Abstract
Background
Limited data exist describing real‐world treatment of de novo and recurrent HER2‐positive metastatic breast cancer (MBC).
Materials and Methods
The Systemic Therapies for HER2‐Positive Metastatic Breast Cancer Study (SystHERs) was a fully enrolled (2012–2016), observational, prospective registry of patients with HER2‐positive MBC. Patients aged ≥18 years and ≤6 months from HER2‐positive MBC diagnosis were treated and assessed per their physician's standard practice. The primary endpoint was to characterize treatment patterns by de novo versus recurrent MBC status, compared descriptively. Secondary endpoints included patient characteristics, progression‐free and overall survival (PFS and OS, by Kaplan‐Meier method; hazard ratio [HR] and 95% confidence interval [CI] by Cox regression), and patient‐reported outcomes.
Results
Among 977 eligible patients, 49.8% (n = 487) had de novo and 50.2% (n = 490) had recurrent disease. A higher proportion of de novo patients had hormone receptor–negative disease (34.9% vs. 24.9%), bone metastasis (57.1% vs. 45.9%), and/or liver metastasis (41.9% vs. 33.1%), and a lower proportion had central nervous system metastasis (4.3% vs. 13.5%). De novo patients received first‐line regimens containing chemotherapy (89.7%), trastuzumab (95.7%), and pertuzumab (77.8%) more commonly than recurrent patients (80.0%, 85.9%, and 68.6%, respectively). De novo patients had longer median PFS (17.7 vs. 11.9 months; HR, 0.69; 95% CI, 0.59–0.80; p < .0001) and OS (not estimable vs. 44.5 months; HR, 0.55; 95% CI, 0.44–0.69; p < .0001).
Conclusion
Patients with de novo versus recurrent HER2‐positive MBC exhibit different disease characteristics and survival durations, suggesting these groups have distinct outcomes. These differences may affect future clinical trial design. Clinical trial identification number. NCT01615068 (http://clinicaltrials.gov).
Implications for Practice
SystHERs was an observational registry of patients with HER2‐positive metastatic breast cancer (MBC), which is a large, modern, real‐world data set for this population and, thereby, provides a unique opportunity to study patients with de novo and recurrent HER2‐positive MBC. In SystHERs, patients with de novo disease had different baseline demographics and disease characteristics, had superior clinical outcomes, and more commonly received first‐line chemotherapy and/or trastuzumab versus those with recurrent disease. Data from this and other studies suggest that de novo and recurrent MBC have distinct outcomes, which may have implications for disease management strategies and future clinical study design.
Keywords: HER2‐positive metastatic breast cancer, De novo, Recurrent, Registry, SystHERs
Short abstract
The SystHERs breast cancer study was a fully enrolled, prospective registry study that explored contemporary treatment patterns and outcomes in patients with HER2‐positive metastatic breast cancer (MBC), resulting in one of the largest real‐world datasets for this population and providing a unique opportunity to assess patients with de novo and recurrent HER2‐positive MBC. This article reports baseline characteristics, treatment patterns, patient‐reported outcomes, and clinical outcomes in these patient subsets.
Introduction
More than 150,000 U.S. women are estimated to have metastatic breast cancer (MBC) 1, with ∼20% positive for human epidermal growth factor receptor 2 (HER2) 2. Although HER2‐positive MBC was historically associated with adverse outcomes, the approval of trastuzumab for the treatment of HER2‐positive MBC in 1998 shifted its prognosis from poor to favorable relative to HER2‐negative MBC 3. Differences in disease characteristics and clinical outcomes have also been reported for patients with metastatic disease observed at first breast cancer diagnosis (i.e., de novo MBC) versus patients diagnosed with metastatic disease after an early breast cancer (EBC) diagnosis (i.e., recurrent MBC) 4, 5, 6. In registHER, an observational registry study of patients with HER2‐positive MBC, patients with de novo MBC had a lower incidence of lung metastases; higher incidences of lymph node, bone, or liver metastases; and longer progression‐free survival (PFS) and overall survival (OS) 7.
Understanding differences between de novo and recurrent MBC could potentially affect clinical care and clinical trial design. However, changes in clinical practice after the 2003–2006 registHER enrollment period may have shifted the epidemiology, characteristics, and treatment patterns of patients with MBC. The U.S. approval of trastuzumab for the adjuvant treatment of EBC in late 2006, for example, is likely to have decreased the number of patients who might otherwise have developed MBC. Additional significant advances in HER2‐targeted therapy have followed, including approval of lapatinib for MBC in 2007, pertuzumab for MBC in 2012 and neoadjuvant use in 2013, and the trastuzumab‐chemotherapy conjugate trastuzumab emtansine (T‐DM1) for MBC in 2013. The addition of first‐line pertuzumab to trastuzumab and a taxane, in particular, was shown to prolong PFS and OS in patients with HER2‐positive MBC 8, 9, 10 and currently is a standard of care for this population. As clinical trials may not adequately characterize patients with de novo versus recurrent MBC because of restrictive eligibility criteria, real‐world data are needed to fill this gap in the modern era of HER2‐targeted therapies.
The Systemic Therapies for HER2‐Positive Metastatic Breast Cancer Study (SystHERs) was a fully enrolled, prospective registry study that explored contemporary treatment patterns and outcomes in patients with HER2‐positive MBC. SystHERs is one of the largest modern, real‐world data sets for this population, representing a unique opportunity to assess patients with de novo and recurrent HER2‐positive MBC. We report baseline characteristics, treatment patterns, patient‐reported outcomes (PROs), and clinical outcomes in these patient subsets.
Materials and Methods
Study Design and Participants
SystHERs (NCT01615068) was a prospective, U.S.‐based, multicenter, observational cohort study that enrolled patients from 138 sites from June 2012 to June 2016. Patients were treated and assessed per their physician's standard practice. The primary study endpoint was to characterize treatment patterns. Secondary endpoints included clinical outcomes, patient characteristics, and PROs. Additional details for study design and sample size rationale have been published 11.
Eligible patients were aged ≥18 years with HER2‐positive MBC diagnosed within 6 months of enrollment. HER2‐positivity was determined by the treating physician and could be based on any HER2‐positive result from the primary breast tumor (EBC or MBC) or metastatic lesions. SystHERs was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation E6 Guidelines for Good Clinical Practice, U.S. Food and Drug Administration regulations, and applicable local laws. The study protocol was approved at each participating study site by the site's ethics committee or institutional review board (IRB); a central IRB was used for sites that did not have an IRB. Patients provided written informed consent for the use of medical records.
Assessments and Statistical Methods
Investigators reported MBC diagnosis type for each patient at enrollment. De novo MBC was defined as initial breast cancer diagnosis with distant metastases observed concurrently or confirmed within 90 days. Hormone receptor–positive disease was defined as institutionally specified estrogen receptor (ER)–positive and/or progesterone receptor (PR)–positive tumors in EBC or MBC.
Baseline and disease characteristics and PROs were collected at enrollment. In patients with recurrent disease, EBC disease history and treatments were collected retrospectively at enrollment, when available. MBC treatments, disease progression, and clinical outcomes were investigator‐determined and captured quarterly from clinical notes, patient charts, diagnostic tests, and laboratory findings. PROs were reported quarterly. First‐line treatment was defined as any therapy received for MBC up to first disease progression. Patients could consent to quarterly survival follow‐up in the event of study discontinuation.
PROs and treatment patterns were compared descriptively between de novo and recurrent MBC cohorts. For the comparison of baseline patient and disease characteristics, Fisher's exact test was used for categorical variables, and the Wilcoxon rank‐sum test was used for continuous variables. PFS (MBC diagnosis to first investigator‐assessed disease progression or death, whichever occurred first) and OS (MBC diagnosis to death) were estimated using the Kaplan‐Meier product‐limit method and compared across cohorts with a log‐rank test. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated using Cox regressions. The data cutoff date was October 3, 2017.
Results
Patients
In total, 1,028 patients were initially identified to meet study inclusion criteria during the enrollment period (June 2012 to June 2016), and 1,005 were enrolled, for a refusal rate of 2.2% (23/1,028). Twenty‐eight patients did not meet eligibility criteria upon review, in most cases because the patients did not have metastatic disease. Of the remaining 977 patients, 49.8% (n = 487) had de novo and 50.2% (n = 490) had recurrent MBC. At data cutoff, 60.2% (293/487) and 47.8% (234/490) of patients remained on study in the de novo and recurrent cohorts, respectively. Median follow‐up duration was 27.8 months (range, 0.4–64.7 months) from MBC diagnosis.
At baseline, patients with de novo MBC were younger and had higher body mass index (BMI), lower incidence of hormone receptor–positive disease, lower incidence of central nervous system (CNS) metastasis, and higher incidences of bone and liver metastasis compared with patients with recurrent MBC (Table 1). Baseline cardiovascular disease history and risk factors were generally similar between the two cohorts (supplemental online Table 1).
Table 1.
Baseline demographic, patient, and disease characteristics
| Characteristic | All eligible(n = 977) | De novo(n = 487) | Recurrent(n = 490) | p value |
|---|---|---|---|---|
| Median age at MBC diagnosis, years (range) | 56 (21–90) | 55 (21–89) | 58 (28–90) | <.001 |
| Age group at MBC diagnosis, n (%) | .02 | |||
| <50 years | 287 (29.4) | 158 (32.4) | 129 (26.3) | |
| 50–69 years | 562 (57.5) | 277 (56.9) | 285 (58.2) | |
| ≥70 years | 128 (13.1) | 52 (10.7) | 76 (15.5) | |
| Race, n (%) | .42 | |||
| White | 766 (78.4) | 374 (76.8) | 392 (80.0) | |
| Black/African American | 151 (15.5) | 83 (17.0) | 68 (13.9) | |
| Asian | 13 (1.3) | 5 (1.0) | 8 (1.6) | |
| Other | 29 (3.0) | 13 (2.7) | 16 (3.3) | |
| Not reported/unknown | 18 (1.8) | 12 (2.5) | 6 (1.2) | |
| Ethnicity, n (%) | .59 | |||
| Not Hispanic/Latino | 845 (86.5) | 422 (86.7) | 423 (86.3) | |
| Hispanic/Latino | 94 (9.6) | 49 (10.1) | 45 (9.2) | |
| Not reported/unknown | 38 (3.9) | 16 (3.3) | 22 (4.5) | |
| BMI, n (%) | .03 | |||
| <30 | 581 (59.5) | 273 (56.1) | 308 (62.9) | |
| ≥30 | 385 (39.4) | 209 (42.9) | 176 (35.9) | |
| Missing | 11 (1.1) | 5 (1.0) | 6 (1.2) | |
| ECOG PS, n (%) | .15 | |||
| 0 | 460 (47.1) | 233 (47.8) | 227 (46.3) | |
| 1 | 365 (37.4) | 185 (38.0) | 180 (36.7) | |
| 2 | 67 (6.9) | 30 (6.2) | 37 (7.6) | |
| 3 | 8 (0.8) | 1 (0.2) | 7 (1.4) | |
| Unknown/missing | 77 (7.9) | 38 (7.8) | 39 (8.0) | |
| Hormone receptor status,a n (%) | <.001 | |||
| ER‐ and/or PR‐positive | 685 (70.1) | 317 (65.1) | 368 (75.1) | |
| ER‐ and PR‐negative | 292 (29.9) | 170 (34.9) | 122 (24.9) | |
| Visceral disease,b n (%) | 649 (66.4) | 315 (64.7) | 334 (68.2) | .25 |
| Number of metastatic sites at MBC diagnosis, n (%) | .06 | |||
| 1 | 417 (42.7) | 207 (42.5) | 210 (42.9) | |
| 2 | 258 (26.4) | 143 (29.4) | 115 (23.5) | |
| ≥3 | 302 (30.9) | 137 (28.1) | 165 (33.7) | |
| EBC diagnosis to MBC diagnosis, n (%) | NA | |||
| <24 months | NA | NA | 114 (23.3) | |
| ≥24 months | NA | NA | 373 (76.1) | |
| Missing | NA | NA | 3 (0.6) | |
| Selected metastatic sites, n (%) | ||||
| Bone | 503 (51.5) | 278 (57.1) | 225 (45.9) | <.001 |
| Liver | 366 (37.5) | 204 (41.9) | 162 (33.1) | .005 |
| Lung | 310 (31.7) | 144 (29.6) | 166 (33.9) | .15 |
| CNS | 87 (8.9) | 21 (4.3) | 66 (13.5) | <.001 |
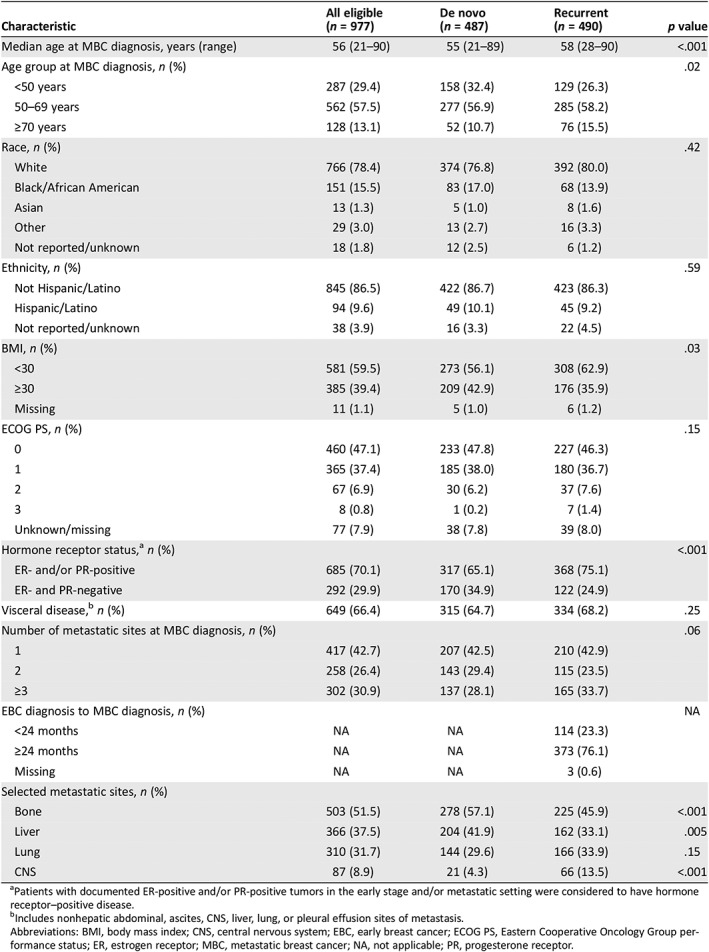
Patients with documented ER‐positive and/or PR‐positive tumors in the early stage and/or metastatic setting were considered to have hormone receptor–positive disease.
Includes nonhepatic abdominal, ascites, CNS, liver, lung, or pleural effusion sites of metastasis.
Abbreviations: BMI, body mass index; CNS, central nervous system; EBC, early breast cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; MBC, metastatic breast cancer; NA, not applicable; PR, progesterone receptor.
Breast Cancer History in Patients with Recurrent Disease
In patients with recurrent disease, median time from EBC to MBC diagnoses was 42.8 months (range, 4–452 months). Of 430 patients with retrospective (neo)adjuvant systemic treatment data, most received chemotherapy (90.7% [390/430]) and/or HER2‐targeted therapy (65.8% [283/430]), most commonly a taxane (82.6% [355/430]) and trastuzumab (65.8% [283/430]), respectively (supplemental online Table 2).
In patients with recurrent disease and known ER and PR status in both primary (EBC) and metastatic tissue, receptor status discordance was observed in 18.6% (68/365) and 30.3% (103/340) of cases, respectively (Table 2). Tumor ER status converted from ER‐positive primary to ER‐negative metastatic in 14.2% of patients (52/365), versus 4.4% (16/365) who converted from ER‐negative to ER‐positive. Similarly, tumor PR status more commonly converted from PR‐positive primary to PR‐negative metastatic (24.1% [82/340]) than from PR‐negative to PR‐positive (6.2% [21/340]). Finally, of recurrent patients with known HER2 status in primary and metastatic tissue, 25.8% (82/318) had HER2‐negative or HER2‐equivocal primary tumors and HER2‐positive metastatic tumors. Conversion from HER2‐negative primary to HER2‐positive metastatic tissue was higher in patients with hormone receptor–positive (26.4% [46/174]) versus hormone receptor–negative disease (10.3% [9/87]).
Table 2.
ER, PR, and HER2 receptor status in primary (early breast cancer) versus metastatic tumors in patients with recurrent HER2‐positive metastatic breast cancer (n = 490)
| Primary tumor status | Metastatic tumor status | |||
|---|---|---|---|---|
| Positive | Negative | Equivocal | Unknown | |
| ER status, n (%) | ||||
| Positive | 205 (41.8) | 52 (10.6) | 66 (13.5) | |
| Negative | 16 (3.3) | 92 (18.8) | 32 (6.5) | |
| Unknown | 20 (4.1) | 4 (0.8) | 3 (0.6) | |
| PR status, n (%) | ||||
| Positive | 90 (18.4) | 82 (16.7) | 51 (10.4) | |
| Negative | 21 (4.3) | 147 (30.0) | 60 (12.2) | |
| Unknown | 18 (3.7) | 15 (3.1) | 6 (1.2) | |
| HER2 status, n (%) | ||||
| Positive | 214 (43.7) | 6 (1.2) | 9 (1.8) | 93 (19.0) |
| Negative | 49 (10.0) | 0 (0) | 1 (0.2) | 1 (0.2) |
| Equivocal | 33 (6.7) | 0 (0) | 6 (1.2) | 3 (0.6) |
| Unknown | 71 (14.5) | 0 (0) | 4 (0.8) | 0 (0) |
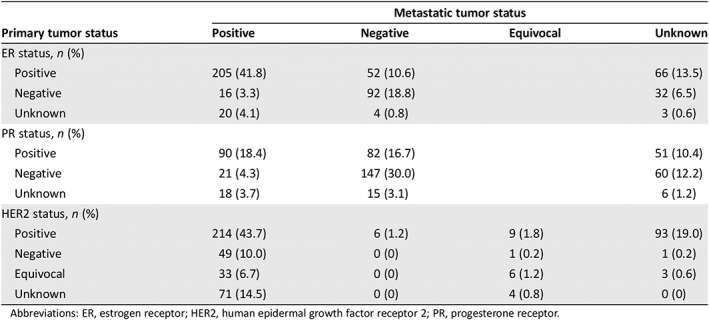
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Treatment Patterns
Among all patients, 97.0% (948/977) had received first‐line systemic treatment for MBC by the data cutoff (Table 3). Patients with de novo MBC received first‐line chemotherapy more commonly than those with recurrent MBC (89.7% [437/487] vs. 80.0% [392/490], respectively), but hormonal therapy was administered in similar proportions to both groups (43.3% [211/487] vs. 41.4% [203/490], respectively).
Table 3.
| Treatment | All eligible (n = 977), n (%) | De novo (n = 487), n (%) | Recurrent (n = 490), n (%) |
|---|---|---|---|
| Treatment by patients with any first‐line exposure | |||
| Patients treated with first‐line therapy for MBC | 948 (97.0) | 477 (97.9) | 471 (96.1) |
| Patients treated with any first‐line HER2‐targeted therapy for MBC | 923 (94.5) | 471 (96.7) | 452 (92.2) |
| Patients treated with any first‐line chemotherapy for MBC | 829 (84.9) | 437 (89.7) | 392 (80.0) |
| Patients treated without first‐line chemotherapy for MBC | 119 (12.2) | 40 (8.2) | 79 (16.1) |
| Pertuzumab + trastuzumab regimens | |||
| Any pertuzumab + trastuzumab | 712 (72.9) | 377 (77.4) | 335 (68.4) |
| Pertuzumab + trastuzumab only | 14 (1.4) | 6 (1.2) | 8 (1.6) |
| Pertuzumab + trastuzumab without chemotherapy | 37 (3.8) | 15 (3.1) | 22 (4.5) |
| Pertuzumab + trastuzumab + any chemotherapy | 675 (69.1) | 362 (74.3) | 313 (63.9) |
| Pertuzumab + trastuzumab + any taxanec | 650 (66.5) | 357 (73.3) | 293 (59.8) |
| Pertuzumab + trastuzumab + any platinum compound | 67 (6.9) | 53 (10.9) | 14 (2.9) |
| Pertuzumab + trastuzumab + any hormonal therapy | 285 (29.2) | 158 (32.4) | 127 (25.9) |
| Pertuzumab + trastuzumab + any aromatase inhibitor | 224 (22.9) | 123 (25.3) | 101 (20.6) |
| Pertuzumab + trastuzumab + tamoxifen | 60 (6.1) | 44 (9.0) | 16 (3.3) |
| Pertuzumab + trastuzumab + hormonal therapy without chemotherapy | 23 (2.4) | 9 (1.8) | 14 (2.9) |
| Pertuzumab + trastuzumab + chemotherapy + hormonal therapy | 262 (26.8) | 149 (30.6) | 113 (23.1) |
| Concurrent | 60 (6.1) | 25 (5.1) | 35 (7.1) |
| Sequential | 202 (20.7) | 124 (25.5) | 78 (15.9) |
| Trastuzumab‐based regimens not containing pertuzumab | |||
| Any trastuzumab without pertuzumab | 175 (17.9) | 89 (18.3) | 86 (17.6) |
| Trastuzumab without pertuzumab + any chemotherapy | 118 (12.1) | 68 (14.0) | 50 (10.2) |
| Trastuzumab without pertuzumab + any taxanec | 88 (9.0) | 55 (11.3) | 33 (6.7) |
| Trastuzumab + docetaxel | 35 (3.6) | 27 (5.5) | 8 (1.6) |
| Trastuzumab + paclitaxel | 51 (5.2) | 30 (6.2) | 21 (4.3) |
| Trastuzumab without pertuzumab + any platinum compound | 41 (4.2) | 30 (6.2) | 11 (2.2) |
| Trastuzumab without pertuzumab + any hormonal therapy | 95 (9.7) | 46 (9.4) | 49 (10.0) |
| Trastuzumab + any aromatase inhibitor | 77 (7.9) | 36 (7.4) | 41 (8.4) |
| Trastuzumab + tamoxifen | 14 (1.4) | 9 (1.8) | 5 (1.0) |
| Trastuzumab without pertuzumab + chemotherapy + hormonal therapy | 45 (4.6) | 26 (5.3) | 19 (3.9) |
| Concurrent | 10 (1.0) | 7 (1.4) | 3 (0.6) |
| Sequential | 35 (3.6) | 19 (3.9) | 16 (3.3) |
| Other HER2‐targeted therapies | |||
| Any T‐DM1 | 71 (7.3) | 20 (4.1) | 51 (10.4) |
| Any lapatinib | 42 (4.3) | 12 (2.5) | 30 (6.1) |
| Lapatinib + hormonal therapy | 15 (1.5) | 6 (1.2) | 9 (1.8) |
| Regimens without HER2‐targeted therapy | |||
| Hormonal therapy only | 17 (1.7) | 2 (0.4) | 15 (3.1) |
| Chemotherapy only | 5 (0.5) | 3 (0.6) | 2 (0.4) |
| Chemotherapy + hormonal therapy only | 3 (0.3) | 1 (0.2) | 2 (0.4) |
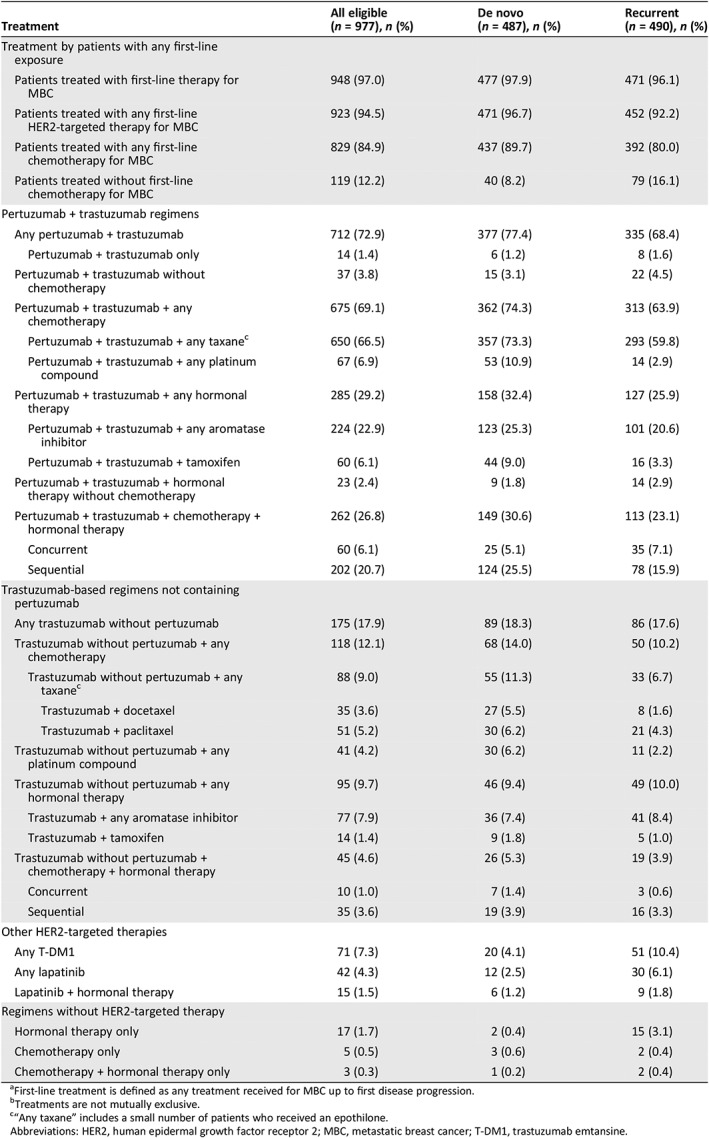
First‐line treatment is defined as any treatment received for MBC up to first disease progression.
Treatments are not mutually exclusive.
“Any taxane” includes a small number of patients who received an epothilone.
Abbreviations: HER2, human epidermal growth factor receptor 2; MBC, metastatic breast cancer; T‐DM1, trastuzumab emtansine.
First‐line HER2‐targeted therapy was administered to 96.7% [471/487] and 92.2% [452/490] of de novo and recurrent patients, respectively, with disparities in trastuzumab (95.7% [466/487] vs. 85.9% [421/490], respectively) and pertuzumab use (77.8% [379/487] vs. 68.6% [336/490]). Despite these differences, the most common treatment regimen in both cohorts was pertuzumab + trastuzumab + taxane (± hormonal therapy), administered to 73.3% (357/487) and 59.8% (293/490) of de novo and recurrent patients, respectively. Administration of sequential chemotherapy and hormonal therapy in combination with pertuzumab + trastuzumab was more common in both de novo and recurrent patients (de novo: 83.2% [124/149]; recurrent: 69.0% [78/113]) compared with concurrent chemotherapy and hormonal therapy (de novo: 16.8% [25/149]; recurrent: 31.0% [35/113]). Patients with recurrent MBC received first‐line regimens containing T‐DM1 (10.4% [51/490]) or lapatinib (6.1% [30/490]), or hormonal therapy alone (3.1% [15/490]), more commonly than patients with de novo disease (4.1% [20/487], 2.5% [12/487], and 0.4% [2/487], respectively).
Patient‐Reported Outcomes
PRO questionnaires were completed at enrollment, and completion rates were similar between the de novo and recurrent cohorts (supplemental online Table 3). At enrollment, patients with de novo versus recurrent MBC reported similar or slightly more favorable scores on all measures, including those assessing quality of life, impairment in daily activities, and cognitive dysfunction (supplemental online Table 3).
Clinical Outcomes
By data cutoff, 63.4% (309/487) of the de novo and 73.9% (362/490) of the recurrent cohort had progressed or died, and 24.4% (119/487) and 38.8% (190/490) had died, respectively. Median PFS from MBC diagnosis was 17.7 months (95% CI, 16.0–19.7) and 11.9 months (95% CI, 11.0–13.2) in patients with de novo and recurrent disease, respectively (HR, 0.69; 95% CI, 0.59–0.80; p < .0001; Fig. 1A). Median OS was 44.5 months (95% CI, 40.1–51.7) in the recurrent cohort and was not yet estimable in the de novo cohort (HR, 0.55; 95% CI, 0.44–0.69; p < .0001; Fig. 1B). Among patients with recurrent disease, lower PFS and OS were observed in those with shorter disease‐free intervals using a 24‐month cut point (supplemental online Fig. 1A and B).
Figure 1.
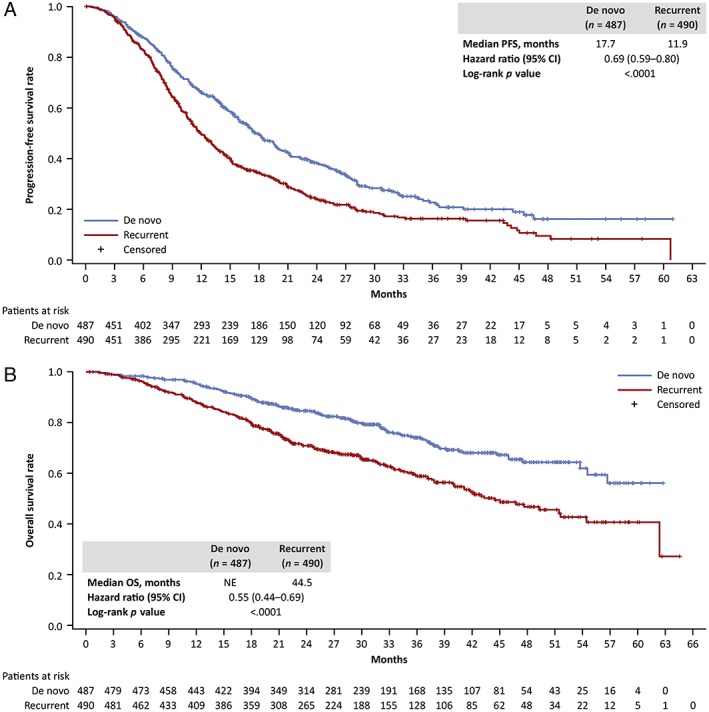
Survival by de novo versus recurrent metastatic breast cancer. (A): Progression‐free survival. (B): Overall survival. Abbreviations: CI, confidence interval; NE, not estimable; OS, overall survival; PFS, progression‐free survival.
In both the de novo and recurrent cohorts, hormone receptor–positive disease was associated with longer median first‐line PFS and OS than hormone receptor–negative disease (supplemental online Table 4A). Median PFS and OS were also higher in patients who received first‐line HER2‐targeted therapy versus those who did not, with the largest differences observed in the de novo cohort (supplemental online Table 4B). Patients who received first‐line chemotherapy did not significantly differ in median PFS or OS from those who did not, with the exception of higher PFS in the de novo cohort (supplemental online Table 4C).
Discussion
In this analysis of real‐world data from the SystHERs registry, patients with HER2‐positive de novo MBC had longer median PFS and OS than those with recurrent MBC. The most favorable survival outcomes were observed in patients with hormone receptor–positive de novo MBC, and the poorest in those with hormone receptor–negative recurrent disease, which may reflect prior exposure to therapy and selection for resistant disease upon relapse. Compared with the recurrent cohort, a higher proportion of patients with de novo MBC were aged <50 years, had BMI ≥30, had hormone receptor–negative MBC, and had baseline bone or liver metastasis, whereas patients with recurrent MBC more commonly had CNS metastasis. First‐line pertuzumab + trastuzumab + taxane, a standard of care for patients with HER2‐positive MBC, was administered more commonly to patients with de novo versus recurrent disease.
Clinical outcomes for both de novo and recurrent MBC have improved over the past decade. In SystHERs, patients with de novo and recurrent MBC had a median PFS of 17.7 months and 11.9 months, respectively, versus 12.1 months and 9.3 months in registHER 7, which enrolled patients prior to the approval of pertuzumab, lapatinib, and T‐DM1 for MBC and trastuzumab and pertuzumab for EBC. Findings were similar for OS (medians of not estimable and 44.5 months [SystHERs] vs. 41.7 and 32.8 months [registHER] for de novo and recurrent cohorts, respectively) 7. These outcomes notably exceed those observed prior to U.S. approval of trastuzumab (1998): median survival for patients with de novo MBC was only 23 months from 1987 to 1993 12. Continued advances in treatments and clinical care, including improved management of adverse events and comorbidities, may further enhance outcomes in de novo and recurrent patients.
Most patients received first‐line HER2‐targeted therapy and/or chemotherapy, both of which were administered to a slightly higher proportion of patients with de novo versus recurrent MBC. In particular, patients with de novo MBC received pertuzumab + trastuzumab + taxane more commonly, and T‐DM1 and lapatinib less commonly, than patients with recurrent MBC. These differences may reflect, in part, differences in baseline characteristics and prior treatment administration. For example, patients with recurrent disease were older and had a higher incidence of CNS metastasis at MBC diagnosis, perhaps because of the sanctuary nature of the CNS. However, the clear differences in sites of presenting disease, hormone receptor status, and other features suggest an intrinsic biological difference between de novo and recurrent disease. Additionally, presumed trastuzumab resistance upon disease recurrence may have influenced first‐line trastuzumab use for MBC. In patients with systemic (neo)adjuvant treatment data, 65.8% received trastuzumab for EBC. First‐line trastuzumab‐based regimens for MBC appear to remain an effective treatment option after prior trastuzumab exposure and relapse 13, 14; however, in SystHERs, 85.9% of patients with recurrent disease received trastuzumab versus 95.7% of those with de novo MBC. As trastuzumab and taxanes are now commonly used in EBC, future analyses should directly assess clinical outcomes from first‐line pertuzumab + trastuzumab + taxane treatment versus other regimens, such as T‐DM1, in patients with prior trastuzumab or taxane exposure. Interestingly, we observed that OS was significantly higher in patients who received first‐line HER2‐targeted therapy versus those who did not in both the de novo and recurrent cohorts, but we did not observe a similar trend in patients who did versus did not receive first‐line chemotherapy.
In patients with recurrent disease, we observed discordance in ER and PR status between primary and metastatic tumors in 18.6% and 30.3% of patients, respectively. ER‐positive and PR‐positive primary tumors more commonly converted to ER‐negative and PR‐negative metastatic tumors (ER, 14.2%, and PR, 24.1%) compared with the converse (ER, 4.4%, and PR, 6.2%). Although conversion from HER2‐negative or HER2‐equivocal primary to HER2‐positive metastatic was more common than the converse, this was likely due to exclusion of patients with HER2‐negative metastatic lesion biopsies. In patients with conversion from HER2‐negative primary to HER2‐positive metastatic tumors, conversion was higher in patients with hormone receptor–positive versus hormone receptor–negative disease. It is plausible that subclonal expansion and selective pressures of specific treatments in EBC may have influenced the direction of biomarker conversions, although noncentralized testing may also contribute to these differences. High receptor conversion rates observed in this study and others 15 support repeat testing to guide treatment selection in patients with recurrent MBC.
A recent retrospective cohort study of a U.S.‐based institutional registry database found that the ratio of patients with de novo to recurrent HER2‐positive MBC has increased over time 6, potentially because of increased use of screening scans and a reduction in recurrences since the availability of HER2‐targeted (neo)adjuvant therapies. In SystHERs, 49.8% patients had de novo and 50.2% patients had recurrent MBC, versus 33% and 67% in the registHER study, respectively 7. However, the Flatiron database of real‐world electronic health records reported that, of patients diagnosed with HER2‐positive MBC from 2011 to 2016, 37% had de novo and 63% had recurrent disease 16. The discrepancy with SystHERs data may be due to continued shifts in the proportions of de novo and recurrent patients, differences in sampling and study design (prospective vs. retrospective), or ascertainment bias at enrollment (for example, a proclivity to consider enrollment for patients new to a clinic or practice versus returning patients, who may be more likely to have recurrent MBC).
Notably, SystHERs was a prospective study, had a rigorous approach to ensure recruitment of all eligible patients, enrolled patients from academic and community sites across the U.S., and included a broader patient population than typically seen with the strict exclusion criteria of clinical trials. These strengths provide the possibility of generalizing our observations of treatment patterns and clinical outcomes across patients with HER2‐positive MBC. However, interpretation of our findings are limited by the follow‐up time available for OS analyses, differences in baseline characteristics between cohorts, and variability in investigator reporting, particularly related to reporting of disease progression. Furthermore, whereas MBC data were captured prospectively, EBC data were obtained retrospectively from the patients' prior medical records and may have been incomplete. Treatment selection related to comorbidities may limit conclusions regarding the impact of specific therapies on disease outcomes. Most limitations from this analysis are consistent with those of observational studies in general.
Conclusion
As an observational registry of patients with HER2‐positive MBC, SystHERs provides a large, modern, real‐world data set for this population and, thereby, presents a unique opportunity to study patients with de novo and recurrent HER2‐positive MBC. In SystHERs, patients with de novo disease had different baseline demographics and disease characteristics, had superior clinical outcomes, and more commonly received first‐line chemotherapy and/or trastuzumab versus those with recurrent disease. Data from this and other studies suggest that de novo and recurrent MBC have distinct outcomes, which may inform future clinical study design and could point to biological differences.
Author Contributions
Conception/design: Debu Tripathy, Adam Brufsky, Melody Cobleigh, Mohammad Jahanzeb, Peter A. Kaufman, Ginny Mason, Joyce O'Shaughnessy, Hope S. Rugo, Sandra M. Swain, Denise A. Yardley, Laura Chu, Haocheng Li, Vincent Antao, Sara A. Hurvitz
Provision of study material or patients: Debu Tripathy, Adam Brufsky, Melody Cobleigh, Mohammad Jahanzeb, Peter A. Kaufman, Ginny Mason, Joyce O'Shaughnessy, Hope S. Rugo, Sandra M. Swain, Denise A. Yardley, Sara A. Hurvitz
Collection and/or assembly of data: Debu Tripathy, Adam Brufsky, Melody Cobleigh, Mohammad Jahanzeb, Peter A. Kaufman, Ginny Mason, Joyce O'Shaughnessy, Hope S. Rugo, Sandra M. Swain, Denise A. Yardley, Laura Chu, Haocheng Li, Vincent Antao, Sara A. Hurvitz
Data analysis and interpretation: Debu Tripathy, Adam Brufsky, Melody Cobleigh, Mohammad Jahanzeb, Peter A. Kaufman, Ginny Mason, Joyce O'Shaughnessy, Hope S. Rugo, Sandra M. Swain, Denise A. Yardley, Laura Chu, Haocheng Li, Vincent Antao, Sara A. Hurvitz
Manuscript writing: Debu Tripathy, Adam Brufsky, Melody Cobleigh, Mohammad Jahanzeb, Peter A. Kaufman, Ginny Mason, Joyce O'Shaughnessy, Hope S. Rugo, Sandra M. Swain, Denise A. Yardley, Laura Chu, Haocheng Li, Vincent Antao, Sara A. Hurvitz
Final approval of manuscript: Debu Tripathy, Adam Brufsky, Melody Cobleigh, Mohammad Jahanzeb, Peter A. Kaufman, Ginny Mason, Joyce O'Shaughnessy, Hope S. Rugo, Sandra M. Swain, Denise A. Yardley, Laura Chu, Haocheng Li, Vincent Antao, Sara A. Hurvitz
Disclosures
Debu Tripathy: Pfizer, Novartis (C/A), Novartis (RF); Adam Brufsky: F. Hoffmann‐La Roche/Genentech, Novartis, Pfizer, Sandoz, AstraZeneca, Amgen, Eli Lilly & Co. (C/A, other—travel support); Melody Cobleigh: F. Hoffmann‐La Roche/Genentech (C/A, RF); Mohammad Jahanzeb: F. Hoffmann‐La Roche/Genentech (C/A), Puma (SAB, other—Data and Safety Monitoring Board); Peter A. Kaufman: F. Hoffmann‐La Roche/Genentech (C/A, RF); Joyce O'Shaughnessy: AbbVie Inc., Agendia, Amgen Biotechnology, AstraZeneca, Bristol‐Myers Squibb, Celgene Corporation, Eisai, Genentech, Genomic Health, GRAIL, Immunomedics, Heron Therapeutics, Ipsen Biopharmaceuticals, Jounce Therapeutics, Eli Lilly & Co., Merck, Myriad, Novartis, Ondonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Seattle Genetics, Syndax Pharmaceuticals (C/A); Hope S. Rugo: Merck, Mylan, Puma, Eli Lilly & Co., Pfizer (other—travel), F. Hoffmann‐La Roche/Genentech, Pfizer, Novartis, Eli Lilly & Co., OBI Pharma, Macrogenics, Merck (RF); Sandra M. Swain: Athenex, Daiichi‐Sanyo, Eli Lilly & Co., F. Hoffmann‐La Roche/Genentech, Genomic Health, Inivata, Ltd., Novartis, Pieris Pharmaceuticals, Tocagen (C/A), F. Hoffmann‐La Roche/Genentech, Pfizer, Merrimack (RF), Athenex, Daiichi‐Sanyo, Eli Lilly & Co., F. Hoffmann‐La Roche/Genentech, Inivata, Ltd., Novartis, Pieris Pharmaceuticals, Caris Life Sciences, NanoString Technologies, AstraZeneca, Bristol‐Myers Squibb (other—nonfinancial support), AstraZeneca (other—Independent Data Monitoring Committee member); Denise A. Yardley: Novartis, Genentech (C/A), F. Hoffmann‐La Roche/Genentech, Novartis (RF); Laura Chu: Genentech (E), F. Hoffmann‐La Roche/Genentech (OI); Haocheng Li: F. Hoffmann‐La Roche/Genentech (E); Vincent Antao: Genentech (E), F. Hoffmann‐La Roche/Genentech (OI); Sara A. Hurvitz: Novartis, Eli Lilly & Co., OBI Pharma (other—travel support), F. Hoffmann‐La Roche/Genentech, Novartis, GlaxoSmithKline, Boehringer Ingelheim, Sanofi, Pfizer, Amgen, OBI Pharma, Puma Biotechnology, Dignitana, Bayer, BioMarin, Eli Lilly & Co., Merrimack, Daiichi‐Sankyo, Immunomedics, Macrogenics, Pieris, Seattle Genetics (RF). All authors received nonfinancial support from F. Hoffmann‐La Roche in the form of medical writing support for this manuscript. Ginny Mason indicated no additional financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Tables
Acknowledgments
The authors are grateful to the patients, families, and investigators who participated in SystHERs. We would also like to thank Musa Mayer (http://advancedbc.org) for her work as part of the SystHERs steering committee; the SystHERs team, including clinical operations leads Michelle Usher (F. Hoffmann‐La Roche/Genentech, Inc.) and Sandy Lam (F. Hoffmann‐La Roche/Genentech, Inc.); Bongin Yoo (F. Hoffmann‐La Roche/Genentech, Inc.) for his contributions to the statistical analysis; Allen Lee (Everest Clinical Research Services, Inc.) for his assistance with the statistical analysis; and Bokai Xia (F. Hoffmann‐La Roche/Genentech, Inc.) for his statistical programming expertise. Third‐party writing assistance was provided by Sabrina Hom, Ph.D., of Ashfield Healthcare Communications (a UDG Healthcare plc company) and funded by F. Hoffmann‐La Roche/Genentech, Inc. F. Hoffmann‐La Roche/Genentech, Inc. funded the SystHERs study and participated in the study design, data collection, data analysis, data interpretation, and writing of this report.
Results from a similar analysis of interim data were presented in part at the Thirty‐Seventh Annual CTRC‐AACR San Antonio Breast Cancer Symposium, December 9–13, 2014, in San Antonio, TX, as follows: Debu Tripathy, Adam Brufsky, Melody Cobleigh et al. Increasing proportion of de novo compared with recurrent HER2‐positive metastatic breast cancer: Early results from the Systemic Therapies for HER2‐Positive Metastatic Breast Cancer Registry study. In: Proceedings of the Thirty‐Seventh Annual CTRC‐AACR San Antonio Breast Cancer Symposium: December 9–13, 2014; San Antonio, TX. Cancer Res. 2015;75(suppl 9):P3‐07‐14A.
Disclosure of potential conflicts of interest may be found at the end of this article.
References
- 1. Mariotto AB, Etzioni R, Hurlbert M et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howlader N, Altekruse SF, Li CI et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dawood S, Broglio K, Buzdar AU et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional‐based review. J Clin Oncol 2010;28:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dawood S, Broglio K, Ensor J et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 2010;21:2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lobbezoo DJ, van Kampen RJ, Voogd AC et al. Prognosis of metastatic breast cancer: Are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer 2015;112:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malmgren JA, Mayer M, Atwood MK et al. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990‐2010. Breast Cancer Res Treat 2018;167:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yardley DA, Kaufman PA, Brufsky A et al. Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2‐positive metastatic breast cancer. Breast Cancer Res Treat 2014;145:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baselga J, Cortés J, Kim SB et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swain SM, Kim SB, Cortés J et al. Pertuzumab, trastuzumab, and docetaxel for HER2‐positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncol 2013;14:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swain SM, Baselga J, Kim SB et al. Pertuzumab, trastuzumab, and docetaxel in HER2‐positive metastatic breast cancer. N Engl J Med 2015;372:724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tripathy D, Rugo HS, Kaufman PA et al. The SystHERs registry: An observational cohort study of treatment patterns and outcomes in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer. BMC Cancer 2014;14:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andre F, Slimane K, Bachelot T et al. Breast cancer with synchronous metastases: Trends in survival during a 14‐year period. J Clin Oncol 2004;22:3302–3308. [DOI] [PubMed] [Google Scholar]
- 13. Negri E, Zambelli A, Franchi M et al. Effectiveness of trastuzumab in first‐line HER2+ metastatic breast cancer after failure in adjuvant setting: a controlled cohort study. The Oncologist 2014;19:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambertini M, Ferreira AR, Poggio F et al. Patterns of care and clinical outcomes of first‐line trastuzumab‐based therapy in HER2‐positive metastatic breast cancer patients relapsing after (neo)adjuvant trastuzumab: An Italian multicenter retrospective cohort study. The Oncologist 2015;20:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schrijver WAME, Suijkerbuijk KPM, van Gils CH et al. Receptor conversion in distant breast cancer metastases: a systematic review and meta‐analysis. J Natl Cancer Inst 2018;110:568–580. [DOI] [PubMed] [Google Scholar]
- 16. Chu L, Yoo B, Carrigan G et al. How do real‐world treatment patterns compare to guideline recommendations for first‐line metastatic breast cancer patients in US community clinics? Cancer Res 2017;77(suppl 4):P5‐08‐24A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Tables


