cyminum revealed that cell numbers of LA7 can achieve 109 CFU/ml in the presence of 0.02% (v/v) of the given essential oil after 9 h fermentation. The same procedure was employed in the preparation of the fourth soy milk group in the present study.[15]
Animals
16-week-old male Wistar rats weighing 185 ± 25 g raised in the animal house of Isfahan School of Pharmacy and Pharmaceutical Sciences were used. The rats were housed five per cage under standard conditions, including ambient temperature (22 ± 2°C) at 45-55% relative humidity, 12h each of dark and light cycle, and fed a standard pellet rat diet [Table 1] (Behparvar Company, Tehran, Iran), with free access to water.
Table 1.
The content of the standard pellet rat diet*
| Content | Percent** |
|---|---|
| Protein | 19.5-20.5 |
| Fat | 3.5-4.5 |
| Fiber | 4-4.5 |
| Ashe | Maximum 10 |
| Calcium | 0.95-1 |
| Phosphorus | 0.65-0.7 |
| Salt | 0.5-0.55 |
| Moisture | Maximum 10 |
| Lysine | 1.15 |
| Methionine | 0.33 |
| Threonine | 0.72 |
| Tryptophan | 0.25 |
| Energy | 16.16-17 |
*Declared by producer: Behparvar Company, Tehran, Iran. **Data presented in ranges show minimum and maximum values
Streptozotocin-nicotinamide-induced diabetic rats
Diabetes was induced in overnight-fasted rats by administering a single intraperitoneal injection of freshly prepared streptozatocin (STZ) (Sigma Aldrich Company, Germany) at 50 mg/kg bw; subsequently, after 15 min 100 mg/kg of nicotinamide (NA) (Sigma Aldrich Company, Germany) in 0.1 M citrate buffer (pH = 4.5)[16,17] was injected to each rat. Diabetes was confirmed in the STZ-NA treated rats by measuring FBS levels after one weak of induction. Rats with FBS greater than 200 mg/dl were considered diabetic.[16] Then the rats were randomly divided into five separate groups (n = 10 each): of Control group (C group), Soy milk group (SM group), Probiotic soy milk group (PSM group), Soy milk containing essential oil of C.cyminum group (SMC group) and Probiotic soy milk containing essential oil of C.cyminum group (PSMC group). The study was carried out in accordance with the guidelines of the Research Ethics Committee for animal experiments set forth by Isfahan University of Medical Sciences, Isfahan, Iran.
Experimental design
During the one-month study period, the C, SM, PSM, SMC, PSMC groups of rats were respectively gavaged 1 ml/day of physiological saline, plain soy milk, probiotic soy milk, soy milk containing essential oil of C. cyminum and probiotic soy milk containing essential oil of C. cyminum samples. Blood samples were collected using a microcapillary tube from orbital sinus plexus under light ether anesthesia after overnight starvation17 on the 1st, 10th, 20th and 30th days of the study. FBS (mg/dl) was measured using a glucometer (Easy Gluco™, Berlin, Germany). For analysis of blood lipids, serum was obtained through 20-min centrifugation at 3000 rpm and stored in a freezer at - 80°C until analysis. TC (mg/dl), TG (mg/dl), HDL-C (mg/dl) and LDL-C (mg/dl) concentrations were determined by using a commercial kit (Biosystems, Spain) on an A15-Autoanalyser set (Biosystems, Barcelona, Spain). The body weight of rats was measured at 10-day intervals (Sartoruse balance, Germany). For insurance of probiotic replacement, the fecal samples were collected from C, SM and PSM groups on the 1st, 15th and 30th days of the experiment. The population of Lactobacilli was calculated by using the pour plate count method on MRS agar and incubated anaerobically under 5% CO2 at 37°C for 48 h (BioTek Instruments Inc., USA).
Statistical analyses
The data are represented as the mean ± standard error of the mean. The statistical analysis was performed using IBM SPSS Statistics version 20. The formula also calculated the percent change for each variable (E – B)/B × 100, where E is the ‘end of treatment’ values and B is the baseline values. Between-group comparison of percent change values was done by one-way analysis of variance (ANOVA) followed by post-hoc LSD multiple range tests. Repeated measures ANOVA was used to compare the mean values within groups at different measurement times. Also, this statistical test (repeated measures ANOVA) was used to examine interaction effects of group and time on the FBS, TC, TG, LDL-C and HDL-C. Differences with P < 0.05 were considered significant.
Results
The mean values of FBS, TC, TG, LDL-C and HDL-C and the corresponding percent changes in all studied groups during the study period were presented in Table 2. Since the baseline for some studied factors was indicated to be relatively different between the groups, the mean comparison analysis was carried out on the mean percent changes for all the studied factors in addition to the average raw data obtained in every sampling time interval (10th, 20th and 30th days of intervention).
Table 2.
Serum levels of biochemical parameters in the treatment groups at 10 day intervals
| Biochemical factors | Group | Baseline | 10th day of intervention | Percent change# in 10 days | 20th day of intervention | Percent change# in 20 days | 30th day of intervention | Percent change# in 30 days | Ptime‡ | Pgroup‡ | Ptime*group‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FBS (mg/dl) | C | 340.7±37.76 | 367.6±41.64 | 7.98±4.91 | 400.6±49.13 | 16.81±6.27 | 417.1±46.27 | 23.76±7.62 | <0.0001 | <0.05 | 0.01 |
| SM | 273.9±32.85 | 236.9±25.35a | -10.90±4.44a | 190.2±22.1a* | -24.32±8.61a | 198.9±19.62a* | -20.69±7.81a | ||||
| PSM | 400±71.61 | 299.16±51.59 | -23.06±6.76a | 224.66±41.14a* | -32.27±10.30a | 184.66±35.05a* | -48.16±9.53ab | ||||
| SMC | 368±58.31 | 301.55±42.44 | -14.64±7.00a | 272.33±38.12a | -21.11±8.07a | 239±34.2a* | -29.38±9.21a | ||||
| PSMC | 378±37.94 | 306.37±33.61 | -18.87±2.45a | 270.12±37.35a* | -29.57±3.36a | 184.12±14.97a*† | -49.93±2.81ab | ||||
| TG (mg/dl) | C | 109.44±12.46 | 125.89±14.86 | 14.03±4.50 | 134.55±15.81 | 22.30±5.13 | 142.78±16.14 | 27.83±5.59 | <0.05 | >0.05 | 0.008 |
| SM | 127.55±18.97 | 106.44±17.06 | -18.04±4.31a | 93.22±13.62a | -23.95±6.34a | 97.55±9.31a | -6.73±11.87 | ||||
| PSM | 139.33±23.38 | 130.33±27.89 | 4.43±22.89 | 120±24.16 | 4.71±32.06 | 79.66±5.69a | -18.54±32.8 | ||||
| SMC | 107.78±12.82 | 108.44±9 | 8.63±11.03 | 99.22±5.46 | 3.71±13.34 | 91.89±12.88a | -12.67±23.74 | ||||
| PSMC | 119.87±15.25 | 85.75±7.49 | -26.14±4.05ac | 77.87±8.98a | -32.06±7.14a | 62.5±10.29ab* | -43.44±9.89a | ||||
| TC (mg/dl) | C | 70.14±3.76 | 73.79±6.83 | 4.27±6.12 | 72.57±9.54 | 6.06±16.67 | 75.14±7.51 | 7.53±10.1 | <0.05 | >0.05 | 0.636 |
| SM | 66.4±5.13 | 68±2.27 | 7.83±8.83 | 59.3±3.07 | -4.46±10.49 | 61.2±3.13a | -0.32±13.22 | ||||
| PSM | 71±7.97 | 69.66±6.71 | -0.84±2.54 | 61.33±4.32 | -11.30±4.45 | 59.16±6.38a | -15.84±3.52 | ||||
| SMC | 74.44±4.2 | 71.88±4.02 | -0.89±7.89 | 63.88±2.85* | -12.19±5.99 | 60.77±2.14a*† | -15.89±6.01 | ||||
| PSMC | 78.37±4.85 | 71.87±5.04 | -7.45±5.46 | 63.75±3.73* | -17.99±3.6 | 58.12±2.98a*† | -24.64±4.36a | ||||
| LDL-C (mg/dl) | C | 14.29±3.11 | 14.62±2.84 | 11.48±7.66 | 15.3±3.08 | 19.54±11.25 | 16.68±3.09 | 28.98±9.32 | <0.05 | >0.05 | 0.006 |
| SM | 9.33±1.65 | 10.16±0.98 | 31.28±17.99 | 7.1±0.63a | 4.14±25.59 | 7.2±0.63a | 3.18±25.87 | ||||
| PSM | 15.33±3.96 | 13.56±3.82 | -8.65±12.14b | 10.01±4.48 | -35.60±17.28a | 10.67±4.48a | -35.81±9.7a | ||||
| SMC | 8.81±1.07 | 6.74±1.44a | -27.16±6.93ab | 6.58±0.89a | -24.04±6.85 | 6.12±0.89a | -26.98±10.88a | ||||
| PSMC | 13.77±2.76 | 8.89±1.76 | -30.71±9.23ab | 7.96±1.61a* | -28.99±11.97b | 8.31±1.61a* | -31.26±12.36a | ||||
| HDL-C (mg/dl) | C | 31.83±2.44 | 31.87±1.84 | 1.02±2.95 | 30.86±2.15 | -2.68±2.24 | 31.68±1.05 | -5.97±5.78 | <0.0001 | >0.05 | 0.02 |
| SM | 30.71±3.33 | 34.23±2.13 | 16.18±8.69 | 32.14±1.89 | 9.03±7.76 | 33.33±2.5 | 12.59±8.59 | ||||
| PSM | 32.66±2.44 | 38.38±1.87ac | 20.71±11.05 | 39.48±2.14abd* | 22.50±6.31 | 41.71±2.45ab* | 28.9±5.61a | ||||
| SMC | 32±1.75 | 33.2±1.47 | 4.68±3.97 | 35.34±1.54 | 11.31±3.51 | 36.99±1.09* | 17.51±5.07 | ||||
| PSMC | 28.91±2.58 | 35.29±1.65* | 30.24±15.17a | 33.77±1.81 | 25.37±15.88a | 38.26±1.94* | 40.08±14.39ab |
C=Control; SM=Soy milk; PSM=Probiotic soy milk; SMC=Soy milk containing essential oil of C. cyminum; PSMC=Probiotic soy milk containing essential oil of C. cyminum. Values are presented as Mean±SEM. #Percent change is calculated relative to baseline. One-way ANOVA: aP<0.05 vs. control in the same columns; bP<0.05 vs. SM in the same columns; cP<0.05 vs. SMC group in the same columns; dP<0.05 vs. PSMC group in the same columns. Repeated measures ANOVA: *P<0.05 vs. baseline in the same row; †P<0.05 vs. after 10 days of intervention in the same row; ‡Ptime, and Pgroup: Fixed factor; ‡Ptime*group: Interaction effect
Changes in FBS levels
As shown in Table 2, the results of the one-way ANOVA analysis showed that percent change values of FBS in the studied groups related to the all times are significantly different (P < 0.05). The findings of the repeated measures ANOVA test showed that mean values of this parameter (FBS) in different times of all studied groups are significantly different (P < 0.0001). In addition, interaction effects of group and time represented a significant impact on the FBS (P = 0.01).
FBS levels decrease over time in all conditions compared to baseline, except for control. Just after 10 days of the intervention, a significant decrease in FBS in all treated groups was raveled compared to the control group, and this trend continued to the 20th day of the study (P < 0.05). However, at the end of the experiment, PSM and PSMC showed the most reducing percent change in serum blood glucose (-48.16 ± 9.53% and -49.93 ± 2.81%, respectively), suggesting that concerning blood glucose reduction, probiotic inclusion was even more effective than the used extract.
Changes in TG levels
Based on the one-way ANOVA analysis in Table 2, there was not a significant difference in percent change values of TG in the studied groups related to the all times (P > 0.05). The repeated measures ANOVA test revealed that mean values of TG in different times of all studied groups are significantly different (P < 0.05). Furthermore, interaction effects of group and time demonstrated a significant impact on the TG (P = 0.008).
Regarding the change in TG, among the studied rat groups, PSMC exhibited a rapid, significant reduction in TG after ten days of the study, and the occurred difference this parameter with other groups remained steady all through the study period (P < 0.05). At the end of the study, although, all the interventions showed significant downward TC values than the control group (P < 0.05), comparison of the percent changes in these parameters limited the active treatment to just PSMC (-43.44 ± 9.89%).
Serum TC changes
In Table 2, one-way ANOVA analysis revealed that percent change values of TC in the studied groups related to the all times are not significantly different (P > 0.05). On the other hand, the findings of the repeated measures ANOVA test showed that mean values of this parameter (TC) in different times of all studied groups are significantly different (P < 0.05). The interaction effects analysis of the group and time revealed a not significant impact on the TG (P = 0.636).
As one can see in Table 2, the difference in TC levels followed the same trend as TG. Nevertheless, the significant variations occurred at a later time; after 20 days of study TC levels in the SMC and PSMC groups showed substantial reductions compared with the TC level at baseline (P < 0.05). At the end of the intervention, the highest percent change in TC level was observed in the PSMC group (24.64 ± 4.36%), which was significantly different from the control group (P < 0.05).
Changes in serum LDL-C
One-way ANOVA analysis depicts that percent change values of LDL-C in the studied groups related to the all times were not significantly different (P > 0.05) [Table 2]. The repeated measures ANOVA test revealed that the mean values of LDL-C in different times of all studied groups are significantly different (P < 0.05). Furthermore, interaction effects of group and time revealed a significant impact on the LDL-C (P = 0.006).
Concerning the LDL-C level, all the four treated groups achieved improvement throughout the study. However, meaningful differences were observed in the groups that received mixed formulations in that soon after ten days of intervention, PSM, SMC, and PSMC indicated reduced LDL-C in comparison to the control and SM group. Interestingly, at the end of the study, with the highest percent changes which was observed in the PSM group (- 35.81 ± 9.7%), PSMC, SMC, and PSM groups significantly showed less LDL-C level as compared with the control group (P < 0.05). While the intake of plain soy milk in the SM group failed to impose such a reduction.
Changes in serum HDL-C
As shown in Table 2, the result of the one-way ANOVA analysis demonstrated that percent change values of HDL-C in the studied groups related to the all times are not significantly different (P > 0.05). In addition, the findings of the repeated measures ANOVA test showed that mean values of this parameter (HDL-C) in different times of all studied groups are significantly different (P < 0.0001). In addition, interaction effects of group and time depicted a significant impact on the HDL-C (P = 0.02).
As depicted in Table 2, during the study, all study groups, except control showed a rise in HDL-C in a way that at the end of the intervention, three of the four treated groups including PSM, SMC and PSMC showed significant changes in HDL-C from the baseline (P < 0.05). However, comparing the groups, PSM and PSMC revealed the most effective treatments. HDL-C percent changes levels of the PSMC group were different compared with that in control all through the study time. Also, at the end of the experiment, with the highest HDL- C percent change value, this group was different compared with that of SM groups either (40.08 ± 14.39% vs. 12.59 ± 8.59%).
Changes in body weight
Figure 1 shows the weight of the rats during the experiment. The body weight in the PSMC group was different from the control group after ten days of the experiment. These differences were also observed at the end of the experiment. Also at this time, there was a significant difference between the weight of the SM and PSM groups (P < 0.05). The weight of the SMC group was different from the control group after 20 days of intervention. At the end of the experiment, the weight of the PSM, SMC, and PSMC groups was different from the baseline. In terms of percent changes, all groups were different from the C group (C = 1.28 ± 5.46%; SM = 12.27 ± 5.38%; PSM = 28.22 ± 5.83%; SMC = 20.04 ± 7.32%; PSMC = 29.46 ± 2.82%), and the weight of the PSMC group differed from the SM group. The greatest percent change was observed in the PSMC group (29.46 ± 2.82%).
Figure 1.
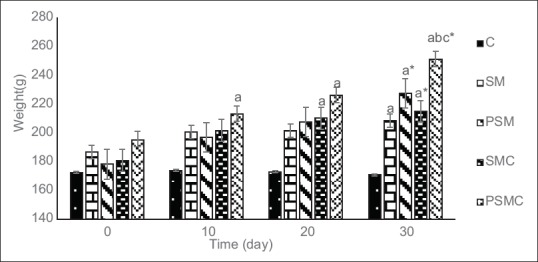
Body weight (g) in the treatment groups at 10 day intervals. C = Control; SM = Soy milk; PSM = Probiotic soy milk; SMC = Soy milk containing essential oil of C. cyminum; PSMC = Probiotic soy milk containing essential oil of C. cyminum. Values are presented as Mean ± SEM. aP < 0.05 vs. control in the same time. bP < 0.05 vs. SM group in the same time. cP < 0.05 vs. PSM group in the same time. *P < 0.05 vs. baseline in the same group
Changes in fecal Lactobacilli
Based on the one-way ANOVA analysis in Table 3, there was a significant difference in percent change values of fecal Lactobacilli in the studied groups related to the all times (P < 0.0001). The repeated measures ANOVA test revealed that mean values of fecal Lactobacilli in different times of all studied groups are significantly different (P < 0.0001). Furthermore, interaction effects of group and time demonstrated a significant impact on the fecal Lactobacilli (P < 0.0001).
Table 3.
Faecal lactobacilli counts (Log cfu/g) in the treatment groups at 15 day intervals
| Group | Baseline | 15th day of intervention | 30th day of intervention | Percent change | P time‡ | P group‡ | P time*group‡ |
|---|---|---|---|---|---|---|---|
| C | 8.61±0.18 | 8.40±0.19 | 8.48±0.11 | -1.22±1.51 | <0.0001 | <0.0001 | <0.0001 |
| SM | 8.46±0.17 | 8.38±0.17 | 8.61±0.12 | 1.92±1.61 | |||
| PSM | 8.85±0.29 | 9.59±0.27ab | 10.81±0.25ab*† | 22.47±6.40ab |
C=Control; SM=Soy milk; PSM=Probiotic soy milk. Values are presented as Mean±SEM. One-way ANOVA: aP<0.05 vs. control in the same columns; bP<0.05 vs. SM group in the same columns. Repeated measures ANOVA: * P<0.05 vs. baseline in the same row; †P<0.05 vs. after 15 days of intervention in the same row; ‡Ptime, and Pgroup: Fixed factor; ‡Ptime*group: Interaction effect
Table 3 reports the number of fecal Lactobacilli. After 15 days of intervention, the number of fecal Lactobacilli in the PSM group was different from the control and SM group. This difference was also observed at the end of the intervention. In this time, Lactobacilli counts in the PSM group were different compared with pre-intervention and at 15 days. The highest percent change in the number of bacteria was observed in the PSM group (22.47 ± 6.40%), which was also different from the other two groups.
Discussion
The findings of the present study showed that all applied treatments were to some extent beneficial to the serum blood parameters of STZ-induced diabetic rats during one month. However, the supposition of this study has been confirmed in that fermented soy milk, added with C. cyminum extract is more effective than each of the treatments.
In Table 4, the results of the most similar animal studies to the present work are summarized. SM group revealed improvement in blood glucose and TG in comparison with the control group. These results are consistent with the results of Tsai et al. in that in obese subjects with type 2 diabetes, soy polysaccharide significantly managed postprandial serum glucose concentrations and TG levels.[18] The regulatory effects of soy milk products on FBS and serum lipid parameters were associated with its flavones, genistein, protein and polysaccharides.[18,19,20,21]
Table 4.
Animal studies in which soy components, soymilk, fermented soymilk or Cuminum cyminum were used as dietary supplements for diabetes management in animal models
| Author | Animal model | Compound | Amount and duration | Fasting blood glucose* | TG* | TC* | LDL-C* | HDL-C* | Body weight* | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Lu et al. | Diabetic-STZ rats | high-isoflavone soy protein | 20%/For 8 weeks | Significantly decreased | ND | ND | ND | Significantly increased | Significantly increased | 21 |
| Lee et al. | Diabetic-STZ rats | genistein supplement | 600 mg/kg diet/For 3 weeks | Significantly decreased - 19.6** | Significantly decreased | Significantly decreased | ND | Significantly increased | ND | 19 |
| isolated soy protein supplement | 200 g/kg /For 3 weeks | Significantly decreased -24.9 | Significantly decreased | Significantly decreased | ND | Significantly increased | ND | |||
| Marazza et al. | Diabetic-STZ mice | soymilk | 5 ml/day/For 28 day | Significantly decreased | Significantly decreased | Significantly decreased | Significantly decreased | No effect | Significantly increased | 10 |
| soymilk fermented with Lactobacillus rhamnosus CRL981 | 5 ml/day/For 28 day | Significantly decreased - 40.2 | Significantly decreased | Significantly decreased | Significantly decreased -20 | No effect | Significantly increased | |||
| Mohammadi Sartang et al. | Diabetic-STZ rats | Soymilk | 1 mL/day/For 28 days | Significantly decreased - 35.8 | Significantly decreased | Significantly decreased | No effect | Significantly increased 20.4 | Significantly increased + 18.54 | 30 |
| fermented soymilk (by Bifidobacterium lactis) | 1 mL/day/For 28 days | Significantly decreased - 39.3 | Significantly decreased | Significantly decreased | No effect | Significantly increased 24.8 | Significantly increased + 19.02 | |||
| fermented soymilk fortified with omega-3 | 1 mL/day/For 28 days | Significantly decreased -47.2 | Significantly decreased -39.30 | Significantly decreased -20.80 | No effect | Significantly increased 14.8 | Significantly increased + 21.48 | |||
| Willatgamuwa et al. | Diabetic-STZ rats | Cumin powder | 1.25% of diet/For 8 weeks | Significantly decreased - 50 | ND | ND | ND | ND | A slight increasing | 40 |
| Jagtap et al. | Diabetic-STZ rats | Metthanolic extract of seeds of C.cyminum | 200 mg/day/For 28 days | Significantly decreased | ND | ND | ND | ND | Significantly increased | 14 |
| Metthanolic extract of seeds of C.cyminum | 400 mg/day/For 28 days | Significantly decreased | ND | ND | ND | ND | Significantly increased | |||
| Metthanolic extract of seeds of C.cyminum | 600 mg/day/For 28 days | Significantly decreased | ND | ND | ND | ND | Significantly increased | |||
| Srivsatava et al. | High fructose fed diabetic- STZ rats. | Ethanolic extract of seeds of C. cyminum | 250 mg/Kg body weight/For 14 days | Significantly decreased | Significantly decreased -21.04 | Significantly decreased - 22.7 | Significantly decreased - 16.9 | Significantly increased + 12.2 | ND | 37 |
| Present study | Diabetic STZ-NA rats | SM | 1 ml/day/For one month | Significantly decreased -44.45 | -34.56 | -7.85 | -25.8 | +18.56 | Significantly increased + 10.99 | - |
| PSM | 1 ml/day/For one month | Significantly decreased -71.92 | -46.37 | -23.42 | Significantly decreased - 64.79 | Significantly increased + 34.87 | Significantly increased + 26.94 | |||
| SMC | 1 ml/day/For one month | Significantly decreased -53.14 | -40.5 | -23.47 | Significantly decreased - 58.96 | +23.48 | Significantly increased + 18.76 | |||
| PSMC | 1 ml/day/For one month | Significantly decreased -73.69 | Significantly decreased - 71.27 | Significantly decreased - 32.17 | Significantly decreased - 60.24 | Significantly increased + 46.05 | Significantly increased + 28.18 |
C=Control; SM=Soy milk; PSM=Probiotic soy milk; SMC=Soy milk containing essential oil of C. cyminum; PSMC=Probiotic soy milk containing essential oil of C. cyminum; ND=Not determined; *Comparison with the diabetic control group; **Percent change compared to the diabetic control group.
In another study, Rossi showed soy milk consumption by hypercholesterolemic rabbits increased HDL-C levels, while, this effect was not observed in the SM group in our study.[22]
Several mechanisms are suggested to bear advantages of soy component to diabetes subjects; inhibition of intestinal glucose uptake via a reduction in sodium-dependent glucose transporter, and beta-cell secretion promotion leading to a reduction in postprandial hyperglycemia.[23] The isolated soy protein was found to be able to decrease the expression of sterol regulatory element-binding protein binding (SREBP), followed by increased adiponectin levels and reduced triglyceride levels.[9] Also, soy peptides expression of LDL-C receptors in the liver is referred for decreased levels of serum cholesterol.[24]
Soy milk isoflavones can regulate glucose and lipid metabolism through the regulation of gene expression related to nuclear receptors of peroxisome proliferator-activated receptor (PPAR), increases in lipoprotein clearance and HDL-C biogenesis.[9,25]
Despite the significant difference of weight in SM group compared to control which appeared clearly at the end of the study, this group did not put weight from their baseline. Administration of nutritious soy milk in the treated groups provided them with the additional source of energy that resulted in their weight gain in comparison to the control group. Protein supply was mentioned to be responsible for weight gain in STZ-diabetic rats receiving soy milk.[26]
The effects of soy consumption on body weight changes are controversial. While soy milk played a role in the regulation of body weight through increasing energy expenditure in male rats[27] and phytoestrogen content of soy reduced fat accumulation,[28] It was reported that isoflavones in soy milk improved metabolism in diabetic rats and led to their weight gain. Meanwhile, a human study published on the ineffectiveness of soy protein daily intake on body weight changes.[29]
Using soymilk-containing probiotic bacteria usually brings up enhanced beneficial effects on blood glucose and serum lipid parameters compared to soymilk.
Inconsistent to our results, using a diabetes animal model induced with streptozotocin, Mrraza indicated that the fermented soymilk with Lactobacillus rhamnosus CRL981 gave rise to more reduction in blood glucose levels than unfermented soymilk.[10]
Regarding percent change in serum lipid parameters, comparison of PSM with SM group showed no superiority of the fermented product. This is consistent with the results of the two other studies[10,30] in that, fermented soy milk induced no more significant difference on the lipid parameters of STZ-diabetic rats than the plain soy milk.
Lactobacillus plantarum A7 (KC 355240) is a native strain with proven probiotic properties.[31,32] In our previous study, this strain of bacteria has the potential to decrease serum TC, LDL-C, and TG in mice.[31] It was reported that soy milk fermented with another L. plantarum strain, Lactobacillus plantarum P-8, produced a regulation effect in serum lipid profile hyperlipidemic rats.[33] Probiotic bacteria have hypolipidaemic effects by several probable mechanisms[34] and some studies, confirmed the positive physiological function of Lactobacillus strains belonging to, L. plantarum species. Xie et al. study showed that supplementation of Lactobacillus plantarum 9-41-A and Lactobacillus fermentum M1-16 had beneficial effects on TG, TC and LDL-C in serum, enhancing effects on intestinal Lactobacillus and Bifidobacterium bacteria and reductions in body weight in rats fed a high cholesterol diet.[35]
In our study, examination of fecal samples of the control group, SM and PSM were carried out to investigate the activity and efficiency of cultured bacteria in the PSM group. The result showed that PSM group had the highest number of lactobacilli in feces, suggesting that probiotic microorganism replacement promotes Lactobacilli growth in the rat intestine.
Despite a great deal of evidence on the effects of fermented soy milk on glucose metabolism,[5,10] using a combination of fermented soymilk with a natural ingredient was rarely investigated. Ju and Han showed the hypoglycemic effect of fermented soymilk extracts added with 5% bokbunja (FSB) in STZ-induced diabetic mice. Although their study was different from us in design, we share a common conclusion of the superior effects of the combined formula of fermented soy milk with a natural ingredient.[5] In another study carried out by Mohammadi Sartang et al., the addition of omega three oil to fermented soy milk did not produce extra beneficial effects on serum blood glucose and lipid.[30]
Our results concerning soy milk with C. cyminum (SMC), showed that this treatment had an advantage over the FBS and LDL-C of the tested STZ-diabetic rats.
The effect of 5% cumin powder on blood glucose levels resulted in a 17% reduction in the treated STZ-induced group than STZ-control in one study.[36] In two other studies, administration 200-600 mg/kg metabolic extract C. cyminum seeds in STZ rats caused 17- 60% reduction in blood glucose from the control group.[14,37] Using approximately 200 mg extract per kg of soymilk, 50% reduction of fasting blood sugar from the control group in our study supports other studies results [Table 4]. The beneficial effect of C. Cyminum on serum blood lipid parameters was also examined. Although, our results confirmed the efficiency of C. cyminum on these parameters for FBS and LDL-C, The significance of the results obtained by this group is that as a control to the fermented product (PSMC), this treatment includes innate potential which enhanced through fermentation. The same reason can be given for using fermented soy milk with identified beneficial effects which were a plant extract free control to PSMC. This treatment showed superior effect than other therapies for managing diabetes complications. Meanwhile, the results from the body weight data showed that PSMC group was more effective in weight gain than the other groups. This is a limitation of our study that after treating the rats with STZ, their weight loss was not recorded. In doing so, the increase in weight in the treated groups could have cleared that this defect was controlled by applied treatments. Marazza et al. concluded that treating the STZ induced mice with soymilk fermented with L. rhamnosus CRL981 prevented the weight loss that appeared in the diabetic control group.[10] Weight loss in diabetes-induced rats was associated with dehydration, loss of body fat and protein catabolism caused by increased catabolic reactions.[38,39,40]
Conclusions
Probiotic soy milk using LA7 added with essential oil of C. cyminum create a more significant reduction in FBS, TG and TC levels, enhances HDL-C levels and has beneficial effects on weight gain in comparison with probiotic soy milk and soy milk containing C. cyminum essential oil in diabetic rats. Besides, PSMC decreased LDL-C levels. It seems that the combination of probiotics and essential oils is a robust functional supplement for diabetes management in an animal model.
Financial support and sponsorship
This study was conducted as an MSc research thesis (code 293112, approval date: 27 December 2015), ethically assessed and approved by research ethics board acting under the graduate office at the Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the staff of Food Security Research Center for their financial support. They are also grateful to Mrs. Marziyeh Aghababaei and Parastoo Tavassoli for their assistance in this project. This study was approved by the School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (code 293112).
References
- 1.American diabetes association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:81–90. [Google Scholar]
- 2.Guariguata L, Whiting D, Hambleton I, Beagley J, Linnenkamp U, Shaw J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2011;93:1092–101. doi: 10.3945/ajcn.110.007187. [DOI] [PubMed] [Google Scholar]
- 5.Ju HE, Han JS. Hypoglycemic Effect of Fermented Soymilk Added with Bokbunja (Rubus coreanus Miquel) in Diabetic Mice. Food Sci Biotechnol. 2010;19:1041–6. [Google Scholar]
- 6.Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81:397–408. doi: 10.1093/ajcn.81.2.397. [DOI] [PubMed] [Google Scholar]
- 7.Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35:1981–5. doi: 10.2337/dc12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Yan L, Wang J, Zhang Q, Zhou Q, Sun T, et al. Fermentation characteristics of six probiotic strains in soymilk. Ann Microbiol. 2012;62:1473–83. [Google Scholar]
- 9.Kim Y, Yoon S, Lee SB, Han HW, Oh H, Lee WJ, et al. Fermentation of soy milk via lactobacillus plantarum improves dysregulated lipid metabolism in rats on a high cholesterol diet. PLoS One. 2014;9:e88231. doi: 10.1371/journal.pone.0088231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marazza JA, LeBlanc JG, de Giori GS, Garro MS. Soymilk fermented with Lactobacillus rhamnosus CRL981 ameliorates hyperglycemia, lipid profiles and increases antioxidant enzyme activities in diabetic mice. J Funct Foods. 2013;5:1848–53. [Google Scholar]
- 11.Bomba A, Brandeburova A, Ricanyova J, Strojny L, Chmelarova A, Szabadosova V, et al. The role of probiotics and natural bioactive compounds in modulation of the common molecular pathways in pathogenesis of atherosclerosis and cancer. Biologia. 2012;67:1–13. [Google Scholar]
- 12.Allahghaderi T, Rasooli I, Owlia P, Jalali-Nadooshan M, Ghazanfari T, Taghizadeh M, et al. Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J Food Sci. 2010;75:54–61. doi: 10.1111/j.1750-3841.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 13.Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N. Hypolipiddemic effect of cuminum cyminum L. on alloxan induced diabetic rats. Pharmacol Res. 2002;46:251–5. doi: 10.1016/s1043-6618(02)00131-7. [DOI] [PubMed] [Google Scholar]
- 14.Jagtap AG, Patil PB. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food Chem Toxicol. 2010;48:2030–6. doi: 10.1016/j.fct.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Babashahi M, Mirlohi M, Ghiasvand R, Torki-Baghbadorani S. Evaluation of response surface methodology to predict optimum growth conditions for Lactobacillus plantarum A7 (KC 355240) in probiotic soy milk containing essential oil of Cuminum cyminum. Recent Pat Food Nutr Agric. 2016;8:1–6. doi: 10.2174/2212798408666161026161105. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Kagan L, Mager DE. Population pharmacodynamic modeling of exenatide after 2-week treatment in STZ/NA diabetic rats. J Pharm Sci. 2013;102:3844–51. doi: 10.1002/jps.23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petchi R, Vijaya C, Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin - nicotinamide induced diabetic wistar rats. J Tradit Complement Med. 2014;4:108–17. doi: 10.4103/2225-4110.126174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai A, Vinik A, Lasichak A, Lo G. Effects of soy polysaccharide on postprandial plasma glucose, insulin, glucagon, pancreatic polypeptide, somatostatin, and triglyceride in obese diabetic patients. Am J Clin Nutr. 1987;45:596–601. doi: 10.1093/ajcn/45.3.596. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006;79:1578–84. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 21.Lu MP, Wang R, Song X, Chibbar R, Wang X, Wu L, et al. Dietary soy isoflavones increase insulin secretion and prevent the development of diabetic cataracts in streptozotocin-induced diabetic rats. Nutr Res. 2008;28:464–71. doi: 10.1016/j.nutres.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Rossi EA, Vendramini RC, Carlos IZ, Ueiji IS, Squinzari MM, Silva SI, Jr, et al. Effects of a novel fermented soy product on the serum lipids of hypercholesterolemic rabbits. Arq Bras Cardiol. 2000;74:213–6. doi: 10.1590/s0066-782x2000000300003. [DOI] [PubMed] [Google Scholar]
- 23.Vedavanam K, Srijayanta S, O’Reilly J, Raman A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE) Phytother Res. 1999;13:601–8. doi: 10.1002/(sici)1099-1573(199911)13:7<601::aid-ptr550>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Cho SJ, Juillerat MA, Lee CH. Cholesterol lowering mechanism of soybean protein hydrolysate. J Agric Food Chem. 2007;55:10599–604. doi: 10.1021/jf071903f. [DOI] [PubMed] [Google Scholar]
- 25.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133:1238–43. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 26.Shim JY, Kim KO, Seo BH, Lee HS. Soybean isoflavone extract improves glucose tolerance and raises the survival rate in streptozotocin-induced diabetic rats. Nutr Res Pract. 2007;1:266–72. doi: 10.4162/nrp.2007.1.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmen FA, Mercado CP, Zavacki AM, Huang SA, Greenway AD, Kang P, et al. Soy protein diet alters expression of hepatic genes regulating fatty acid and thyroid hormone metabolism in the male rat. J Nutr Biochem. 2010;21:1106–13. doi: 10.1016/j.jnutbio.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol. 2009;304:30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy a longitudinal randomized clinical trial. Diabetes Care. 2008;31:648–54. doi: 10.2337/dc07-2065. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi Sartang M, Mazloomi SM, Tanideh N, Rezaian Zadeh A. The effects of probiotic soymilk fortified with omega-3 on blood glucose, lipid profile, haematological and oxidative stress, and inflammatory parameters in streptozotocin nicotinamide-induced diabetic rats. J Diabetes Res. 2015;2015:1–9. doi: 10.1155/2015/696372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fazeli H, Moshtaghian j, Mirlohi M, Shirzadi M. Reduction in serum lipid parameters by incorporation of a native strain of Lactobacillus Plantarum A7 in Mice. J Diabetes Metab Disord. 2010;9:1–7. [Google Scholar]
- 32.Mirlohi M, Soleimanian Zad S, Dokhani S, Sheikh Zeinodin M. Microbial and physiochemical changes in yoghurts containing different Lactobacillus delbrueckii subsp. bulgaricus strains in association with Lactobacillus plantarum as an adjunct culture. Int J Dairy Technol. 2014;67:246–54. [Google Scholar]
- 33.Wang Z, Bao Y, Zhang Y, Zhang J, Yao G, Wang S, et al. Effect of soymilk fermented with Lactobacillus plantarum p-8 on lipid metabolism and fecal microbiota in experimental hyperlipidemic rats. Food Biophys. 2013;8:43–9. [Google Scholar]
- 34.Salaj R, Stofilova J, Soltesova A, Hertelyova Z, Hijova E, Bertkova I, et al. The effects of two lactobacillus plantarum strains on rat lipid metabolism receiving a high fat diet. Sci World J. 2013;2013:1–7. doi: 10.1155/2013/135142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie N, Cui Y, Yin YN, Zhao X, Yang JW, Wang ZG, et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement Altern Med. 2011;11:1–11. doi: 10.1186/1472-6882-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar PA, Reddy PY, Srinivas P, Reddy GB. Delay of diabetic cataract in rats by the antiglycating potential of cumin through modulation of α-crystallin chaperone activity. J Nutr Biochem. 2009;20:553–62. doi: 10.1016/j.jnutbio.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Srivsatava R, Srivastava SP, Jaiswal N, Mishra A, Maurya R, Srivastava AK. Antidiabetic and antidyslipidemic activities of Cuminum cyminum L. in validated animal models. Med Chem Res. 2011;20:1656–66. [Google Scholar]
- 38.Al-Shamaony L, Al-Khazraji SM, Twaij HA. Hypoglycaemic effect of Artemisia herba alba II Effect of a valuable extract on some blood parameters in diabetic animals. J Ethnopharmacol. 1994;43:167–71. doi: 10.1016/0378-8741(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 39.Hakim ZS, Patel BK, Goyal RK. Effects of chronic ramipril treatment in streptozotocin-induced diabetic rats. Indian J Physiol Pharmacol. 1997;41:353–60. [PubMed] [Google Scholar]
- 40.Willatgamuwa SA, Platel K, Saraswathi G, Srinivasan K. Antidiabetic influence of dietary cumin seeds (Cuminum cyminum) in streptozotocin induced diabetic rats. Nutr Res. 1998;18:131–42. [Google Scholar]


