Summary
Dendritic cells (DCs) are essential for generating T‐cell‐based immune responses through sensing of potential inflammatory and metabolic cues in the local environment. However, there is still limited insight into the processes defining the resultant DC phenotype, including the type of early transcriptional changes in pro‐inflammatory cues towards regulatory or type 2 immune‐based cues induced by a variety of exogenous and endogenous molecules. Here we compared the ability of a selected number of molecules to modulate the pro‐inflammatory phenotype of lipopolysaccharide (LPS) and interferon‐γ (IFN‐γ)‐stimulated human monocyte‐derived DCs towards an anti‐inflammatory or regulatory phenotype, including Ascaris suum body fluid [helminth pseudocoelomic fluid (PCF)], the metabolites succinate and butyrate, and the type 2 cytokines thymic stromal lymphopoietin and interleukin‐25. Our data show that helminth PCF and butyrate treatment suppress the T helper type 1 (Th1)‐inducing pro‐inflammatory DC phenotype through induction of different transcriptional programs in DCs. RNA sequencing indicated that helminth PCF treatment strongly inhibited the Th1 and Th17 polarizing ability of LPS + IFN‐γ‐matured DCs by down‐regulating myeloid differentiation primary response gene 88 (MyD88)‐dependent and MyD88‐independent pathways in Toll‐like receptor 4 signaling. By contrast, butyrate treatment had a strong Th1‐inhibiting action, and transcripts encoding important gut barrier defending factors such as IL18, IL1B and CXCL8 were up‐regulated. Collectively, our results further understanding of how compounds from parasites and gut microbiota‐derived butyrate may exert immunomodulatory effects on the host immune system.
Keywords: Ascaris suum, dendritic cells, type 2 immune response
This study examines the immunoregulatory potential of different exogenous and endogenous molecules on pro‐inflammatory human monocyte‐derived dendritic cells (moDCs) with the focus of identifying early transcriptional regulators of pro‐inflammatory cues. Body fluid from Ascaris suum was found to potently down‐regulate specific T helper type 1‐ and type 17‐related pathways in pro‐inflammatory moDCs, which overlapped, but also segregated from those induced by the intestinal metabolite butyrate. These results improve our understanding of how compounds from parasites may exert immunomodulatory effects on the host immune system.

Abbreviations
- COX2
cyclooxygenase‐2
- DCs
dendritic cells
- HSF1
heat shock factor 1
- IFN‐γ
interferon‐γ
- LPS
lipopolysaccharide
- MyD88
myeloid differentiation primary response gene 88
- PAMPs
pathogen‐associated molecular patterns
- PCF
pseudocoelomic fluid
- PD‐L1
programmed death‐ligand 1
- Rab7b
Ras related in brain 7b
- Th1
type 1 helper
- Th17
type 17 helper
- Th2
type 2 helper
- TLRs
Toll‐like receptors
- Treg
regulatory T cells
- TSLP
thymic stromal lymphopoietin
Introduction
Dendritic cells (DCs) are specialized antigen‐presenting cells that initiate and direct a variety of specific T‐cell responses. This depends on expression of pattern recognition receptors on DCs, including Toll‐like receptors (TLRs), nucleotide‐binding oligomerization domain‐like receptors and RIG‐I‐like receptors,1 activation of which leads to initiation of distinct transcriptional programs. Different classes of pathogen‐associated molecular patterns (PAMPs) induce different types of T helper cell responses, where effective responses to intracellular and virus‐based PAMPs promote T helper type 1 (Th1), fungi and extracellular bacterial PAMPs promote T helper type 17 (Th17), and helminth parasites induce type 2 (Th2) responses.1 Additionally, the influence of transforming growth factor‐β during the interaction with the DC induces the differentiation of naive T helper cells towards regulatory T (Treg) cells,2, 3 which play important roles in limiting immunopathologies and maintaining immune homeostasis.
In addition to PAMPs, intestinal DCs can also sense specific endogenous signals originating from the intestinal epithelium in response to PAMP stimulation, including thymic stromal lymphopoietin (TSLP), interleukin‐25 (IL‐25) and IL‐33. TSLP has been reported to influence DCs to promote Th2 responses,4 and IL‐33 seems to further strengthen TSLP‐DC‐mediated Th2 responses5 as well as inducing Treg cell immune responses.6 Additionally, succinate, an intermediate metabolite of the Krebs cycle, and butyrate, a fiber‐derived gut bacterial metabolite, have both been reported to induce immune regulatory mechanisms in PAMP‐exposed DCs.7, 8
Due to the strong Th2/Treg responses induced by helminths,9, 10 deliberate infection with helminths has been extensively explored as a therapy for various inflammatory disorders.11 However, helminth infections per se may not be necessary to exploit helminth‐mediated host protection because helminth‐derived products themselves have proven effective in relieving pro‐inflammatory conditions.12, 13, 14, 15, 16 Several studies have addressed the mode of immunomodulation induced by different helminth‐derived products, which appear to possess an ability to down‐regulate gene expression of TLR4 signaling‐associated molecules such as MYD88, IRAK2, IRF8, JUN, RELA and TLR4,17 thereby inhibiting synthesis of pro‐inflammatory cytokines (IL‐12p70, tumor necrosis factor‐α (TNF‐α)) and chemokines (Chemokine (C‐C motif) ligand 5 (CCL5, also known as RANTES), and macrophage inflammatory protein‐1β (CCL4)).18 Furthermore, helminth‐derived products have been reported to increase the levels of SOCS1 and PTPN6 mRNAs as well as their cognate proteins19 or to strongly induce OX40L and programmed death‐ligand 1 (PD‐L1) surface expression.14, 19, 20 Collectively, these modulations result in a DC phenotype that is able to promote Th2 polarization, as confirmed by DC–T‐cell co‐culturing experiments.14, 18
As described above, DCs can sense diverse molecular signals that skew Th1‐polarizing DCs towards another phenotype, which, dependent on the molecule, can result in Th2, Th17 or Treg differentiation of naive T helper cells. However, detailed insights into the specific molecular mechanisms whereby Th1–PAMP‐exposed DCs are skewed to these other T helper cell‐based responses are lacking; in particular, there is limited knowledge into Th2‐promoting programs21, 22 that are of importance for the generation of immunological memory to helminth infections. To increase this understanding, we here compared the extent to which exogenous helminth‐derived compounds from Ascaris suum as well as endogenous bacterial metabolites and type 2 immune response‐mediating cytokines were able to skew DCs exposed to Th1‐inducing PAMPs towards an anti‐inflammatory or type 2/regulatory phenotype. The results described herein show that helminth‐derived compounds hold a strong potential to down‐regulate both Th1 and Th17 immune responses, thereby furthering our understanding of helminth‐driven immune regulation.
Materials and methods
Helminth pseudocoelomic fluid and bitter melon extract preparation
Fresh, adult A. suum worms obtained from the intestine of pigs at a local slaughterhouse (Danish Crown, Ringsted, Denmark), were initially rinsed in water and sterile phosphate‐buffered saline (PBS), and then kept in sterile PBS during the whole procedure of pseudocoelomic fluid (PCF) preparation. Worms were decapitated and PCF was collected by gently massaging the outside of the cuticle, while wearing sterilized gloves, as described in ref. 23, and PCF was centrifuged at 3000 g for 20 min (to separate the debris and other contents like eggs, damaged tissue etc.). The supernatant was then sterile filtered through a 0·2‐μm syringe filter and stored at −80°C until use. The PCF was tested negative for contamination with lipopolysaccharide (LPS) as described in23. Average dry‐matter content of the filtered PCF was determined based on freeze–drying.
Generation and stimulation of human monocyte‐derived dendritic cells
Human peripheral blood mononuclear cells were isolated from buffy coats from human donors using Ficoll‐Paque gradient centrifugation. CD14+ monocytes were isolated from peripheral blood mononuclear cells by magnetic‐activated cell sorting (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in complete medium consisting of RPMI‐1640 (Lonza, Basel, Switzerland; BE12‐167F) supplemented with 10% heat‐inactivated fetal bovine serum (Gibco, Grand Island, NY, USA; 10270‐098), 2 mm l‐glutamine (Lonza, BE17‐605E), 50 µm 2‐mercaptoethanol (Gibco, 21985‐0223), 1% penicillin‐streptomycin at 37° and 5% CO2. During the differentiation stage, complete medium was added 150 U/ml recombinant human IL‐4 (Miltenyi, 130‐093‐922) and 160 U/ml recombinant human granulocyte–macrophage colony‐stimulating factor (Miltenyi, 130‐093‐866) to obtain monocyte‐derived DCs (moDCs).24 Medium was replaced after 3 days of culturing, and moDCs were used 6 days after the start of culture. The cells were on average >70% CD1a‐positive, and with <3% CD14+ CD16+ cells, based on flow cytometry as in ref. 24, and responded with high levels of IL‐12p70 production upon stimulation with LPS and interferon‐γ (IFN‐γ) (mean IL‐12p70 production of 4641·8 pg/ml; SD = 688·1 pg/ml). Cells were harvested and rested for 1 hr at 37° and 5% CO2 before stimulation. At this point, moDCs were supplemented with complete medium containing TSLP (to a final concentration of 25 ng/ml; Miltenyi, 130‐106‐271), IL‐25 (25 ng/ml; Miltenyi, 130‐115‐646), TSLP + IL‐25 (both 25 ng/ml), helminth PCF (100 μg dry matter/ml, tested in different doses in pre‐study experiments), butyrate (2 mm; Sigma, St Louis, MO; B5887), or succinate (0·5 mm and 2 mm, di‐sodium succinate hexahydrate [S7594751; Merck, Darmstadt, Germany]). Unstimulated cells were supplemented with complete medium alone. Immediately afterwards, medium containing Escherichia coli O26:B6 LPS (final concentration of 100 ng/ml; Sigma, L2654) and IFN‐γ (10 ng/ml; R&D Systems, Minneapolis, MN; 285‐IF‐100) was added. Cells were stimulated for 4 or 20 h at 37°, 5% CO2 and 95% humidity.
Cytokine enzyme‐linked immunosorbent assay
After stimulation for 20 hr, the cell‐free culture supernatants were harvested and frozen at −80° until further analysis. IL‐12p70 levels were determined using enzyme‐linked immunosorbent assay (ELISA; R&D Systems, DY1270) according to the manufacturer's protocol.
Flow cytometry
The moDCs were collected after harvesting of culture supernatants, washed in PBS + 0·1% NaN3 + 1% fetal bovine serum (FACS buffer) (280 g for 5 min, 5°), incubated for 10 min with Fc‐receptor block (2% heat‐inactivated human serum in FACS buffer), and stained with fluorophore‐conjugated antibodies in FACS buffer for 30 min, washed and analyzed immediately on a FACS Canto II (BD Biosciences, San Jose, CA) with a three laser (405, 488 and 633 nm) configuration. Antibodies used for flow cytometry were CD1a‐fluorescein isothiocyanate (eBiosciences, San Diego, CA; 11‐0149‐42), HLA‐DR‐Peridinin chlorophyll protein‐Cy5.5 (BD Biosciences, 347402), CD14‐phycoerythrin‐Cy7 (eBiosciences, 25‐0149‐42), CD86‐V450 (BD 560357), CD40‐allophycicyanin (BD Biosciences, 555591). Cells were kept at 4° throughout the staining procedure.
RNA extraction, RT‐qPCR and RNA sequencing
The transcriptional profile of stimulated human moDCs was evaluated after 4 hr of stimulation. Upon 4 hr of stimulation, cells were immersed and stored in RNAprotect Cell Reagent (Qiagen, Hilden, Germany) at −80° until further analysis. RNA was isolated using the RNeasy micro kit (Qiagen) according to the manufacturer's instructions. Total RNA concentration was measured using a Qubit 2.0 fluorometer (ThermoFisher Scientific, Waltham, MA) and quality was checked using a high‐sensitivity RNA Pico chip (Agilent Bioanalyzer) according to the manufacturer's protocol. cDNA was prepared by reverse transcription of 5·6 μl of purified RNA. Resulting cDNA was amplified using the Smart‐seq2 protocol25 with minor modifications, and using the primers and reagents listed in the Supplementary material (Table S1). In brief, the following conditions for cDNA synthesis were used: incubation of the reverse transcription RT Master mix and input RNA (72° for 3 min), addition of template‐switching oligo master mix, reverse transcription (42° for 90 min, followed by 10 cycles of 50° for 2 min, 42° for 2 min, and finally inhibition of the reaction at 70° for 15 min. The cDNA amplification was performed by incubating the cDNA and cDNA preamplification master mix at 98° for 3 min, followed by 19 cycles of 98° for 15 seconds, 67° for 20 seconds and 72° for 6 min, with a final extension at 72° for 5 min using the PCR cycler and reagents (see Supplementary material, Table S1). The cDNA Obtained was purified using AMPure XP beads (Beckman Coulter, Pasadena, CA; A63881), quantified using Qubit HS dsDNA Assay Kit (Life Technologies, Carlsbad, CA), and checked for quality and library size using a High‐Sensitivity DNA chip (Agilent Bioanalyzer, Santa Clara, CA).
For RNA sequencing, sequencing libraries from amplified cDNA from moDCs were generated using the Nextera XT DNA library preparation kit with multiplexing primers according to the manufacturer's protocol (Illumina, San Diego, CA; FC‐131‐1096). Resultant cDNA libraries were purified, quantified and checked for library fragment size distributions as described above. cDNA libraries with insert sizes of 200–700 bp were used for paired‐end sequencing on the Illumina NovaSeq 6000 sequencing platform (Novogene Corporation, Beijing, China). Cutadapt (v1·15) was used to remove sequencing adapters and low‐quality reads from the de‐multiplexed raw data. STAR (v2.6.0) and RSEM26 were used to map and align the clean reads and perform transcript quantification, respectively. After mapping transcripts to gene symbols, we obtained a gene count matrix with 17 220 genes and removed the least abundant transcripts for the 31 genes with duplicate gene symbols, resulting in a list of 17 189 genes. To remove low‐abundance transcripts, the data set was filtered to only include genes that were present in at least three samples with at least five reads, resulting in a set containing 8312 genes. Differentially expressed genes were identified using SAM‐seq v3·027 using a two‐class paired analysis with 20 re‐samplings and 100 permutations. Pathway gene enrichment analysis was performed using the Reactome Pathway Database.28 Sequencing data are available at the NCBI Gene Expression Omnibus (GEO: GSE137229).
Statistics and data analysis
Data analyses were performed using flowjo software (v10.0.7; Treestar, Ashland, OR), prism 8.0.2 (GraphPad Software, Graphpad, San Diego, CA) and R v3.5.2 (R Core Team, Vienna, Austria (2018)). While using prism, one‐way analysis of variance was performed followed by the Holm–Sidak method for multiple comparisons with *P < 0·05, **P < 0·01, ***P < 0·001. For RNA‐seq differential gene expression and gene set enrichment analysis, q‐values < 0·1 were considered as statistically significant.
Results
Helminth PCF and butyrate suppress LPS + IFN‐γ‐induced IL‐12p70 secretion in moDCs while TSLP and IL‐25 have no effect
Initially, we compared an exogenous helminth‐derived compound from A. suum, endogenous bacterial metabolites and type 2 immune response‐mediating cytokines for their ability to skew DCs exposed to Th1‐inducing PAMPs towards an anti‐inflammatory or type 2/regulatory phenotype. The endogenous epithelial‐derived cytokines IL‐25, IL‐33 and TSLP are all regarded as important modifiers of the gut environment. We therefore aimed to test for their capacity to regulate the Th1‐pro‐inflammatory program in moDCs. However, before initiation of the studies we first analyzed a set of our own RNA‐seq data from unstimulated moDCs (n = 9) for expression of mRNAs encoding the receptors for the cytokines IL‐25 (IL17RB), IL‐33 receptor (IL1RL1) and TSLP (CRLF2). We identified expression of the receptors for IL‐25 (IL17RB) and TSLP (CRLF2) in moDCs, but were unable to detect expression of mRNA encoding the IL‐33 receptor (IL1RL1) (see Supplementary material, Fig. S1a). Based on these results, we therefore included IL‐25 and TSLP in the panel of stimuli.
We next compared the immunomodulatory potential of the compounds in LPS‐ and IFN‐γ‐activated moDCs (Fig. 1a). We used human moDCs for the study, because they represent the only DCs to express TLR4, required for a full response to the bacterial Th1‐promoting PAMP LPS.29 Escherichia coli LPS is present in the small intestine during normal conditions30 and may act as an activating PAMP of intestinal monocytes, turning them into moDCs. As we have experienced that moDCs from some donors are refractory to IL‐12p70 production after LPS stimulation, we co‐stimulated moDCs with IFN‐γ, which allows for a full activation of the Toll–interleukin‐1 receptor‐domain‐containing adaptor‐inducing interferon‐β and nuclear factor‐κB responses via the IFN‐γ receptor even in CD1a‐low moDCs.24, 31 LPS + IFN‐γ‐matured moDCs classically attain a pro‐inflammatory phenotype with an increased cell‐surface expression of maturation and co‐stimulatory markers such as HLA‐DR, CD80, CD86 and CD40, as well as production of high amounts of IL‐12p70, which is needed to promote Th1 polarization.32 We first screened the potential for helminth PCF, butyrate, TSLP, IL‐25 and TSLP + IL‐25 in combination to suppress the LPS + IFN‐γ‐induced Type 1 immune profile of high IL‐12p70, as low levels of IL‐12p70 are a prerequisite for any compound with Th1–PAMP skewing potential that may promote the DCs to develop into a Th2, Th17 or Treg phenotype.32 Among those compounds, only helminth PCF and butyrate were able to significantly inhibit LPS + IFN‐γ‐induced IL‐12p70 secretion (q < 0·001), whereas TSLP and IL‐25, individually or in combination, failed to do so (Fig. 1b). Similarly, only helminth PCF (q < 0·03) and butyrate (q < 0·001), enhanced CD86 expression levels, and only butyrate led to decreased expression of CD40 (q < 0·001; Fig. 1b). We also tested if the complex helminth PCF solution would hold immune‐activating components in itself by stimulating moDCs with helminth PCF alone. We obtained similar stimulation profiles to those seen for unstimulated (immature) moDCs (see Supplementary material, Fig. S1b), indicating than no Th1‐promoting PAMPs are present in the helminth PCF solution.
Figure 1.
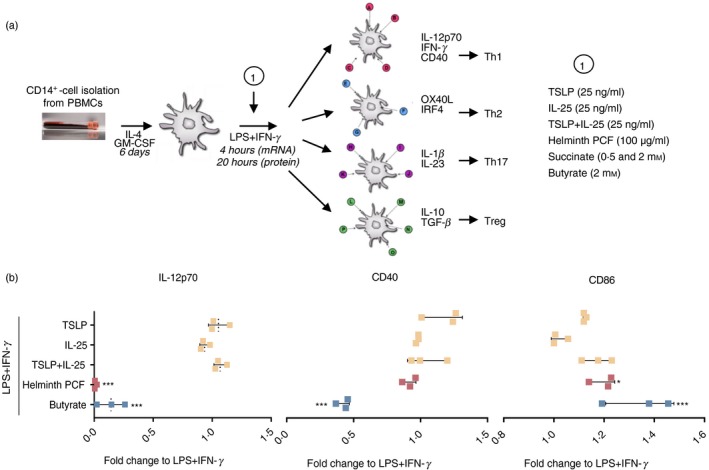
Helminth pseudocoelomic fluid (PCF) and butyrate suppress lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ)‐induced interleukin‐12p70 (IL‐12p70) secretion in monocyte‐derived dendritic cells (moDCs). (a) Overview of study design: (i) isolation of CD14+ monocytes from human blood peripheral blood mononuclear cells, (ii) differentiation of monocytes into dendritic cells (moDCs) using recombinant human IL‐4 and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) for 6 days, (iii) stimulation of moDCs with indicated compounds (1), (iv) collection and preparation of samples for RNA‐seq, flow cytometry and ELISA analyses. End‐point measures are the type of transcripts (mRNA; RNA‐seq, 4 hr of stimulation), surface marker expression (flow cytometry, 20 hr) and cytokines (proteins, 20 hr) produced by the stimulated moDCs. The figure also displays the specific molecules guiding naive T helper cell polarization into different effector T cells. GM‐CSF, granulocyte–macrophage colony‐stimulating factor; LPS, lipopolysaccharide; IFN‐γ, interferon‐γ; PCF, pseudocoelomic fluid; Th1, type 1 T helper cells; Th2, type 2 T helper cells; Th17, type 17 T helper cells; Tregs, regulatory T cells. (b) Concentration of secreted IL‐12p70 in cell‐free culture supernatant and expression of the co‐stimulatory molecules CD40 and CD86 in moDCs stimulated with the indicated compounds relative to LPS + IFN‐γ only. Final concentrations of the compounds were as follows: IL‐25: 25 ng/ml, TSLP: 25 ng/ml, PCF: 100 μg dry matter/ml, Butyrate: 2 mm. Levels of IL‐12p70, CD40 and CD86 in LPS + IFN‐γ‐stimulated moDCs were: IL‐12p70: 4641·8 ± 688·1 pg/ml, CD40: 1647 ± 361 MFI, CD86: 2424 ± 311 MFI. MFI, median fluorescence intensity, mean ± SD. The experiment was performed using moDCs from three different donors. *P < 0·05, ***P < 0·001 by one‐way analysis of variance and Dunnett's post‐test for multiple comparisons to LPS + IFN‐γ.
These data indicated that helminth PCF and butyrate exhibited differential immunomodulatory properties in pro‐inflammatory moDCs and may hold specific potentials to be further addressed. On the contrary, the epithelial‐derived cytokines IL‐25 and TSLP showed no immunomodulatory properties in pro‐inflammatory moDCs.
Helminth PCF predominantly suppresses Th1 and Th17 programs in pro‐inflammatory moDCs
To explore the regulation of the genes involved in the early process of rewiring of pro‐inflammatory moDCs by helminth PCF and butyrate, we used RNA‐seq to generate transcriptional profiles of moDCs stimulated with LPS + IFN‐γ alone or in combination with helminth PCF or butyrate for 4 hr. Helminth PCF resulted in significant down‐regulation of the expression of 131 genes in LPS + IFN‐γ‐activated moDCs (Fig. 2a), whereas no genes exhibited significantly up‐regulated expression, suggesting that helminth PCF exerts general immune suppressing effects in Type 1‐activated moDCs. Many of the genes whose expression was down‐regulated by helminth PCF encode immune‐polarizing and modifying cytokines (e.g. IL12A, IL12B, IL23A, IFNB1) and chemokines (e.g. CCL3, CCL4, CXCL1, CXCL2), surface receptors (e.g. TLR4, PTGER4), cytoplasmic receptors (e.g. NOD2), transcription factors/regulators (e.g. IRF8, NFKB1, NFKBIZ), enzymes (e.g. IDO1, PTGS2) and molecules involved in intracellular signaling (e.g. IRAK2, TRAF1). Several of the down‐regulated genes also encode proteins involved in moDC maturation and stimulatory activity, antigen presentation (e.g. CTSS, LAMP3) and apoptosis (e.g. GOS2, BIRC2, BCL2A1) (Fig. 2b; and see Supplementary material, Data S1).
Figure 2.
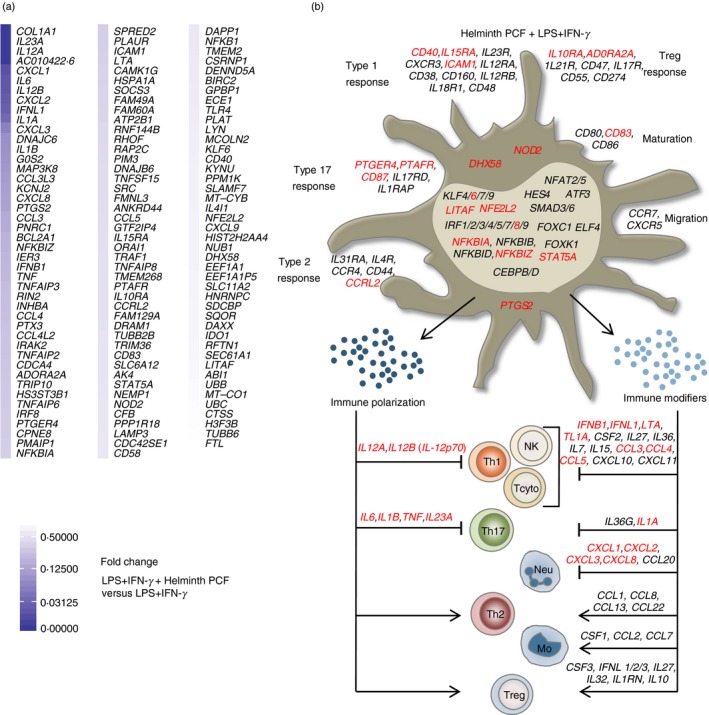
Helminth pseudocoelomic fluid (PCF) predominantly targets T helper type 1 (Th1) and Th17 polarizing abilities in pro‐inflammatory monocyte‐derived dendritic cells (moDCs). (a) Differentially expressed genes arranged by fold change in PCF + lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) ‐stimulated moDCs compared with LPS + IFN‐γ only. All genes (131) with a q‐value < 0·1 are shown. (b) Predicted phenotype of moDCs based on their transcriptional profile. The illustration focuses mainly on molecules from moDCs involved in the polarizing signals provided to naive T helper cells. Shown are the differentially regulated genes with a q‐value < 0·1. Black: genes up‐regulated by LPS + IFN‐γ (relative to unstimulated moDCs), Red: genes down‐regulated by PCF + LPS + IFN‐γ (relative to LPS + IFN‐γ only). Th1, type 1 T helper cells; NK, natural killer cells; Tcyto, cytotoxic T cells; Th17, type 17 T helper cells; Neu, neutrophils; Th2, type 2 T helper cells; Mo, monocytes; Tregs, regulatory T cells.
Helminth PCF did not regulate the expression of genes involved in moDC migration (e.g. CCR7 and CXCR5), suggesting that helminth PCF‐exposed moDCs probably maintain their ability to migrate to the nearby lymph nodes and prime antigen‐specific T cells. Helminth PCF treatment slightly decreased mRNA expression of the co‐stimulatory molecule CD40 and the maturation marker CD83 without affecting the transcription levels of the two other co‐stimulatory molecules CD80 and CD86. The flow cytometric analysis showed a significant increase of cell surface expression of CD86 in helminth PCF‐treated moDCs (Fig. 1b), which was not identified at the mRNA level, perhaps due to the low fold increase and/or presence of preformed, surface‐recycled CD86.
The RNA‐seq analysis revealed a down‐regulation of expression by helminth PCF of genes that are crucial for DC‐mediated Th1 and/or Th17 immune responses (Fig. 2a). The combined transcriptional profile of helminth PCF‐exposed moDCs, in terms of their ability to promote specific naive T helper cell polarization and further immune amplification, is provided in Fig. 2(b). From this, it appears that helminth PCF‐treated moDCs hold a limited potential to polarize and amplify Th1 and Th17 immune responses. This conclusion is based on the significant down‐regulation of transcripts of IL12A, IL12B, IL1B, IL23A and IL6 by helminth PCF. The IL12A and IL12B genes encode the subunits of the cytokine IL‐12p70, while IL23A and IL12B encode subunits of IL‐23. IL‐12p70 promotes Th1 polarization when provided as signal 3 from the DC to a naive CD4+ T cell, whereas IL‐1β in combination with IL‐23 and IL‐6 promotes Th17 polarization in naïve human T‐cells.33 At 4 hr upon activation, helminth PCF also down‐regulated expression of genes encoding transcription factors or transcriptional regulators associated with Th1 and Th17 polarization. In the normal Type 1 polarizing moDCs, LPS binding to TLR4 brings multiple transcription factors and transcription regulators into action, including nuclear factor‐κB, AP‐1, IKBα, IKBζ, interferon regulatory factor 3 (IRF3), KLF6, LITAF and NFE2L2, which are involved in promoting Th1 and/or Th17 immune responses.34, 35, 36, 37, 38 Furthermore, LPS‐stimulated IRF8 is associated with enhanced Th1 and Th17 immune response levels39 through a positive feedback loop involving type 1 IFN signaling.40 Helminth PCF significantly down‐regulated transcripts of IRF8, NFKB1, NFKBIZ, KLF6 and LITAF genes in Type 1 polarizing moDCs, further supporting progression into a non‐Th1 and/or Th17 polarizing phenotype. Furthermore, helminth PCF exposure decreased moDC transcript levels for the cytokine‐encoding genes IFNB1, IFNL1, LTA, TNF, TNFSF15 and IL1A 33, 41, 42, 43, 44 and the chemokine‐encoding genes CCL3, CCL4, CCL5, CXCL1, CXCL2, CXCL3 and CXCL8,45, 46 which are all associated with promotion of type 1 and/or type 17 immune responses.
Notably, transcripts of STAT5A and CCRL2, which have been associated with DC‐mediated Th2 immune responses46, 47 were also down‐regulated in helminth PCF‐treated moDCs. However, expression of IRF4, which is critical for DC‐mediated Th2 differentiation,48 remained unaffected by helminth PCF. Similarly, expression of genes encoding many Th2 chemo‐attractants (e.g. CCL1, CCL8, CCL13, CCL22)46 remained unaffected by helminth PCF treatment. The mRNA levels of PTGS2, which encodes the enzyme cyclooxygenase‐2 (COX‐2), was among the genes most down‐regulated by helminth PCF treatment (Fig. 2a; see Supplementary material, Data S1). Recently, the COX‐2 product prostaglandin E2 has been shown to be involved in DC‐mediated Th2 polarization in an autocrine manner20 and has also been shown to induce expression of the Th2 and Treg chemo‐attractant CCL22 in inflammatory DCs.49 However, CCL22 mRNA expression was not affected in helminth PCF‐treated moDCs. These findings indicate that helminth PCF treatment might hold distinct immunoregulatory properties that also modulate DC‐mediated Th2 and/or Treg cell polarization.
Previous studies have shown that helminth‐derived products inhibit LPS‐induced DC activation by suppressing TLR4 signaling pathways.17, 50 Using microarray and RT‐PCR techniques, Klaver et al.17 showed that soluble products of the pig helminth Trichuris suis decreased transcript levels of various components of MyD88‐dependent (IRAK2, JUN and RELA) and type 1 IFN signaling (IFNB, IRF8 and GBP2) molecules in LPS‐stimulated moDCs. Additionally, T. suis soluble products induced expression of the protein Ras related in brain 7b, which associated with simultaneous reduction of TLR4 surface expression.17 Similarly, we found that helminth PCF also modified expression of genes involved in TLR4 signaling. Levels of mRNA encoding MAP3K8, A20, IRAK2, SOCS3 and TRAF1, all of which are involved in TLR4 signaling37, 51 were significantly down‐regulated by helminth PCF in LPS + IFN‐γ‐activated moDCs.
Lipopolysaccharide‐stimulated DCs have been shown to be unresponsive to re‐stimulation with LPS or LPS + IFN‐γ, whereas they are fully capable of being activated by CD40L, activated T cells, and their products.52 Here we identified that helminth PCF treatment down‐regulated expression of mRNA encoding several surface molecules (CD40, IL15RA, ICAM1, PTGER4, PTAFR, CD87, IL10RA, ADORA2A and CCRL2) in LPS+ IFN‐γ‐activated moDCs, suggesting that helminth PCF treatment might prevent re‐stimulation of moDCs by subsequent signals associated with either initiation and/or amplification of different immune responses. Given that helminth PCF down‐regulated IL15RA transcript levels, it is possible that helminth PCF‐treated moDCs will have less efficient natural killer cell‐activating properties. Interleukin‐15Rα is important for the Type 1 response, as IL‐15Rα can enhance the anti‐tumor properties of natural killer or CD8+ T cells through trans‐presentation when bound to IL‐15.53 Similarly, helminth PCF treatment down‐regulated expression of PTAFR, encoding the receptor of platelet‐activating factor, which can promote Th17 immune responses via IL‐6 induction in moDCs,54 indicating that helminth PCF‐treated moDCs might evade further activation by platelet‐activating factor.
To further examine the immunomodulatory properties of helminth PCF in pro‐inflammatory DCs, we went on to perform a pathway analysis including all the significantly down‐regulated genes. Using the Reactome pathway database,28 we found that genes whose expression was down‐regulated by helminth PCF were enriched in 10 pathways (Table 1). Most of them are involved in inflammatory processes, further supporting the idea that helminth PCF exerts immunosuppressing effects in pro‐inflammatory moDCs. Particularly, helminth PCF treatment down‐regulated the expression of genes involved in inflammatory signaling pathways associated with different cytokines and chemokines as well as cellular stress conditions. Additionally, PCF treatment also seemed to down‐regulate the expression of genes encoding elements of heat‐shock response pathways (DNAJB6 and HSPA1A), which are activated in response to cellular stress inducers, e.g. pathogen infections.
Table 1.
Down‐regulated pathways (q‐value < 0·01) obtained by gene set enrichment analysis on genes from human monocyte‐derived dendritic cells (moDCs) stimulated with helminth pseudocoelomic fluid (PCF) + lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) versus LPS + IFN‐γ alone
| Pathway1 | P‐value | q‐value | Genes involved |
|---|---|---|---|
| Interleukin‐10 signaling | 1·11 × 10−16 | 1·77 × 10−13 | CXCL8, IL10RA, CCL3L3, PTAFR, CXCL1, CXCL3, PTGS2, CXCL2, TNF, ICAM1, IL1A, IL6, CCL5, IL1B, CCL4, CCL3, IL12B, IL12A |
| Interleukin‐4 and Interleukin‐13 signaling | 1·11 × 10−10 | 8·82 × 10−8 | SOCS3, IL1A, IL6, CXCL8, IL23A, IL1B, IL12B, IL12A, PTGS2, TNF, ICAM1 |
| Signaling by interleukins | 4·47 × 10−8 | 2·38 × 10−5 | CXCL8, CCL3L3, PTAFR, CXCL1, NOD2, CXCL3, PTGS2, CXCL2, TNF, ICAM1, SOCS3, IFNL1, UBB, IRAK2, CCL5, CCL4, UBC, CCL3, IL12B, IL12A, MAP3K8, STAT5A, LYN, IL15RA, IL10RA, NFKB1, NFKBIA, IL1A, IL6, IL23A, IL1B, TMEM2 |
| Attenuation phase | 6·95 × 10−8 | 2·76 × 10−5 | DNAJB6, UBB, HSPA1A |
| Cytokine signaling in immune system | 8·52 × 10−7 | 2·72 × 10−4 | CD40, CXCL8, CCL3L3, PTAFR, CXCL1, NOD2, CXCL3, PTGS2, CXCL2, TNF, ICAM1, SOCS3, IFNL1, UBB, IRAK2, CCL5, CCL4, UBC, CCL3, IL12B, IL12A, MAP3K8, STAT5A, LYN, IL15RA, TNFSF15, IFNB1, IL10RA, NFKB1, NFKBIA, IL1A, IL6, IL23A, IL1B, TMEM2, LTA, IRF8, BIRC2 |
| Senescence‐associated secretory phenotype (SASP) | 1·57 × 10−6 | 4·16 × 10−4 | IL1A, IL6, HIST2H2AA4, CXCL8, UBB, H3F3B, IL1B, UBC, NFKB1 |
| Immune system | 2·04 × 10−6 | 4·23 × 10−4 | CD40, CXCL8, TNFAIP6, TNFAIP3, CXCL1, CXCL3, CXCL2, TNF, CTSS, ICAM1, SEC61A1, TUBB6, DHX58, IL12B, IL12A, MAP3K8, IL15RA, PLAUR, RHOF, RAP2C, EEF1A1, IL1A, RNF144B, TUBB2B, IL23A, IL1B, LTA, IRF8, ORAI1, TLR4, CFB, BIRC2, FTL, SRC, CCL3L3, PTAFR, DAPP1, NOD2, PTGS2, RFTN1, SOCS3, SDCBP, IFNL1, UBB, IRAK2, CCL5, CCL4, UBC, CCL3, SLAMF7, CD58, STAT5A, LYN, TNFSF15, IFNB1, IL10RA, NFKB1, NFKBIA, COL1A1, IL6, TMEM2, ABI1, TRIM36, PTX3, HSPA1A |
| HSF1 activation | 2·12 × 10−6 | 4·23 × 10−4 | EEF1A1, DNAJB6, UBB, HSPA1A |
| HSF1‐dependent transactivation | 5·67 × 10−6 | 1·0 × 10−3 | DNAJB6, UBB, HSPA1A |
| Chemokine receptors bind chemokines | 6·25 × 10−5 | 9·94 × 10−3 | CXCL9, CXCL8, CCL5, CCRL2, CXCL1, CXCL3, CXCL2 |
Abbreviations: HSF1, heat‐shock factor 1.
Reactome Pathway Database (https://reactome.org/).
Helminth PCF and butyrate modify gene expression in pro‐inflammatory moDCs differently
Butyrate has previously been shown to exert immunomodulatory effects in LPS‐stimulated moDCs,7 to be associated with improving homeostasis at mucosal sites,55 and promoting Treg cell development at mucosal sites in mouse models.56 As butyrate is acknowledged to hold these important immunoregulatory properties, and as it, similarly to helminth PCF, was found to act as an immunomodulator of LPS+IFN‐γ‐matured moDCs (Fig. 1b), we sought to compare the immunomodulatory properties of helminth PCF and butyrate to analyze differences and similarities between the two.
Butyrate treatment of LPS + IFN‐γ‐activated moDCs resulted in an extensive regulation of the transcriptional profile with a total of 3480 genes being significantly regulated, out of which expression of 1703 was up‐regulated and expression of 1777 genes was down‐regulated (Fig. 3a), indicating that butyrate treatment exerted a broader immunomodulation on pro‐inflammatory moDCs in comparison with helminth PCF treatment (131 genes, Fig. 2a). We found 101 regulated genes to be shared between butyrate‐ and helminth PCF‐treated moDCs (Fig. 3a). Out of these genes, butyrate treatment up‐regulated expression of 10 genes and down‐regulated expression of 91 genes compared with the LPS + IFN‐γ‐activated moDCs, whereas expression of all these 101 genes was significantly down‐regulated by helminth PCF treatment (see Supplementary material, Data S1).
Figure 3.
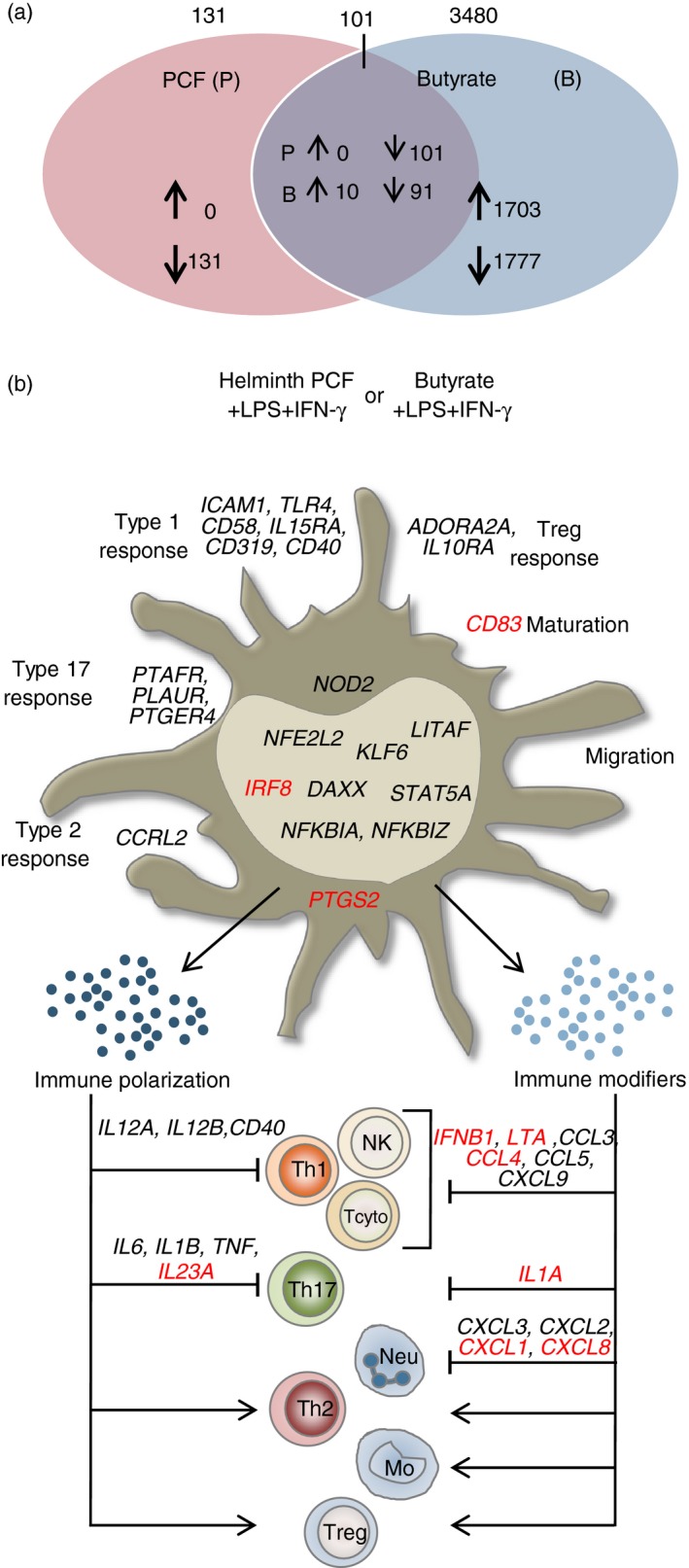
Helminth pseudocoelomic fluid (PCF) and butyrate modify gene expression in lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) ‐stimulated monocyte‐derived dendritic cells (moDCs) differently. (a) Venn diagram displaying unique and shared regulated genes (q value < 0·1) in helminth PCF + LPS + IFN‐γ‐ and butyrate + LPS + IFN‐γ‐stimulated moDCs relative to LPS + IFN‐γ only. (b) Distinct regulation of immune relevant genes in helminth PCF + LPS + IFN‐γ‐ and butyrate + LPS + IFN‐γ‐stimulated moDCs capable of modifying DC‐guided T helper cell polarization and immune cell trafficking. Black: genes down‐regulated by both helminth PCF and butyrate, Black in bold: genes up‐regulated by butyrate, but down‐regulated by PCF, Red: genes down‐regulated by PCF uniquely but not regulated by butyrate. Mo, monocytes; Neu, neutrophils; NK, natural killer cells; Tcyto, cytotoxic T cells; Th1, type 1 T helper cells; Th17, type 17 T helper cells; Th2, type 2 T helper cells; Tregs, regulatory T cells.
Focusing on the immune relevant genes regulated by helminth PCF and butyrate, we found multiple transcripts of genes that encode proteins involved in promoting Th1 and/or Th17 immune responses via moDCs to be down‐regulated (Fig. 3b). In the context of Th1 immune responses, butyrate treatment down‐regulated expression of mRNA encoding the Th1‐polarizing effector molecules IL12A, IL12B and CD40, which is coherent with the flow cytometric and ELISA analyses (Fig. 1b), and the Th1 immune amplifying chemokine‐encoding genes CCL5 and CXCL9. Although IL12A expression was suppressed in both helminth PCF‐ and butyrate‐treated moDCs, the down‐regulation of the other four genes was stronger for butyrate‐treated moDCs. In contrast, expression of mRNA encoding the monocyte‐ and lymphocyte‐attracting chemokine gene CCL3 was up‐regulated in butyrate treatment but down‐regulated with helminth PCF treatment. However, helminth PCF treatment also down‐regulated expression of IFNB1, LTA and CCL4 mRNA, suggesting that although both helminth PCF and butyrate might be able to inhibit Th1 polarization, helminth PCF specifically down‐regulated a broader set of immunological effector molecules and might be more efficient in limiting amplification of Th1 immune responses.
We observed striking differences between helminth PCF‐ and butyrate‐induced immunomodulation in relation to Th17 immune responses. Whereas helminth PCF down‐regulated expression of most of the genes associated with either Th17 polarization (IL6, IL1B and IL23A) or amplification of Th17 immune responses (IL1A, CXCL1, CXCL2, CXCL3 and CXCL8), butyrate treatment only significantly down‐regulated expression of IL6. Furthermore, butyrate treatment up‐regulated expression of a few genes known to be involved in either Th17 polarization (IL1B) or amplification (CXCL3 and CXCL8). Most of the genes encoding transcription factors and transcription regulators down‐regulated by helminth PCF treatment were also down‐regulated by butyrate except for IRF8, which is associated with enhanced Th1 and Th17 immune responses levels.39 Notably, the transcript levels of NFKBIZ were up‐regulated by butyrate treatment and down‐regulated by helminth PCF. Increased activity of the product of NFKBIZ, IκB‐ζ, has been associated with enhanced Th17 polarization and immune amplification.36 However, as IL12B, which is essential for formation of the Th17 amplification cytokine IL‐23, is strongly down‐regulated by butyrate, it is rather unlikely that butyrate‐treated moDCs effectively promote Th17 immune responses. Finally, we noted that helminth PCF uniquely down‐regulated PTGS2 transcript levels, whereas no such effect was observed in response to butyrate treatment. Similarly, mRNA expression of the maturation marker gene CD83 was only down‐regulated by helminth PCF treatment.
Succinate is unable to down‐regulate IL‐12p70 secretion in LPS + IFN‐γ‐activated moDCs
Succinate has previously been reported to be secreted by helminths57 and to exert immunoregulatory properties on poly(I:C) co‐stimulated human DCs.8 We therefore speculated whether succinate could be involved in mediating the immune suppressive effects of helminth PCF. LPS + IFN‐γ‐activated moDCs were co‐stimulated with 0·5 and 2 mm of succinate. We found that succinate even at the highest concentration failed to induce significant immunomodulatory changes in IL‐12p70 production and CD40 and CD86 expression in LPS + IFN‐γ‐activated moDCs (Fig. 4). Similarly, 2 mm succinate did not induce any significant transcriptional changes in LPS + IFN‐γ‐activated moDCs (data not shown). These findings indicate that succinate is unable to transcriptionally reprogram LPS + IFN‐γ‐matured DCs, and further emphasize that the immunosuppressive effects of helminth PCF on LPS + IFN‐γ‐activated moDCs may be mediated by other compounds than succinate.
Figure 4.
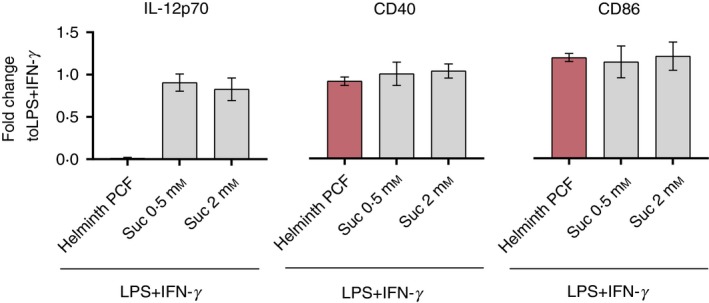
Succinate is unable to down‐regulate lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) ‐induced interleukin‐12p70 (IL‐12p70) secretion in monocyte‐derived dendritic cells (moDCs). Concentration of secreted IL‐12p70 in cell‐free culture supernatant and expression of co‐stimulatory molecules CD40 and CD86 on moDCs stimulated with helminth pseudocoelomic fluid (PCF) as in Fig. 1, and indicated succinate (suc) concentrations relative to LPS + IFN‐γ only. Graphs display mean ± SD.
Discussion
The overall aim of our study was to explore the early transcriptional changes induced by helminth PCF, the bacterial metabolites succinate and butyrate, and the Type 2 immune response‐mediating cytokines TSLP and IL‐25 on Type 1‐promoted inflammatory moDCs. Our exploratory analysis of the transcriptional data presented herein suggests that helminth PCF holds a strong potential to down‐regulate Th1 and Th17 immune responses, whereas we only detected down‐regulation of Th1 immune responses during LPS‐ and IFN‐γ‐induced inflammatory conditions by butyrate.
Previous studies have examined the immunomodulatory effects of helminth PCF on human moDCs reporting that helminth PCF conditioning suppresses pro‐inflammatory cytokine production (IL‐12p70, IL‐6, IL‐23 and TNF‐α) in LPS‐stimulated moDCs.58, 59 Our analysis of the early transcriptional profile of helminth PCF‐treated LPS + IFN‐γ‐matured moDCs supports these previous findings and suggests that helminth PCF may interfere with TLR4 signaling to exert its immunosuppressive effects, as indicated by identification of decreased expression of molecules downstream of TLR4 activation. Our results showed that helminth PCF down‐regulated expression of genes encoding components of predominantly the MyD88‐dependent but also the MyD88‐independent TLR4 signaling cascade like IRAK2,60 MAP3K8,61 IFNB and IRF8.40 Predominant down‐regulation of the MyD88‐dependent pathway is supported by the observation that helminth PCF treatment down‐regulated TNF, IL6, IL12A, IL12B and PTGS2 transcript levels, which are primarily induced after MyD88‐dependent pathway activation.62 Additionally, helminth PCF treatment also seemed to further down‐regulate the IFN‐β induction pathway as supported by down‐regulated TRIM36 mRNA expression levels, because TRIM36 is associated with potentiation of IFN‐β expression.63
Understanding DC‐mediated Th2 induction has been a long‐standing focus in immunology and is mainly based on studies wherein soluble egg antigen (SEA) from Schistosoma mansoni and T. suis soluble products were employed to initiate DC‐mediated Th2 immune response.14, 17, 18, 20 In soluble egg antigen, one of the multiple compounds that can induce DC‐mediated Th2 immune responses is omega‐1; a glycosylated T2 RNase that can inhibit pro‐inflammatory protein synthesis, induce strong surface expression of OX40L on DCs and increase the IL‐4/IFN‐γ ratio in DCs and allogenic naive CD4+ T‐cell co‐culturing experiments.18, 19 T2 RNase homologs have been suggested to be present in the A. suum genome,18 implying that helminth PCF might hold similar Th2‐inducing properties, but this notion was previously refuted based on findings that PCF treatment did not alter IL‐4 levels in allogenic naive CD4+ T cells co‐cultured with DCs.58 Moreover, a recent study showed that soluble egg antigen treatment of DCs induced prostaglandin E2 synthesis, resulting in autocrine induced OX40L, which further primed DCs to initiate Th2 responses.20 The observations in our study, and the study by Midttun et al. 58 that helminth PCF down‐regulated PTGS2 transcripts as well as COX‐2 protein levels indicate that helminth PCF‐treated moDCs might not be able to induce prostaglandin E2‐dependent DC‐mediated Th2 responses. However, more studies are needed to confirm this as Ascaris spp. infections indeed are known to induce Th2/Treg immune responses in humans.64, 65 Perhaps under in vivo settings, Ascaris spp. may trigger additional signals to initiate Th2 responses. Furthermore, because helminth PCF is a mixture of multiple antigenic proteins and glycans,66, 67, 68 and we and previous studies did not use fractionated PCF components, it remains possible that the Th2‐inducing components of PCF may be masked by other PCF components exerting differential immunomodulatory effects. To this, it is important to add that transcript levels of the transcription factor IRF4, which is critical for DC‐mediated Th2 differentiation,48 remained unaffected by helminth PCF treatment.
Contrasting the probable induction of Th2 immune responses in humans, two mouse studies strongly support the potential of helminth PCF to induce robust Treg cell responses as well as increased IL‐10 levels during LPS‐induced inflammatory conditions.69, 70 In both studies, mice were injected with 1 μg LPS with or without PCF supplementation into an air pouch and the researchers observed that helminth PCF prevented LPS‐induced leukocyte influx and suppressed pro‐inflammatory cytokine (IL‐1β, TNF‐α and IL‐6) secretion. Furthermore, helminth PCF supplementation has also been shown to induce Treg cells during non‐LPS‐induced inflammatory conditions.15, 16
Although we did not focus on the chemical composition of the helminth PCF, it seems that both protein69, 70 and glycan68, 71 structures in PCF could be responsible for their immunoregulatory potential. Notably, the metabolite succinate has been shown to be secreted by the helminth Nippostrongylus brasiliensis.57 Our observation that succinate did not exert any immunomodulatory effects on LPS + IFN‐γ‐activated moDCs suggests that the immunomodulatory effects of helminth‐derived succinate depend on other helminth compounds, or that distinct helminths might use different immunomodulatory mechanisms.
A recent study showed that butyrate treatment of LPS‐matured moDCs suppressed IL‐12p70 secretion and priming of Th1 and Th2 responses.7 Based on the transcriptional profile of butyrate‐treated LPS + IFN‐γ‐matured DCs, we here find that butyrate treatment of moDC does not lead to an immunosuppressive phenotype as strong as PCF treatment. This is suggested based on the increased butyrate‐induced expression of IL18, CXCL8 and IL1B transcript levels,36, 45, 46 which are all related to the Type 17 immune axis. Although we identified a strong down‐regulation of the IL12B, making it difficult to form the Th17‐amplifying cytokine IL‐23, other components of a Th17‐polarizing phenotype are in play, e.g. relayed by the specific up‐regulation of IL1B and NFKBIZ along with unaltered IL1A levels by butyrate treatment. During DC‐mediated Th17 induction, IL‐1β may mediate a positive‐feedback loop in an autocrine/paracrine fashion via IκB‐ζ to promote Th17 polarization.36 However, as the IL‐23 subunit IL12B is strongly down‐regulated by butyrate, and IL‐23 is a cardinal cytokine for Th17 amplification, we conclude that butyrate‐treated pro‐inflammatory moDCs will hold a non‐Th17‐polarizing phenotype, as also supported by Kaisar et al.,7 while promoting an innate based Type 17 environment.
As addressed above, it has previously been shown that butyrate and helminth PCF can promote Treg cell induction in vivo. However, it seems that they induce different Treg cell subsets, as butyrate treatment of DCs induces IL‐10‐producing Type 1 Treg cells,7 whereas helminth PCF induces inducible CD4+ CD25hi Foxp3+ Treg cells.70 Importantly, inducible Foxp3+ Treg cells could be generated via different mechanisms, one of which is dependent on the enzyme indoleamine 2, 3‐dioxygenase (IDO).72 However, our finding that helminth PCF treatment in moDCs led to the down‐regulation of expression of the IDO1 gene (see Supplementary material, Data S1) suggests that this pathway might not be involved in the reported PCF‐induced moDC‐mediated Treg cell induction. Likewise, we found that helminth PCF did not up‐regulate the expression of other core genes involved in the negative regulation of immune responses, e.g. PDL1 encoding PD‐L1. Considering, the PD‐L1–programmed cell death protein 1 axis plays a critical role in the induction of Treg cells as well as being involved in immune evasion,73 the observation that helminth PCF did not up‐regulate PDL1 gene expression suggests that other immune evasive mechanisms might be employed by A. suum.
The finding that TSLP and IL‐25, even when combined, did not exert identifiable immunosuppressive effects in pro‐inflammatory moDCs suggests that either the Type 1‐polarizing mediators LPS in combination with IFN‐γ have more dominating effects than TSLP and/or IL‐25, or that TSLP and IL‐25 might act indirectly on moDCs. Indeed, during Type 2 immune responses in mice, IL‐25 and/or TSLP have been shown to indirectly down‐regulate DC‐mediated Type 1 inflammatory conditions through their effects on ILC2s.74
In conclusion, our results indicate that helminth PCF harbors a potent potential to down‐regulate genes involved in the progression of Th1 and Th17 responses by moDCs, suggesting possible prophylactic and therapeutic applications of helminth PCF against Th1‐ and/or Th17‐dependent autoimmune diseases. By contrast, butyrate induced a more diverse regulatory phenotype in DCs that might favor intestinal homeostasis during normal conditions. Collectively, our results contribute to our understanding of the mechanisms by which helminth A. suum and the gut microbiota‐derived metabolite butyrate modulate moDC‐mediated immune responses.
Disclosures
The authors have no conflicts of interest to disclose.
Author contributions
PA, KK and SB conceived and designed the study and PA and DA performed the experiments. PA and DA analyzed the data: PA for ELISA and flow cytometry gating, DA for RNA library prep and JMM for RNA‐seq analysis. ARW provided the PCF. PA, JMM and SB wrote the paper and KK, ARW and DA revised it. All authors approved the manuscript.
Supporting information
Fig. S1. (a) mRNA expression level of indicated cytokine receptors in immature and mature [lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) ‐stimulated] monocyte‐derived dendritic cells (moDCs), n = 9. (b) Concentration of secreted interleukin‐12p70 in cell‐free culture supernatant from moDCs stimulated with or without LPS + IFN‐γ and helminth pseudocoelomic fluid (PCF), relative to control (immature moDCs), n = 3.
Table S1. Reagents and primers used for reverse transcription and cDNA amplification.
Data S1. List of differently regulated genes in dendritic cells stimulated with Helminth pseudocoelomic fluid (PCF) + lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) or Butyrate + LPS + IFN‐γ versus LPS + IFN‐γ alone.
Acknowledgements
This project was funded by the Technical University of Denmark (DTU) and The Danish Research Foundation via the Molecular Pathology PhD Program at DTU. The authors would like to thank Lisbeth Buus Rosholm and Ditte Hørling Liboriussen for their help with moDC stimulations and RT‐qPCR data generation.
References
- 1. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015; 16:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boks MA, Kager‐Groenland JR, Haasjes MSP, Zwaginga JJ, van Ham SM, ten Brinke A. IL‐10‐generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction—a comparative study of human clinical‐applicable DC. Clin Immunol 2012; 142:332–42. [DOI] [PubMed] [Google Scholar]
- 3. Coombes JL, Siddiqui KRR, Arancibia‐Cárcamo CV, Hall J, Sun C‐M, Belkaid Y et al A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF‐β and retinoic acid‐dependent mechanism. J Exp Med 2007; 204:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N et al TSLP‐activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murakami‐Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A. IL‐33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int 2014; 63:443–55. [DOI] [PubMed] [Google Scholar]
- 6. Pastille E, Wasmer MH, Adamczyk A, Vu VP, Mager LF, Phuong NNT et al The IL‐33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol 2019; 12:990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaisar MMM, Pelgrom LR, van der Ham AJ, Yazdanbakhsh M, Everts B. Butyrate conditions human dendritic cells to prime type 1 regulatory T cells via both histone deacetylase inhibition and G protein‐coupled receptor 109A signaling. Front Immunol 2017;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido‐Perrig N et al Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol 2008; 9:1261–9. [DOI] [PubMed] [Google Scholar]
- 9. Maizels RM. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin Microbiol Infect 2016; 22:481–6. [DOI] [PubMed] [Google Scholar]
- 10. Grencis RK. Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu Rev Immunol 2015; 33:201–25. [DOI] [PubMed] [Google Scholar]
- 11. Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis 2014; 14:1150–62. [DOI] [PubMed] [Google Scholar]
- 12. Radovic I, Gruden‐Movsesijan A, Ilic N, Cvetkovic J, Mojsilovic S, Devic M et al Immunomodulatory effects of Trichinella spiralis‐derived excretory–secretory antigens. Immunol Res 2015; 61:312–25. [DOI] [PubMed] [Google Scholar]
- 13. Matisz CE, Leung G, Reyes JL, Wang A, Sharkey KA, McKay DM. Adoptive transfer of helminth antigen‐pulsed dendritic cells protects against the development of experimental colitis in mice. Eur J Immunol 2015; 45:3126–39. [DOI] [PubMed] [Google Scholar]
- 14. Kuijk LM, Klaver EJ, Kooij G, van der Pol SMA, Heijnen P, Bruijns SCM et al Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 2012; 51:210–8. [DOI] [PubMed] [Google Scholar]
- 15. Rocha FA, Leite AK, Pompeu MM, Cunha TM Jr, WA, Soares FM, et al Protective effect of an extract from Ascaris suum in experimental arthritis models. Infect Immun 2008; 76:2736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McConchie BW, Norris HH, Bundoc VG, Trivedi S, Boesen A, Urban JF et al Ascaris suum‐derived products suppress mucosal allergic inflammation in an interleukin‐10‐independent manner via interference with dendritic cell function. Infect Immun 2006; 74:6632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klaver EJ, Kraan TVDP, Laan LC, Kringel H, Cummings RD, Bouma G et al Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun 2015; 16:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Everts B, Hussaarts L, Driessen NN, Meevissen MHJ, Schramm G, van der Ham AJ et al Schistosome‐derived omega‐1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med 2012; 209:1753–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klaver EJ, Kuijk LM, Lindhorst TK, Cummings RD, van Die I. Schistosoma mansoni soluble egg antigens induce expression of the negative regulators SOCS1 and SHP1 in human dendritic cells via interaction with the mannose receptor. PLoS ONE 2015; 10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaisar MMM, Ritter M, del Fresno C, Jónasdóttir HS, van der Ham AJ, Pelgrom LR et al Dectin‐1/2–induced autocrine PGE2 signaling licenses dendritic cells to prime Th2 responses. PLoS Biol 2018; 16:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim B, Kim TH. Fundamental role of dendritic cells in inducing Th2 responses. Korean J Intern Med 2018; 33:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Na H, Cho M, Chung Y. Regulation of Th2 cell immunity by dendritic cells. Immune Netw 2016; 16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jakobsen SR, Myhill LJ, Williams AR. Effects of Ascaris and Trichuris antigens on cytokine production in porcine blood mononuclear and epithelial cells. Vet Immunol Immunopathol 2019; 211:6–9. [DOI] [PubMed] [Google Scholar]
- 24. Søndergaard JN, Brix S. Isolation of IL‐12p70‐competent human monocyte‐derived dendritic cells. J Immunol Methods 2012; 386:112–6. [DOI] [PubMed] [Google Scholar]
- 25. Picelli S, Faridani OR, Björklund ÅK, Winberg G, Sagasser S, Sandberg R. Full‐length RNA‐seq from single cells using Smart‐seq2. Nat Protoc 2014; 9:171–81. [DOI] [PubMed] [Google Scholar]
- 26. Li B, Dewey CN. RSEM: Accurate transcript quantification from RNA‐seq data with or without a reference genome. BMC Bioinformatics 2011; 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J, Tibshirani R. Finding consistent patterns: a nonparametric approach for identifying differential expression in RNA‐Seq data. Stat Methods Med Res 2013; 22:519–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P et al The reactome pathway knowledge base. Nucleic Acids Res 2018; 46(D1):D649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ et al Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol 2014; 15:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zmora N, Zilberman‐Schapira G, Suez J, Mor U, Dori‐Bachash M, Bashiardes S et al Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018; 174:1388–1405.e21. [DOI] [PubMed] [Google Scholar]
- 31. Søndergaard JN, Laursen JM, Rosholm LB, Brix S. Mycobacterium tuberculosis promotes Th17 expansion via regulation of human dendritic cells toward a high CD14 and low IL‐12p70 phenotype that reprograms upon exogenous IFN‐γ . Int Immunol 2014; 26:705–16. [DOI] [PubMed] [Google Scholar]
- 32. Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T et al Cutting edge: different toll‐like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal‐regulated kinase‐mitogen‐activated protein kinase and c‐Fos. J Immunol 2003; 171:4984–9. [DOI] [PubMed] [Google Scholar]
- 33. Gianello V, Salvi V, Parola C, Moretto N, Facchinetti F, Civelli M et al The PDE4 inhibitor CHF6001 modulates pro‐inflammatory cytokines, chemokines and Th1‐ and Th17‐polarizing cytokines in human dendritic cells. Biochem Pharmacol 2019; 163:371–80. [DOI] [PubMed] [Google Scholar]
- 34. Goodman WA, Omenetti S, Date D, Di Martino L, De Salvo C, Kim GD et al KLF6 contributes to myeloid cell plasticity in the pathogenesis of intestinal inflammation. Mucosal Immunol 2016; 9:1250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rothe T, Gruber F, Uderhardt S, Ipseiz N, Rössner S, Oskolkova O et al 12/15‐lipoxygenase‐mediated enzymatic lipid oxidation regulates DC maturation and function. J Clin Invest 2015; 125:1944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cardone M, Dzutsev AK, Li H, Riteau N, Gerosa F, Shenderov K et al Interleukin‐1 and interferon‐γ orchestrate β‐glucan‐activated human dendritic cell programming via IκB‐ζ modulation. PLoS ONE 2014; 9:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008; 42:145–51. [DOI] [PubMed] [Google Scholar]
- 38. Myokai F, Takashiba S, Lebo R, Amar S. A novel lipopolysaccharide‐induced transcription factor regulating tumor necrosis factor gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci USA. 1999; 96:4518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qiu Y, Yu H, Zhu Y, Ye Z, Deng J, Su W et al Hypermethylation of interferon regulatory factor 8 (IRF8) confers risk to Vogt‐Koyanagi‐Harada Disease. Sci Rep 2017; 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tailor P, Tamura T, Kong HJ, Kubota T, Kubota M, Borghi P et al The feedback phase of type I interferon induction in dendritic cells requires interferon regulatory factor 8. Immunity 2007; 27:228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Syedbasha M, Egli A. Interferon lambda: modulating immunity in infectious diseases. Front Immunol 2017; 8:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foucher ED, Blanchard S, Preisser L, Descamps P, Ifrah N, Delneste Y et al IL‐34‐ and M‐CSF‐induced macrophages switch memory T cells into Th17 cells via membrane IL‐1α . Eur J Immunol 2015; 45:1092–102. [DOI] [PubMed] [Google Scholar]
- 43. Patil S, Fribourg M, Ge Y, Batish M, Tyagi S, Hayot F et al Single‐cell analysis shows that paracrine signaling by first responder cells shapes the interferon‐β response to viral infection. Sci Signal 2015; 8:ra16. [DOI] [PubMed] [Google Scholar]
- 44. Suresh M, Lanier G, Large MK, Whitmire JK, Altman JD, Ruddle NH et al Role of lymphotoxin in T‐cell responses during an acute viral infection. J Virol 2002; 76:3943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Del Fresno C, Saz‐Leal P, Enamorado M, Wculek SK, Martínez‐Cano S, Blanco‐Menéndez N et al DNGR‐1 in dendritic cells limits tissue damage by dampening neutrophil recruitment. Science 2018; 362:351–6. [DOI] [PubMed] [Google Scholar]
- 46. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014; 32:659–702. [DOI] [PubMed] [Google Scholar]
- 47. Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K et al The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol 2013; 14:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL et al Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun 2013; 4:2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res 2008; 68:5972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Liempt E, van Vliet SJ, Engering A, García Vallejo JJ, Bank CMC, Sanchez‐hernandez M et al Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C‐type lectins and suppress TLR‐induced dendritic cell activation. Mol Immunol 2007; 44:2605–15. [DOI] [PubMed] [Google Scholar]
- 51. Kawasaki T, Kawai T. Toll‐like receptor signaling pathways. Front Immunol 2014; 5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abdi K, Singh NJ, Matzinger P. Lipopolysaccharide‐activated dendritic cells: “Exhausted” or alert and waiting? J Immunol 2012; 188:5981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van den Bergh JMJ, Van Tendeloo VFI, Smits ELJM. Interleukin‐15: New kid on the block for antitumor combination therapy. Cytokine Growth Factor Rev 2015; 26:15–24. [DOI] [PubMed] [Google Scholar]
- 54. Hamel‐Côté G, Gendron D, Rola‐Pleszczynski M, Stankova J. Regulation of platelet‐activating factor‐mediated protein tyrosine phosphatase 1B activation by a Janus kinase 2/calpain pathway. PLoS ONE 2017; 12:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S et al Metabolite‐sensing receptors GPR43 and GPR109A facilitate dietary fibre‐induced gut homeostasis through regulation of the inflammasome. Nat Commun 2015; 6:1–15. [DOI] [PubMed] [Google Scholar]
- 56. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504:446–50. [DOI] [PubMed] [Google Scholar]
- 57. Nadjsombati MS, McGinty JW, Lyons‐Cohen MR, Jaffe JB, DiPeso L, Schneider C et al Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 2018; 49:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Midttun HLE, Acevedo N, Skallerup P, Almeida S, Skovgaard K, Andresen L et al Ascaris suum infection downregulates inflammatory pathways in the pig intestine in vivo and in human dendritic cells in vitro . J Infect Dis 2018; 217:310–9. [DOI] [PubMed] [Google Scholar]
- 59. Summan A, Nejsum P, Williams AR. Modulation of human dendritic cell activity by Giardia and helminth antigens. Parasite Immunol 2018; 40:1–5. [DOI] [PubMed] [Google Scholar]
- 60. Keating SE, Maloney GM, Moran EM, Bowie AG. IRAK‐2 participates in multiple Toll‐like receptor signaling pathways to NFκB via activation of TRAF6 ubiquitination. J Biol Chem 2007; 282:33435–43. [DOI] [PubMed] [Google Scholar]
- 61. van Riet E, Everts B, Retra K, Phylipsen M, van Hellemond JJ, Tielens AGM et al Combined TLR2 and TLR4 ligation in the context of bacterial or helminth extracts in human monocyte derived dendritic cells: molecular correlates for Th1/Th2 polarization. BMC Immunol 2009; 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin YC, Huang DY, Chu CL, Lin YL, Lin WW. The tyrosine kinase syk differentially regulates toll‐like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci Signal 2013; 6:1–13. [DOI] [PubMed] [Google Scholar]
- 63. Versteeg GA, Rajsbaum R, Teresa Sánchez‐Aparicio M, Maestre AM, Valdiviezo J, Shi M et al The E3‐ligase TRIM family of proteins regulates signaling pathways triggered by innate immune pattern‐recognition receptors. Immunity 2013; 38:384–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cooper PJ, Chico ME, Sandoval C, Nutman TB. Atopic phenotype is an important determinant of immunoglobulin E ‐ mediated inflammation and expression of T helper cell type 2 cytokines to ascaris antigens in children exposed to ascariasis. J Infect Dis 2004; 190:1338–46. [DOI] [PubMed] [Google Scholar]
- 65. Cooper PJ, Chico ME, Sandoval C, Espinel I, Guevara A, Kennedy MW et al Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis 2000; 182:1207–13. [DOI] [PubMed] [Google Scholar]
- 66. Franchini GR, Pór JL, Ibáñez M, Rey MF, Bélgamo JA, Smith BO et al The unusual lipid binding proteins of parasitic helminths and their potential roles in parasitism and as therapeutic targets. Prostaglandins Leukot Essent Fat Acids 2015; 93:31–6. [DOI] [PubMed] [Google Scholar]
- 67. Chehayeb JF, Robertson AP, Martin RJ, Geary TG. Proteomic analysis of adult Ascaris suum fluid compartments and secretory products. PLoS Negl Trop Dis 2014; 8:e2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pöltl G, Kerner D, Paschinger K, Wilson IBH. N‐Glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues. FEBS J 2007; 274:714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Antunes MFP, Titz TO, Batista IFC, Marques‐Porto R, Oliveira CF, Alves De Araujo CA et al Immunosuppressive PAS‐1 is an excretory/secretory protein released by larval and adult worms of the ascarid nematode Ascaris suum . J Helminthol 2015; 89:367–74. [DOI] [PubMed] [Google Scholar]
- 70. Titz TO, de Araújo CAA, Enobe CS, Rigato PO, Oshiro TM, de Macedo‐Soares MF Ascaris suum infection modulates inflammation: implication of CD4+ CD25high Foxp3+ T cells and IL‐10. Parasite Immunol 2017; 39:e12453. [DOI] [PubMed] [Google Scholar]
- 71. Favoretto BC, Casabuono AAC, Portes‐Junior JA, Jacysyn JF, Couto AS, Faquim‐Mauro EL. High molecular weight components containing N‐linked oligosaccharides of Ascaris suum extract inhibit the dendritic cells activation through DC‐SIGN and MR. Mol Immunol 2017; 87:33–46. [DOI] [PubMed] [Google Scholar]
- 72. Kushwah R, Hu J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci 2011; 1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Qin W, Hu L, Zhang X, Jiang S, Li J, Zhang Z et al The diverse function of PD‐1/PD‐L pathway beyond cancer. Front Immunol 2019; 10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Halim TYF, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG et al Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol 2016; 17:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (a) mRNA expression level of indicated cytokine receptors in immature and mature [lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) ‐stimulated] monocyte‐derived dendritic cells (moDCs), n = 9. (b) Concentration of secreted interleukin‐12p70 in cell‐free culture supernatant from moDCs stimulated with or without LPS + IFN‐γ and helminth pseudocoelomic fluid (PCF), relative to control (immature moDCs), n = 3.
Table S1. Reagents and primers used for reverse transcription and cDNA amplification.
Data S1. List of differently regulated genes in dendritic cells stimulated with Helminth pseudocoelomic fluid (PCF) + lipopolysaccharide (LPS) + interferon‐γ (IFN‐γ) or Butyrate + LPS + IFN‐γ versus LPS + IFN‐γ alone.


