Abstract
Background
Many patients with chronic obstructive pulmonary disease (COPD) still experience daily symptoms, exacerbations, and accelerated lung function decline, even when receiving maximal combined treatment with inhaled long-acting bronchodilators and corticosteroids. Novel treatment options are needed for these patients.
Phosphodiesterases (PDEs) are enzymes that impact a range of cellular functions by modulating levels of cyclic nucleotides, and there is evidence to suggest that combined inhibition of PDE3 and PDE4 can have additive (or perhaps synergistic) effects. This study investigated the efficacy and safety of ensifentrine, a first-in-class dual inhibitor of PDE 3 and 4, in patients with COPD.
Methods
This randomised, double-blind, placebo-controlled, parallel-group, dose-ranging study recruited patients with COPD, post-bronchodilator forced expiratory volume in 1 s (FEV1) 40–80% predicted and FEV1/forced vital capacity ratio ≤ 0.7. Patients were randomised equally to inhale nebulised ensifentrine 0.75, 1.5, 3 or 6 mg or placebo, all twice daily. Primary outcome: placebo-adjusted difference in peak FEV1 (assessed over 3 h) at Week 4.
Results
The study took place between July 2017 and February 2018. Of 405 patients randomly assigned to medication, 375 (92.6%) completed the study. For peak FEV1 at Week 4, all four ensifentrine doses were superior to placebo (p ≤ 0.0001) with least squares mean differences of 146 (95% CI 75–216), 153 (83–222), 200 (131–270) and 139 (69–210) mL for ensifentrine 0.75, 1.5, 3 and 6 mg, respectively. Respiratory symptoms (assessed using the Evaluating Respiratory Symptoms questionnaire) were also significantly improved with all ensifentrine doses at Week 4. Adverse events were reported by 33.3, 44.4, 35.4 and 36.3% patients with ensifentrine 0.75, 1.5, 3 and 6 mg, respectively, and 39.2% with placebo.
Conclusions
In this four-week Phase IIb study, all four ensifentrine doses significantly improved bronchodilation and symptoms, with a dose-ranging effect from 0.75 to 3 mg twice daily, and all doses well tolerated. The study supports the continuing development of ensifentrine in COPD.
Trial registration
EudraCT 2016–005205-40, registered 30 May 2017.
Keywords: Phosphodiesterase inhibitors, Chronic obstructive pulmonary disease, Spirometry, Symptoms, Safety
Background
Characteristic features of chronic obstructive pulmonary disease (COPD) are airflow obstruction and persistent inflammation [1]. Inhaled long-acting bronchodilators and corticosteroids have been shown to improve symptoms and health-related quality of life, and to reduce exacerbation rates in patients with COPD [1]. However, many patients still experience daily symptoms [2–4], exacerbations [5–7], and accelerated lung function decline [8], even when receiving maximal combined treatment with these inhaled drugs. Novel treatment options are needed for these patients.
Phosphodiesterases (PDEs) are enzymes that impact a range of cellular functions by modulating levels of cyclic nucleotides. PDE3 regulates cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) concentrations in airway smooth muscle, such that inhibition results in airway smooth muscle relaxation [9–11]. PDE4 regulates cAMP concentrations and is involved in inflammatory cell activation; consequently, inhibition has anti-inflammatory effects [9–11]. There is evidence to suggest that combined inhibition of PDE3 and PDE4 can have additive (or perhaps synergistic) effects with respect to both anti-inflammatory and bronchodilator activity [9].
Ensifentrine is an inhaled first-in-class dual inhibitor of PDE3 and PDE4 that has previously demonstrated bronchodilator and anti-inflammatory efficacy in proof of concept studies in healthy volunteers, and in patients with asthma or COPD [12–15]. In patients with COPD, lung function and safety data had previously been obtained from up to 6 days treatment, although with no evaluation of symptoms [15]. Delivery of ensifentrine by inhalation aims to minimise the systemic adverse effects that have been associated with oral administration of PDE inhibitors [16].
This randomised, double-blind, placebo-controlled, parallel-group Phase IIb study aimed to investigate the efficacy, in terms of lung function and symptoms, and safety of 4 weeks dosing of a range of ensifentrine doses in patients with COPD who did not receive any concomitant long-acting bronchodilator therapy for COPD.
Methods
Study design
Patients meeting the inclusion/exclusion criteria at screening entered a 7–14-day run-in period during which long acting bronchodilator therapy was washed out; inhaled corticosteroids were continued at the same dose from ≥4 weeks prior to screening and throughout the study (Additional file 1: Figure S1). After the run-in, patients were randomised equally to five treatment groups, to inhale twice daily nebulised ensifentrine 0.75, 1.5, 3 or 6 mg or placebo. Patients then attended the study centre weekly for 4 weeks. Rescue salbutamol was permitted, but not within 8 h before spirometry assessments.
The study was approved by independent ethics committees at each institution, and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice (ICH/CPMP/135/95). The study is registered at ClinicalTrials.gov (NCT03443414) and EudraCT (2016–005205-40).
Patients
Eligible patients were: males or females aged 40–75 years, inclusive; diagnosed with COPD for at least 1 year; clinically stable for at least 4 weeks; post-bronchodilator forced expiratory volume in 1 s (FEV1) 40–80% predicted normal and FEV1/forced vital capacity ratio ≤ 0.7; and current or former smokers with smoking history of at least 10 pack-years. To remain eligible, pre-dose FEV1 at randomisation was to be ±20% of the screening value. All patients provided written informed consent prior to any study-related procedure. Full inclusion and exclusion criteria are in the additional file.
Procedures
At the randomisation visit, baseline (pre-dose) data were collected for spirometry (FEV1), St. George’s Respiratory Questionnaire – COPD Specific (SGRQ-C), Baseline Dyspnoea Index, and Medical Research Council dyspnoea scale (MRC). Spirometry was assessed pre-dose and up to 3 h post-dose on Weeks 1, 2 and 3, and up to 12 h post-dose on Day 1 and Week 4. At Week 4, Transition Dyspnoea Index (TDI), SGRQ-C, MRC and patient’s global assessment of change were assessed pre-dose. The patient’s global assessment of change is a study-specific questionnaire in which patients were asked, “Compared with prior to the study start, how do you feel your breathing is?”, graded on a scale of 1 = much worse to 5 = much better, with 3 = no change. Daily throughout the study (including between screening and randomisation) patients used an e-diary to record rescue medication use and COPD symptoms (using the Evaluating Respiratory Symptoms [E-RS™:COPD] questionnaire). Vital signs (blood pressure and pulse rate) and 12-lead electrocardiograms were assessed pre- and post-dose on all visits, with Holter monitor data collected over the 24-h periods prior to randomisation (for baseline) and the Week 4 visit. Adverse events were captured over the study duration.
Outcomes
The primary objective was to investigate the placebo-corrected effect of ensifentrine on change from baseline in peak FEV1 (assessed over 0–3 h) on Week 4. Secondary efficacy variables were: morning trough FEV1 (measured 15 min pre-dose) on Weeks 1–4; average FEV1 over 0–12 h on Day 1 and Week 4; average FEV1 over 0–3 h on Day 1 and Weeks 1–4; peak FEV1 on Day 1 and Weeks 1–3; E-RS™:COPD total score and average daily use of rescue medication over Weeks 1, 2, 3 and 4; and TDI, SGRQ-C total score (mean and percentage of responders), MRC and patient’s global assessment of change after 4 weeks. Ensifentrine safety was assessed by adverse events, electrocardiogram data, Holter monitoring, laboratory safety tests and vital signs.
Randomisation and masking
Patients were assigned to treatment groups in accordance with a randomisation list generated by the sponsor’s contract research organisation. Patients, investigators, site staff and sponsor personnel were masked to treatment assignment for the duration of the study, with the exceptions of the sponsor’s clinical supply chain lead, and of designated unblinded personnel at the sites, who, because the active and placebo treatments did not visually match, administered the in-clinic dose to patients and were not to take part in any of the study assessments.
Statistical analysis
Peak FEV1 standard deviation was estimated to be 250 mL. With a two-sided test at a 5% significance level and 80 evaluable patients per group, it was estimated that there would be 80% power to detect a true difference of 111 mL between any two treatments. This detectable limit was considered sufficient to identify a minimal effective dose of ensifentrine. Thus 80 patients per group were to be randomised.
The primary endpoint (Week 4 peak FEV1) was analysed using a restricted maximum likelihood-based mixed model for repeated measured (MMRM), including fixed effects for treatment, visit and treatment by visit interaction, patient as random effect, baseline value as covariate and covariance structure by visit. Ensifentrine–placebo differences with 95% confidence intervals and corresponding two-sided p-values were calculated. To control for the familywise error rate, a fixed-sequence testing strategy was employed, with the highest ensifentrine dose tested vs placebo. If a statistically significant difference was found at the two-sided α level of 5%, the testing proceeded with the next lower dose. If a test was non-significant, testing stopped and the remaining null hypotheses accepted.
A similar mixed model for repeated measured (MMRM) method was used to analyse most of the secondary efficacy endpoints, with the same hierarchical testing within endpoint, although endpoints were tested independently. The average FEV1 endpoints were calculated using the linear trapezoidal method as the area under the curve divided by the length of the time interval of interest. E-RS™:COPD total score data and rescue medication use were averaged to give weekly scores. The percentage of SGRQ-C responders, defined as patients with an improvement from baseline of ≥4 units, was analysed using a logistic regression model adjusting for treatment and country and giving the odds for being a responder as outcome, treatment differences expressed as the odds ratio.
The efficacy data were analysed in the full analysis set, which comprised all randomised patients who received at least one dose of study medication and had at least one post-treatment efficacy assessment. Safety analyses were in the safety population, which was all randomised patients who received at least one dose of study medication.
Results
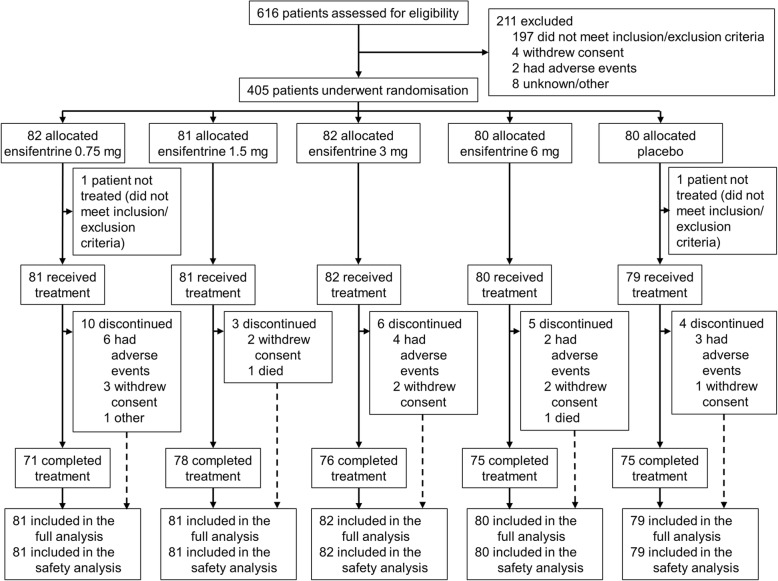
The study took place between 10 July 2017 and 7 February 2018 in 47 study centres across six countries (Bulgaria [8], Czech Republic [2], Germany [16], Poland [13], Romania [7], and United Kingdom [1]). We recruited 616 patients, of whom 405 were eligible to be randomly assigned to medication, with 375 (93%) completing the study (Fig. 1). Compliance to treatment was high, with the median duration of exposure being 29.0 days in all five groups. Baseline characteristics of the recruited patients are shown in Table 1.
Fig. 1.
Patient flow through the study
Table 1.
Baseline demographics and disease characteristics (all randomised analysis set)
| Ensifentrine | Placebo (N = 80) | ||||
|---|---|---|---|---|---|
| 0.75 mg (N = 82) | 1.5 mg (N = 81) | 3 mg (N = 82) | 6 mg (N = 80) | ||
| Age, years | 63.6 (7.05) | 63.4 (6.40) | 62.5 (6.51) | 62.9 (6.73) | 63.5 (6.44) |
| Sex, n (%) | |||||
| Male | 56 (68) | 46 (57) | 45 (55) | 48 (60) | 50 (63) |
| Female | 26 (32) | 35 (43) | 37 (45) | 32 (40) | 30 (38) |
| Race, n (%) | |||||
| White | 82 (100) | 81 (100) | 82 (100) | 80 (100) | 80 (100) |
| Post-bronchodilator FEV1 | |||||
| L | 1.67 (0.464) | 1.60 (0.466) | 1.62 (0.441) | 1.63 (0.474) | 1.69 (0.493) |
| % predicted | 56.0 (10.34) | 56.0 (9.83) | 55.6 (10.18) | 55.3 (9.47) | 56.0 (9.89) |
| FEV1 reversibility, % | 10.4 (10.13) | 11.0 (12.32) | 12.9 (13.53) | 12.3 (11.14) | 11.7 (10.60) |
| Smoking status, n (%) | |||||
| Current | 50 (61) | 40 (49) | 47 (57) | 42 (53) | 43 (54) |
| Ex | 32 (39) | 41 (51) | 35 (43) | 38 (48) | 37 (46) |
| Smoking history, pack-years | 44.7 (21.27) | 43.7 (21.98) | 41.8 (19.05) | 37.3 (16.75) | 43.3 (20.21) |
| Chronic bronchitis, n (%) | 48 (59) | 43 (53) | 56 (68) | 58 (73) | 46 (58) |
| SGRQ-C | 49.9 (17.36) | 43.4 (17.06) | 42.1 (18.78) | 44.1 (15.02) | 42.3 (17.07) |
| BDI | 5.9 (1.44) | 6.4 (1.81) | 6.4 (1.43) | 6.4 (1.16) | 6.4 (1.38) |
| MRC | 2.8 (0.82) | 2.5 (0.79) | 2.5 (0.79) | 2.6 (0.73) | 2.5 (0.75) |
| MRC score, n (%) | |||||
| < 2 | 4 (5) | 8 (10) | 6 (7) | 4 (5) | 4 (5) |
| ≥ 2 | 78 (95) | 73 (90) | 76 (93) | 76 (95) | 76 (95) |
| Rescue medication, puffs per daya | 1.5 (1.80) | 1.5 (1.84) | 1.9 (2.14) | 1.9 (2.13) | 1.5 (1.88) |
| E-RS:COPD™b | 13.6 (6.77) | 12.3 (6.05) | 12.0 (6.03) | 12.2 (6.29) | 11.5 (6.23) |
| Concomitant ICS use, n (%) | 33 (40) | 36 (44) | 29 (35) | 32 (40) | 28 (35) |
Data are mean (standard deviation) unless specified otherwise
aAvailable baseline data from 81, 81, 82, 80 and 79 patients, respectively
bAvailable baseline data from 75, 75, 77, 77 and 77, respectively
Abbreviations: FEV1 forced expiratory volume in 1 s, SGRQ-C St George’s Respiratory Questionnaire – COPD Specific, BDI Baseline Dyspnoea Index, MRC Medical Research Council dyspnoea scale, E-RS:COPD™ Evaluating Respiratory Symptoms in COPD questionnaire, COPD chronic obstructive pulmonary disease, ICS inhaled corticosteroid
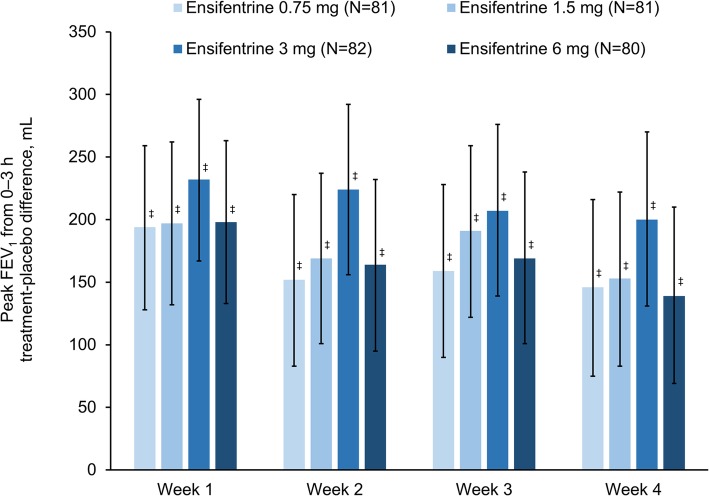
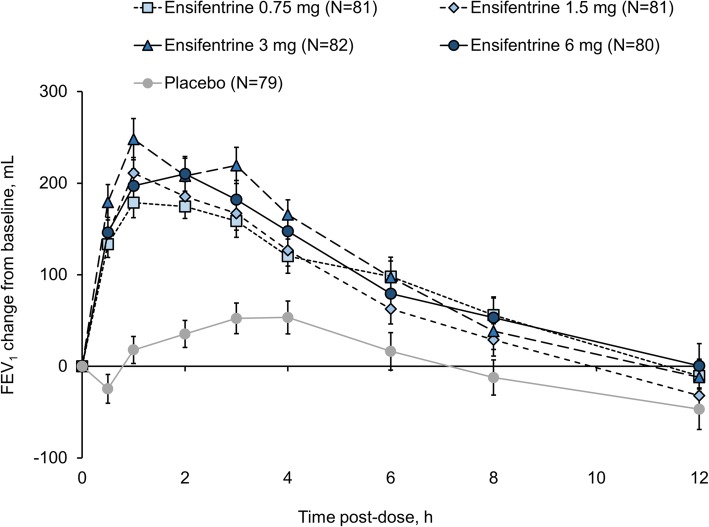
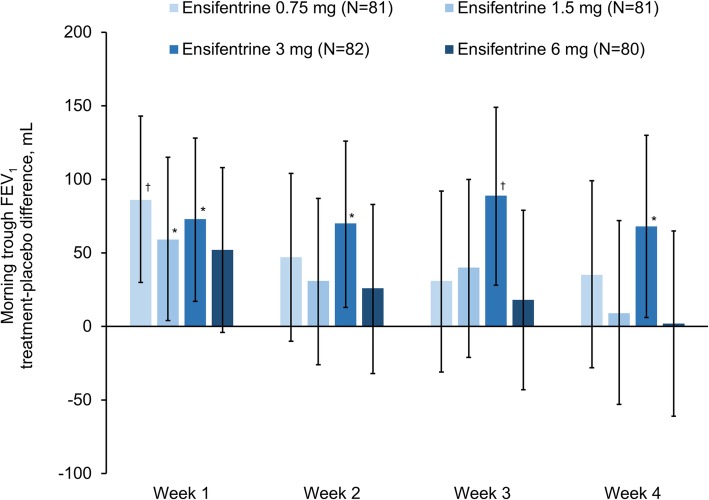
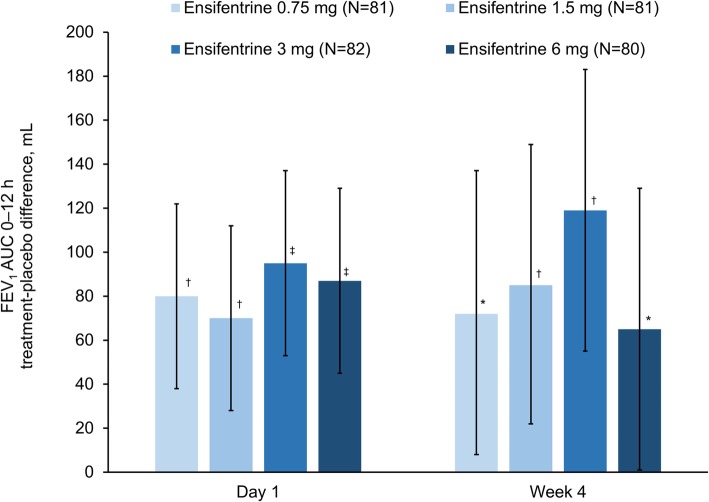
For the primary endpoint (peak FEV1 on Week 4), all four ensifentrine doses were superior to placebo (p < 0.001) with differences between 139 and 200 mL (Fig. 2). Following the first dose on Day 1, there was an immediate increase in FEV1 in all four ensifentrine groups (Fig. 3), with peak FEV1 on Day 1 significantly higher for all ensifentrine doses compared to placebo, and differences vs placebo of 153 (95% confidence interval 105, 201), 158 (110, 206), 207 (159, 256), and 173 (125, 221) mL for ensifentrine 0.75, 1.5, 3 and 6 mg (all p < 0.001). Peak FEV1 was also significantly higher for all ensifentrine doses compared to placebo after 1, 2 and 3 weeks (p < 0.001; Fig. 2), whereas for morning (pre-dose) trough FEV1, only ensifentrine 3 mg was consistently statistically superior to placebo, with differences ranging between 68 and 89 mL (Fig. 4). Average FEV1 over 12 h for all ensifentrine doses was superior to placebo, both immediately following the first dose (Day 1) and after four weeks, for example with a difference vs placebo of 119 mL for the 3 mg dose at Week 4 (Fig. 5). Similarly, average FEV1 over 0–3 h for all ensifentrine doses was superior to placebo at all visits (Additional file 1: Table S1). In general, a dose-response effect on FEV1 parameters was apparent for ensifentrine doses between 0.75 mg and 3 mg.
Fig. 2.
Peak FEV1 between 0 and 3 h post-dose (full analysis set). Data are least squares means treatment–placebo differences and 95% confidence intervals. ‡p < 0.001 vs placebo. Least squares mean changes from baseline in the placebo group (N = 79) were 36, 50, 42 and 57 mL at Weeks 1, 2, 3 and 4, respectively. Abbreviation: FEV1, forced expiratory volume in 1 s
Fig. 3.
Individual timepoint FEV1 on Day 1 (full analysis set). Data are mean and standard error of the mean. Abbreviation: FEV1, forced expiratory volume in 1 s
Fig. 4.
Morning trough FEV1 (full analysis set). Data are least squares means treatment–placebo differences and 95% confidence intervals. *p < 0.05; †p < 0.01 vs placebo. Least squares mean changes from baseline in the placebo group (N = 79) were −56, −32, −49 and −28 mL at Weeks 1, 2, 3 and 4, respectively
Fig. 5.
Average FEV1 over 0–12 h (full analysis set). Data are least squares means treatment–placebo differences and 95% confidence intervals. *p < 0.05; †p < 0.01; ‡p < 0.001 vs placebo. Least squares mean changes from baseline in the placebo group (N = 79) were 8 and −33 mL on Day 1 and at Week 4, respectively. Abbreviation: FEV1, forced expiratory volume in 1 s
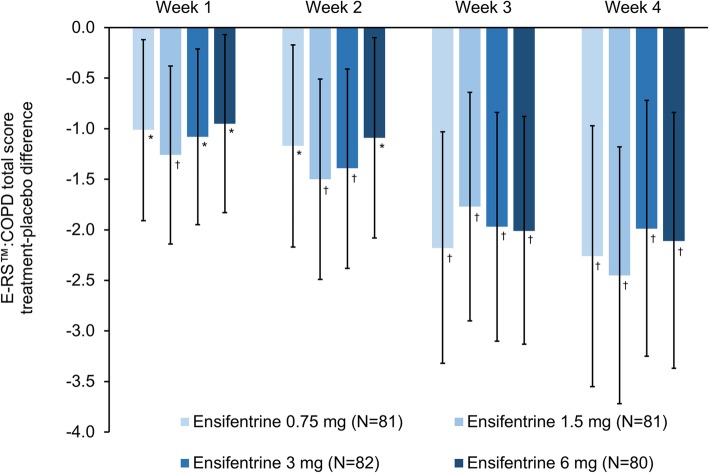
There was a progressive improvement in symptoms over the duration of the study for all four doses compared to placebo as measured by daily E-RS™:COPD total score (Fig. 6). For TDI focal score, all four ensifentrine doses were superior to placebo at Week 4, with differences ranging from 1.11 to 1.64 (Table 2). Compared with patients receiving placebo, after four weeks there was a numerical improvement vs placebo in both mean SGRQ-C total score and the percentage of responders with all four ensifentrine doses, although the differences vs placebo were not statistically significant (Table 2 and Additional file 1: Table S2). Similarly, at Week 4 there was a numerical improvement vs placebo in MRC total score for all ensifentrine doses (Additional file 1: Table S3), whereas for the patient’s global assessment of change, all ensifentrine doses were statistically superior to placebo at this timepoint (Additional file 1: Table S3). Rescue medication use was numerically lower with all four ensifentrine doses compared with placebo over all four periods, with the reduction statistically significant for the 1.5, 3 and 6 mg doses in the Week 2, 3 and 4 analyses (Additional file 1: Table S4).
Fig. 6.
E-RS™:COPD total score (full analysis set). Data are least squares means treatment–placebo differences and 95% confidence intervals. *p < 0.05; †p < 0.01 vs placebo. Least squares mean changes from baseline in the placebo group (N = 79) were 0.38, 0.57, 1.11 and 1.19 at Weeks 1, 2, 3 and 4, respectively. Abbreviation: ERS:COPD™, Evaluating Respiratory Symptoms in COPD questionnaire
Table 2.
TDI focal score and SGRQ-C total score after 4 weeks (full analysis set)
| Ensifentrine dose | TDI focal score | SGRQ-C total score | ||
|---|---|---|---|---|
| Mean (SD) | Treatment–placebo difference (95% confidence interval); p value | Mean (SD) | Treatment–placebo difference (95% confidence interval); p value | |
| 6 mg (N = 80) | 1.39 (2.179) | 1.11 (0.16 to 2.06); 0.022 | 41.5 (15.20) | −2.67 (− 6.26 to 0.91); 0.143 |
| 3 mg (N = 82) | 1.55 (3.436) | 1.19 (0.25 to 2.14); 0.014 | 40.1 (15.93) | −2.29 (−5.96 to 1.37); 0.220 |
| 1.5 mg (N = 81) | 1.92 (3.221) | 1.64 (0.69 to 2.59); 0.001 | 41.4 (16.24) | −2.85 (−6.46 to 0.76); 0.121 |
| 0.75 mg (N = 81) | 1.49 (2.810) | 1.29 (0.32 to 2.25); 0.009 | 45.1 (14.95) | −2.22 (−5.87 to 1.42); 0.231 |
| Placebo (N = 79) | 0.37 (3.220) | 43.5 (16.99) | ||
Abbreviations: TDI, Transition Dyspnoea Index; SGRQ-C, St George’s Respiratory Questionnaire – Chronic Obstructive Pulmonary Disease Specific
The overall proportion of patients experiencing adverse events was similar with all five treatments (Table 3). Occurrence was not related to ensifentrine dose, and the majority of events were mild or moderate in severity. The only adverse events considered related to study medication that occurred in more than two patients with any treatment were cough, dyspnoea and productive cough; again, occurrence was not related to ensifentrine dose (Table 3). Seven patients had a serious adverse event (Table 3), only two of whom experienced events considered by the investigators to be related to study medication: one patient receiving ensifentrine 1.5 mg, who committed suicide (although the patient had significant personal and financial stress factors); and one patient receiving ensifentrine 0.75 mg who had a medical history of hepatic cirrhosis (and so did not meet study eligibility criteria), and who experienced haemorrhage of oesophageal varices (which resolved after study medication was interrupted), hepatic cirrhosis and hepatic encephalopathy. A second patient died during the study, with the death being of unknown cause, but considered unrelated to study medication.
Table 3.
Adverse events, overall and most common (safety analysis set)
| Ensifentrine | Placebo (N = 79) | ||||
|---|---|---|---|---|---|
| 0.75 mg (N = 81) | 1.5 mg (N = 81) | 3 mg (N = 82) | 6 mg (N = 80) | ||
| Any adverse event | 27 (33) | 36 (44) | 29 (35) | 29 (36) | 31 (39) |
| Headache | 4 (5) | 4 (5) | 7 (9) | 4 (5) | 3 (4) |
| Worsening of COPD symptoms | 5 (6) | 5 (6) | 3 (4) | 3 (4) | 6 (8) |
| Cough | 4 (5) | 4 (5) | 6 (7) | 1 (1) | 1 (1) |
| Nasopharyngitis | 2 (2) | 4 (5) | 4 (5) | 5 (6) | 7 (9) |
| Hypertension | 2 (2) | 1 (1) | 4 (5) | 3 (4) | 1 (1) |
| Nausea | 3 (4) | 2 (2) | 2 (2) | 0 | 2 (3) |
| Dyspnoea | 3 (4) | 1 (1) | 1 (1) | 1 (1) | 5 (6) |
| Productive cough | 0 | 3 (4) | 1 (1) | 0 | 0 |
| Any treatment-related adverse event | 8 (10) | 11 (14) | 12 (15) | 8 (10) | 10 (13) |
| Cough | 2 (2) | 1 (1) | 4 (5) | 1 (1) | 1 (1) |
| Dyspnoea | 1 (1) | 0 | 0 | 0 | 3 (4) |
| Productive cough | 0 | 3 (4) | 0 | 0 | 0 |
| Any severe adverse event | 4 (5) | 1 (1) | 2 (2) | 1 (1) | 2 (3) |
| Any serious adverse event | 2 (2) | 2 (2) | 1 (1) | 1 (1) | 1 (1) |
| Any serious treatment-related adverse event | 1 (1) | 1 (1) | 0 | 0 | 0 |
| Any adverse event leading to drug discontinuation | 6 (7) | 1 (1) | 4 (5) | 2 (3) | 2 (3) |
| Any adverse event leading to death | 0 | 1 (1) | 0 | 1 (1) | 0 |
Data are n (%). The most common adverse events and drug-related adverse events are those reported in more than two patients in any group
There was no relationship between ensifentrine dose and adverse events leading to withdrawal from the study. Six patients withdrew from the study due to adverse events considered related to study medication: three patients in the ensifentrine 0.75 mg group (hepatic cirrhosis [one patient]; vomiting, headache and nausea [one patient]; dyspnoea [one patient]); one patient in the ensifentrine 1.5 mg group (suicide); and one patient each in the ensifentrine 6 mg and placebo groups (worsening of COPD symptoms).
Changes from baseline in electrocardiogram parameters were minimal, and were similar across treatment groups, with no electrocardiogram-related serious adverse events or adverse events leading to premature withdrawal. Overall, no trends by treatment or clinically meaningful changes were observed in any Holter electrocardiogram parameter during the study. Finally, there were no treatment-related trends in any of the laboratory tests or vital sign parameters.
Discussion
Overall, all four ensifentrine doses were more effective than placebo, with a dose-dependent response between 0.75 and 3 mg for peak and average FEV1. Bronchodilation was apparent from the first dose, with all ensifentrine doses providing immediate improvements in lung function from Day 1, and minimal further improvements in ensifentrine–placebo differences from Week 1 to Week 4. Despite being only a four-week study, ensifentrine also provided symptomatic improvements versus placebo in terms of E-RS™:COPD total score and TDI focal score. Differences versus placebo for all four ensifentrine doses at Week 4 were close to, or exceeded, the E-RS™:COPD total score minimum clinically important difference (MCID) of 2 units, and exceeded the TDI focal score MCID of 1 unit. Whereas the effects of ensifentrine on bronchodilation were apparent from the first dose, there was a gradual improvement in symptoms (E-RS™:COPD). This further highlights the often poor association between FEV1 and patient reported outcomes, and in this case indicates that the improvement of persistent symptoms follows a gradual time course rather than a rapid improvement. Longer studies are planned to further evaluate this progressive symptomatic improvement.
The rapid bronchodilation observed is consistent with previous short-term ensifentrine studies in COPD, and with preclinical evidence of the direct effect of ensifentrine on large and small airways, including increased mucociliary clearance and bronchial relaxation [17–19]. In particular, ensifentrine had a substantial effect on peak FEV1, with differences vs placebo at Week 4 between 139 and 200 mL; these improvements were consistent with the effects on FEV1 averaged over 12 h. The more progressive improvement in symptoms (especially breathlessness) could, at least in part, be due to a reduction in hyperinflation. Indeed, in a number of studies of inhaled long-acting bronchodilators, maximal improvements in FEV1 parameters were observed early in the study, whereas improvements in hyperinflation tended to be more progressive, with the maximal effect not observed for several weeks [20, 21]. In previous short-term studies, ensifentrine significantly reduced residual volume, a marker of hyperinflation, compared with placebo, and the addition of ensifentrine to salbutamol, ipratropium or tiotropium significantly reduced residual volume compared with salbutamol, ipratropium or tiotropium alone [13]. Although not assessed in the current study, improvements in residual volume are not detected by FEV1 changes, and are likely to have occurred in at least a subgroup of patients. Another factor contributing to the symptomatic improvement, and also explaining the gradually increasing effect on these endpoints, could be the anti-inflammatory effect of ensifentrine, as demonstrated in preclinical studies [18] and in a clinical study [12], although again this was not directly assessed here.
The lung function data suggest a dose-response up to 3 mg that plateaus at higher doses (6 mg, although this dose was still effective). This lung function dose-response was not mirrored in the symptom data, where all ensifentrine doses showed similar effects on symptoms measured by TDI and E-RS™:COPD, and with the ensifentrine 6 mg dose not worse than 3 mg for these endpoints. A previous crossover study of 3 days treatment with ensifentrine in addition to tiotropium showed that 6 mg had greater effects compared to 1.5 mg on lung function [13]. However, in a six-day parallel-group study the lung function effects of ensifentrine 6 mg did not differentiate from 1.5 mg [15]. These differing lung function results may reflect more between-patient variability in lung function responses that can occur in parallel-group studies. Overall, the available data suggest that ensifentrine doses of 3 and 6 mg are likely to be at the top of the dose-response curves.
This study was not powered to show statistically significant improvements in the MRC dyspnoea scale or in the SGRQ-C total score or responder analysis, even though there were clear numerical improvements in these parameters versus placebo. The MRC is relatively insensitive to change [22], and it is possible that the study duration was insufficient to demonstrate a clear impact on SGRQ-C. Importantly, however, all four ensifentrine doses demonstrated a good overall safety and tolerability profile, with no dose-limiting safety signals observed. The overall frequency of adverse events was no greater with any ensifentrine dose than placebo, and the incidence of treatment-related adverse events was low. Importantly for this novel class of therapy, no gastrointestinal or cardiovascular treatment-related adverse events were observed. Furthermore, there were no treatment-related trends for any of the laboratory tests or vital sign parameters.
A limitation of the current study is the relatively short treatment duration. Although the differential effect of ensifentrine on lung function and symptoms in this study may suggest the presence of anti-inflammatory activity (with an immediate improvement in lung function, but a delayed impact on symptoms), the four-week study duration was insufficient to fully evaluate the anti-inflammatory effect of ensifentrine, especially on exacerbations – or to distinguish between bronchodilator and anti-inflammatory effects. Furthermore, although we did not apply any inclusion criteria to select patients with symptomatic COPD (other than requiring patients to be clinically stable), we acknowledge that the majority of the patients recruited had relatively high symptom scores (approximately 95% had MRC ≥2 at baseline), and so the use of one or two long-acting bronchodilators would be indicated. The current study did not evaluate the effect of ensifentrine in combination with such therapies, or compare ensifentrine to other treatments. However, the use of ensifentrine in combination with other bronchodilators has been evaluated in previous short-term studies in patients with COPD [13]. In these earlier studies, the addition of a single dose of ensifentrine 6 mg to salbutamol increased peak FEV1 by 108 mL compared with salbutamol alone (p < 0.01), and the addition of ensifentrine to ipratropium increased peak FEV1 by 94 mL (p < 0.01). Furthermore, after 3 days of treatment, the addition of ensifentrine 1.5 mg or 6 mg to tiotropium increased peak FEV1 by 104 and 127 mL, respectively (both p < 0.01).
In conclusion, in this four-week Phase IIb study all four doses of ensifentrine significantly improved bronchodilation and symptoms compared with placebo, with a dose-response from 0.75 to 3 mg twice daily, and all doses being well tolerated. Overall, the results suggest that ensifentrine has a novel dual mechanism of action and support the continuing development of ensifentrine in COPD.
Supplementary information
Additional file 1: Figure S1. Study design. Table S1. Average FEV1 over 0–3 h (full analysis set). Table S2. Percentage of patients with ≥4 unit improvement in SGRQ-C total score after four weeks (full analysis set). Table S3. Medical Research Council dyspnoea score and patient global assessment of change after four weeks (full analysis set). Table S4. Rescue medication use (full analysis set).
Acknowledgements
Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
The authors would like to thank the investigators and patients at the investigative sites for their support of this study. Writing support was provided by David Young of Young Medical Communications and Consulting Ltd. This support was funded by Verona Pharma plc.
Authors’ contributions
The study was conceived and designed by DS and BTM, with support from FJM. BTM oversaw the conduct of the study. The data were acquired by DS and HW, analysed by TB, and interpreted by all authors. The manuscript was revised for intellectual content and approved for publication by all authors.
Funding
This study was funded by Verona Pharma plc. Verona Pharma plc was responsible for the design and analysis of the study, oversaw its conduct and was responsible for study report preparation.
Availability of data and materials
The data from this study are available on request, following submission of a valid research protocol to the corresponding author.
Ethics approval and consent to participate
The study was approved by independent ethics committees at each institution. All patients provided written informed consent prior to any study-related procedure.
Consent for publication
Not applicable.
Competing interests
DS reports personal fees from Verona during the conduct of the study. Outside the submitted work, he reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Theravance, and Verona.
FJM reports having supported the parent Verona study. Outside the submitted work, he reports personal fees and non-financial support from the American College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, Canadian Respiratory Network, Chiesi, ConCert, Dartmouth, Genentech, GlaxoSmithKline, Inova Fairfax Health System, Miller Communications, National Association for Continuing Education, Novartis, Pearl Pharmaceuticals, PeerView Communications, Physicians Education Resource, Potomac, Prime Communications, Puerto Rican Respiratory Society, Sunovion, Teva, Theravance, and the University of Alabama Birmingham, non-financial support from Gilead, Nitto, ProterrixBio and Zambon, personal fees from American Thoracic Society, Columbia University, France Foundation, Integritas, MD Magazine, Methodist Hospital Brooklyn, New York University, Patara/Respivant, PlatformIQ, Rare Disease Healthcare Communications, Rockpointe, UpToDate, WebMD/MedScape and Western Connecticut Health Network, grants from the National Institutes of Health (NIH), and other from Afferent/Merck, Bayer, Biogen, Bridge Biotherapeutics, ProMedior, Prometic and Veracyte.
HW reports personal fees from AstraZeneca, Bayer, BerlinChemie, Boehringer Ingelheim, GlaxoSmithKline, Novartis and Roche, all outside the submitted work.
TB reports personal fees from Verona, both during the conduct of the study and outside the submitted work.
BTM is an employee of Verona, the sponsor of the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12931-020-1307-4.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. [Google Scholar]
- 2.Punekar YS, Mullerova H, Small M, Holbrook T, Wood R, Naya I, et al. Prevalence and burden of dyspnoea among patients with chronic obstructive pulmonary disease in five European countries. Pulm Ther. 2016;2:59–72. doi: 10.1007/s41030-016-0011-5. [DOI] [Google Scholar]
- 3.Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37:264–272. doi: 10.1183/09031936.00051110. [DOI] [PubMed] [Google Scholar]
- 4.Gruenberger J-B, Vietri J, Keininger DL, Mahler DA. Greater dyspnea is associated with lower health-related quality of life among European patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:937–944. doi: 10.2147/COPD.S123744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas M, Radwan A, Stonham C, Marshall S. COPD exacerbation frequency, pharmacotherapy and resource use: an observational study in UK primary care. COPD. 2014;11:300–309. doi: 10.3109/15412555.2013.841671. [DOI] [PubMed] [Google Scholar]
- 6.Punekar YS, Shukla A, Müllerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis. 2014;9:65–73. doi: 10.2147/COPD.S54417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhamane A, Moretz C, Zhou Y, Burslem K, Saverno K, Jain G, et al. COPD exacerbation frequency and its association with health care resource utilization and costs. Int J Chron Obstruct Pulmon Dis. 2015;10:2609–2618. doi: 10.2147/COPD.S90148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 9.Banner KH, Press NJ. Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease. Br J Pharmacol. 2009;157:892–906. doi: 10.1111/j.1476-5381.2009.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer J, Philpott AJ, van Amsterdam RG, Shahid M, Zaagsma J, Nicholson CD. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br J Pharmacol. 1992;106:1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page C. P., Spina D. Phosphodiesterases as Drug Targets. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. Phosphodiesterase Inhibitors in the Treatment of Inflammatory Diseases; pp. 391–414. [DOI] [PubMed] [Google Scholar]
- 12.Franciosi LG, Diamant Z, Banner KH, Zuiker R, Morelli N, Kamerling IMC, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med. 2013;1:714–727. doi: 10.1016/S2213-2600(13)70187-5. [DOI] [PubMed] [Google Scholar]
- 13.Singh D, Abbott-Banner K, Bengtsson T, Newman K. The short term bronchodilator effects of the dual PDE3 and PDE4 inhibitor RPL554 in COPD. Eur Respir J. 2018;52:1801074. doi: 10.1183/13993003.01074-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjermer L, Abbott-Banner K, Newman K. Efficacy and safety of a first-in-class inhaled PDE3/4 inhibitor (ensifentrine) vs salbutamol in asthma. Pulm Pharmacol Ther. 2019;58:101814. doi: 10.1016/J.PUPT.2019.101814. [DOI] [PubMed] [Google Scholar]
- 15.Singh D, Abbott-Banner KH, Reid F, Newman K. A phase I, randomised, double blind, placebo controlled, study to assess the safety, tolerability and pharmacokinetics of multiple inhaled doses of RPL554 administered by nebuliser to healthy male subjects and stable COPD patients. Am J Respir Crit Care Med. 2016;193:A6838. doi: 10.1164/rccm.201511-2212ED. [DOI] [Google Scholar]
- 16.Watz H, Bagul N, Rabe KF, Rennard S, Alagappan VK, Román J, et al. Use of a 4-week up-titration regimen of roflumilast in patients with severe COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:813–822. doi: 10.2147/COPD.S154012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calzetta L, Page CP, Spina D, Cazzola M, Rogliani P, Facciolo F, et al. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther. 2013;346:414–423. doi: 10.1124/jpet.113.204644. [DOI] [PubMed] [Google Scholar]
- 18.Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one] J Pharmacol Exp Ther. 2006;318:840–848. doi: 10.1124/jpet.105.099192. [DOI] [PubMed] [Google Scholar]
- 19.Turner MJ, Matthes E, Billet A, Ferguson AJ, Thomas DY, Randell SH, et al. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am J Physiol Cell Mol Physiol. 2016;310:L59–L70. doi: 10.1152/ajplung.00324.2015. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell DE, Casaburi R, Vincken W, Puente-Maestu L, Swales J, Lawrence D, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med. 2011;105:1030–1036. doi: 10.1016/j.rmed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 21.O’Donnell DEE, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 22.Glaab T, Vogelmeier C, Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res. 2010;11:79. doi: 10.1186/1465-9921-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Study design. Table S1. Average FEV1 over 0–3 h (full analysis set). Table S2. Percentage of patients with ≥4 unit improvement in SGRQ-C total score after four weeks (full analysis set). Table S3. Medical Research Council dyspnoea score and patient global assessment of change after four weeks (full analysis set). Table S4. Rescue medication use (full analysis set).
Data Availability Statement
The data from this study are available on request, following submission of a valid research protocol to the corresponding author.