Abstract
Management of melanoma has been revolutionized by the use of immune checkpoint inhibitors. Immune system changes associated with aging may affect the efficacy of immune‐based therapies. Using the National Cancer Database, we evaluated the impact of age on the receipt and efficacy of modern immunotherapies in patients with metastatic melanoma. We identified 11,944 patients from 2011–2015, of whom 25% received immunotherapy. Older (≥60 years), compared with younger, patients were less likely to receive immunotherapy (odds ratio, 0.69; 95% confidence interval [CI], 0.61–0.78; p < .001). Immunotherapy was associated with a survival benefit in both younger and older patients (<60 years: hazard ratio [HR], 0.64; 95% CI, 0.57–0.72; p < .001; ≥60 years: HR, 0.55; 95% CI, 0.50–0.60; p < .001). Importantly, there was a statistically significant interaction between age and survival with immunotherapy, where a greater benefit was observed for older patients (p interaction = 0.013). Further work studying the age‐related response to immunotherapy is warranted.
Short abstract
Recent evidence suggests that patient age may have an important effect on patient response to immunotherapies. This article evaluates the effect of age on the receipt and efficacy of modern immunotherapies in patients with metastatic melanoma.
Therapies targeting immune checkpoints such as cytotoxic T lymphocyte‐associated protein‐4 (CTLA‐4), programmed death protein‐1 (PD‐1), and programmed death ligand (PD‐L1) have revolutionized the management of metastatic melanoma 1, 2. Although immune checkpoint inhibitors (ICIs) in metastatic melanoma have shown remarkable improvements in survival, the impact of patient age on the efficacy of ICIs remains unclear 3, 4, 5, 6. Furthermore, older patients may be undertreated because of concerns about treatment related toxicities and are in general under‐represented in oncology clinical trials 7, 8. Given age‐related disparities in cancer care and the expanding role of ICIs, it is important to determine whether patient age modifies the effectiveness of these treatments. We used the National Cancer Database (NCDB) to evaluate the receipt and efficacy of immunotherapies based on age, specifically younger versus older than 60 years. Although the use of an age threshold is somewhat arbitrary, 60 years was chosen to be in line with a recent preclinical, multi‐institutional study investigating this issue 6.
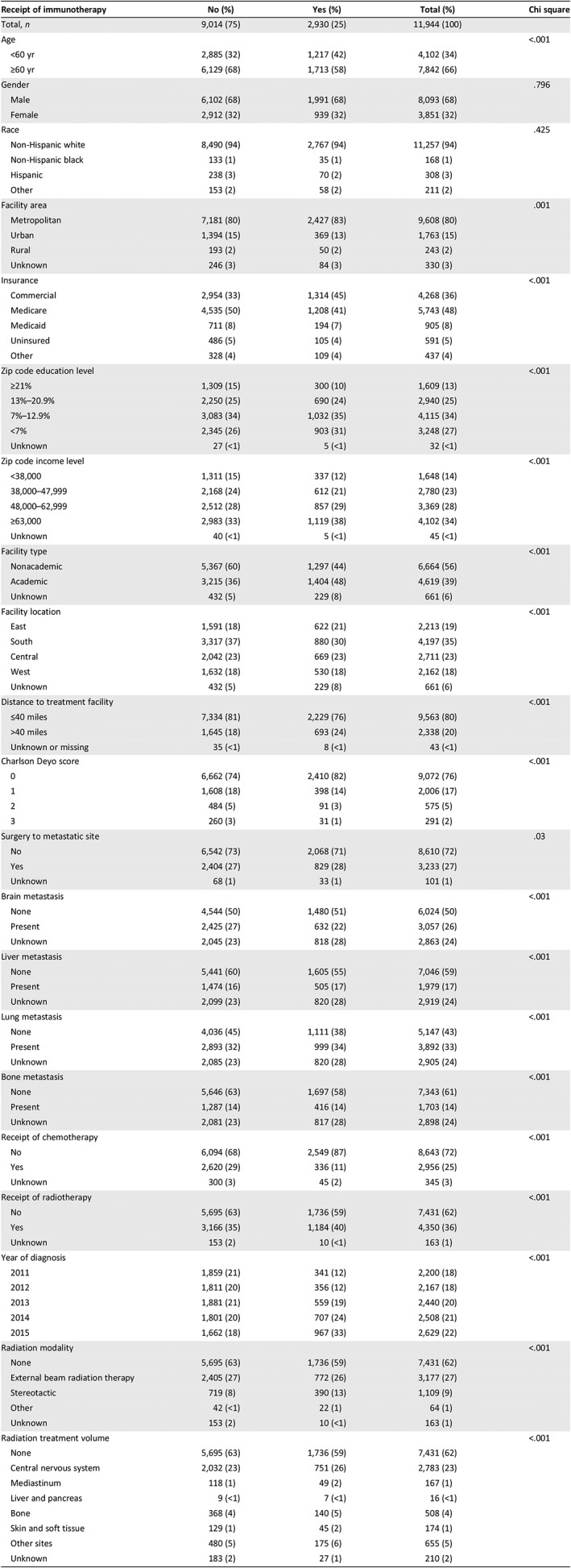
Inclusion criteria consisted of patients ≥18 years with cutaneous stage IV (metastatic) melanoma diagnosed between 2011 and 2015. We only included patients diagnosed from 2011 onwards, as the first modern ICI (ipilimumab) was approved for metastatic melanoma in that year. Patients were excluded if the receipt of immunotherapy or disease stage was unknown or missing. A total of 11,944 patients met the inclusion criteria. Of this cohort, 2,930 (25%) patients received immunotherapy. The median age of the cohort was 66 years (range, 18–90), with 4,102 (34%) patients aged <60 years and 7,842 (66%) patients aged ≥60 years. Detailed baseline characteristics are provided in Table 1.
Table 1.
Baseline patient characteristics
| Receipt of immunotherapy | No (%) | Yes (%) | Total (%) | Chi square |
|---|---|---|---|---|
| Total, n | 9,014 (75) | 2,930 (25) | 11,944 (100) | |
| Age | <.001 | |||
| <60 yr | 2,885 (32) | 1,217 (42) | 4,102 (34) | |
| ≥60 yr | 6,129 (68) | 1,713 (58) | 7,842 (66) | |
| Gender | .796 | |||
| Male | 6,102 (68) | 1,991 (68) | 8,093 (68) | |
| Female | 2,912 (32) | 939 (32) | 3,851 (32) | |
| Race | .425 | |||
| Non‐Hispanic white | 8,490 (94) | 2,767 (94) | 11,257 (94) | |
| Non‐Hispanic black | 133 (1) | 35 (1) | 168 (1) | |
| Hispanic | 238 (3) | 70 (2) | 308 (3) | |
| Other | 153 (2) | 58 (2) | 211 (2) | |
| Facility area | .001 | |||
| Metropolitan | 7,181 (80) | 2,427 (83) | 9,608 (80) | |
| Urban | 1,394 (15) | 369 (13) | 1,763 (15) | |
| Rural | 193 (2) | 50 (2) | 243 (2) | |
| Unknown | 246 (3) | 84 (3) | 330 (3) | |
| Insurance | <.001 | |||
| Commercial | 2,954 (33) | 1,314 (45) | 4,268 (36) | |
| Medicare | 4,535 (50) | 1,208 (41) | 5,743 (48) | |
| Medicaid | 711 (8) | 194 (7) | 905 (8) | |
| Uninsured | 486 (5) | 105 (4) | 591 (5) | |
| Other | 328 (4) | 109 (4) | 437 (4) | |
| Zip code education level | <.001 | |||
| ≥21% | 1,309 (15) | 300 (10) | 1,609 (13) | |
| 13%–20.9% | 2,250 (25) | 690 (24) | 2,940 (25) | |
| 7%–12.9% | 3,083 (34) | 1,032 (35) | 4,115 (34) | |
| <7% | 2,345 (26) | 903 (31) | 3,248 (27) | |
| Unknown | 27 (<1) | 5 (<1) | 32 (<1) | |
| Zip code income level | <.001 | |||
| <38,000 | 1,311 (15) | 337 (12) | 1,648 (14) | |
| 38,000–47,999 | 2,168 (24) | 612 (21) | 2,780 (23) | |
| 48,000–62,999 | 2,512 (28) | 857 (29) | 3,369 (28) | |
| ≥63,000 | 2,983 (33) | 1,119 (38) | 4,102 (34) | |
| Unknown | 40 (<1) | 5 (<1) | 45 (<1) | |
| Facility type | <.001 | |||
| Nonacademic | 5,367 (60) | 1,297 (44) | 6,664 (56) | |
| Academic | 3,215 (36) | 1,404 (48) | 4,619 (39) | |
| Unknown | 432 (5) | 229 (8) | 661 (6) | |
| Facility location | <.001 | |||
| East | 1,591 (18) | 622 (21) | 2,213 (19) | |
| South | 3,317 (37) | 880 (30) | 4,197 (35) | |
| Central | 2,042 (23) | 669 (23) | 2,711 (23) | |
| West | 1,632 (18) | 530 (18) | 2,162 (18) | |
| Unknown | 432 (5) | 229 (8) | 661 (6) | |
| Distance to treatment facility | <.001 | |||
| ≤40 miles | 7,334 (81) | 2,229 (76) | 9,563 (80) | |
| >40 miles | 1,645 (18) | 693 (24) | 2,338 (20) | |
| Unknown or missing | 35 (<1) | 8 (<1) | 43 (<1) | |
| Charlson Deyo score | <.001 | |||
| 0 | 6,662 (74) | 2,410 (82) | 9,072 (76) | |
| 1 | 1,608 (18) | 398 (14) | 2,006 (17) | |
| 2 | 484 (5) | 91 (3) | 575 (5) | |
| 3 | 260 (3) | 31 (1) | 291 (2) | |
| Surgery to metastatic site | .03 | |||
| No | 6,542 (73) | 2,068 (71) | 8,610 (72) | |
| Yes | 2,404 (27) | 829 (28) | 3,233 (27) | |
| Unknown | 68 (1) | 33 (1) | 101 (1) | |
| Brain metastasis | <.001 | |||
| None | 4,544 (50) | 1,480 (51) | 6,024 (50) | |
| Present | 2,425 (27) | 632 (22) | 3,057 (26) | |
| Unknown | 2,045 (23) | 818 (28) | 2,863 (24) | |
| Liver metastasis | <.001 | |||
| None | 5,441 (60) | 1,605 (55) | 7,046 (59) | |
| Present | 1,474 (16) | 505 (17) | 1,979 (17) | |
| Unknown | 2,099 (23) | 820 (28) | 2,919 (24) | |
| Lung metastasis | <.001 | |||
| None | 4,036 (45) | 1,111 (38) | 5,147 (43) | |
| Present | 2,893 (32) | 999 (34) | 3,892 (33) | |
| Unknown | 2,085 (23) | 820 (28) | 2,905 (24) | |
| Bone metastasis | <.001 | |||
| None | 5,646 (63) | 1,697 (58) | 7,343 (61) | |
| Present | 1,287 (14) | 416 (14) | 1,703 (14) | |
| Unknown | 2,081 (23) | 817 (28) | 2,898 (24) | |
| Receipt of chemotherapy | <.001 | |||
| No | 6,094 (68) | 2,549 (87) | 8,643 (72) | |
| Yes | 2,620 (29) | 336 (11) | 2,956 (25) | |
| Unknown | 300 (3) | 45 (2) | 345 (3) | |
| Receipt of radiotherapy | <.001 | |||
| No | 5,695 (63) | 1,736 (59) | 7,431 (62) | |
| Yes | 3,166 (35) | 1,184 (40) | 4,350 (36) | |
| Unknown | 153 (2) | 10 (<1) | 163 (1) | |
| Year of diagnosis | <.001 | |||
| 2011 | 1,859 (21) | 341 (12) | 2,200 (18) | |
| 2012 | 1,811 (20) | 356 (12) | 2,167 (18) | |
| 2013 | 1,881 (21) | 559 (19) | 2,440 (20) | |
| 2014 | 1,801 (20) | 707 (24) | 2,508 (21) | |
| 2015 | 1,662 (18) | 967 (33) | 2,629 (22) | |
| Radiation modality | <.001 | |||
| None | 5,695 (63) | 1,736 (59) | 7,431 (62) | |
| External beam radiation therapy | 2,405 (27) | 772 (26) | 3,177 (27) | |
| Stereotactic | 719 (8) | 390 (13) | 1,109 (9) | |
| Other | 42 (<1) | 22 (1) | 64 (1) | |
| Unknown | 153 (2) | 10 (<1) | 163 (1) | |
| Radiation treatment volume | <.001 | |||
| None | 5,695 (63) | 1,736 (59) | 7,431 (62) | |
| Central nervous system | 2,032 (23) | 751 (26) | 2,783 (23) | |
| Mediastinum | 118 (1) | 49 (2) | 167 (1) | |
| Liver and pancreas | 9 (<1) | 7 (<1) | 16 (<1) | |
| Bone | 368 (4) | 140 (5) | 508 (4) | |
| Skin and soft tissue | 129 (1) | 45 (2) | 174 (1) | |
| Other sites | 480 (5) | 175 (6) | 655 (5) | |
| Unknown | 183 (2) | 27 (1) | 210 (2) |
A multivariable logistic regression model was used to evaluate the patterns of receipt of immunotherapy. Compared with patients <60 years of age, those ≥60 years were significantly less likely to receive immunotherapy (odds ratio [OR], 0.69; 95% confidence interval [CI], 0.61–0.78; p < .001; supplemental online Table 1). We also noted that this gap in receiving immunotherapy between younger and older patients decreased with time. In the time period between 2011 and 2013, patients ≥60 years were 35% less likely to receive immunotherapy compared with those <60 years (OR, 0.65; 95% CI, 0.55–0.77; p < .001). However, in the time period between 2014 and 2015, older patients were 27% less likely to receive immunotherapy compared with their younger counterparts (OR, 0.72; 95% CI, 0.61–0.86; p < .001). Although it is hard to accurately know the reason for these differences, we speculate that it is likely a combination of increased physician comfort with toxicity management as well as use of immunotherapies with more favorable toxicity profiles (i.e., CTLA‐4 vs. PD‐1 agents).
To determine if the hazards of death associated with immunotherapy was dependent on age, a multivariable Cox proportional hazards model with an interaction term between receipt of immunotherapy (yes or no) and age (<60 and ≥60 years) was used (covariates included in the multivariable model are listed in supplemental online Table 2). This model was also used to derive hazards of death associated with receipt of immunotherapy for patients <60 years and ≥60 years. Both younger and older patients were noted to derive a significant benefit from immunotherapy. Receipt of immunotherapy was associated with a decreased hazard of death in patients <60 years (HR, 0.64; 95% CI, 0.57–0.72; p < .001) and in those ≥60 years (HR, 0.55; 95% CI, 0.50–0.60; p < .001). Importantly, there was a statistically significant interaction between age and survival with immunotherapy; with a greater benefit observed for older patients (p interaction = 0.013, Table 2). Propensity score (PS)–weighted multivariable analysis with robust variance estimation was used to further adjust for potential confounding 9. After PS‐weighted analysis, older age remained associated with a greater survival benefit (Table 2, covariates used for PS analysis are listed in supplemental online Table 2). Additional sensitivity analyses using age thresholds of 55, 65, and 70 years also yielded consistent results (supplemental online Table 3).
Table 2.
Overall survival associated with immunotherapy in patients with metastatic melanoma
| Age | Multivariable | PS analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | p interaction | HR (95% CI) | p value | p interaction | |
| <60 yr | 0.64 (0.57–0.72) | <.001 | 0.66 (0.57–0.75) | <.001 | ||
| ≥60 yr | 0.55 (0.50–0.60) | <.001 | 0.55 (0.50–0.61) | <.001 | ||
| Total | 0.013 | 0.036 | ||||
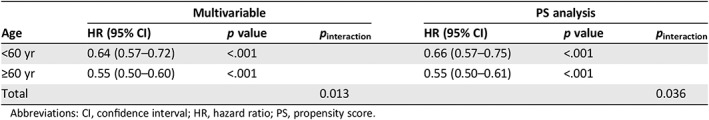
Abbreviations: CI, confidence interval; HR, hazard ratio; PS, propensity score.
In this study, we used a real world cohort of patients from a national cancer registry to evaluate the receipt and efficacy of modern immunotherapy in adults ≥60 years with metastatic melanoma compared with those <60 years. Our results showed that older patients (≥60 years) were less likely to receive immunotherapy than their younger counterparts (<60 years). We also observed that immunotherapy conferred a statistically significantly larger survival benefit for older patients with metastatic melanoma as compared with younger patients. These results remained consistent using both a multivariable regression model and PS‐weighted analysis after controlling for a wide range of measured confounders, as well as on sensitivity analysis using different age thresholds.
Our finding of greater survival benefit with immunotherapy in older patients is substantiated by clinical and preclinical data 5, 6. Notably, a recent multi‐institutional study demonstrated that patients ≥60 years derived greater benefit with anti–PD‐1 therapy 6. Furthermore, the investigators noted that the likelihood of response increased with age and observed a progression free survival benefit of 13% for each increasing decade of life. A similar preferential response to PD‐1 blockade was seen in aged mice, potentially due to a higher population of regulatory T cells observed in younger mice, which are known to suppress antitumor immune response.
There are, however, data from two recent meta‐analyses that included patients treated with modern ICIs that failed to show a difference in OS between younger versus older adults 3, 4. Although the reasons for these discrepant results are unclear, our study may further inform the discussion because of several inherent strengths. This data set is the one of the largest to date, analyzing the impact of immunotherapy in over 10,000 diverse patients derived from a real‐world sample from across the U.S. Also, the older patients typically included in clinical trials tend to be those with a good performance status; however, our study simulates real‐life practice patterns in which older patients often have functional limitations and multiple illnesses.
Our study is subject to certain limitations. First, given its retrospective nature, there is inherent selection bias. We performed PS‐weighted analysis adjusting for a wide range of measured confounders to minimize this bias. Secondly, the NCDB does not detail the type of immunotherapy used, and therefore, specific conclusions about the nature of agents used, such as CTLA‐4 inhibitors, PD‐(L)1 inhibitors, interleukin‐2, and interferon‐α, cannot be drawn. To counter this limitation, we only included patients treated in the time period following approval of the first ICI agent (ipilimumab) in 2011 for metastatic melanoma. Additional limitations include lack of information about BRAF mutational status as well as unavailability or incomplete recording of various prognostic factors such as lactate dehydrogenase level or ulceration (>80% patients coded as unknown). Finally, we lacked information about dosage, schedules, time on treatment, medication adherence, and toxicity, which may have affected outcomes.
In summary, we demonstrate that in the era of ICIs, patients older than 60 years were less likely to receive immunotherapy. Additionally, although patients both younger and older than 60 years had improved survival outcomes with immunotherapy, the latter derived a greater survival benefit in our analysis. Given the widespread use of immunotherapy across numerous malignancies and an aging cancer population, further research is needed to better understand the impact of age on the anticancer immune response.
Disclosures
Tara C. Mitchell: Merck, Bristol‐Myers Squibb, Array (SAB), Merck, Bristol‐Myers Squibb (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Robert C, Schachter J, Long G V et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 2. Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elias R, Giobbie‐Hurder A, Mccleary NJ et al. Efficacy of PD‐1 & PD‐L1 inhibitors in older adults: A meta‐analysis. J Immunother Cancer 2018;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishijima TF, Muss HB, Shachar SS et al. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta‐analysis. Cancer Treat Rev 2016;45:30–37. [DOI] [PubMed] [Google Scholar]
- 5. Perier‐Muzet M, Gatt E, Péron J et al. Association of immunotherapy with overall survival in elderly patients with melanoma. JAMA Dermatol 2018;154:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kugel CH, 3rd , Douglass SM, Webster MR et al. Age correlates with response to anti‐PD1, reflecting age‐related differences in intratumoral effector and regulatory t‐cell populations. Clin Cancer Res. 2018:5347–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis JH, Kilgore ML, Goldman DP et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003;21:1383–1389. [DOI] [PubMed] [Google Scholar]
- 8. Scher KS, Hurria A. Under‐representation of older adults in cancer registration trials: Known problem, little progress. J Clin Oncol 2012;30:2036–2038. [DOI] [PubMed] [Google Scholar]
- 9. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables


