Abstract
Background
Wolbachia pipientis are bacterial endosymbionts of arthropods currently being implemented as biocontrol agents to reduce the global burden of arboviral diseases. Some strains of Wolbachia, when introduced into Aedes aegypti mosquitoes, reduce or block the replication of RNA viruses pathogenic to humans. The wAlbB strain of Wolbachia was originally isolated from Aedes albopictus, and when transinfected into Ae. aegypti, persists in mosquitoes under high temperature conditions longer than other strains. The utility of wAlbB to block a broad spectrum of RNA viruses has received limited attention. Here we test the ability of wAlbB to reduce or block the replication of a range of Flavivirus and Alphavirus species in cell culture.
Methods
The C6/36 mosquito cell line was stably infected with the wAlbB strain using the shell-vial technique. The replication of dengue, West Nile and three strains of Zika (genus Flavivirus), and Ross River, Barmah Forest and Sindbis (genus Alphavirus) viruses was compared in wAlbB-infected cells with Wolbachia-free controls. Infectious virus titres were determined using either immunofocus or plaque assays. A general linear model was used to test for significant differences in replication between flaviviruses and alphaviruses.
Results
Titres of all viruses were significantly reduced in cell cultures infected with wAlbB versus Wolbachia-free controls. The magnitude of reduction in virus yields varied among virus species and, within species, also among the strains utilized.
Conclusion
Our results suggest that wAlbB infection of arthropods could be used to reduce transmission of a wide range of pathogenic RNA viruses.
Keywords: Arbovirus, Mosquito, Dengue, Zika, Ross River Virus, West Nile, Sindbis
Background
Mosquito-borne viruses contribute significantly to the global burden of infectious diseases. Two genera of viruses responsible for significant numbers of human disease cases are Flavivirus and Alphavirus. Dengue viruses (DENV) are the most important human pathogens among the flaviviruses (family Flaviviridae), causing an estimated 390 million infections annually among the more than 2.5 billion people at risk of infection [1, 2]. Zika virus (ZIKV) causes a mild febrile illness in adults and may result in foetal loss during pregnancy and congenital neural malformations in babies [3, 4]. West Nile virus (WNV) can cause encephalitis and is now endemic in Europe and North America [5, 6]. The Australian strain of WNV, Kunjin virus (WNVKUN), also can cause encephalitis [7]. Within the genus Alphavirus (family Togaviridae), Ross River virus (RRV) and Barmah Forest virus (BFV) are two of the most common infections occurring in Australia and cause arthralgia and myalgia [8]. RRV also has caused outbreaks of disease in the Pacific, resulting in tens of thousands of cases [9]. Sindbis virus (SINV) infections are associated with a rash and mild fever in humans and have caused disease outbreaks in northern Europe [10, 11].
Transinfection of mosquito vector populations with Wolbachia has been proposed as an arbovirus biocontrol measure that may be self-sustaining and environmentally friendly [12]. Wolbachia are obligate intracellular bacteria that have evolved diverse ways to manipulate reproduction in their arthropod hosts in order to invade host populations [13, 14]. It is estimated that between 40–60% of all insect species are infected with diverse strains of Wolbachia [15, 16]. When transinfected into Aedes aegypti mosquitoes, some Wolbachia strains block the replication and transmission of viruses such as dengue, Zika and chikungunya (CHIKV) [17–22]. The pathogen-blocking ability of Wolbachia has resulted in this biocontrol agent being trialled in the field in at least 12 countries (http://www.worldmosquitoprogram.org), with the aim of making native mosquito populations refractory to arbovirus transmission [22–24].
The ability of Wolbachia to block pathogen replication depends, in part, on the strain of bacteria being used [25, 26]. Stable infections have been established in Ae. aegypti with several strains, including wMelPop [27] and wMel [18], both of which are native to Drosophila melanogaster. wMelPop over-replicates in its hosts and is highly effective in restricting replication and transmission of a broad range of human arboviruses, including DENV [17, 20], CHIKV [17, 28], yellow fever virus [17, 28] and WNV [19]. However, wMelPop is unlikely to invade and persist in wild populations due to its reduction of host fitness [26, 29–31]. wMel blocks the replication of DENV [18, 22, 32], ZIKV [33, 34] and CHIKV [35], without significantly reducing mosquito fitness [18]. It is also able to invade and persist in mosquito populations [23, 24, 36]. However, wMel can be lost from the mosquito host when exposed to heat stress [37, 38], potentially reducing the extent of virus blocking and slowing the spread of Wolbachia through a vector population.
The Wolbachia strain wAlbB, isolated from Ae. albopictus mosquitoes, has been found to be more stable than wMelPop and wMel under high heat conditions both in the laboratory [38] and the field [39]. At high temperatures, wAlbB transinfected into Ae. aegypti mosquitoes, exhibited a high and stable density of bacteria, and high maternal transmission fidelity [38–40]. wAlbB has invaded caged populations of Ae. aegypti [41], blocks DENV transmission in at least 40% of mosquitoes [20, 40] and is currently being tested in the field in Malaysia [42].
Preliminary results from releases in Malaysia suggest that wAlbB can persist in field mosquitoes, be maintained at high frequencies, and may significantly reduce dengue incidence [43]. Despite wAlbB holding significant promise as a biocontrol agent, its ability to block the replication of a broad range of human arboviruses has not been systematically tested. Here, we test the ability of wAlbB to block the replication of several flaviviruses and alphaviruses in mosquito cell lines.
Methods
Mosquito cells and infection with wAlbB
The Ae. albopictus cell line C6/36 [44] was maintained at 28 °C in RPMI-1640 medium containing 25 Mm HEPES (Sigma-Aldrich, Castle Hill, Australia), supplemented with 10% v/v heat-inactivated foetal bovine serum (FBS, Gibco, Mt. Waverely, Australia) and 1% v/v l-glutamine (Invitrogen, Carlsbad, USA). The wAlbB-infected cell line, designated C6/36.wAlbB, was generated by introducing wAlbB from Aa23 Ae. albopictus cells [45] into C6/36 cells using the shell vial technique, according to previously published methods [46, 47]. C6/36.wAlbB cells were maintained in 2:1 mixture of RPMI-1640 media buffered with HEPES (Sigma-Aldrich) and Schneider’s Drosophila Modified medium (Lonza, Basel, Switzerland), supplemented with 10% v/v FBS and 1% v/v l-glutamine. Preliminary experiments (data not shown) indicated Schneider’s Drosophila Modified medium (Lonza, Basel, Switzerland) was necessary for maintenance of wAlbB in cell culture. All insect cells were maintained at 28 °C and subcultured in maintenance media at a 1:3 ratio once each week for C6/36.wAlbB cells and 1:5 ratio twice a week for C6/36 controls.
Fluorescent in situ hybridization (FISH) for wAlbB detection
C6/36.wAlbB cells and C6/36 control cells without Wolbachia were seeded into duplicate wells in chambered slides (Bio-Basic, Ontario, Canada) and incubated for 24 h at 28 °C. Cell monolayers were washed with sterile phosphate buffered saline (PBS), fixed with ice-cold 4% paraformaldehyde (PFA) (VWR Alfa, BioStrategy, Tingalpa, Australia) at 4 °C for 30 min and then washed three times in 0.1 M phosphate buffer. The cells were dehydrated by sequential immersion of the slides, at 2 min intervals, in 70%, 95% and 100% v/v ethanol/water at room temperature. Hybridization was conducted overnight at 37 °C in a humidified container with hybridization cocktail II + 50% formamide (BioBasic, Ontario, Canada) containing 100 ng/µl of Cy5 labelled, Wolbachia-specific 16S rRNA W2 oligonucleotide probe (5ʹ-CY5-CTT CTG TGA GTA CCG TCA TTA TC-3ʹ) [48], synthesized at IDT DNA (Singapore). After hybridization, the slides were rinsed in 1× SSC buffer containing 10mM dithiothreitol (DTT) (AppliChem GmbH Germany), and then twice in 0.5× SSC buffer containing 10 mM DTT. All washes were performed at 55 °C for 15 min each. Cells were then stained with 0.5 µg/ml DAPI (Sigma-Aldrich, Castle Hill, Australia) and images captured on a Zeiss epifluorescent microscope at 100× magnification. Signals from five separate microscope fields from 3 independent cell culture samples were analysed.
Virus species and strains
WNVKUN (MRM 16 strain), RRV (T48), BFV (16313) and SINV (MRM39) were obtained from the World Health Organisation Collaborating Centre for Arbovirus Reference and Research at Queensland University of Technology, Australia. We used DENV serotype 2 strain ET300 (GenBank: EF440433) as a representative strain of dengue. The following strains of Zika virus were used: a Brazilian isolate (GenBank: KU365780), the French Polynesian isolate H/PF/2013 (GenBank: KJ776791) and the African genotype reference strain MR766. All virus stocks were propagated in C6/36 cells maintained as described above but with FBS supplementation reduced to 2%. Culture supernatant was harvested 2 days following infection of cells with SINV, 3 days after RRV and BFV infections, and 4 days after WNVKUN infections. Supernatants were harvested 4 days post-infection (dpi) for ZIKV strain KU365780 and 5 dpi for ZIKV strains MR766 and H/PF/2013, and DENV-2 ET300. Cell debris was removed from culture supernatants by centrifugation at 4000×g for 10 min at 4 °C and virus concentrated by ultrafiltration through a 100 kDa filter in an Amicon filter device (Merck Milipore, Massachusetts, USA) according to the manufacturer’s instructions. The concentrate was aliquoted into sterile 2 ml cryovials before freezing at – 80 °C.
Virus infection experiments
C6/36 and C6/36.wAlbB cells were seeded into 24-well plates at 2.5 × 105 cells per well and allowed to attach for 24 h at 28 °C. Infection with each virus strain was performed in triplicate wells, at multiplicities of infection (MOI) of 0.1, 1 or 10 in FBS-free RPMI-1640 medium (Sigma-Aldrich, Castle Hill, Australia). The virus was allowed to adsorb for 2 h before the inoculum was removed, the monolayers were washed twice with sterile PBS and then incubated at 28 °C in fresh maintenance media [RPMI-1640 containing 25 mM HEPES (Sigma-Aldrich) supplemented with 2% FBS (Gibco) and 1% Glutamax (Sigma-Aldrich)]. Supernatants were harvested from three independent replicate wells every 24 h for 8 days from cultures infected with flaviviruses. Because alphaviruses replicate much faster than flaviviruses, supernatants for these viruses were sampled every 8 h up to 48 h post-infection (8, 16, 24, 32, 40 and 48 h), then every 24 h until day 6 (72, 96, 120 and 144 h) and finally at day 8 (192 h).
Plaque and immunofocus assays to determine virus titres
Infectious virus titres were determined using either plaque or immunofocus assays on Vero (African green monkey kidney) cells maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) containing 5% v/v foetal bovine serum (FBS, Gibco) and 1% l-glutamine (Invitrogen, Carlsbad, USA) at 37 °C in an atmosphere of 5% v/v CO2/air. Cells were seeded in 24-well plates at 2.0 × 105 cells per well and incubated overnight at 37 °C. Confluent monolayers were infected with 200 µl of serial ten-fold dilutions of virus for 2 h at 37 °C, with gentle rocking every 15 min. A 1 ml overlay (1:1 v/v) consisting of 8% w/v carboxy-methyl cellulose (CMC, Sigma-Aldrich) and Medium 199 (Sigma-Aldrich) was added to each well and plates incubated at 37 °C in an atmosphere of 5% v/v CO2/air. After the desired length of incubation (i.e. 2 days for SINV, 3 days for RRV and BFV, 4 days for KUNV and ZIKV KU365780, and 5 days for ZIKV MR766 and P13F/251013-18), overlay media was removed and cell monolayers were washed twice in PBS. Cells then were stained with 300 µl of 0.05% w/v Crystal violet in 1% v/v formaldehyde and PBS for 1 h, rinsed with water, dried and plaques counted.
As DENV did not produce plaques reliably with the protocol above, infectious titres were determined using immunofocus assay. Initial steps were performed as above before proceeding with the following modifications. Five days post infection, the CMC overlay was removed, and cell monolayers fixed with ice-cold (1:1 v/v) acetone-methanol (Thermo Fisher Scientific, Brisbane, Australia). Blocking was performed by the addition of 200 µl of 5% w/v skim milk powder in PBS for 1 h at 37 °C. DENV-infected cells were detected using the anti-Flavivirus monoclonal antibody 4G2 (TropBio, Cairns, Australia) as the primary antibody, followed by horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, USA) as a secondary. Infectious foci were detected using SigmaFast with DAB (Sigma-Aldrich), after the manufacturer’s instructions. Plaque and immunofocus assays were performed in duplicate for each sample.
Analyses
Virus titres were log10-transformed and general linear models were used to test for statistically significant differences. The Chi-square test of association, Fisher’s exact test, and a general linear model were used to compare the results from cell lines separately for each time point and for each MOI. Statistical analyses were performed using the IBM SPSS Statistics software (version 23.0) (SPSS Inc., Chicago, USA) and GraphPad Prism Version 7.00 (GraphPad Software, La Jolla, California USA, 2008). To enable graphing of virus titre values of 0 (no plaques), 1 was added to all values and the resulting number log10-transformed.
Results
Stable infection of C6/36 cells with Wolbachia strain wAlbB
The presence of Wolbachia in the cytoplasm of C6/36.wAlbB cells was confirmed using FISH (Fig. 1a). The density of wAlbB in the cytoplasm of infected C6/36 cells was less than 40% in early cell passages (P 1-20; data not shown), as found by other groups [49]. However, by passage 40, the percentage of cells containing wAlbB had increased from approximately 60% in passage 28 to more than 95% (P < 0.01 by Mann Whitney test; Fig. 1b).
Fig. 1.

Detection of Wolbachia wAlbB by Fluorescent in situ hybridization of C6/36.wAlbB cells. a Carbocyanine5-labelled oligonucleotide probe corresponding to nucleotide sequences in Wolbachia 16S rRNA within the cytoplasm of the host cell (red). Cell nuclei stain blue with DAPI. b Proportion of cells containing Wolbachia wAlbB detectable by FISH between passages 28 and 40. Images were taken at a magnification of 100×. Error bars represent the standard deviation of the mean of three independent cell culture samples. Statistical significance was calculated by Mann Whitney test (P < 0.05, denoted by **)
Wolbachia strain wAlbB blocks Flavivirus replication in vitro
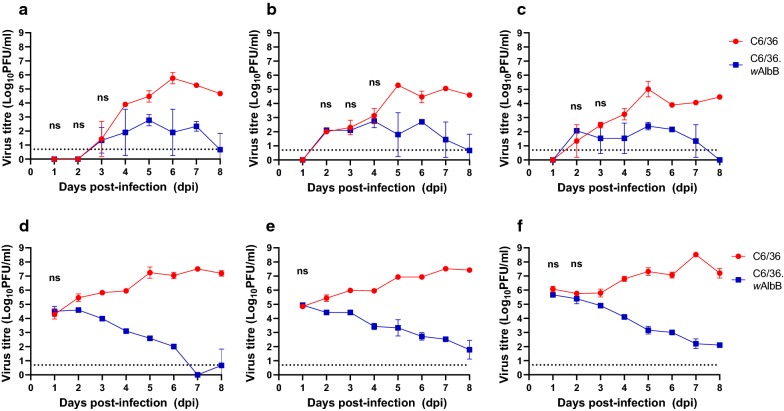
All flaviviruses tested replicated to lower titres in C6/36.wAlbB cells compared to Wolbachia-free C6/36 controls, regardless of MOI. Although titres from Wolbachia-infected and control cells were similar at early time points (1–3 dpi, Fig. 2), titres of DENV produced in C6/36.wAlbB were reduced by an average of 2–3 logs by 8 days post-infection (dpi) (Fig. 2a–c). Titres of WNVKUN were reduced by almost 5 logs, particularly at later time points during infection (6–8 dpi) (Fig. 2d–f), although virus remained detectable until the end of the experiment. Only with ZIKV did we observe a complete cessation in replication due to wAlbB presence (Fig. 3). Replication of ZIKV African strain MR766 was reduced to a point where no infectious virus particles could be detected by plaque assay, except for 1 dpi post-infection and at the high MOI of 10 (Fig. 3a–c). Titres of Brazilian strain ZIKV-KU365780 were reduced by at least 6 logs at 8 dpi across all MOI (Fig. 3d–f). For the French Polynesian strain H/PF/2013, initial replication in C6/36.wAlbB cells resulted in virus titres comparable to titres from control C6/36 cells, but titres became undetectable at 3 dpi (Fig. 3g–i). For both Brazilian and French Polynesian ZIKV strains, we observed that the higher the MOI the longer it took before infectious virus disappeared from C6/36.wAlbB cells.
Fig. 2.
Kinetics of virus production following infection of C6/36 and C6/36-wAlbB cells with DENV 2 strain ET300 and WNVKUN at MOI of 0.1 (a, d), 1 (b, c) and 10 (c, f). Means and standard deviations (error bars) for each time point are shown (n = 3 wells per time point). Abbreviations: PFU, plaque forming unit; ns, virus yields that are not significantly different (P > 0.05) between cell lines. Limit of detection for the plaque assay is shown as a dotted line
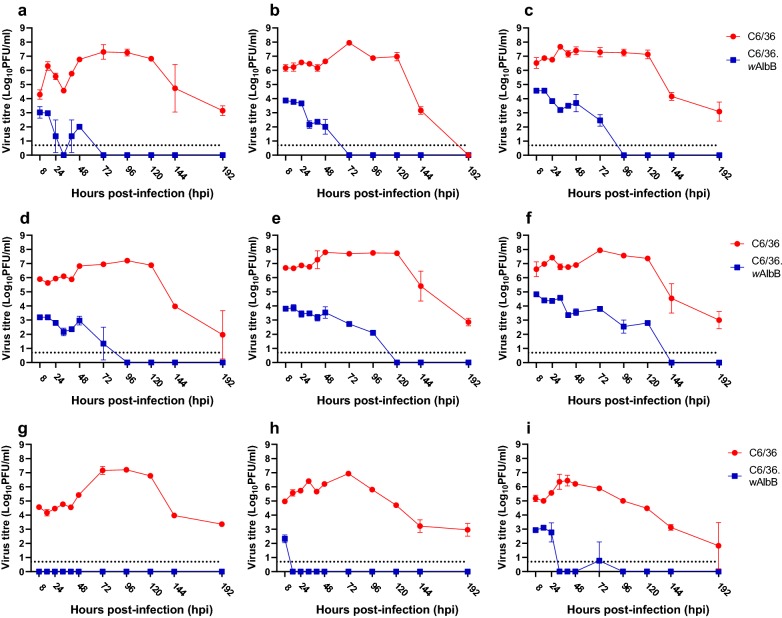
Fig. 3.
Kinetics of virus production following infection of C6/36 and C6/36.wAlbB cells with three strains of ZIKV at MOI of 0.1, 1 and 10 (left to right). African strain MR766 (a–c), Brazilian strain KU365780 (d–f), and French Polynesian strain H/PF/2013 (g–i). Means and standard deviations (error bars) for each time-point are shown (n = 3 wells per time-point). Abbreviations: PFU, plaque forming unit; ns, virus yields that are not significantly different (P > 0.05) between cell lines. Limit of detection for the plaque assay is shown as a dotted line
Wolbachia strain wAlbB blocks Alphavirus replication in vitro
The replication of the three alphaviruses tested was reduced in C6/36.wAlbB cells compared to controls, across all MOI (Fig. 4). The magnitude of Wolbachia-mediated blocking of BFV (Fig. 4a–c) and SINV (Fig. 4d–f) replication increased with time. For BFV, this ranged from a reduction of 1–2 logs at 8–24 h post-infection (hpi) to more than 4 logs at 72–144 hpi in wAlbB-infected cells versus controls. BFV and SINV could be detected in culture supernatants for longer post-infection at high MOI rather than low MOI, although all viruses had disappeared from supernatants of wAlbB-infected cells by 144 hours into the experiment. At the MOI of 0.1, SINV could not be detected at 96 hpi; however, at the MOI of 10, replication was detected for a further 48 hours. RRV was largely undetectable at MOI of 0.1 and 1 (Fig. 4g–i), except for 8 hpi at MOI 1. However, at MOI of 10, infectious virus was detected until 32 hpi and thereafter only re-appeared at 72 hpi (Fig. 4i). There were no significant differences (general linear model F(1, 6) = 2.33, P = 0.18) in the extent of Wolbachia-mediated blocking between flaviviruses and alphaviruses.
Fig. 4.
Kinetics of virus production following infection of C6/36 and C6/36.wAlbB cells with the alphaviruses BFV (a–c), SINV (d–f) and RRV (g–i) in C6/36 at MOI of 0.1, 1 and 10. Means and standard deviations (error bars) for each time-point are shown (n = 3 wells per time-point). Abbreviations: PFU, plaque forming unit. Limit of detection for the plaque assay is shown as a dotted line
Discussion
A large body of evidence has now accumulated documenting the ability of transinfected Wolbachia to block virus replication [50–52]. Although most reports have concerned the field-released wMel strain, the ability of wAlbB to block virus replication is being increasingly explored. Our results show that yields of infectious virus from a range of flaviviruses were consistently reduced in wAlbB-infected C6/36 cells versus Wolbachia-free cells. Our data are consistent with previous reports of the ability of wAlbB to block ZIKV in other cell lines [53], although we observed much stronger blocking in the C6/36 system compared to this earlier report. It is also consistent with previous reports of DENV [40] and WNVKUN [54] blocking in mosquitoes. Our data, using the C6/36 cell line background, confirm that the RNAi response is not an absolute requirement for Wolbachia-mediated blocking [55] since these cells are defective in this pathway [56]. Despite the reduction in virus replication observed due to Wolbachia, infectious DENV and WNVKUN were produced and remained detectable in most treatments until the end of the experiment. By contrast, ZIKV levels rapidly fell below levels of detection for most MOI treatments and virus strains. The results suggest the blocking effect of wAlbB may be stronger for ZIKV than DENV, similar to observations from Ae. aegypti mosquitoes [40].
Significant blocking in wAlbB-infected cells was also observed for RRV, BFV and SINV compared to uninfected cells. This is similar to other studies utilizing alphaviruses, such as Semliki Forest virus [57]. In contrast to DENV and WNVKUN, infectious yields of alphaviruses in wAlbB-infected cells fell to undetectable levels much earlier in the experiment compared to control cells. The speed at which alphavirus stopped being produced in Wolbachia-infected cells was a function of inoculum size, with high MOI treatments producing detectable virus for much longer than low MOIs. For both BFV and SINV, we observed a ~ 24 h delay in the time taken for the MOI 10 infection to become undetectable in comparison to the MOI 1 infection. This delay due to higher initial inoculum was also observed with ZIKV, particularly the Asian genotype strains. Interestingly, the same pattern was not observed for DENV or WNVKUN. These data suggest that, for some viruses, the block hypothesized to occur early in infection, possibly at the virus translation stage [57–59], may be delayed if the initial virus population is large. A possible explanation is that a large starting population size allows the virus to partially overcome the initial challenge imposed by Wolbachia in these cells. However, subsequent cycles of infection may be hampered by low numbers of progeny viruses and the ability of Wolbachia to reduce the infectivity of these progeny [58, 60], ultimately causing extinction of the virus.
Our data show that differences in the ability of wAlbB to block viruses is related to individual virus species and strains rather than broader taxonomic groupings such as genera or families. For example, among the alphaviruses, RRV production was undetectable for most time points while BFV production was reduced at later time points (72–96 hpi). Within the flaviviruses, a similar pattern was observed for ZIKV, whereby the prototype strain MR766 was undetectable at most time points but Asian genotype strains persisted much longer, and, in some cases, infectious virus briefly rebounded from almost zero levels. These brief rebounds were also observed for the three alphaviruses, as well as WNVKUN, and were not always a function of high initial MOI. Subtle replication differences among virus species and strains [61] may result in varying abilities of arboviruses to persist and, potentially evade the blocking effect of Wolbachia.
Conclusions
Our results have implications for using wAlbB to control arboviruses. As wMelPop appears unable to become established in wild mosquito populations [26] and wMel may not survive at high temperatures in the field [62], alternative strains of Wolbachia need to be considered for biocontrol. Invasion of wAlbB-infected Ae. aegypti has been achieved for a small area in Malaysia [52] and has been associated with a reduction in the incidence of dengue in an endemic area [43]. Our study adds to the growing body of evidence that wAlbB is able to inhibit a wide range of mosquito-borne viruses and supports the case for a broader virus surveillance programmes in areas where the strain is being evaluated to determine whether it has an impact on diseases other than dengue.
Acknowledgements
We thank Professor Jason Rasgon (Pennsylvania State University) for the gift of the wAlbB-infected Aa23 cell line, Mrs Debbie Phillips (Queensland University of Technology) for technical assistance and Dr Louise Marquart and Mr Lachlan Webb (QIMR Berghofer) for their help with statistical analysis. We thank Professor Paul Young (The University of Queensland) for the DENV ET300 strain, and Drs Van-Mai Cao-Lormeau (Institute Louis Malarde), Julian Druce (Victorian Infectious Diseases Reference Laboratory) and Dr Pedro Vasconcelos for Zika virus strains. We would also like to acknowledge the Ian Potter Foundation (Australia) for an equipment grant enabling the purchase of a fluorescent microscope at QUT.
Abbreviations
- BFV
Barmah Forest virus
- CHIKV
chikungunya virus
- CMC
carboxymethylcellulose
- DENV
dengue virus
- DTT
dithiothreitol
- FBS
fetal bovine serum
- FISH
fluorescent in situ hybridization
- MOI
multiplicity of infection
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- RRV
Ross River virus
- SINV
Sindbis virus
- WNVKUN
West Nile virus (Kunjin strain)
- ZIKV
Zika virus
Authors’ contributions
OE performed experiments, analyzed data and wrote the first draft of the manuscript. JGA contributed materials. All authors contributed to conceptualizing the project, and writing and editing the manuscript. All authors read and approved the final manuscript.
Funding
The study was funded by a National Health and Medical Research Council grant to FDF (APP1020817) and internal Queensland University of Technology (QUT) and Institute for Health and Biomedical Innovation (IHBI) grants. OE was funded by the government of Nigeria through the TeTFund Scholarship and a QUT part-scholarship.
Availability of data and materials
All data is presented within the paper and materials are available upon reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(Suppl. 12):S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 4.Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubalek Z. European experience with the West Nile virus ecology and epidemiology: could it be relevant for the New World? Viral Immunol. 2000;13:415–426. doi: 10.1089/vim.2000.13.415. [DOI] [PubMed] [Google Scholar]
- 6.Marfin AA, Gubler DJ. West Nile encephalitis: an emerging disease in the United States. Clin Infect Dis. 2001;33:1713–1719. doi: 10.1086/322700. [DOI] [PubMed] [Google Scholar]
- 7.Russell RC. Vectors vs. humans in Australia—who is on top down under? An update on vector-borne disease and research on vectors in Australia. J Vector Ecol. 1998;23:1–46. [PubMed] [Google Scholar]
- 8.Tesh RB. Arthritides caused by mosquito-borne viruses. Annu Rev Med. 1982;33:31–40. doi: 10.1146/annurev.me.33.020182.000335. [DOI] [PubMed] [Google Scholar]
- 9.Aaskov JG, Mataika JU, Lawrence GW, Rabukawaqa V, Tucker MM, Miles JA, Dalglish DA. An epidemic of Ross River virus infection in Fiji, 1979. Am J Trop Med Hyg. 1981;30:1053–1059. doi: 10.4269/ajtmh.1981.30.1053. [DOI] [PubMed] [Google Scholar]
- 10.Dunstan RA, Seed CR, Keller AJ. Emerging viral threats to the Australian blood supply. Aust N Z J Public Health. 2008;32:354–360. doi: 10.1111/j.1753-6405.2008.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurkela S, Rätti O, Huhtamo E, Uzcátegui NY, Nuorti JP, Laakkonen J, et al. Sindbis virus infection in resident birds, migratory birds, and humans, Finland. Emerg Infect Dis. 2008;14:41–47. doi: 10.3201/eid1401.070510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iturbe-Ormaetxe I, Walker T, O’Neill SL. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011;12:508–518. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 14.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 15.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira CD, Goncalves DS, Baton LA, Shimabukuro PH, Carvalho FD, Moreira LA. Broader prevalence of Wolbachia in insects including potential human disease vectors. Bull Entomol Res. 2015;105:305–315. doi: 10.1017/S0007485315000085. [DOI] [PubMed] [Google Scholar]
- 17.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 19.Hussain M, Lu G, Torres S, Edmonds JH, Kay BH, Khromykh AA, Asgari S. Effect of Wolbachia on replication of west nile virus in a mosquito cell line and adult mosquitoes. J Virol. 2013;87:851–858. doi: 10.1128/JVI.01837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousson L, Zouache K, Arias-Goeta C, Raquin V, Mavingui P, Failloux AB. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl Trop Dis. 2012;6:e1989. doi: 10.1371/journal.pntd.0001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 24.OʼNeill SL, Ryan PA, Turley AP, Wilson G, Retzki K, Iturbe-Ormaetxe I, et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2018;2:36. doi: 10.12688/gatesopenres.12844.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson NM, Kien DT, Clapham H, Aguas R, Trung VT, Chau TN, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7:279ra237. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, OʼNeill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 28.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMeniman CJ, OʼNeill SL. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl Trop Dis. 2010;4:e748. doi: 10.1371/journal.pntd.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeap HL, Mee P, Walker T, Weeks AR, OʼNeill SL, Johnson P, et al. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics. 2011;187:583–595. doi: 10.1534/genetics.110.122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross PA, Endersby NM, Hoffmann AA. Costs of three Wolbachia infections on the survival of Aedes aegypti larvae under starvation conditions. PLoS Negl Trop Dis. 2016;10:e0004320. doi: 10.1371/journal.pntd.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye YH, Carrasco AM, Frentiu FD, Chenoweth SF, Beebe NW, van den Hurk AF, et al. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci Rep. 2016;6:28792. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aliota MT, Walker EC, Uribe Yepes A, Velez ID, Christensen BM, Osorio JE. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis. 2016;10:e0004677. doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, et al. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulrich JN, Beier JC, Devine GJ, Hugo LE. Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl Trop Dis. 2016;10:e0004873. doi: 10.1371/journal.pntd.0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 2017;13:e1006006. doi: 10.1371/journal.ppat.1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross PA, Ritchie SA, Axford JK, Hoffmann AA. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis. 2019;13:e0007357. doi: 10.1371/journal.pntd.0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14:e1006815. doi: 10.1371/journal.ppat.1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 42.Ritchie SA, van den Hurk AF, Smout MJ, Staunton KM, Hoffmann AA. Mission Accomplished? We need a guide to the ‛post releaseʼ world of Wolbachia for Aedes-borne disease control. Trends Parasitol. 2018;34:217–226. doi: 10.1016/j.pt.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Nazni WA, Hoffmann AA, Noor Afizah A, Cheong YL, Mancini MV, Golding N, et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr Biol. 2019;29:4241–4248. doi: 10.1016/j.cub.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Igarashi A. Isolation of a Singhʼs Aedes albopictus cell clone sensitive to dengue and chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- 45.OʼNeill SL, Pettigrew MM, Sinkins SP, Braig HR, Andreadis TG, Tesh RB. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol. 1997;6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- 46.Frentiu FD, Robinson J, Young PR, McGraw EA, OʼNeill SL. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS ONE. 2010;5:e13398. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobson SL, Marsland EJ, Veneti Z, Bourtzis K, OʼNeill SL. Characterization of Wolbachia host cell range via the in vitro establishment of infections. Appl Environ Microbiol. 2002;68:656–660. doi: 10.1128/AEM.68.2.656-660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xi Z, Dean JL, Khoo C, Dobson SL. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem Mol Biol. 2005;35:903–910. doi: 10.1016/j.ibmb.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raquin V, Valiente Moro C, Saucereau Y, Tran FH, Potier P, Mavingui P. Native Wolbachia from Aedes albopictus blocks chikungunya virus infection in cellulo. PLoS ONE. 2015;10:e0125066. doi: 10.1371/journal.pone.0125066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caragata EP, Dutra HL, Moreira LA. Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol. 2016;32:207–218. doi: 10.1016/j.pt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Lindsey ARI, Bhattacharya T, Newton ILG, Hardy RW. Conflict in the intracellular lives of endosymbionts and viruses: a mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses. 2018;10:141. doi: 10.3390/v10040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross PA, Turelli M, Hoffmann AA. Evolutionary ecology of Wolbachia releases for disease control. Annu Rev Genet. 2019;53:93–116. doi: 10.1146/annurev-genet-112618-043609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultz MJ, Isern S, Michael SF, Corley RB, Connor JH, Frydman HM. Variable inhibition of Zika virus replication by different Wolbachia strains in mosquito cell cultures. J Virol. 2017;91:e00339-17. doi: 10.1128/JVI.00339-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joubert DA, Walker T, Carrington LB, De Bruyne JT, Kien DH, Hoang Nle T, et al. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terradas G, McGraw EA. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr Opin Insect Sci. 2017;22:37–44. doi: 10.1016/j.cois.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl Trop Dis. 2010;4:e856. doi: 10.1371/journal.pntd.0000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rainey SM, Martinez J, McFarlane M, Juneja P, Sarkies P, Lulla A, et al. Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathog. 2016;12:e1005536. doi: 10.1371/journal.ppat.1005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhattacharya T, Newton ILG, Hardy RW. Wolbachia elevates host methyltransferase expression to block an RNA virus early during infection. PLoS Pathog. 2017;13:e1006427. doi: 10.1371/journal.ppat.1006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schultz MJ, Tan AL, Gray CN, Isern S, Michael SF, Frydman HM, Connor JH. Wolbachia wStri blocks Zika virus growth at two independent stages of viral replication. MBio. 2018;9:e00738-18. doi: 10.1128/mBio.00738-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koh C, Audsley MD, Di Giallonardo F, Kerton EJ, Young PR, Holmes EC, McGraw EA. Sustained Wolbachia-mediated blocking of dengue virus isolates following serial passage in Aedes aegypti cell culture. Virus Evol. 2019;5:vez012. doi: 10.1093/ve/vez012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willard KA, Demakovsky L, Tesla B, Goodfellow FT, Stice SL, Murdock CC, Brindley MA. Zika virus exhibits lineage-specific phenotypes in cell culture, in Aedes aegypti mosquitoes, and in an embryo model. Viruses. 2017;9:383. doi: 10.3390/v9120383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ross PA, Hoffmann AA. Continued susceptibility of the wMel Wolbachia infection in Aedes aegypti to heat stress following field deployment and selection. Insects. 2018;9:78. doi: 10.3390/insects9030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is presented within the paper and materials are available upon reasonable request.