Abstract
Background:
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by the three main symptom domains including inattention, hyperactivity, and impulsivity. Recent findings suggested that nutrients might play an important role in the pathology of ADHD. The present study aimed to examine the effects of Vitamin D and magnesium supplementation on behavior problems in children with ADHD.
Methods:
This double-blind, randomized controlled clinical trial study was conducted on 66 children with ADHD in Clinic of Noor and Ali Asghar Hospital in Isfahan, Iran, in 2016. Children were randomly allocated to receive both Vitamin D (50,000 IU/week) and magnesium (6 mg/kg/day) supplements (n = 33) or placebos (n = 33) for 8 weeks. Conners’ Parent Rating Scale was used to evaluate children's behavior at baseline and at the end of the study.
Results:
After 8 weeks of Vitamin D consumption as well as magnesium, the serum levels of 25-hydroxy-Vitamin D3 and magnesium increased significantly in the intervention group compared with placebo group. Supplementation with Vitamin D and magnesium caused a significant decrease in conduct problems, social problems, and anxiety/shy scores; but it had no significant effect on psychosomatic problems score.
Conclusions:
Vitamin D and magnesium supplementation in children with ADHD was effective on conduct problems, social problems, and anxiety/shy scores compared with placebo intake, but it did not affect psychosomatic problem scores, significantly.
Keywords: Attention-deficit disorder with hyperactivity, magnesium, randomized clinical trial, Vitamin D
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a serious neurodevelopmental condition characterized by the three main symptom domains including inattention, hyperactivity, and impulsivity.[1] The estimated prevalence of ADHD is between 5% and 7% in schoolchildren worldwide.[2,3] In Iran, its prevalence is 5.03% among boys and 2.79% among girls.[4] ADHD impairs school functioning and academic achievement profoundly.[5] It is revealed that IQ in children with ADHD is lower than their peer.[6,7] Furthermore, learning disorders (LDs) are more common in youth with ADHD.[8] Lower IQ and LD accompanied by ADHD symptoms are major reasons for academic underachievement.[6,7,8] These impairments can affect children's quality of life and impose substantial costs on their family, health-care services, and educational systems worldwide.[5,9]
ADHD is a complex disorder influenced by genetic and environmental factors.[10] Prenatal exposures such as maternal smoking, psychosocial and dietary factors are some of these environmental factors.[10,11] Recent findings suggested that nutrients might play an important role in the pathology of ADHD.[12,13] Moreover, some studies showed that there were associations between nutrient levels and ADHD behaviors.[14] Based on recent studies, magnesium and Vitamin D levels might contribute to symptomatology of ADHD.[15,16,17]
Magnesium is an essential element to maintain the body performance.[18] Many observational studies showed that the serum magnesium level in ADHD children was lower than controls.[19,20,21] There are few interventional studies that assay the effect of magnesium intake on ADHD-related outcomes.[15] Magnesium-B6 supplementation in children with ADHD resulted in a significant improvement in ADHD symptoms.[22,23,24] Magnesium supplementation in combination with polyunsaturated fatty acids and zinc decreased inattention, hyperactivity, and impulsivity in children with ADHD.[25] Magnesium supplementation along with standard treatment improved inattention, hyperactivity, impulsivity, opposition, and conceptual level in children with ADHD.[26,27]
Vitamin D is a fat-soluble vitamin which is necessary for calcium metabolism, growth, and development of musculoskeletal system and prevention of many chronic diseases.[28]
Recently, it has been shown that Vitamin D deficiency is more prevalent in children with ADHD compared to healthy children.[16,17] To the best of our knowledge, there is only one interventional study that examines the effect of Vitamin D supplementation on ADHD in children.[29] In this study, Vitamin D supplementation caused a significant improvement in ADHD evening symptoms.
Recent studies suggest possible interactions between Vitamin D and magnesium.[30,31,32,33,34] Vitamin D supplementation could elevate serum magnesium levels.[30,31] Furthermore, serum levels of Vitamin D might be affected by magnesium intake.[32] In addition, magnesium might affect Vitamin D metabolism.[33,34]
Therefore, the aim of the present study is to determine the effect of Vitamin D and magnesium supplementation on behavior problems in children with ADHD.
Methods
This double-blind, randomized controlled clinical trial study was conducted on 74 children with ADHD (22 girls and 52 boys) in Clinic of Noor and Ali Asghar Hospital in Isfahan, Iran, from March 10 to May 22, 2016. Samples were calculated with a confidence interval of 95% and 80% power of the test (S = 24, d = 8.2). The study was approved by Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran. This trial was registered at Iranian Registry of Clinical Trials as IRCT2016030326886N1.
The inclusion criteria were: (1) ADHD diagnosis using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM IV): DSM IV diagnoses ADHD based on ADHD-related symptoms. A minimum of six out of nine symptoms of inattention and a minimum of six out of nine symptoms of hyperactivity/impulsivity in two or more settings (e.g., home and school) is associated with ADHD diagnosis;[1] (2) age between 6 and 12 years old; (3) signing informed written consents by children's parents to participate their children in the study; (4) the serum 25-hydroxy-Vitamin D (25-OH-Vitamin D) level <30 ng/dl;[35] and (5) the serum Mg <2.3 mg/dl.[36] The exclusion criterion was: Having any other diseases, for example, comorbid psychiatric disorders like autism spectrum disorders (ASD).
Despite our recommendation, not to use multivitamin-multimineral supplements, eight children had consumed multivitamins with Vitamin D. Therefore, they were excluded from the study before random allocation. Participants (n = 66) randomly allocated to either a supplement group (n = 33) or a placebo group (n = 33) using block randomization after stratification for sex. The block size was two and concealed from the executer. An independent person made random allocation cards using computer-generated sequence and used sequentially numbered, sealed, opaque envelopes to conceal the allocation. Neither the researcher nor the participants were aware of the two allocated groups. Children in supplement group received Vitamin D (50,000 IU/week) in the form of oral pearls and magnesium (6 mg/kg/day)[18] in the form of oral tablets. Children in placebo group received Vitamin D (edible paraffin oil) and magnesium (microcrystalline cellulose and stearic acid) placebos which were similar in appearance, color, and taste to these supplements and produced in the School of Pharmacy, Isfahan University of Medical Sciences. The follow-up period lasted eight weeks. Supplements were given to the participants by an independent person in the recruitment center. Both groups received drug medication (Ritalin). Participant's compliance was measured through compare the serum levels of Vitamin D and magnesium before and after the supplementation in two groups.[37]
Detailed information about sex, age, and taking medications was collected by a checklist.
Anthropometric measurements
Height was measured in a standing position, without shoes and by a tape measure with a precision of 0.5 cm. Weight was determined with minimal clothing, without shoes and by analog scale with a precision of 100 g. Body mass index (BMI) was calculated by weight in kilograms divided by height in meters squared.
Assessment of behavior problems
Children's behavior problems (conduct problems, social problems, anxiety/shy, and psychosomatic problems) were scored and evaluated using the Conners’ Parent Rating Scale-48 (CPRS-48). This questionnaire is a 4-point Likert scale (not at all = 0, just a little = 1, pretty much = 2, and very much = 3) which includes 48 questions to evaluate the symptoms.[38] This scale has been validated in Iran.[39] CPRS-48 questionnaire was filled by children's parents at baseline and at the end of the trial period.
Biochemical measurement
Serum levels of 25-OH-Vitamin D and magnesium were measured at baseline and at the end of the trial period. Serum levels of 25-OH-Vitamin D were measured by enzyme-linked immunosorbent assay (ELISA) method with a commercially available ELISA kit from Immundiagnostik AG, Bensheim, Germany. Serum 25-OH-Vitamin D levels were categorized as: deficient (serum levels <10 ng/ml); insufficient (serum levels between 10 and 30 ng/ml); and sufficient (serum levels >30 ng/ml).
Serum levels of magnesium were measured by an autoanalyzer (Hitachi 917, Roche Diagnostics® GmbH, Mannheim, Germany) using a commercially available kit.
Statistical analysis
Normality of variables distribution was assessed by Kolmogorov–Smirnov test. As the outcome variables were not normally distributed, we used nonparametric tests. The Wilcoxon signed-rank test was used for comparing intragroup changes. The Mann-Whitney test was used for comparing intergroup changes. Chi-square test was used to assess the significance level of qualitative variables include gender and BMI between the two groups. Participant's compliance was measured through the comparison of the mean changes in serum levels of 25-OH-Vitamin D and magnesium in the intervention group with the placebo group during the study. For all analyses, SPSS 19 (SPSS, Inc., Chicago, IL, USA) was used. P = 0.05 considered as significance level.
Results
Among 74 children with ADHD, eight children were excluded from the study due to consuming multivitamins with Vitamin D before randomization. None of the participants lost to follow-up; therefore, 66 participants completed the study and were included in the final analysis [Figure 1].
Figure 1.
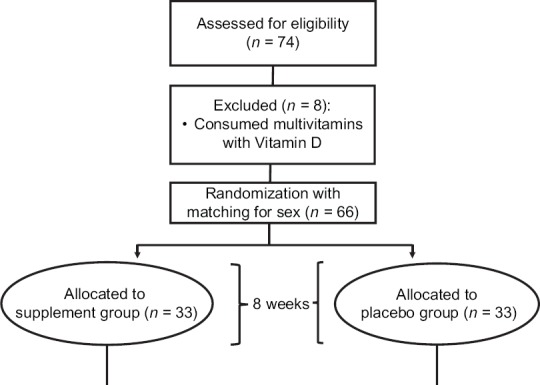
Participants’ flow diagram
Mean values for age, sex, BMI, and other general characteristics of participants represented in Table 1. No significant difference was found in terms of age, sex, BMI, and Ritalin dosage between intervention and placebo groups. Baseline characteristics of conduct problems, social problems, psychosomatic problems, and anxiety/shy scores were not significant in the two groups [Table 2]. Furthermore, there were not any significant differences between the serum levels of 25-OH-vitamin D and magnesium at baseline in the two groups [Figures 2 and 3].
Table 1.
General characteristics of the study participantsa
| Intervention (n=33) | Control (n=33) | Pb | |
|---|---|---|---|
| Age (years) | 9.06±1.76 | 9.15±1.46 | 0.80 |
| Weight (kg) | 31.33±9.93 | 31.17±8.82 | 0.96 |
| Height (cm) | 129.46±11.12 | 129.34±9.48 | 0.87 |
| BMI (kg/m²), n (%) | |||
| Underweight | 3 (9.10) | 1 (3.00) | 0.66 |
| Normal weight | 17 (51.50) | 21 (63.60) | |
| Overweight | 8 (24.20) | 7 (21.20) | |
| Obese | 5 (15.20) | 4 (12.10) | |
| Sex, n (%) | |||
| Boy | 23 (69.7) | 23 (69.7) | 0.99 |
| Girl | 10 (30.3) | 10 (30.3) | |
| Ritalin dose (mg/kg) | 31.33±9.93 | 31.21±8.81 | 0.93 |
aData are presented as means±SD other than those specified, bFor comparison of numerical values Mann–Whitney test, and for qualitative values, Fisher’s exact test has been used. BMI=Body mass index, SD=Standard deviation
Table 2.
The effect of Vitamin D and magnesium supplementation in children with attention-deficit hyperactivity disorder based on Conners̕ parent rating scalea
| Scales | Intervention (n=33) | Control (n=33) | Pc | Pd | Pe | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8th week | Changeb | Baseline | 8th week | Changeb | ||||
| Conduct problems score | 16.91±1.78 | 13.61±1.45 | −3.30±0.51 | 17.24±1.62 | 17.48±1.56 | 0.24±0.16 | 0.69 | 0.05 | 0.001 |
| Social problems score | 10.24±1.08 | 8.03±0.96 | −2.21±0.51 | 10.42±1.03 | 10.61±1.00 | 0.18±0.19 | 0.86 | 0.07 | 0.001 |
| Psychosomatic problems score | 8.79±0.90 | 8.55±0.84 | −0.24±0.16 | 9.00±0.88 | 9.03±0.88 | 0.03±0.06 | 0.81 | 0.69 | 0.20 |
| Anxiety-shy score | 8.24±0.65 | 6.82±0.55 | −1.42±0.25 | 8.06±0.61 | 8.09±0.60 | 0.03±0.06 | 0.86 | 0.09 | 0.001 |
aReported values are means±SEM, bCalculated by subsidizing values at 8th week from values at baseline, cComparison of values at baseline between the two groups using Mann–Whitney test, dComparison of values at 8th week between the two groups using Mann–Whitney test, eComparison of values changes between the two groups using Mann–Whitney test, c,d,eThe P=0.05 considered as significance level. SEM=Standard error of mean
Figure 2.
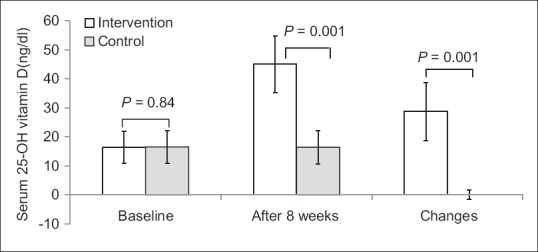
Serum 25-hydroxy-Vitamin D levels of participants at study baseline and end of trial. P values obtained from Mann–Whitney test. P value for comparison of changes between the two groups was 0.001
Figure 3.
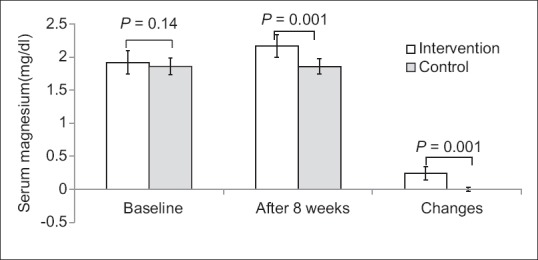
Serum magnesium levels of participants at study baseline and end of trial. P values obtained from Mann–Whitney test. P value for comparison of changes between the two groups was 0.001
After 8 weeks, the serum 25-OH-Vitamin D level increased significantly in the intervention group compared with the placebo group (28.71 ± 10.06 vs. 0.07 ± 1.59; P = 0.001). Furthermore, the serum magnesium level increased in the intervention group compared with the placebo group (0.24 ± 0.1 vs. 0.006 ± 0.03; P = 0.001), significantly [Figures 2 and 3]. Serum 25-OH-Vitamin D and magnesium levels were changed significantly in the intervention group (P = 0.001). However, there were not any significant changes in placebo group.
Supplementation with Vitamin D and magnesium caused a significant decrease in conduct problems score (−3.30 ± 0.51 vs. 0.24 ± 0.16; P = 0.001), social problems score (−2.21 ± 0.51 vs. 0.18 ± 0.19; P = 0.001), and anxiety/shy score (−1.42 ± 0.25 vs. 0.03 ± 0.06; P = 0.001) in intervention group compared with placebos. However, supplementation had no significant effect on psychosomatic problems score (−0.24 ± 0.16 vs. 0.03 ± 0.06; P = 0.20) [Table 2].
According to Table 1, there were not any significant differences between intervention and placebo group for age, BMI, and Ritalin dose. In addition, we assessed the correlation of these confounders with outcome variables, and we did not find any significant correlation. Thus, we did not consider these variables as confounder. Sex was matched during the study. All children were monitored for clinical and laboratory evidence of toxicity or other adverse events. No adverse effects of Vitamin D and magnesium supplementation were reported at the end of this study.
Discussion
In the present study, supplementation with Vitamin D and magnesium in children with ADHD decreased conduct problems, social problems, and anxiety/shy scores compared with placebo intake, however, did not affect psychosomatic problems scores, significantly. To the best of our knowledge, this study is the first clinical trial to examine the effects of Vitamin D and magnesium supplementation on behavior problems in children with ADHD.
ADHD is the most clinically manifested neuropsychiatric condition which contains three diagnostic symptoms: Lack of attention, impulsivity, and hyperactivity. It is a complex disorder that is caused by genetic and environmental factors.[40] Recently, it has been revealed that dietary factors (such as Vitamin D or magnesium) play an important role among environmental factors.[16,40] Global prevalence of ADHD is about 5% in children.[1] ADHD has a high comorbidity with other psychiatric diseases such as ASD and LD.[8,41] The economic costs, especially on education and health care of children with ADHD with or without comorbid conditions, are substantial.[41,42]
To the best of our knowledge, except one adjunctive therapy,[29] there are no other studies on the effect of Vitamin D as a treatment alone or in combination with other nutrients on the severity of symptoms in children with ADHD although low 25-OH-Vitamin D3 levels have been associated with ADHD in observational studies.[16,17,29] Mohammadpour et al. conducted a randomized, double-blind placebo-controlled trial on 62 children with ADHD aged 5–12 years. They prescribed either 2000 IU Vitamin D or placebo per day in addition to methylphenidate for 8 weeks. Severity of symptoms was measured through CPRS-Revised(s), ADHD rating scale-IV, and Weekly Parent Ratings of Evening and Morning Behavior. Serum Vitamin D levels and ADHD evening symptoms improved significantly in the intervention group compared with the placebo group after 8 weeks. Small sample size, short duration of follow-up, and low doses of Vitamin D are limitations of this study.[29] Furthermore, psychosomatic problems were not assessed in this study.
Vitamin D affects various brain functions directly through its nuclear receptors (Vitamin D receptor or VDR) scattered throughout the central nervous system. Experimental and in vitro studies revealed that Vitamin D involved in several pathways in the brain including synthesis of neurotrophic agents, regulation of numerous neurotransmitters, neuroprotection, and neuroimmunomodulation. In humans, cortex, cerebellum, and limbic system, the three important parts of the brain, are involved in the behavior and are targeted by VDR. Thus, VDR polymorphisms could lead to psychiatric conditions such as ADHD, depression, and anxiety.[43,44]
We found only three interventional studies which used magnesium as a treatment and CPRS as an assessment tool in ADHD.[23,26,27] Magnesium was used in combination with other nutrients or along with standard treatment in these studies. In addition, these studies had some limitations including very small sample size and lack of blinding. Moreover, none of these three studies were assessed our subscales such as psychosomatic problems in the present study.[23,26,27]
Magnesium has an important role in regulating of various brain functions (e.g., behavior). Activity of numerous neurotransmitters such as dopamine, norepinephrine, and serotonin is dependent on magnesium. Furthermore, release of n-methyl-d-aspartate-induced norepinephrine is inhibited by magnesium.[18] Thus, as it previously has been shown, magnesium might be useful as a therapeutic agent in the treatment of ADHD.[22,23,24,25,26,27]
There is an association between serum magnesium and Vitamin D levels.[45] Vitamin D supplementation could improve serum levels of magnesium.[30,31] Similarly, magnesium intake might be associated with reduced risks of vitamin D deficiency and insufficiency.[32] Besides, magnesium could affect vitamin D production. The synthesis of 24,25(OH) 2D3 and 1,25(OH) 2D3 might be modulated by magnesium in vivo and in vitro.[33,34] Furthermore, Vitamin D and magnesium affect similar areas of the brain involved in behavior.[18,43]
Our study had several limitations: First, this study did not have a 2 × 2 factorial design to assess the effect of either Vitamin D or magnesium supplementation on the study's variables, separately. Therefore, only the effect of combination of Vitamin D and magnesium supplements was evaluated. This could be due to our small sample size. In addition, dietary intakes of magnesium and Vitamin D were not assessed and duration of follow-up was short.
Conclusions
Vitamin D and magnesium supplementation in children with ADHD was effective on conduct problems, social problems, and anxiety/shy scores compared with placebo intake but did not affect psychosomatic problems scores, significantly.
Financial support and sponsorship
Funding source of this study was supported by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors take thankful pleasure in acknowledging the unsparing assistance of all participants. This study was extracted from MSc thesis which was approved by the School of Nutrition and Food Science, Isfahan University of Medical Sciences (code: IR. MUI. REC.1394.3.961).
References
- 1.Sadock BJ, Sadock VA. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. 9th ed. London: Williams & Wilkins; 2009. pp. 3561–72. [Google Scholar]
- 2.Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics. 2015;135:e994–1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- 3.Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43:434–42. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammadi MR, Ahmadi N, Salmanian M, Asadian-Koohestani F, Ghanizadeh A, Alavi A, et al. Psychiatric disorders in Iranian children and adolescents. Iran J Psychiatry. 2016;11:87–98. [PMC free article] [PubMed] [Google Scholar]
- 5.Klassen AF, Miller A, Fine S. Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics. 2004;114:e541–7. doi: 10.1542/peds.2004-0844. [DOI] [PubMed] [Google Scholar]
- 6.Frazier TW, Youngstrom EA, Glutting JJ, Watkins MW. ADHD and achievement: Meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. J Learn Disabil. 2007;40:49–65. doi: 10.1177/00222194070400010401. [DOI] [PubMed] [Google Scholar]
- 7.Biederman J, Fried R, Petty C, Mahoney L, Faraone SV. An examination of the impact of attention-deficit hyperactivity disorder on IQ: A large controlled family-based analysis. Can J Psychiatry. 2012;57:608–16. doi: 10.1177/070674371205701005. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw SP. Externalizing behavior problems and academic underachievement in childhood and adolescence: Causal relationships and underlying mechanisms. Psychol Bull. 1992;111:127–55. doi: 10.1037/0033-2909.111.1.127. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. MedGenMed. 2006;8:12. [PMC free article] [PubMed] [Google Scholar]
- 10.Thapar A, Langley K, Asherson P, Gill M. Gene-environment interplay in attention-deficit hyperactivity disorder and the importance of a developmental perspective. Br J Psychiatry. 2007;190:1–3. doi: 10.1192/bjp.bp.106.027003. [DOI] [PubMed] [Google Scholar]
- 11.Mash EJ, Barkley RA. Assessment of Childhood Disorders. 4th ed. New York: Guilford Publications; 2012. [Google Scholar]
- 12.Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PR, et al. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled trial. Nutrition. 2012;28:670–7. doi: 10.1016/j.nut.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Nigg JT, Lewis K, Edinger T, Falk M. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry. 2012;51:86–97. doi: 10.1016/j.jaac.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold LE, Bozzolo H, Hollway J, Cook A, DiSilvestro RA, Bozzolo DR, et al. Serum zinc correlates with parent- and teacher – Rated inattention in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15:628–36. doi: 10.1089/cap.2005.15.628. [DOI] [PubMed] [Google Scholar]
- 15.Ghanizadeh A. A systematic review of magnesium therapy for treating attention deficit hyperactivity disorder. Arch Iran Med. 2013;16:412–7. [PubMed] [Google Scholar]
- 16.Goksugur SB, Tufan AE, Semiz M, Gunes C, Bekdas M, Tosun M, et al. Vitamin D status in children with attention-deficit-hyperactivity disorder. Pediatr Int. 2014;56:515–9. doi: 10.1111/ped.12286. [DOI] [PubMed] [Google Scholar]
- 17.Kamal M, Bener A, Ehlayel MS. Is high prevalence of Vitamin D deficiency a correlate for attention deficit hyperactivity disorder? Atten Defic Hyperact Disord. 2014;6:73–8. doi: 10.1007/s12402-014-0130-5. [DOI] [PubMed] [Google Scholar]
- 18.Gröber U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7:8199–226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archana E, Pai P, Prabhu BK, Shenoy RP, Prabhu K, Rao A, et al. Altered biochemical parameters in saliva of pediatric attention deficit hyperactivity disorder. Neurochem Res. 2012;37:330–4. doi: 10.1007/s11064-011-0616-x. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud MM, El-Mazary AA, Maher RM, Saber MM. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital J Pediatr. 2011;37:60. doi: 10.1186/1824-7288-37-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogovitsina OR, Levitina EV. Neurological aspect of clinical symptoms, pathophysiology and correction in attention deficit hyperactivity disorder. Zh Nevrol Psikhiatr Im S S Korsakova. 2006;106:17–20. [PubMed] [Google Scholar]
- 22.Mousain-Bosc M, Roche M, Polge A, Pradal-Prat D, Rapin J, Bali JP, et al. Improvement of neurobehavioral disorders in children supplemented with magnesium-Vitamin B6. I. Attention deficit hyperactivity disorders. Magnes Res. 2006;19:46–52. [PubMed] [Google Scholar]
- 23.Mousain-Bosc M, Roche M, Rapin J, Bali JP. Magnesium VitB6 intake reduces central nervous system hyperexcitability in children. J Am Coll Nutr. 2004;23:545S–8S. doi: 10.1080/07315724.2004.10719400. [DOI] [PubMed] [Google Scholar]
- 24.Nogovitsina OR, Levitina EV. Effect of MAGNE-B6 on the clinical and biochemical manifestations of the syndrome of attention deficit and hyperactivity in children. Eksp Klin Farmakol. 2006;69:74–7. [PubMed] [Google Scholar]
- 25.Huss M, Völp A, Stauss-Grabo M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems – An observational cohort study. Lipids Health Dis. 2010;9:105. doi: 10.1186/1476-511X-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starobrat-Hermelin B. The effect of deficiency of selected bioelements on hyperactivity in children with certain specified mental disorders. Ann Acad Med Stetin. 1998;44:297–314. [PubMed] [Google Scholar]
- 27.El Baza F, AlShahawi HA, Zahra S, AbdelHakim RA. Magnesium supplementation in children with attention deficit hyperactivity disorder. Egypt J Med Hum Genet. 2016;17:63–70. [Google Scholar]
- 28.Holick MF. Sunlight and Vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadpour N, Jazayeri S, Tehrani-Doost M, Djalali M, Hosseini M, Effatpanah M, et al. Effect of Vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: A randomized, double-blind, placebo-controlled trial. Nutr Neurosci. 2018;21:202–9. doi: 10.1080/1028415X.2016.1262097. [DOI] [PubMed] [Google Scholar]
- 30.Al-Daghri NM, Alkharfy KM, Khan N, Alfawaz HA, Al-Ajlan AS, Yakout SM, et al. Vitamin D supplementation and serum levels of magnesium and selenium in type 2 diabetes mellitus patients: Gender dimorphic changes. Int J Vitam Nutr Res. 2014;84:27–34. doi: 10.1024/0300-9831/a000190. [DOI] [PubMed] [Google Scholar]
- 31.Farhanghi MA, Mahboob S, Ostadrahimi A. Obesity-induced magnesium deficiency can be treated by Vitamin D supplementation. J Pak Med Assoc. 2009;59:258–61. [PubMed] [Google Scholar]
- 32.Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, et al. Magnesium, Vitamin D status and mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013;11:187. doi: 10.1186/1741-7015-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risco F, Traba ML. Influence of magnesium on the in vitro synthesis of 24,25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3. Magnes Res. 1992;5:5–14. [PubMed] [Google Scholar]
- 34.Risco F, Traba ML, de la Piedra C. Possible alterations of the in vivo 1,25(OH) 2D3 synthesis and its tissue distribution in magnesium-deficient rats. Magnes Res. 1995;8:27–35. [PubMed] [Google Scholar]
- 35.Holick MF. Vitamin D and health: evolution, biologic functions, and recommended dietary intakes for Vitamin D. Clin Rev Bone Miner Metab. 2009;7:2–19. [Google Scholar]
- 36.Drueke TB, Lacour B. Magnesium homeostasis and disorders of magnesium metabolism. In: Feehally J, Floege J, Johnson RJ, editors. Comprehensive Clinical Nephrology. Philadelphia: Mosby; 2007. pp. 136–8. [Google Scholar]
- 37.Zhang Z, Peluso MJ, Gross CP, Viscoli CM, Kernan WN. Adherence reporting in randomized controlled trials. Clin Trials. 2014;11:195–204. doi: 10.1177/1740774513512565. [DOI] [PubMed] [Google Scholar]
- 38.Gianarris WJ, Golden CJ, Greene L. The conners’ parent rating scales: A critical review of the literature. Clin Psychol Rev. 2001;21:1061–93. doi: 10.1016/s0272-7358(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 39.Shahaeian A, Shahim S, Bashash L, Yousefi F. Standardisation, factor analysis and reliability of the conners’ parent rating scales for 6- to 11-years-old children in shiraz. Q J Psychol Stud. 2007;3:97–120. [Google Scholar]
- 40.Heilskov Rytter MJ, Andersen LB, Houmann T, Bilenberg N, Hvolby A, Mølgaard C, et al. Diet in the treatment of ADHD in children – A systematic review of the literature. Nord J Psychiatry. 2015;69:1–8. doi: 10.3109/08039488.2014.921933. [DOI] [PubMed] [Google Scholar]
- 41.Peacock G, Amendah D, Ouyang L, Grosse SD. Autism spectrum disorders and health care expenditures: The effects of co-occurring conditions. J Dev Behav Pediatr. 2012;33:2–8. doi: 10.1097/DBP.0b013e31823969de. [DOI] [PubMed] [Google Scholar]
- 42.Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51:990–100200. doi: 10.1016/j.jaac.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Annweiler C, Schott AM, Berrut G, Chauviré V, Le Gall D, Inzitari M, et al. Vitamin D and ageing: Neurological issues. Neuropsychobiology. 2010;62:139–50. doi: 10.1159/000318570. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB, et al. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26:551–4. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- 45.Kelishadi R, Ataei E, Ardalan G, Nazemian M, Tajadini M, Heshmat R, et al. Relationship of serum magnesium and Vitamin D levels in a nationally-representative sample of Iranian adolescents: The CASPIAN-III study. Int J Prev Med. 2014;5:99–103. [PMC free article] [PubMed] [Google Scholar]


