Abstract
Lessons Learned
HyperAcute Renal immunotherapy was well tolerated and demonstrated antitumor activity in patients requiring salvage‐line treatment for metastatic renal cell carcinoma (mRCC).
HyperAcute Renal immunotherapy was safely administered with concomitant salvage‐line treatments for mRCC, and it may be a candidate for inclusion in novel combinations for salvage treatment of mRCC because of its unique mechanism of action.
Background
HyperAcute Renal (HAR) immunotherapy exploits a naturally occurring barrier to xenotransplantation and zoonotic infections in humans to immunize patients against metastatic renal cell carcinoma (mRCC) cells. HAR consists of two allogeneic renal cancer cell lines genetically modified to express α(1,3)Gal, to which humans have an inherent pre‐existing immunity.
Methods
Patients with refractory mRCC were eligible for this phase I dose‐escalation trial. Concomitant treatment was permitted after the initial 2 months of HAR monotherapy. HAR was injected intradermally weekly for 4 weeks then biweekly for 20 weeks, totaling 14 immunizations. The primary endpoint was safety and determination of a maximum tolerated dose (MTD).
Results
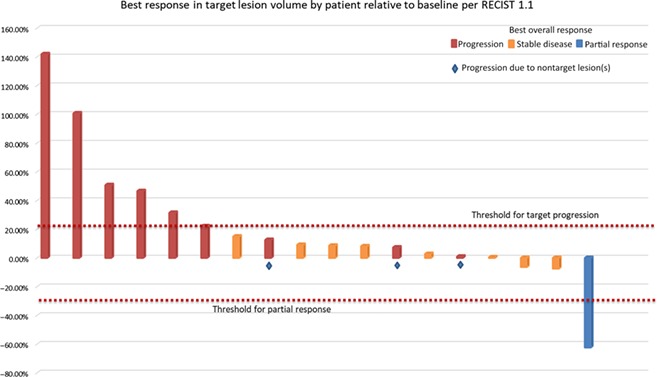
Among 18 patients enrolled, two grade 3 adverse events (AEs) were attributed to HAR, lymphopenia and injection site reaction, and no grade 4/5 AEs occurred. The recommended phase II dose (RP2D) was 300 million cells. One patient had a partial response and eight patients had stable disease, for a disease control rate of 50% (9/18). Median overall survival with low‐dose HAR was 14.2 months and was 25.3 months with high‐dose HAR.
Conclusion
In pretreated mRCC, HAR immunotherapy was well tolerated and demonstrated antitumor activity. HAR immunotherapy may be a candidate for inclusion in novel combinations for salvage treatment of mRCC.
Discussion
The treatment landscape for mRCC is changing quickly, and combinations of immune checkpoint inhibitors and/or angiogenesis‐targeted treatments are now being used as first‐line treatment for mRCC. As these drugs move to the first‐line setting, there is growing need for salvage‐line mRCC treatments with novel mechanisms of action. HAR is a novel immunotherapy that consists of two allogeneic renal cancer cell lines genetically engineered to produce α‐galactosyl epitopes (αGal). Humans do not produce αGal yet have antibodies against it, and these antibodies are the basis for hyperacute‐driven rejection of tumors. To date, HyperAcute immunotherapy has been studied in pancreatic cancer, castration‐refractory prostate cancer, non‐small cell lung cancer, and melanoma. Herein, we report the first study of HAR immunotherapy in patients with mRCC.
In this phase I study, 18 patients with recurrent or refractory mRCC and a clear cell component received weekly intradermal injections of HAR for 4 weeks followed by biweekly injections for 20 weeks. Concomitant treatment with other approved agents was permitted after the initial 2 months of HAR monotherapy. The primary endpoint was safety and determination of MTD. Secondary endpoints were objective response rate (ORR) and progression‐free survival (PFS). The RP2D was set at 300 million cells intradermally without dose‐limiting toxicity (DLT). The most common AEs attributed to HAR were injection site reactions (100%), fatigue (16.7%), and lymphopenia (16.7%). The majority of these events were grade 1 or 2. Two grade 3 AEs attributed to HAR were reported, lymphopenia and injection site pain. The ORR was 5.6%, with one patient having a partial response (PR) to treatment, and the disease control rate was 50% (9/18; Fig. 1). Median PFS was 2.1 months (range 1.6–23.6 months) in all patients. Median overall survival (OS) was 14.2 months with low‐dose HAR (range 3.6–21.6 months) and was 25.3 months with high‐dose HAR (range 5.8–29.3 months).
Figure 1.
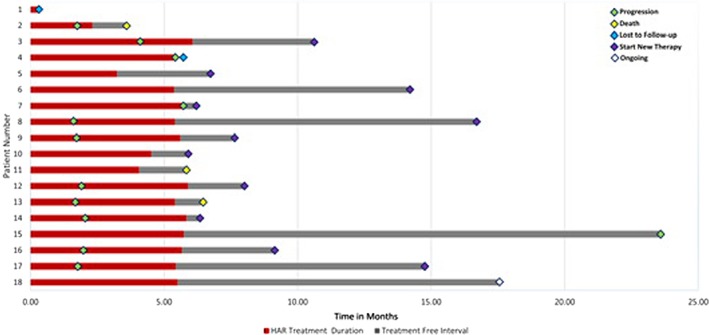
HAR immunotherapy response duration by individual patient.
Abbreviation: HAR, HyperAcute Renal.
In this study, HAR immunotherapy appears safe and well tolerated in patients with mRCC, and as anticipated, the most common AEs were local injection site reactions. Although this trial was designed to assess the safety of HAR immunotherapy, we also report underpowered efficacy findings. One patient has experienced a durable PR that is ongoing at the time of data cutoff, and another eight patients had stable disease with HAR immunotherapy. Although the median PFS for HAR immunotherapy was short at 2.1 months, this was expected based on HAR's immunologic mechanism of action. Additionally, median OS was 25.3 months in the 14 patients who received high‐dose HAR immunotherapy, which is similar to contemporary salvage‐line therapy for mRCC. These findings suggest that HAR immunotherapy may be a candidate for inclusion in further investigations of novel combinations for salvage treatment of mRCC.
Trial Information
- Disease
Renal cell carcinoma – clear cell
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
1 prior regimen
- Type of Study – 1
Phase I
- Type of Study – 2
3+3
- Primary Endpoint
Safety
- Primary Endpoint
Maximum tolerated dose
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
- Dose cohorts will initially consist of three eligible patients each and will be expanded to six patients if a DLT is seen. If MTD is not reached in dose cohort 2, 300 million cells will be declared the RP2D and the cohort will be expanded to enroll a total of 14 patients at that dose level.
- Investigator's Analysis
Drug tolerable, hints of efficacy
Drug Information
- Drug 1
- Generic/Working Name
Alpha‐1,3‐galactosyltransferase‐expressing allogeneic renal cell carcinoma immunotherapy
- Trade Name
HyperAcute Renal immunotherapy
- Company Name
NewLink Genetics Corporation
- Drug Type
Vaccine
- Drug Class
Immune therapy
- Dose
300,000,000 units (U) per flat dose
- Route
Intradermal injection
- Schedule of Administration
Weekly for 4 weeks followed by biweekly for 10 weeks
Dose Escalation Table
| Dose level | Dose of drug: Alpha‐1,3‐galactosyltransferase‐expressing allogeneic renal cell carcinoma immunotherapy | No. enrolled | No. evaluable for toxicity |
|---|---|---|---|
| 1 | 150,000,000 cells | 4 | 4 |
| 2 | 300,000,000 cells | 14 | 14 |

Patient Characteristics
- Number of Patients, Male
14
- Number of Patients, Female
4
- Age
Median (range): 63 (44–78)
- Number of Prior Systemic Therapies
Median (range): 1 (0–4)
Primary Assessment Method
- Title
New assessment
- Number of Patients Enrolled
18
- Number of Patients Evaluable for Toxicity
18
- Number of Patients Evaluated for Efficacy
18
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 1 (6%)
- Response Assessment SD
n = 8 (44%)
- Response Assessment PD
n = 9 (50%)
- Response Assessment OTHER
n = 0 (0%)
- (Median) Duration Assessments PFS
2.1 months
- (Median) Duration Assessments OS
14.2
- Outcome Notes
Median PFS was 2.1 months (range 1.6–23.6 months) in all patients. Median OS was 14.2 months with low‐dose HAR (range 3.6–21.6 months) and was 25.3 months with high‐dose HAR (range 5.8–29.3 months).
Adverse Events
| Adverse event by SOC/PT | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | All grades, n (%) |
|---|---|---|---|---|---|
| Hematologic | 2 (11) | 2 (11) | 1 (6) | 0 (0) | 4 (22) |
| Anemia | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 1 (6) |
| Leukopenia | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Lymphopenia | 1 (6) | 1 (6) | 1 (6) | 0 (0) | 3 (17) |
| General | 16 (89) | 4 (22) | 1 (6) | 0 (0) | 18 (100) |
| Fatigue | 2 (11) | 1 (6) | 0 (0) | 0 (0) | 3 (17) |
| Induration | 2 (11) | 0 (0) | 0 (0) | 0 (0) | 2 (11) |
| Injection site bruising | 6 (33) | 0 (0) | 0 (0) | 0 (0) | 6 (33) |
| Injection site erythema | 15 (83) | 2 (11) | 0 (0) | 0 (0) | 17 (94) |
| Injection site extravasation | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Injection site induration | 10 (56) | 0 (0) | 0 (0) | 0 (0) | 10 (56) |
| Injection site irritation | 2 (11) | 0 (0) | 0 (0) | 0 (0) | 2 (11) |
| Injection site edema | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Injection site pain | 10 (56) | 2 (11) | 1 (6) | 0 (0) | 13 (72) |
| Injection site pruritus | 14 (78) | 1 (6) | 0 (0) | 0 (0) | 15 (83) |
| Injection site reaction | 2 (11) | 0 (0) | 0 (0) | 0 (0) | 2 (11) |
| Injection site scab | 2 (11) | 0 (0) | 0 (0) | 0 (0) | 2 (11) |
| Injection site swelling | 11 (61) | 3 (17) | 0 (0) | 0 (0) | 14 (78) |
| Injection site warmth | 6 (33) | 1 (6) | 0 (0) | 0 (0) | 7 (39) |
| Pain | 4 (22) | 0 (0) | 0 (0) | 0 (0) | 4 (22) |
| Nervous system | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Headache | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Skin | 3 (17) | 0 (0) | 0 (0) | 0 (0) | 3 (17) |
| Blister | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Pruritus | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Rash | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Urticaria | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Vascular | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 1 (6) |
| Hypotension | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 1 (6) |
| Total | 17 (94) | 7 (39) | 2 (11) | 0 (0) | 18 (100) |
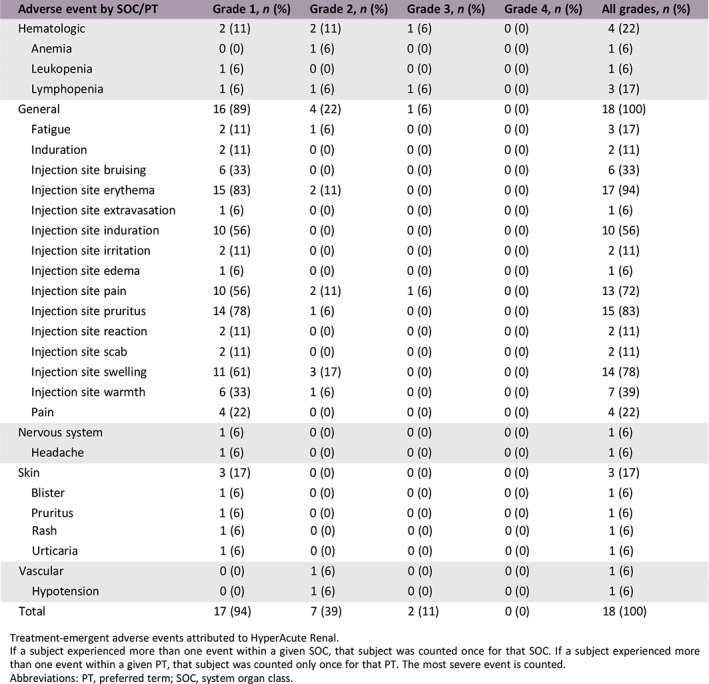
Treatment‐emergent adverse events attributed to HyperAcute Renal.
If a subject experienced more than one event within a given SOC, that subject was counted once for that SOC. If a subject experienced more than one event within a given PT, that subject was counted only once for that PT. The most severe event is counted.
Abbreviations: PT, preferred term; SOC, system organ class.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Drug tolerable, hints of efficacy
Combinations of immune checkpoint inhibitors (ICIs) and/or angiogenesis‐targeted treatments are now used as first‐line treatment for metastatic renal cell carcinoma (mRCC) 1, 2, 3. There is a growing need for salvage‐line mRCC treatments with novel mechanisms of action. mRCC is considered an immunogenic tumor based on its history of spontaneous regressions of metastases to localized therapy, responses to high‐dose interleukin‐2, and the recent success of ICIs 4, 5. Thus, novel immunotherapies are of special interest for patients with mRCC.
HyperAcute Renal (HAR) exploits a natural barrier to xenotransplantation in humans in an attempt to immunize patients against their own mRCC cells. HAR immunotherapy consists of two allogeneic renal cancer cell lines that have been genetically engineered by retrovirus transduction to express the murine enzyme α(1,3)‐galactosyltransferase (αGT), which produces α‐galactosyl epitopes (αGal). αGal is expressed on most mammalian cells, except for humans and Old World monkeys, and αGal epitopes are the basis for hyperacute‐driven rejection of tumors 6. In humans, intestinal bacterial flora stimulate the production of antibodies against αGal epitopes 7. When these naturally acquired antibodies bind to αGal, it results in activation of complement via the classical pathway and induces antibody‐dependent cell‐mediated cytotoxicity (ADCC) via natural killer cells 8, 9, 10, 11. αGal HyperAcute technology has been demonstrated to induce anticancer activity and has been previously studied in pancreatic cancer, castration‐refractory prostate cancer, non‐small cell lung cancer, and melanoma 12, 13, 14. Herein, we report the first study of HAR immunotherapy in patients with mRCC.
In this multi‐institution phase I study, 18 patients with recurrent or refractory mRCC and a clear cell component received weekly intradermal injections of HAR for 4 weeks followed by biweekly injections for 10 weeks. Following institutional review board approval at each institution, all patients provided informed consent. The first four patients received the starting dose of 150 million cells, and then 14 patients received the escalated dose of 300 million cells. Concomitant treatment with other approved agents was permitted after the initial 2 months of HAR monotherapy. The primary endpoint was safety and determination of a maximum tolerated dose. Secondary endpoints are objective response rate (ORR) and progression‐free survival (PFS).
The recommended phase II dose (RP2D) was set at 300 million cells intradermally with no dose‐limiting toxicity (DLT). The most common adverse events (AEs) attributed to HAR were injection site reactions (100%), fatigue (16.7%), and lymphopenia (16.7%; supplemental online Table 1). The majority of these events were grade 1 or 2. Two grade 3 AEs attributed to HAR were reported, lymphopenia and injection site pain. No grade 4 or 5 adverse events were study related. At data cutoff in September of 2018, seven patients (39%) remained alive. The ORR was 5.6%, with one patient having a partial response (PR) to treatment, and the disease control rate was 50% (9/18; Fig. 1). Median PFS was 2.1 months (range 1.6–23.6 months) in all patients. Median overall survival (OS) was 14.2 months with low‐dose HAR (range 3.6–21.6 months) and was 25.3 months with high‐dose HAR (range 5.8–29.3 months). Thirteen patients (72%) received concomitant treatment after 2 months of HAR immunotherapy. The accompanying treatments included nivolumab (12 patients, 67%), cabozantinib (1 patient, 5.6%), and sunitinib (1 patient, 5.6%; Table 1).
In this study, HAR immunotherapy appears safe and well tolerated in patients with mRCC, as the RP2D was reached without any DLTs. As anticipated, the most common AEs were local injection site reactions. In this salvage therapy cohort of patients with mRCC, HAR immunotherapy demonstrated antitumor activity. One patient has experienced a durable PR that is ongoing at the time of data cutoff, and another eight patients had stable disease with HAR immunotherapy. These findings suggest that HAR immunotherapy can be safely combined with other salvage‐line treatments and is worthy of further study in mRCC.
Contemporary salvage‐line treatment for mRCC includes angiogenesis‐targeted treatments (such as cabozantinib, lenvatinib plus everolimus, or axitinib) and the ICI nivolumab 15, 16, 17. However, all of these salvage treatments are now used in the first‐line setting, as combination therapy becomes the new standard of care. The development of first‐line combination therapy has resulted in a pressing need for novel therapeutic classes in salvage‐line treatment of mRCC. HAR immunotherapy is a novel immunotherapy for mRCC that exploits a natural barrier to xenotransplantation and appears safe to combine with pre‐existing salvage treatments. Although this clinical trial was designed to assess the safety of HAR immunotherapy, we also report underpowered efficacy findings. The median PFS for HAR immunotherapy was short at 2.1 months; however, no effect on PFS was expected based on HAR's immunologic mechanism of action. In the 14 patients who received high‐dose HAR immunotherapy, median OS was 25.3 months, which is similar to nivolumab in CheckMate 025 (25.0 months) 16. Because patients received concomitant systemic therapies, it is difficult to elucidate the efficacy of HAR immunotherapy independently of these concomitant therapies. However, this study did establish acceptable safety and feasibility of combining HAR immunotherapy with other approved systemic therapies in mRCC. To accurately assess efficacy, a much larger cohort of patients with mRCC will be needed.
Although the trial met its primary endpoint by demonstrating safety in patients with mRCC, we would consider changing the administration of concomitant therapies if we were to repeat the trial. Per the trial protocol, investigators could administer a concomitant treatment after an initial 2 months of HAR monotherapy. The delay in giving concomitant treatment was necessary to establish the safety of HAR immunotherapy in these patients; however, it could have compromised the synergistic efficacy of HAR immunotherapy and concomitant systemic therapy, such as nivolumab. We hypothesize that HAR immunotherapy and nivolumab could have a synergistic stimulatory effect on the immune system's response to tumor cells. When nivolumab binds to programmed cell death protein 1 in vitro, it potently enhances T‐cell activation and cytokine production, but it does not result in ADCC or complement‐dependent cytotoxicity 18, 19. In contrast, HAR immunotherapy activates complement and mediates ADCC. Theoretically, closer administration of nivolumab and HAR immunotherapy could result in improved efficacy through simultaneous engagement of multiple arms of the immune system.
In pretreated mRCC, HAR immunotherapy was well tolerated and demonstrated preliminary evidence of clinical activity. Because of its unique mechanism, HAR immunotherapy may be a candidate for inclusion in novel combinations for salvage treatment of mRCC.
Disclosures
Charles Drake: Compugen, ImmunExcite, NexImmune, Potenza Therapeutics, Tizona Therapeutics, Inc. (OI), Agenus, Astellas Medivation, AstraZeneca/MedImmune, Bristol‐Myers Squibb (Inst), Compugen, Dendreon, ImmuneXcite, Janssen Oncology, Eli Lilly, Merck, NexImmune, Pfizer, Pierre Fabre, Potenza Therapeutics, Roche/Genentech, Tizona Therapeutics, Inc. (C/A), Aduro Biotech (Inst), Bristol‐Myers Squibb (Inst), Compugen (Inst), Janssen Oncology (Inst) (RF), Patents licensed to BMS (Inst), Patents licensed to Potenza Therapeutics (Inst) (IP); Samuel R. Denmeade: Sophiris Bio (C/A, IP); Yousef Zakharia: Amgen, Roche Diagnostics, Novartis, Jansen, Eisai, Exelixis, Castle Bioscience, Array, Bayer, Pfizer (C/A), NewLink Genetics, Pfizer, Exelixis, Eisai (RF); Benjamin L. Maughan: Bayer, Janssen Oncology, Tempus, Astellas, Exelixis, Bristol‐Meyers Squibb, Peloton Therapeutics (C/A), Bristol‐Meyers Squibb, Clovis (RF, institutional); Eugene Kennedy: CSL Limited, Newlink Genetics (E), Newlink Genetics (OI); Charles J. Link: Newlink Genetics Corporation (E, IP); Nicholas Vahanian: Newlink Genetics (E, OI); Hans Hammers: Bristol‐Meyers Squibb, Merck, Pfizer (C/A), Bristol‐Meyers Squibb, Merck, Novartis, Exelixis, Pfizer, Armo Biosciences (RF); Neeraj Agarwal: Astellas, Astra Zeneca, Argos, BMS, Bayer, Clovis, Eisai, Exelixis, EMD Serono, Ely Lilly, Foundation One, Genentech, Janssen, Merck, Medivation, Novartis, Nektar, Pfizer, Pharmacyclics (C/A), AstraZeneca, Bavarian Nordic, Bristol‐Myers Squibb, Calithera, Celldex, Eisai, Exelixis, Genetech, GlaxoSmithKline), Immunomedics, Janssen, Medivation, Merck, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Sanofi, Takeda, Tracon, Bayer, Clovis, EMD Serono, Ely Lilly, Janssen, Nektar (RF, institutional). Andrew W. Hahn indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Table
Table 1.
Prior lines of treatment, concomitant therapy administered with HAR immunotherapy, and best response by patient
| Patient no. | Best response | Prior lines of treatment | Dose of HAR, cells in millions | Concomitant treatment |
|---|---|---|---|---|
| 1 | SD | 4 | 150 | None |
| 2 | PD | 2 | 150 | None |
| 3 | SD | 1 | 150 | None |
| 4 | SD | 1 | 150 | Nivolumab |
| 5 | SD | 1 | 300 | Nivolumab |
| 6 | PD | 2 | 300 | Nivolumab |
| 7 | SD | 0a | 300 | Nivolumab |
| 8 | PD | 2 | 300 | Nivolumab |
| 9 | PD | 1 | 300 | Nivolumab |
| 10 | SD | 1 | 300 | None |
| 11 | SD | 1 | 300 | Nivolumab |
| 12 | PD | 4 | 300 | Nivolumab |
| 13 | PD | 4 | 300 | Nivolumab; cabozantinib |
| 14 | PD | 1 | 300 | Sunitinib |
| 15 | SD | 1 | 300 | None |
| 16 | PD | 3 | 300 | Nivolumab |
| 17 | PD | 1 | 300 | Nivolumab |
| 18 | PR | 0a | 300 | Nivolumab |
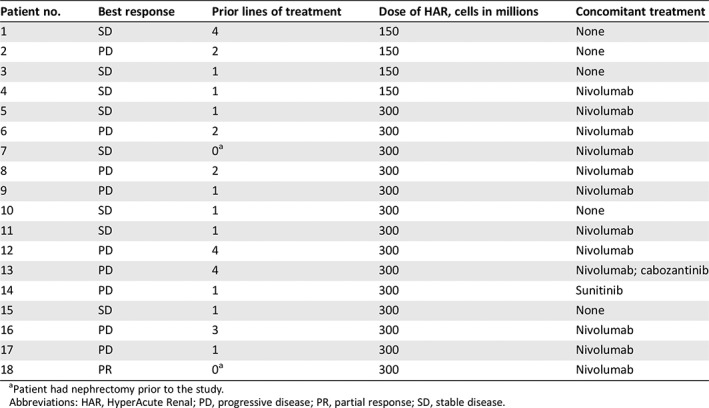
Patient had nephrectomy prior to the study.
Abbreviations: HAR, HyperAcute Renal; PD, progressive disease; PR, partial response; SD, stable disease.
Footnotes
- Sponsor: NewLink Genetics
- Principal Investigators: Hans Hammers, Neeraj Agarwal
- IRB Approved: Yes
Contributor Information
Hans Hammers, Email: hans.hammers@utsouthwestern.edu.
Neeraj Agarwal, Email: neeraj.agarwal@hci.utah.edu.
References
- 1. Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motzer RJ, Penkov K, Haanen J et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg SA, Lotze MT, Muul LM et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine‐activated killer cells and interleukin‐2 or high‐dose interleukin‐2 alone. N Engl J Med 1987;316:889–897. [DOI] [PubMed] [Google Scholar]
- 5. Van de Walle M, Demol J, Staelens L et al. Abscopal effect in metastatic renal cell carcinoma. Acta Clin Belg 2017;72:245–249. [DOI] [PubMed] [Google Scholar]
- 6. Joziasse DH, Oriol R. Xenotransplantation: The importance of the Galalpha1,3Gal epitope in hyperacute vascular rejection. Biochim Biophys Acta 1999;1455:403–418. [DOI] [PubMed] [Google Scholar]
- 7. Galili U, Mandrell RE, Hamadeh RM et al. Interaction between human natural anti‐alpha‐galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun 1988;56:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baumann BC, Forte P, Hawley RJ et al. Lack of galactose‐alpha‐1,3‐galactose expression on porcine endothelial cells prevents complement‐induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol 2004;172:6460–6467. [DOI] [PubMed] [Google Scholar]
- 9. Schaapherder AF, Daha MR, te Bulte MT et al. Antibody‐dependent cell‐mediated cytotoxicity against porcine endothelium induced by a majority of human sera. Transplantation 1994;57:1376–1382. [DOI] [PubMed] [Google Scholar]
- 10. Rossi GR, Mautino MR, Unfer RC et al. Effective treatment of preexisting melanoma with whole cell vaccines expressing α(1,3)‐galactosyl epitopes. Cancer Res 2005;65:10555–10561. [DOI] [PubMed] [Google Scholar]
- 11. Rossi GR, Mautino MR, Awwad DZ et al. Allogeneic melanoma vaccine expressing αGal epitopes induces antitumor immunity to autologous antigens in mice without signs of toxicity. J Immunother 2008;31:545–554. [DOI] [PubMed] [Google Scholar]
- 12. Hardacre JM, Mulcahy M, Small W et al. Addition of algenpantucel‐L immunotherapy to standard adjuvant therapy for pancreatic cancer: A phase 2 study. J Gastrointest Surg 2013;17:94–100; discussion p. 100–101. [DOI] [PubMed] [Google Scholar]
- 13. Hemstreet GP 3rd, Rossi GR, Pisarev VM et al. Cellular immunotherapy study of prostate cancer patients and resulting IgG responses to peptide epitopes predicted from prostate tumor‐associated autoantigens. J Immunother 2013;36:57–65. [DOI] [PubMed] [Google Scholar]
- 14. Riker AI, Rossi GR, Masih P et al. Combination immunotherapy for high‐risk resected and metastatic melanoma patients. Ochsner J 2014;14:164–174. [PMC free article] [PubMed] [Google Scholar]
- 15. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open‐label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 16. Motzer RJ, Hutson TE, Glen H et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open‐label, multicentre trial. Lancet Oncol 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 17. Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 18. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang C, Thudium KB, Han M et al. In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res 2014;2:846–856. [DOI] [PubMed] [Google Scholar]


