Abstract
Background
Non-small-cell lung cancer (NSCLC) is predominant and has low 5-year relative survival rate. Therefore, the mechanisms of NSCLC tumorigenesis must be comprehensively elucidated. MicroRNA-323-3p (miR-323-3p) has been widely explored and found to exert functions in tumorigenesis of several cancer types. However, the expression pattern and biological function of miR-323-3p and the molecular mechanism underlying NSCLC development and progression remain unclear.
Material/Methods
Quantitative reverse-transcription polymerase chain reaction was used to detect the expression of miR-323-3p and TMEFF2 in NSCLC cell lines (A549, NCI-H3255, and H1299) and normal cell line (BEAS-2B). Methylthiazolyl tetrazolium, colony formation, and flow cytometry assays were performed to evaluate the effects of miR-323-3p and TMEFF2 on cell proliferation. Transwell assay was conducted to determine the effects of TMEFF2 on cell migration and invasion. Dual-luciferase reporter assay was used to verify whether TMEFF2 is a target of miR-323-3p. Western blot analysis was performed to analyze protein expression.
Results
The expression of miR-323-3p increased in the 3 NSCLC cell lines (A549, NCI-H3255, and H1299). miR-323-3p regulated cellular progression by directly suppressing TMEFF2 expression and indirectly prohibited the activation of AKT and ERK pathways in NSCLC.
Conclusions
Overall, miR-323-3p was considered a lung cancer oncogene and could be a valuable target for NSCLC therapy.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Cellular Microenvironment; MicroRNAs
Background
Lung cancer is considered the most prevalent cancer with millions of new patients diagnosed every year [1,2]. Non-small-cell lung cancer (NSCLC) accounts for a large proportion of lung cancer cases and include 2 frequent histologic forms: adenocarcinoma (ADC) and squamous cell carcinoma (SCC) [3,4]. ADC is generally located in the distal airway, has a glandular histology, and expresses biomarkers, including thyroid transcription factor 1 and keratin 7, consistent with the origin of the distal lung cancer. SCCs are common in the proximal airway and are often associated with smoking and chronic inflammation. Immunostaining cytokeratin 5 and cyclin 6 and/or transcription factors sry-box 2 (SOX2) and p63 showed that SCCs were significantly different from ADC. Approximately 40% of newly diagnosed NSCLC cases were identified at stages III or IV [5]. Although several clinical trials have been developed and tested in patients with NSCLC, they still have poor prognosis with approximately 4–17% of 5-year overall survival rate [6,7]. The survival rate of NSCLC is far lower than those of other prevalent cancers, such as prostate, breast, melanoma, colon, and rectal cancer, due to late diagnosis and distant metastases [8,9]. Thus, the molecular mechanism underlying NSCLC tumorigenesis and the association between NSCLC and risk factors must be elucidated to provide insights into developing novel therapeutic targets for early diagnosis and treatment of this disease.
MicroRNAs (miRNAs) are factors that negatively regulate the expression of gene [10,11]. By mediating gene expression, miRNAs could play as modulators in the physiological and pathological progression of cancers [12–14]. Increasing lines of evidence revealed numerous downregulated or upregulated miRNAs obtained from various human malignancies, including NSCLC [15,16]. These miRNAs exert functions either as tumor suppressors or promoters depending on the regulated tumor forms and their specific target genes [17]. Hence, miRNA-based medicine can be applied as a novel and promising method for cancer diagnosis and prognosis.
MiR-323-3p is dysregulated in a variety of human cancers, including pancreatic ductal adenocarcinoma [18] and prostate cancer [19,20], as well as in polycystic ovary syndrome [21], Friedreich’s ataxia, and type 2 diabetes mellitus [22,23]. However, the function of miR-323-3p in NSCLC has not been elucidated yet. In the present study, we investigated the expression level and molecular pathogenesis that govern NSCLC formation and progression of miR-323-3p in NSCLC.
Material and Methods
Plasmid construction
The transmembrane protein with EGF-like and 2 follistatin domain (TMEFF2) gene was synthesized using forward primer 5′-CGGGATCCCGATGGTGCTGTGGGAGTCCCCG-3′ and reverse primer 5′-CCCTCGAGGGCTAAAGTTTGGCCCTTGTGA-3′. BamHI and XhoI restriction enzymes were used to digest the polymerase chain reaction (PCR) product. The digested PCR product was ligated to the pcDNA3.1 vector (Invitrogen, CA, USA) to generate a construct pcDNA-TMEFF2. The interference segments were directly synthesized and labeled as si-TMEFF2/si-NC.
Cell culture and treatment
Three cell lines (A549, NCI-H3255, and H1299) were obtained from the Chinese Academy of Cell resource center. Dulbecco’s Modified Eagle Medium (DMEM; Hyclone, Logan, UT, USA) and fetal bovine serum (FBS; Gibco, USA) were used for cell culturation in 5.0% CO2 at 37°C. The A549 cell line was used for the following treatment. The sequences of the hsa-miR-323-3p-related product (mimics and inhibitors) were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). For cell transfection, A549 cells were seeded at 3×105 cells per well in a 6-well plate and cultured for 24 hours or 48 hours. Transient transfection was carried out with lipofectamine 2000 (Invitrogen, USA) following the protocol with the final concentration of 50 nM for miR-323-3p mimics/NC or inhibitors/NC and 50 nM for TMEFF2. At 24 hours or 48 hours after the transfection, the cells were harvested for subsequent experiments: RNA isolation, western blot, and immunofluorescence assay. All experiments were carried out in triplicate.
RNA purification and qRT-PCR
Total RNA was purified from NSCLC or adjacent normal tissue samples by using TRIzol (Invitrogen). The cDNA was generated by a PrimeScript RT-PCR kit (TaKaRa, USA). PCR was achieved by SYBR Green qRT-PCR Master mix (TaKaRa, China). The internal references, namely, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 small nuclear RNA, were used for cellular gene expression and miRNA level, respectively. All amplified primer sequences are shown:
TMEFF2 forward, 5′-TGCAGGATCAGGATCTGGAGATGG-3′;
TMEFF2 reverse, 5′-CCTCGGCATCTTCGTCACATTCTG-3′;
GAPDH forward, 5′-AATGGGCAGCCGTTAGGAAA-3′;
GAPDH reverse, 5′-GCGCCCAATACGACCAAATC-3′;
miR-323-3p forward, 5′-GCGCACATTACACGGTCG-3′;
miR-323-3p reverse, 5′-AGTGCAGGGTCCGAGGTATT-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′;
and U6 reverse, 5′-ACGCTTCACGAATTTGCGTGTC-3′.
The amplification conditions of the reaction included the following: preparation at 95°C for 5 minutes, denaturation at 95°C for 30 seconds, annealing at 60°C for 15 seconds, extension at 72°C for 5 seconds, and extension at 72°C for 10 minutes to terminate the reaction for a total of 40 cycles. All experiments were run in triplicate for each sample. Comparative computed tomography (CT) method (2−ΔΔCT) was used to calculate the expression levels.
Dual-luciferase reporter
A miRNA binding site was found in the 3′UTR of TMEFF2 mRNA based on Targetscan prediction. A dual-luciferase reporter was utilized to demonstrate the binding relationship. The 3′UTR of TMEFF2 (TMEFF2-WT) and its mutant (TMEFF2-Mut) were synthesized and inserted into the psiCHECKTM-2 vector (Promega, Madison, WI, USA). A549 cells were grown and cotransfected, and relative luciferase activity was calculated (Promega, USA). All the experiments were performed independently 3 times.
Methylthiazolyl tetrazolium (MTT) assay
A549 cells in the logarithmic phase were collected, plated in 96-well plates (2000 cells/well), and incubated at 37°C for 24, 48, and 72 hours. Cell proliferation was examined using MTT assay (Sigma-Aldrich, St. Louis, MO, USA). After the cells were incubated with 20 μL of MTT solution (5 mg/mL) for 4 hours, the medium was removed. The cells were then added with 150 μL of dimethyl sulfoxide (DMSO, Sigma-Aldrich) and incubated for additional 10 minutes. Optical density (OD) was calculated at 490 nm wavelength by using a spectrophotometer. All the experiments were carried out independently for 3 times.
Colony formation assay
Population number and proliferation were examined using colony formation assay. A549 cells were digested, counted, and cultured in a 6-well plate (100 cells/well in each well). The cells were stained with 1 mL of 0.1% crystal violet (Sigma-Aldrich, MO, USA). At the end of the experiment, ≥50 cells/colony were counted under a microscope. The size of the colonies was also determined. All experiments were carried out in triplicate.
Cell cycle
Propidium iodide (PI) staining was utilized. At 48 hours after transfection, A549 cells were fixed in 75% ethanol for 24 hours at 4°C. The samples were suspended in 200–500 μL of iced-cold phosphate-buffered saline (PBS) with 400 μL of PI (50 μg/mL) on the next day. The treated cells were filtered by yellow nylon nets and determined by flow cytometry. Cell cycle population was determined using ModFit software. All experiments were carried out in triplicate.
Cell apoptosis
Annexin V fluoresecinisothiocyanate (FITC) staining was applied to detect cell apoptosis. The treated cells were resuspended in 500 μL of annexin V binding buffer supplemented with 5 μL of PI and 5 μL of annexin V-FITC. The samples were examined by flow cytometry analysis, and cell cycle population was determined using FlowJo7.6 software. All experiments were carried out in triplicate.
Transwell assay
Transwell assay was used to determine migration and invasion ability. The swabs were applied to remove cells on the top. Cells on the bottom surface of the membrane were fixed with methanol and stained with 0.5% crystal violet. All experiments were carried out in triplicate.
Protein extraction and western blot analysis
The concentration of the supernatant was determined by the BCA kit (Keygen, Nanjing, China). The treated protein samples were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). Immunoreactive bands were determined by electrochemiluminescence (ECL) plus western blot detection kit (Pierce, Rockford, IL, USA). Relative protein level was standardized to the β-actin level. Antibodies employed in this study were as follows: anti-TMEFF2 (Abcam, Cambridge, MA, USA), anti-AKT, anti-Erk1/2, anti-p-AKT (Ser473), anti-p-Erk1/2 (Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin (Sigma-Aldrich). All experiments were carried out in triplicate.
Statistical analysis
Data were analyzed using GraphPad Prism (San Diego, CA, USA) and reported as mean ± standard deviation (SD). Statistical significance was evaluated with 2-tailed Student’s t-test between 2 groups. P<0.05 was considered statistically significant.
Results
Upregulated miR-323-3p and downregulated TMEFF2 in NSCLC
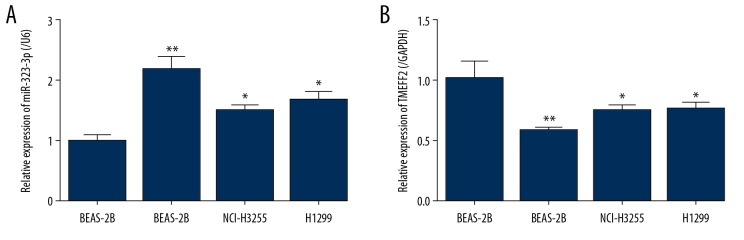
To explore the role of miR323-3p and TMEFF2 in NSCLC, we measured the level of miR-323-3p and TMEFF2 in 3 NSCLC cell lines (A549, NCI-H3255, and H1299). Compared with that in the BEAS-2B cell line, miR-323-3p was remarkably upregulated (Figure 1A) and TMEFF2 was significantly downregulated (Figure 1B) in the 3 NSCLC cell lines. Here, the A549 cell line was selected for further experiments.
Figure 1.
Upregulated miR-323-3p and downregulated TMEFF2 in NSCLC cell lines. (A, B) mRNA level of miR-323-3p and TMEFF2 in 3 NSCLC cell lines (A549, NCI-H3255, and H1299) and normal human lung epithelial cell line BEAS-2B. All the results were reported as mean±standard deviations (SD) (n=3). * P<0.05 and ** P<0.01. miR-323-3p – microRNA-323-3p; TMEFF2 – transmembrane protein with EGF-like and 2 follistatin domain; NSCLC – non-small-cell lung cancer; mRNA – messenger RNA.
MiR-323-3p regulates the proliferation and apoptosis of A549 cells
In A549 cells, miR-323-3p was upregulated in the mimics group (Figure 2A) but downregulated in the group transfected with inhibitor (Figure 2A). We then determined the effects of miR-323-3p on A549 cell proliferation by MTT and colony formation assays. According to the MTT assay, the proliferation rate of A549 cells significantly increased in the miR-323-3p mimics group at 24–72 hours but decreased in the inhibitor group (Figure 2B). This result is consistent with the colony formation assay, which indicated that miR-323-3p promotes the proliferation of A549 cells transfected with miR-323-3p mimics (Figure 2C). We also investigated the role of miR-323-3p on A549 cell cycle and apoptosis by flow cytometry analysis. Relative to the NC group, A549 cells induced the proportion of G1 phase in the miR-323-3p mimics group and fewer cells were arrested in the G1 phase in the inhibitor group (Figure 2D). The apoptotic rate of A549 cells in the miR-323-3p mimics group was markedly suppressed but was elevated significantly in the inhibitor group (Figure 2E). Hence, miR-323-3p promoted the proliferation and suppressed the apoptosis of A549 cells.
Figure 2.
Effect of miR-323-3p on NSCLC cell proliferation and apoptosis. (A) A549 cells were transfected with miR-323-3p mimic or inhibitor to obtain the increased or decreased expression of miR-323-3p. (B, C) Cell proliferation was evaluated by MTT method and colony formation assay. Colonies formed were counted. (D) Cell cycle was measured by flow cytometry and quantified. (E) Apoptosis was evaluated by flow cytometry and quantified. All the results were shown as mean±standard deviation (n=3). * P<0.05, ** P<0.01 and *** P<0.001. miR-323-3p – microRNA-323-3p; NSCLC – non-small-cell lung cancer; mRNA – messenger RNA; MTT – methylthiazolyl tetrazolium.
TMEFF2 is a direct target of miR-323-3p
Bioinformatics tool was utilized to analysis the target genes of miR-323-3p. As shown in Figure 3A, TMEFF2 was selected for further target validation among candidate genes. The TMEFF2-WT 3′-UTR had the binding region of miR-323-3p, while TEMFF2-Mut 3′-UTR missed it. A549 cells were cotransfected with TMEFF2-WT or TMEFF2-Mut construct as well as miR-323-3p mimics or miR-NC. The results revealed that miR-323-3p markedly inhibited the luciferase activities to approximately 50% in the wild-type 3′-UTR of TMEFF2. By contrast, miR-323-3p did not significantly affect the activity in the mutated 3′-UTR of TMEFF2 (Figure 3A). In addition, the effect of miR-323-3p on TMEFF2 protein level was detected by western blot analysis in A549 cells. Overexpression of miR-323-3p significantly prohibited the expression of TMEFF2, while miR-323-3p knockdown markedly promoted it (Figure 3B). These results demonstrated that miR-323-3p directly aimed the 3′-UTR of TMEFF2 and inhibited it in A549 cells.
Figure 3.
TMEFF2 is a novel and direct target of miR-323-3p in A549 cells. (A) Putative binding sequence between miR-323-3p and human TMEFF2 3′-UTR. Luciferase reporter assay was assessed. (B) Effects of miR-323-3p on TMEFF2 expression. The protein expression of TMEFF2 was quantified. All the results were shown as mean±standard deviation (SD) (n=3). * P<0.05 and *** P<0.001. TMEFF2 – transmembrane protein with EGF-like and 2 follistatin domain; miR-323-3p – microRNA-323-3p.
TMEFF2 influences the proliferation, apoptosis, migration, and invasion of A549 cells
A549 cells were transfected with pcDNA-TMEFF2, pcDNA vector, si-TMEFF2, or si-NC. TMEFF2 protein level was determined by western blot analysis. The transfection with pcDNA-TMEFF2 significantly increased TMEFF2 expression compared with that in cells transfected with the vector (Figure 4A). Silencing TMEFF2 markedly decreased its expression compared with the control group. A549 cells were dose-dependently treated for 24, 48, 72, and 96 hours to evaluate cell proliferation. The results showed that ox-TMEFF2 markedly inhibited cell proliferation, and the knockdown of TMEFF2 significantly promoted it at 72 hours (Figure 4B). Importantly, overexpression of TMEFF2 led to a more obvious apoptosis cell ratio than the control group in the flow cytometry analysis (Figure 4C). By contrast, the knockdown of TMEFF2 markedly suppressed cell apoptosis. The Transwell assay results displayed that TMEFF2 markedly suppressed the migration and invasion of A549 cells (Figure 4D, 4E). These findings suggested that TMEFF2 might be a tumor suppressor in NSCLC cell growth and metastasis.
Figure 4.
Effect of TMEFF2 on NSCLC proliferation, apoptosis, migration, and invasion. (A) TMEFF2 expression was evaluated after transfection with pcDNA-TMEFF2 or si-TMEFF2 and quantified. (B) Cell proliferation was examined by MTT method. (C) Cell apoptosis detected by flow cytometry, and apoptosis cells were quantified. Transwell assay indicated the migration (D) and invasion (E) of cells. The migrated or invaded cells were quantified. All the results were reported as mean±standard deviation (SD) (n=3). * P<0.05, ** P<0.01 and *** P<0.001. TMEFF2 – transmembrane protein with EGF-like and 2 follistatin domain; NSCLC – non-small-cell lung cancer; MTT – methylthiazolyl tetrazolium.
MiR-323-3p and TMEFF2 influence AKT and ERK signaling
AKT and ERK signaling often play critical roles in the control of cell survival, proliferation, differentiation, metabolism, and motility and are frequently activated during tumor progression. TMEFF2 regulates the activity of Ras/Raf/MEK/ERK and PI3K/Akt signaling pathways. To reveal the molecular mechanisms of miR-323-3p and TMEFF2 on NSCLC cells, we examined the potential effect of this miRNA and its target on some important signaling pathways by western blot analysis. The transfection with miR-323-3p mimics or si-TMEFF2 significantly downregulated the phosphorylation level of AKT and ERK1/2 without changing the total AKT and ERK expression, suggesting that AKT and ERK signaling pathways were suppressed (Figure 5). By contract, A549 cells transfected with miR-323-3p inhibitor or pcDNA-TMEFF2 possessed remarkably upregulated phosphorylation levels of AKT and ERK1/2 (Figure 5). Thus, these results suggested that miR-323-3p and TMEFF2 could mediate AKT and ERK signaling pathways.
Figure 5.
Effect of miR-323-3p and TMEFF2 on the AKT and ERK signaling pathways. Expression of AKT, p-AKT, ERK, and p-ERK in NSCLC cells transfected with miR-323-3p mimics and inhibitor (A) or pcDNA-TMEFF2 and si-TMEFF2 (B) measured by western blot analysis. The p-AKT/AKT and p-ERK/ERK ratio were quantified. All the results were reported as mean±standard deviation (SD) (n=3). *** P<0.001. miR-323-3p – microRNA-323-3p; TMEFF2 – transmembrane protein with EGF-like and 2 follistatin domain; NSCLC – non-small-cell lung cancer.
TMEFF2 was responsible for the influence of miR-323-3p
Rescue experiments were performed to estimate whether TMEFF2 was responsible for the function of miR-323-3p in NSCLC. The increased TMEFF2 expression significantly reversed the influence of miR-323-3p mimics on the proliferation (Figure 6A), cell cycle (Figure 6B), and apoptosis (Figure 6C) of A549 cells. The decreased expression of TMEFF2 markedly alleviated the inhibitory role of the miR-323-3p inhibitor on cell progression (Figure 7). These findings showed that miR-323-3p and TMEFF2 exerted a critical role in NSCLC pathological process and acted as two regulating targets during tumorigenesis.
Figure 6.
Effects of overexpression of miR-323-3p and TMEFF2 on NSCLC progression. Cell proliferation (A), cycle (B), and apoptosis (C) were evaluated in NSCLC cells transfected with mimic-NC, miR-323-3p mimic, miR-323-3p mimic with pcDNA, or miR-323-3p mimic with pcDNA-TMEFF2. All the results were shown as mean±standard deviation (SD) (n=3). ** P<0.01 and *** P<0.001. miR-323-3p – microRNA-323-3p; TMEFF2 – transmembrane protein with EGF-like and 2 follistatin domain; NSCLC – non-small-cell lung cancer.
Figure 7.
Effects of knockdown of miR-323-3p and TMEFF2 on NSCLC progression. Cell proliferation (A), cycle (B), and apoptosis (C) were detected in NSCLC cells transfected with inhibitor NC, miR-323-3p inhibitor, miR-323-3p inhibitor with si-NC, or miR-323-3p inhibitor with si-TMEFF2. All the results were reported as mean±standard deviation (SD) (n=3). * P<0.05, ** P<0.01 and *** P<0.001. miR-323-3p – microRNA-323-3p; TMEFF2 – transmembrane protein with EGF-like and 2 follistatin domain; NSCLC – non-small-cell lung cancer.
Discussion
In recent years, the number of patients with NSCLC increases every year; NSCLC is typically diagnosed at late stages, has distant metastasis, and has lower survival rate than prostate cancer, breast cancer, melanoma, colon cancer, and rectal cancer [8,9]. NSCLC has poor prognosis, accounting for 4–17% of the 5-year overall survival rate [6,7], and is thus a serious threat to human health. In this regard, the molecular mechanism underlying NSCLC tumorigenesis and the association between NSCLC and their risk factors must be comprehensively investigated to provide insights into developing novel therapeutic targets for early diagnosis and treatment of this disease.
Increasing lines of evidence indicate that miRNAs function as a critical modulator in the tumorigenesis of various human cancers, including NSCLC [24–27]. The abnormal expression of miRNAs in NSCLC might contribute to tumor occurrence and development by acting as a crucial positive or negative regulator [28]. Previous report discovered that the level of miR-323-3p was significantly induced in prostate cancer, IL-22- and IL-17-positive T cells, and cardiomyopathy [19,22,29]. These results revealed that miR-323-3p could be a critical prognostic modulator in tumorigenesis.
A critical finding in this study is the high levels of miR-323-3p in NSCLC cells. Experiments that manipulated miR-323-3p in A549 cells showed that decreasing miR-323-3p expression promoted cell proliferation, induced cell cycle G1 arrest, and inhibited apoptosis. Through target gene prediction based on bioinformatics and whole genome microarray analyses, we showed that TMEFF2 gene is a downstream target of miR-323-3p. TMEFF2 has been identified as a single-pass type I transmembrane protein, and its methylation has been considered as a suppressor in cancer cells, such as NSCLC [30], gastric cancer [31], colorectal cancer [32], renal cell carcinoma [33], and prostate cancer [34]. We also showed that the expression of TMEFF2 was inhibited at the mRNA and protein levels by overexpressing miR-323-3p, and silencing miR-323-3p had the opposite effect. Furthermore, overexpression of TMEFF2 reversed the oncogenic effects induced by miR-323-3p. Experiments in the patient samples showed lower TMEFF2 levels in patients with NSCLC than in the control group. These findings suggest that miR-323-3p promotes cell proliferation, apoptosis, migration, and invasion, at least in part by targeting TMEFF2. The luciferase reporter assay system further demonstrated that miR-323-3p could be a direct target of the mammalian gene TMEFF2 and markedly suppressed its expression.
We further explored the mechanism underlying miR-323-3p promotion of the proliferation and apoptosis of NSCLC cells. Previous studies also reported that AKT and ERK signaling pathways often play critical roles in controlling cell survival, proliferation, differentiation, metabolism, and motility and are frequently activated during tumor progression [35,38]. TMEFF2 activated the AKT and ERK signaling to exert biological functions in the progression of prostate cancer [39]. In this study, overexpression of miR-323-3p or knockdown of TMEFF2 led to a reduction in p-AKT and p-ERK, which suggested that miR-323-3p inhibited AKT/ERK signaling by directly downregulating TMEFF2 expression.
Conclusions
Our findings provide concrete evidence that aberrant expression of miR-323-3p is related to NSCLC development through direct inhibition of TMEFF2 expression. MiR-323-3p can promote NSCLC progression by enhancing cell proliferation, cycle, and apoptosis due to the inhibition of downstream TMEFF2 and AKT/ERK signaling. Thus, the distribution of miR-323-3p in different histological subtypes might provide novel and promising insights into future therapies for human cancers.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:38–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–46. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehurst AW, Bodemann BO, Cardenas J, et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 2007;446:815–19. doi: 10.1038/nature05697. [DOI] [PubMed] [Google Scholar]
- 6.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S–40S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 7.Highlights in NSCLC from the 2014 American Society of Clinical Oncology annual meeting. Clin Adv Hematol Oncol. 2014;12(10 Suppl 18):7–16. [PubMed] [Google Scholar]
- 8.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 9.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 10.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–55. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 11.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 12.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Garzon R, Marcucci G. Potential of microRNAs for cancer diagnostics, prognostication and therapy. Curr Opin Oncol. 2012;24:655–59. doi: 10.1097/CCO.0b013e328358522c. [DOI] [PubMed] [Google Scholar]
- 14.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–22. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Li B, Niu L, et al. miR-592 functions as a tumor suppressor in human non-small cell lung cancer by targeting SOX9. Oncol Rep. 2017;37:297–304. doi: 10.3892/or.2016.5275. [DOI] [PubMed] [Google Scholar]
- 16.Pei K, Zhu JJ, Wang CE, et al. MicroRNA-185-5p modulates chemosensitivity of human non-small cell lung cancer to cisplatin via targeting ABCC1. Eur Rev Med Pharmacol Sci. 2016;20:4697–704. [PubMed] [Google Scholar]
- 17.MaugeriSaccà M, Coppola V, Bonci D, et al. MicroRNAs and prostate cancer: From preclinical research to translational oncology. Cancer J. 2012;18:253–61. doi: 10.1097/PPO.0b013e318258b5b6. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Liu P, Wu H, et al. MicroRNA-323-3p inhibits cell invasion and metastasis in pancreatic ductal adenocarcinoma via direct suppression of SMAD2 and SMAD3. Oncotarget. 2016;7:14912–24. doi: 10.18632/oncotarget.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Q, Yao X, Zheng J. MiR-323 Inhibits prostate cancer vascularization through adiponectin receptor. Cell Physiol Biochem. 2015;36:1491–98. doi: 10.1159/000430313. [DOI] [PubMed] [Google Scholar]
- 20.Gao Q, Zheng J. MicroRNA-323 upregulation promotes prostate cancer growth and docetaxel resistance by repressing p73. Biomed Pharmacother. 2018;97:528–34. doi: 10.1016/j.biopha.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Liu Y, Lv M, et al. MiR-323-3p regulates the steroidogenesis and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting IGF-1. Gene. 2019;683:87–100. doi: 10.1016/j.gene.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Seco-Cervera M, Gonzalez-Rodriquez D, Ibanez-Cabekkos JS, et al. Circulating miR-323-3p is a biomarker for cardiomyopathy and an indicator of phenotypic variability in Friedreich’s ataxia patients. Sci Rep. 2017;7:5237. doi: 10.1038/s41598-017-04996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Li J, Tang W, et al. Adiponectin receptor 1-mediated micro RNA-323-3p regulates functions of the MIN6 cell line via the AMP-activated protein kinase/sirtuin-1 pathway. J Intern Med Res. 2018;46:1693–708. doi: 10.1177/0300060518758584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding M, Qiu TF, Zhou PG. MicroRNA-449a suppresses non-small cell lung cancer. Cell Biochem Biophys. 2015;71:1255–59. doi: 10.1007/s12013-014-0339-0. [DOI] [PubMed] [Google Scholar]
- 25.Pastorkova Z, Skarda J, Andel J. The role of microRNA in metastatic processes of non-small cell lung carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(3):343–57. doi: 10.5507/bp.2016.021. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Sheng Z, Cai Y. Effects of microRNA-513b on cell proliferation, apoptosis, invasion, and migration by targeting HMGB3 through regulation of mTOR signaling pathway in non-small-cell lung cancer. J Cell Physiol. 2019;234:10934–41. doi: 10.1002/jcp.27921. [DOI] [PubMed] [Google Scholar]
- 27.He X, Chen SY, Yang Z, et al. MiR-4317 suppresses non-small cell lung cancer (NSCLC) by targeting fibroblast growth factor 9 (FGF9) and cyclin D2 (CCND2) J Exp Clin Cancer Res. 2018;37:230. doi: 10.1186/s13046-018-0882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 29.Karner J, Wawrzyniak M, Tankov S, et al. Increased microRNA-323-3p in IL-22/IL-17-producing T cells and asthma: A role in the regulation of the TGF-beta pathway and IL-22 production. Allergy. 2017;72:55–65. doi: 10.1111/all.12907. [DOI] [PubMed] [Google Scholar]
- 30.Lee SM, Park JY, Kim DS. Methylation of TMEFF2 gene in tissue and serum DNA from patients with non-small cell lung cancer. Mol Cells. 2012;34:171–76. doi: 10.1007/s10059-012-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun T, Du W, Xiong H, et al. TMEFF2 deregulation contributes to gastric carcinogenesis and indicates poor survival outcome. Clin Cancer Res. 2014;20:4689–704. doi: 10.1158/1078-0432.CCR-14-0315. [DOI] [PubMed] [Google Scholar]
- 32.Azuara D, Rodriguez-Moranta F, De Oca J, et al. Novel methylation panel for the early detection of neoplasia in high-risk ulcerative colitis and Crohn’s colitis patients. Inflamm Bowel Dis. 2013;19:165–73. doi: 10.1002/ibd.22994. [DOI] [PubMed] [Google Scholar]
- 33.Chen E, Zheng F, Yuan X, et al. The effect of TMEFF2 methylation on the tumor stage and survival outcome of clear cell renal cell carcinoma. Cancer Biomark. 2017;19:207–12. doi: 10.3233/CBM-161656. [DOI] [PubMed] [Google Scholar]
- 34.Green T, Chen X, Ryan S, et al. TMEFF2 and SARDH cooperate to modulate one-carbon metabolism and invasion of prostate cancer cells. Prostate. 2013;73:1561–75. doi: 10.1002/pros.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosc. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- 37.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem Sci. 2011;36:320–28. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dangle PP, Zaharieva B, Jia H, et al. Ras-MAPK pathway as a therapeutic target in cancer – emphasis on bladder cancer. Recent Pat Anticancer Drug Discov. 2009;4:125–36. doi: 10.2174/157489209788452812. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Ruiz-Echevarria MJ. TMEFF2 modulates the AKT and ERK signaling pathways. Int J Biochem Mol Biol. 2013;4:83–94. [PMC free article] [PubMed] [Google Scholar]