Abstract
Background
Checkpoint inhibitor therapy is widely known to cause a number of immune‐related adverse events. One rare adverse effect that is emerging is eosinophilic fasciitis, a fibrosing disorder causing inflammatory infiltration of subcutaneous fascia. It is characterized clinically by edema and subsequent induration and tightening of the skin and subcutaneous tissues. The condition is rare, yet at our institutions we have seen four cases in the past 3 years. We describe our 4 cases and review 11 other cases reported in the literature.
Case Presentation
We present four cases of eosinophilic fasciitis following treatment with programmed cell death protein 1 or programmed cell death‐ligand 1 blockade. All patients had extremity involvement with characteristic skin changes ranging from peripheral edema to induration, tightening, and joint limitation. The patients had varying degrees of peripheral eosinophilia. In two of our patients, the diagnosis was made by full‐thickness skin biopsy showing lymphocytic infiltration of the subcutaneous fascia, with CD4+ T cells predominating in one case and CD8+ T cells in the other. In the other two cases, the diagnosis was made on the basis of characteristic imaging findings in the context of clinical features consistent with the diagnosis. All four patients were treated with glucocorticoids with varying degrees of success; immunotherapy had to be discontinued in all four. Patients with advanced melanoma who experienced this adverse effect had either a partial response or a complete response to therapy.
Conclusion
Eosinophilic fasciitis can occur as a result of checkpoint inhibitor therapy. Although a tissue diagnosis is the gold standard, imaging studies may facilitate the diagnosis in the presence of consistent clinical features, but a degree of suspicion is key to recognizing the condition early. Therapy requires a collaborative approach by oncology, rheumatology, and dermatology; physical therapy is an important adjunct in treatment. For advanced melanoma, it may be a good prognostic indicator.
Implications for Practice
It is important for clinicians to recognize that eosinophilic fasciitis is a potential immune‐related adverse event (irAE) as a consequence of immune checkpoint inhibitor therapy. The presentation is quite stereotypical; the diagnosis can be made by imaging in the absence of a full‐thickness skin biopsy. Early intervention is important to limit morbidity. This irAE may be a good prognostic sign among patients with melanoma.
Keywords: Checkpoint inhibitor, Eosinophilic fasciitis, Immune‐related adverse event
Short abstract
Eosinophilic fasciitis is an adverse effect of checkpoint inhibitor therapy. This article describes four cases of this rare adverse event and reviews the eleven cases reported in the literature to date.
Introduction
Checkpoint inhibitors (CPIs) have changed the landscape of cancer therapeutics. Since the approval of ipilimumab, a cytotoxic T‐lymphocyte‐associated‐protein (CTLA)‐4–blocking antibody, for metastatic melanoma in 2011, a number of other CPIs have been approved for a variety of malignancies, including blockers of programmed cell death protein 1 (PD‐1) and its ligand PD‐L1. Under physiologic conditions, upon T‐cell activation, checkpoints are upregulated and engaged in order to limit immune activation and tissue damage. Checkpoint inhibitors allow T cells to remain active against tumor cells 1. The persistent activation of T cells has resulted in many well‐documented immune‐related adverse events (irAEs) affecting multiple organs 2. In rheumatology, the most common irAEs are arthritis, sicca syndrome, polymyalgia rheumatica, and myositis 3.
Eosinophilic fasciitis (EF) is a rare fibrosing condition characterized by erythema, edema, and induration of extremities, often, but not always, accompanied by peripheral eosinophilia. It is considered a scleroderma mimic, in that skin tightening is the prominent symptom, but unlike scleroderma, patients with EF do not have sclerodactyly, Raynaud's phenomenon, telangiectasias, or nailfold capillary changes; conversely, patients with scleroderma do not exhibit a “groove sign” or peau d'orange. EF is characterized by inflammatory infiltration, thickening, and fibrosis involving the fascia 4, 5, 6. In the oft‐cited criteria set proposed by Pinal‐Fernandez in 2014, predicated on the exclusion of systemic sclerosis, the two major criteria for diagnosis are (a) swelling or induration of the skin and subcutaneous tissues and (b) fascial thickening with lymphocytes and macrophages, with or without eosinophilic infiltration on biopsy. If only one major criterion is present, the presence of any two of the following minor criteria can help make the diagnosis: (a) peripheral eosinophilia, (b) hypergammaglobulinemia, (c) muscle weakness, (d) groove sign, and (e) hyperintense fascia on T2‐weighted images by magnetic resonance imaging (MRI) 7.
Although EF is quite rare, we have seen four cases of EF following treatment with a PD‐1 or PD‐L1 inhibitor over a 3‐year period, suggesting that PD‐1/PD‐L1 blockade is a potent trigger of this condition. With the increasing number of indications for CPI therapy, clinicians need to be aware of this entity as a potential irAE, as morbidity can be diminished if recognized early. Here we present our 4 cases and review the additional 11 cases from in the literature.
Case 1
A 48‐year‐old man was referred to rheumatology for a chief complaint of leg stiffness. He had received treatment with atezolizumab (1,200 mg every 3 weeks for 13 doses) in combination with erlotinib as part of a trial for a diagnosis of stage IV lung adenocarcinoma. Six months into treatment, he noted tightness and pain in his upper and lower extremities accompanied by leg swelling. Creatine kinase (CK) was initially elevated to 933 U/L but subsequently normalized without intervention over 2 weeks; absolute eosinophil count (AEC) climbed from 700 to 3,500 three months later. Three months after the onset of his symptoms, atezolizumab was discontinued. Five months after the onset of his symptoms, he was referred to rheumatology. Examination revealed thickening of the skin of the forearms and of the legs below the knees that limited mobility at the elbows, wrists, knees, and ankles. A “groove sign” was noted over the left leg (Fig. 1A) and right forearm. MRI of the left tibia showed mild fascial edema (Fig. 2A). A full‐thickness skin biopsy showed changes consistent with eosinophilic fasciitis: there was striking expansion of the fascia by collagen, hyaluronic acid, and fibrin accompanied by numerous lymphocytes and plasma cells; significant tissue eosinophilia was not observed. CD3 staining revealed the presence of T lymphocytes, predominantly CD8+ T cells, with a CD4‐to‐CD8 ratio of 1:5 (Fig. 3). The patient was treated with prednisone 60 mg/day and methotrexate 20 mg weekly. Methotrexate was discontinued after 1 month because of conflict with further chemotherapy. Over the course of 3 years, although punctuated initially with flares of the skin disease requiring intermittent high‐dose steroids, his skin eventually started to soften. His malignancy progressed but responded to additional surgery and chemotherapy.
Figure 1.
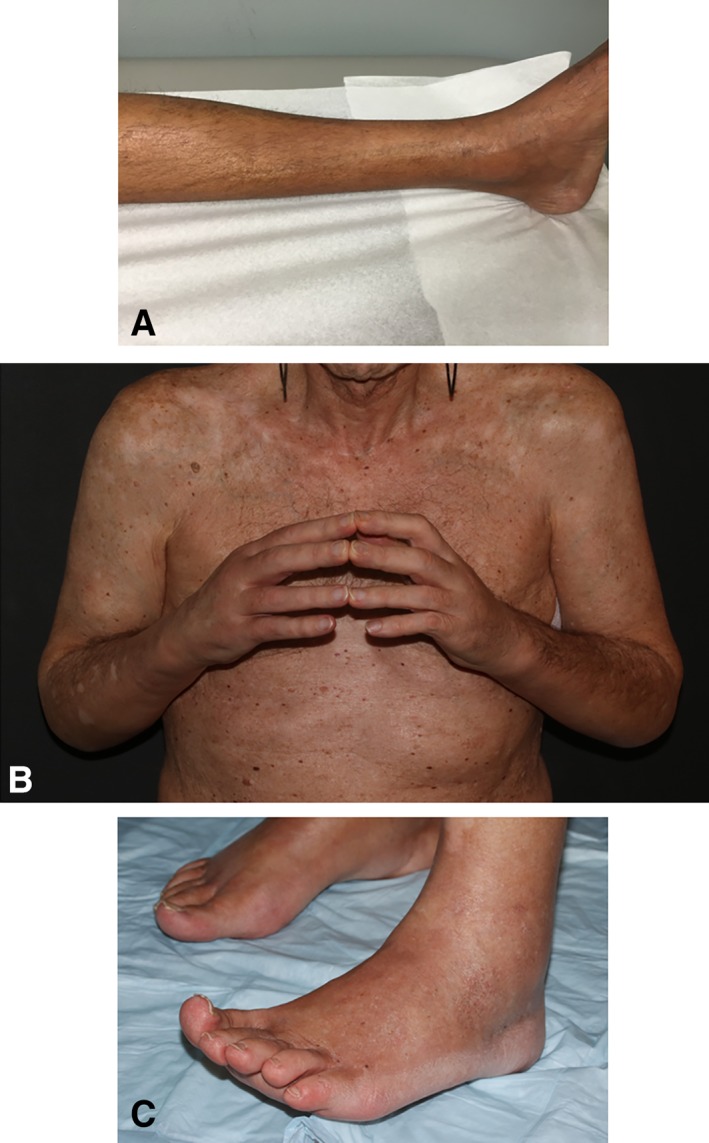
Spectrum of skin findings. (A): Patient 1. Groove sign along a superficial vessel, with swelling around the medial malleolus. (B): Patient 4. Puffiness of the hands and fingers with limited finger extension. (C): Patient 4. Pedal edema most obvious around the lateral malleolus.
Figure 2.
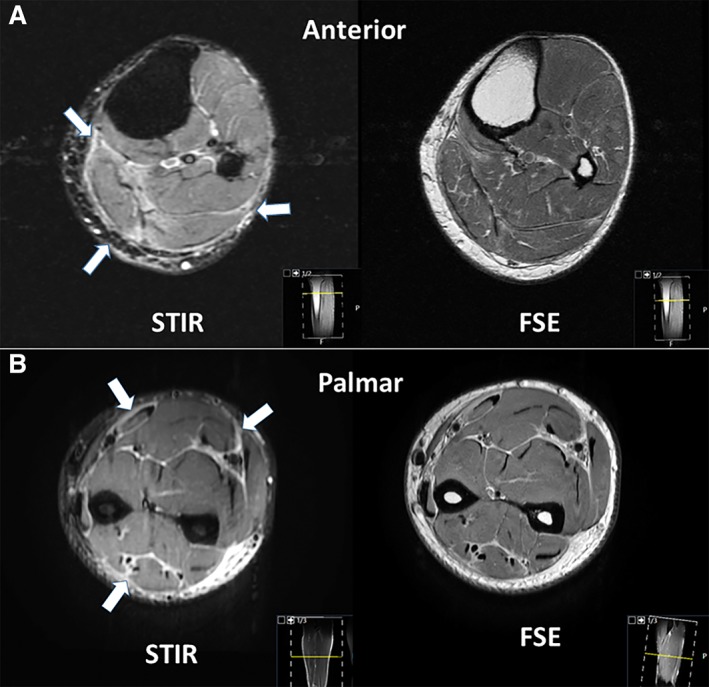
Magnetic resonance imaging findings. (A): Patient 1. Axial section through the proximal leg shows fascial edema (arrows in STIR) and preserved muscle bulk in intermediate‐weighted FSE sequences. (B): Patient 3. Axial section through the mid‐forearm shows fascial edema (arrows in STIR) and preserved muscle bulk in intermediate‐weighted FSE sequences.
Abbreviations: FSE, fast spin echo; STIR, short tau inversion recovery.
Figure 3.
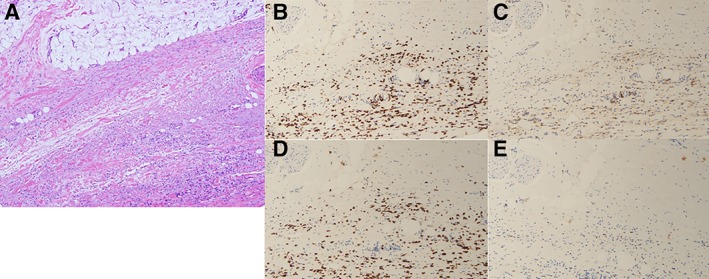
Full‐thickness skin biopsy from patient 1. (A): Hematoxylin and eosin staining at ×400 showing broad, hyalinized collagen bundles within the fascia with deposition of mucin and a lymphocytic and plasma cell infiltrate. (B–E): Immunostains demonstrating CD8+ T‐cell predominance (×200). Lymphocytes are primarily of the T‐cell subset as revealed by the extent of immunoreactivity for CD3 (B). The T cells comprise a mixture of CD4+ T cells (C) and CD8+ T cells (D), with some cells staining positive for granzyme (E).
Case 2
A 71‐year‐old female was evaluated for myalgias and pitting edema following nivolumab therapy. She had been diagnosed with vulvar melanoma 2 years prior to presentation and underwent a vulvar resection. For her first local recurrence, she received radiation therapy. She was later found to have disease metastatic to lymph nodes and lungs, for which she was started on nivolumab (480 mg monthly × three doses). She had a good response to the treatment, with repeat scans demonstrating shrinkage of some lesions and resolution of others. After her third treatment, she developed myalgias involving the shoulders, thighs, and calves, with a pronounced diurnal variation, accompanied by subjective fevers, and pitting edema in the arms and feet. Nivolumab was held. On presentation to rheumatology, she was given a preliminary diagnosis of either polymyalgia rheumatica or relapsing seronegative symmetric synovitis with pitting edema related either to her malignancy or to the nivolumab. She was treated with prednisone 60 mg and infliximab 3 mg/kg initially. On her second visit 1 month later, she was noted to have a taut consistency to her skin in a circumscribed area measuring 3 × 5 cm on each forearm just proximal to the wrists. Punch biopsy of the skin showed only poikiloderma. By her third visit another month later, the waxiness had extended to involve the forearms circumferentially, with tethering and a “woody” texture. Prior to her first visit with rheumatology, her AEC peaked at 2,400; CK was normal. A full‐thickness skin biopsy confirmed the diagnosis of eosinophilic fasciitis, with an inflammatory fibrosing reaction involving the subcutaneous tissue, fascia, and skeletal muscle. The inflammatory cell infiltrate consisted of lymphocytes, plasma cells, and eosinophils. CD3 staining confirmed the presence of T lymphocytes, most of which were CD4+, with a CD4‐to‐CD8 ratio of roughly 4:1 (Fig. 4). The patient continued on prednisone 60 mg/day and was started on methotrexate 15 mg weekly, to which she had a good response. Twelve months after her last dose of nivolumab, her scans reveal no evidence of metastatic melanoma. Methotrexate was discontinued because of medication intolerance, but the patient remains on 5 mg of prednisone, which has halted the progression of skin changes.
Figure 4.
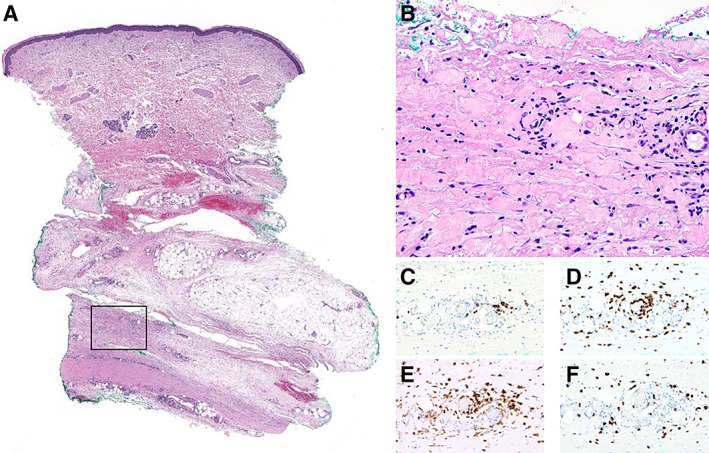
Full‐thickness skin biopsy from patient 2. (A): Fibrosing stromal changes with predominant involvement of deep subcutaneous tissue and fascia is appreciated at low magnification (hematoxylin and eosin [H&E], ×10; square indicating area seen at higher magnification in (B)). (B): Inflammatory infiltrate composed of lymphocytes, plasma cells, and eosinophils is seen in perivascular and interstitial pattern on a background of sclerosis and fibrinoid degenerative changes (H&E, ×400). (C): CD20 immunostain highlights few B cells. (D): CD3 immunostain highlights the lymphocytic infiltrate that corresponds predominantly to T cells. (E): Most T cells are CD4+. CD4 also highlights occasional dendritic cells and histiocytes. (F): CD8+ T cells are also present. (C–F × 200)
Case 3
A 43‐year‐old male was diagnosed with metastatic melanoma. He received pembrolizumab at 2 mg/kg (200 mg) every 3 weeks. Fifteen months (20 doses) later, he developed subjective tightness and swelling of the forearms with some loss of mobility at the wrists, followed later by discomfort in the back of the knees with foot dorsiflexion. He was unable to do pushups, squats, or jumps because of forearm, wrist, and leg tightness. Upon evaluation by rheumatology 4 weeks later, he was noted to have limited wrist mobility bilaterally and swelling of the volar aspect of the forearms. The skin had a normal appearance and the fascia was not “woody.” His CK was normal, and AEC was 700. MRI of the right forearm showed mild tenosynovitis in the flexor and extensor compartments, with fascial edema and relatively maintained muscle signal (Fig. 2B). Pembrolizumab, to which his cancer had had a complete response, was held. Prednisone was initiated at 40 mg daily and subsequently increased to 40 mg twice daily, without a significant response. Mycophenolate mofetil (MMF) 3 grams per day was added to his regimen, and prednisone was tapered. Two months after MMF initiation, while on prednisone 25 mg daily, the patient began to note significant clinical improvement.
Case 4
A 70‐year‐old male with a history of multiple sclerosis on weekly interferon beta 1a was diagnosed with metastatic melanoma and received treatment with pembrolizumab 2 mg/kg (140 mg every 3 weeks for 13 doses), to which his cancer had a partial response. Eight months after initiation of treatment, he developed right foot and right hand swelling and his monitoring abdominal computed tomography scan showed mild edema of the posterior subcutaneous abdominopelvic tissues. AEC was 2,300. Following a 6‐day solumedrol taper starting at 24 mg, his arm and leg swelling improved by about 75%. Four months later, he developed explosive onset diffuse swelling of the hands, arms, feet, and legs accompanied by a profound fatigue and weight loss. He received treatment with 60 mg of prednisone and the anti‐immunoglobulin E (IgE) agent omalizumab (IgE level 229 kU/L; upper limit of normal 214 kU/L), which resulted in minor improvement. Upon presentation to rheumatology, the patient's extremities were tight and woody distally > proximally. He had some puckering of the skin of the upper arms and a positive groove sign. He had limited finger extension and significant ankle edema (Fig. 1B, 1C), with involvement of the skin of the lower abdominal wall. He had limited range of motion in the shoulders, elbows, wrists, fingers, knees, and ankles. Prednisone was increased to 40 mg twice daily, and methotrexate was added to his regimen. The patient's condition progressed despite maximal methotrexate dosing at 25 mg weekly. The patient responded but flared when prednisone was tapered to 30 mg twice daily. His multiple sclerosis precluded treatment with tumor necrosis factor and interleukin (IL)‐6 blockers, so the CTLA‐4 agonist abatacept was added to his regimen.
Data Review
There are 11 reported cases of fasciitis resulting from CPI therapy, although they are variably labeled: 5 are described as “eosinophilic fasciitis,” 8, 9, 10, 11, 12 1 “lymphocytic fasciitis,” with only mild eosinophilia and without an eosinophilic infiltrate within the fascia on biopsy 13, 4 “myofasciitis” (fasciitis with muscle involvement) 14, 15, 16, and 1 “asymptomatic fasciitis.” 17.
Table 1 describes our 4 newly reported cases and summarizes the 11 cases from the literature. Nine had metastatic melanoma, and two each had renal cell carcinoma, urothelial cancers, and non‐small cell lung cancer, reflecting the Food and Drug Administration (FDA)‐approved indications for CPI. Thirteen cases occurred after treatment with a PD‐1 blocker, one of whom received initial combination therapy with a CTLA‐4 blocker in addition to the PD‐1 blockade. One case occurred after PD‐L1 blockade. The case of asymptomatic abdominal fasciitis, published in 2011, is the only one to occur following treatment with a CTLA‐4 blocker alone 17; indeed, the first PD‐1 blocker was not FDA approved until 2014. Onset of symptoms was anywhere from 1.5 to 24 months after initiation of treatment. CPI treatment was discontinued in 11 cases: in 2 patients because of cancer progression and in 9 cases because of the irAE. The status of CPI treatment is not reported in four cases. Most patients were treated with steroids; eight were treated with methotrexate, one with mycophenolate mofetil, and one with abatacept (Table 2). With regard to cancer status, all nine patients with metastatic melanoma had either a complete response (n = 8) or a partial response (n = 1). In the remaining six cases, the malignancy progressed in four, was stable in one, and was unknown in one.
Table 1.
Cases of eosinophilic fasciitis following checkpoint inhibitor therapy: clinical characteristics and malignancy status
| Patient no. | Reference | Sex | Age, years | Malignancy | Checkpoint inhibitor | Onset, months | Cancer status | Checkpoint discontinued |
|---|---|---|---|---|---|---|---|---|
| 1 | Current paper | M | 48 | Stage IV pulmonary adenocarcinoma | Atezolizumab 1,200 mg every 3 weeks × 13 | 6 | Progression | Yes |
| 2 | Current paper | F | 71 | Metastatic melanoma | Nivolumab 480 mg monthly × 3 | 3 | Complete response | Yes |
| 3 | Current paper | M | 43 | Metastatic melanoma | Pembrolizumab 200 mg every 3 weeks × 20 | 15 | Complete response | Yes |
| 4 | Current paper | M | 70 | Metastatic melanoma | Pembrolizumab 140 mg every 3 weeks × 13 | 8 | Partial response | Yes |
| 5 | Khoja et al., 2016 | F | 51 | Metastatic melanoma | Pembrolizumab | 18 | Complete response | Yes |
| 6 | Lidar et al., 2018 | F | 53 | Melanoma | Pembrolizumab | 8 | Complete response | Yes |
| 7 | Andrés‐Lencina et al., 2018 | M | 65 | Stage IV bladder cancer | Nivolumab + ipilimumab × 3 months, then nivolumab alone | 16 | Progression | Yes |
| 8 | Le Tallec et al., 2019 | F | 56 | Stage IV pulmonary adenocarcinoma | Nivolumab | 9 | Stable disease | Not reported |
| 9 | Toussaint et al., 2019 | F | 77 | Metastatic melanoma | Pembrolizumab | 22 | Complete response | Yes |
| 10 | Rischin et al., 2018 | M | 55 | Metastatic melanoma | Nivolumab | 24 | Complete response | Yes |
| 11 | Parker et al., 2018 | F | 43 | Metastatic melanoma | Nivolumab | 15 | Complete response | Not reported |
| 12 | Daoussis et al., 2017 | M | 64 | Renal cell carcinoma | Nivolumab | 10 | Not reported | Not reported |
| 13 | Narvaez et al., 2018 | F | 67 | Metastatic renal cell carcinoma | Pembrolizumab | 2 | Progression | Yes due to cancer progression |
| 14 | Narvaez et al., 2018 | M | 56 | Metastatic urothelial carcinoma | Avelumab | 1.5 | Progression | Yes due to cancer progression |
| 15 | Bronstein et al., 2011 | F | 74 | Melanoma | Ipilimumab | 14 | Complete response | Not reported |
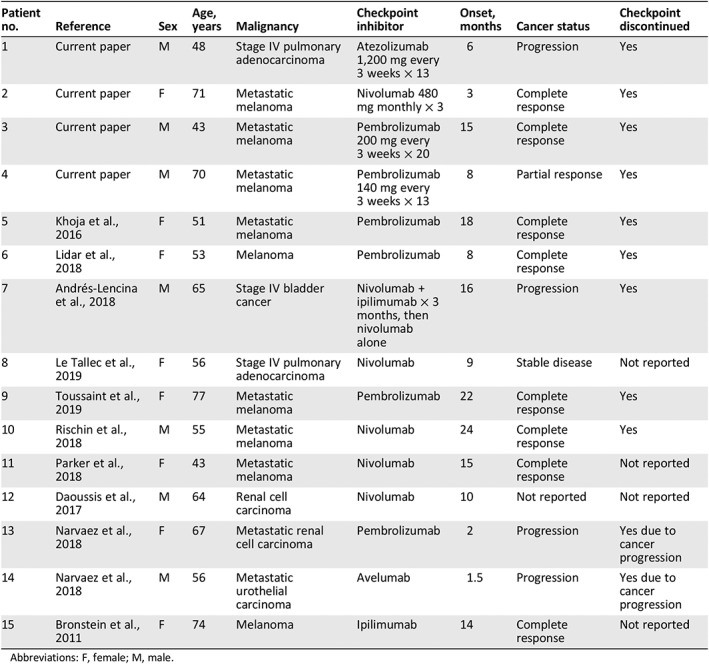
Abbreviations: F, female; M, male.
Table 2.
Laboratory, imaging, histopathology results, and therapy
| Patient no. | Reference | Presentation and physical exam findings | CK, U/L | Peak absolute eosinophil count, per μL | Imaging | Biopsy | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | Current paper | Elbows, wrists, knees, ankles, with tightness, swelling, reduced wrist range of motion, and a positive groove sign in the left leg and right forearm | 933 | 3,500 | MRI left tibia: very mild edema seen diffusely within the fascia | Full‐thickness skin biopsy: striking expansion of the fascia by connective tissue matrix comprising collagen and mucin. Superficial inflammatory cell infiltrate permeating the fascia comprising lymphocytes and plasma cells. CD3 staining demonstrates presence of T lymphocytes, CD8+ T cells predominate | Prednisone 60 mg/day, methotrexate 20 mg/week |
| 2 | Current paper | Pitting edema of hands and feet, myalgias; subsequent skin tightness of the forearms | <20 | 2,400 | None performed | Full‐thickness skin biopsy: fibrosing stromal changes; subcutaneous, fascia, and skeletal muscle infiltration by plasma cell–rich inflammatory infiltrate with eosinophils; CD3+ staining demonstrates presence of T lymphocytes; CD4+ T cells predominate | Prednisone 60 mg/day, methotrexate 15 mg/week |
| 3 | Current paper | Swelling of volar aspect of forearms; limited knee range of motion | 104 | 700 | MRI right forearm: edema within the fascial planes of the extensor palmar compartments, muscle signal relatively maintained; mild wrist extensor and flexor tenosynovitis | None performed | Prednisone 80 mg/day, mycophenolate 3 g/day |
| 4 | Current paper | Fatigue, weight loss, extremity swelling initially; leathery texture of arms and legs; positive groove sign; reduced range of motion in fingers, wrists, elbows, shoulders, knees, and ankles | 2,300 | MRI pelvis: fascial edema in pelvis and proximal thighs, extensive soft tissue edema and proximal abductor muscles bilaterally | None performed | Prednisone 80 mg/day, methotrexate 15 mg/week | |
| 5 | Khoja et al., 2016 | Myalgia, puffiness of the face; thickened and tethered waxy skin on all limbs and abdomen | 28 | 5,240 | MRI right upper limb: marked fascial edema associated with the musculature of the arm, right chest wall involving the latissimus dorsi, serratus anterior, and pectoralis | Full‐thickness skin biopsy: infiltration of dermis with a lymphoeosinophilic infiltrate with scattered eosinophils in the interstitium | Methylprednisolone 1 g/day (for treatment of coincident presumed CNS vasculitis) |
| 6 | Lidar et al., 2018 | Not reported | Not reported | Not reported | PET/CT: increased uptake in soft tissues in legs | Muscle biopsy: eosinophilic fasciitis | Corticosteroid and methotrexate |
| 7 | Andrés‐Lencina et al., 2018 | Brownish‐red plaque with significant induration and tethered waxy skin on the pubis that extended to the left anterior iliac crest region | Not reported | 4,000 | None performed | Full‐thickness skin biopsy: dermal fibrosis with dense hyalinized collagen, extending to the subcutis and fascia. Lymphocytic perivascular infiltration was also observed, with eosinophils that deepened to fascia | Prednisone 100 mg/day; methotrexate 20 mg/week (cyclosporine ineffective) |
| 8 | Le Tallec et al., 2019 | Myalgia, diffuse skin thickening of arms and legs | WNL | 4,140 | MRI left thigh: abnormal linear high signal along the fasciae | Muscle biopsy: marked CD8‐positive inflammatory infiltrate of the fasciae coexisting with eosinophils with an anecdotic muscle involvement, thus ruling out other diagnoses such as myositis | Corticosteroid and methotrexate |
| 9 | Toussaint et al., 2019 | Myalgia, extremity edema; woody induration, peau d'orange, positive groove sign bilateral forearms | 227 | 4,092 | MRI bilateral forearms: superficial and deep fascial thickening, thickened skin, and intra‐/subcutaneous edema | None performed | Prednisone 1 mg/kg/day, methotrexate |
| 10 | Rischin et al., 2018 | Diffuse arthralgia and myalgia; forearms and hands with skin tightness and forearm induration, severe limitation of finger movement | WNL | 600 | MRI forearm: fasciitis surrounding all muscle compartments with no myositis | Left forearm biopsy: florid lymphocytic fasciitis composed of predominantly T cells and macrophages, with scattered plasma cells and perivascular cuffing by lymphocytes in the deep subcutaneous tissue and underlying fascia; CD3+ staining demonstrates presence of T lymphocytes | Prednisolone 50 mg/day, methotrexate 20 mg/week |
| 11 | Parker et al., 2018 | Fatigue and myalgia; proximal muscle weakness; woody feel to skin of forearms, contracture of the left forearm flexor | 75 | WNL | MRI: symmetric fascial thickening and intense STIR signal centered around the muscle fascia of all thigh and calf muscle groups | Full‐thickness skin‐muscle biopsy: fascial and perifascicular inflammatory infiltrate with CD3+ cells; majority of myofibers showed HLA Class‐I immunolabelling | Prednisolone 30 mg/day, IVIg |
| 12 | Daoussis et al., 2017 | Swelling of wrists, knees, ankles, profound crepitus in all involved areas | Not reported | Not reported | MRI knee and ankle: symmetric myofasciitis with associated tenosynovitis | None performed | Methylprednisolone 12 mg/day |
| 13 | Narvaez et al., 2018 | Both patients (patients 13 and 14) are described as having proximal symmetric leg weakness and stiffening of the skin of the lower extremities | Not reported | Not reported | MRI lower extremities: focal changes of myositis and fasciitis of the right gastrocnemius and soleus; subcutaneous edematous changes | None performed | NSAIDs and colchicine; drug withdrawal |
| 14 | Narvaez et al., 2018 | Not reported | Not reported | MRI lower extremities: extensive changes of myositis and less marked changes of fasciitis involving the adductor muscles | None performed | Drug withdrawal | |
| 15 | Bronstein et al., 2011 | Clinically silent | WNL | Not reported | PET/CT: FDG‐avid abdominal fasciitis | None performed | Not reported |
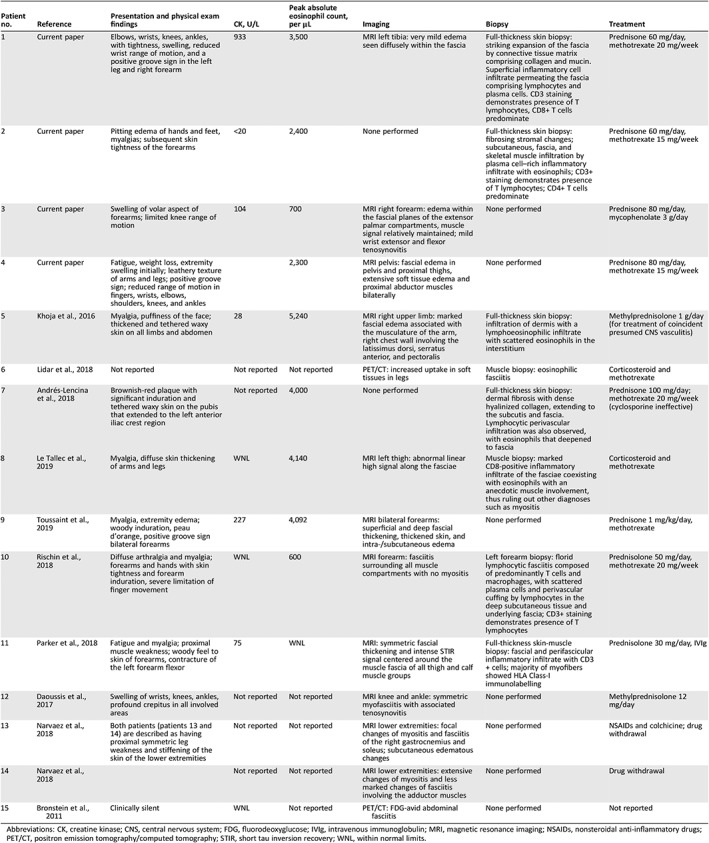
Abbreviations: CK, creatine kinase; CNS, central nervous system; FDG, fluorodeoxyglucose; IVIg, intravenous immunoglobulin; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti‐inflammatory drugs; PET/CT, positron emission tomography/computed tomography; STIR, short tau inversion recovery; WNL, within normal limits.
Patients often presented with myalgias and limb edema, sometimes with systemic symptoms such as fatigue and fever. Physical exam findings include edema, induration, a “woody” or “tethered” quality to the skin, and limitation in joint mobility.
Patients often presented with myalgias and limb edema, sometimes with systemic symptoms such as fatigue and fever. Physical exam findings include edema, induration, a “woody” or “tethered” quality to the skin, and limitation in joint mobility.
In 11 cases, imaging studies, most commonly an extremity MRI, suggested the diagnosis. Eight patients had a tissue diagnosis usually from a full‐thickness skin biopsy that showed dense infiltration of the fascia by lymphocytes and plasma cells, and variable tissue eosinophilia (Table 2). Immunohistochemistry performed in five cases demonstrated a T‐cell–dominant (CD3+) infiltrate. Patients 1 and 8 had a striking predominance of CD8+ T cells, whereas patient 2 had a predominance of CD4+ T cells.
Discussion
When confronted with a patient with myalgia, extremity edema, and fibrosing skin changes in the setting of a malignancy, clinicians have a variety of conditions to consider. Myositis and scleroderma can both be paraneoplastic in nature, as can EF 18, 19, 20. In addition, cancer treatment can itself cause many of these conditions 21.
Of the 15 cases of CPI‐associated EF presented here, all but 1 (Bronstein, 2011) meet the criteria for EF put forth by Pinal‐Fernandez et al. 7. Notably, as Mazori et al. report, neither the absence of peripheral eosinophilia nor the presence of muscle involvement exclude the diagnosis 22. EF in the noncancer setting is characterized histologically by fascial thickening and fibrosis, with an inflammatory infiltrate composed of lymphocytes, plasma cells, and histiocytes. Paradoxically, tissue eosinophilia may be absent 7, 22. Light microscopic assessment is so characteristic that immunohistochemistry is rarely performed, although kappa and lambda staining is sometimes done to rule out a paraneoplastic form of EF in the setting of a plasma cell dyscrasia.
Toquet et al. demonstrate that CD8+ T cells predominate in non‐CPI‐associated EF and that some of these cells contain granzyme B, which might exert a direct cytotoxic effect 23. EF in CPI‐treated patients may result from prolonged CD8+ T‐lymphocyte activation. Indeed, LeTallec et al. demonstrated CD8 predominance in their patient 11, and our first patient had a marked expansion of CD8+ T cells, some of which stained positive for granzyme (Fig. 3). However, this may not be the entire story, as our patient 2 had a CD4+ predominant infiltrate (Fig. 4). Pathology in CPI‐induced myositis, which may share pathogenic mechanisms with EF (as demonstrated by class I major histocompatibility complex staining in Parker et al. 14) especially in patients with EF with muscle involvement, reveals similarly discrepant findings, with CD4 predominance in four cases 24, CD8 predominance in most of the other cases 25, 26, and one case with a CD4‐to‐CD8 ratio of 1:1 27.
Peripheral blood mononuclear cell (PBMC)‐derived IL‐5 has also been implicated in the development of EF 28. IL‐5, which plays a crucial role in eosinophil activation, is largely produced by Th2 cells 29, 30. PD‐1 blockade in vitro has been shown to suppress Th2 responses in prostate cancer and melanoma 31, perhaps accounting for the rarity of eosinophil‐related adverse events. However, tumor cells can themselves produce IL‐5 29. In addition, IL‐5 alone is not sufficient for eosinophil‐lineage commitment in studies of progenitor cells 32. Hollande et al. recently showed in a mouse model of hepatocellular carcinoma and breast cancer that tumor expression of the alarmin IL‐33 was sufficient to activate eosinophil‐mediated antitumor responses 33.
There has been much interest in using eosinophil counts as biomarkers of response to CPI therapy. Multiple studies show that higher baseline eosinophil counts are predictive of better overall survival in patients with melanoma treated with PD‐1 blockade, CTLA‐4 blockade, or a combination 34, 35, 36. An increase in eosinophil count during treatment with ipilimumab in patients with melanoma has also been associated with an improved clinical response 37, 38. Conversely, high eosinophil counts are associated with toxicities 39. In an analysis of 285 patients treated with CPIs, higher eosinophil levels were associated with higher‐grade (grade ≥ 3) cutaneous irAEs 40. In the present series, eight out of nine patients with advanced melanoma had a complete response, and the remaining one patient had a partial response. For advanced cutaneous melanoma, the rate of complete response to nivolumab in clinical trials is 16% 41. It is therefore possible that EF is a byproduct of eosinophilic antitumor activity and a good prognostic marker at least in advanced melanoma.
The treatment of EF can be challenging because of a dearth of available literature. Glucocorticoids are considered first‐line therapy, and patients who do not achieve an adequate response are treated with methotrexate. In a relatively large cohort of 63 patients with EF, Wright et al. found that the mean maximum steroid dose was 51 mg and the mean duration of therapy was 18 months; 64% of patients treated with a combination of methotrexate and prednisone achieved a complete response 42. In one retrospective study of 32 patients, the mean maximum steroid dose was 52 mg, and patients pulsed with 3 days of methylprednisolone 500–1,000 mg/day had better responses 43. In one single‐arm study, high‐dose pulse methotrexate 4 mg/kg monthly was given for 5 months and all 12 patients achieved improved skin scores 44. A variety of other immunomodulators have been used, including azathioprine, sulfasalazine, penicillamine, cyclosporine, rituximab, infliximab, tocilizumab, tofacitinib, and mycophenolate mofetil 5, 6, 45. In the context of CPI‐induced EF, therapies that preferentially inhibit T cells might make sense mechanistically, although there are no data to support this. Abatacept has not been used to treat EF, and its mechanism of action as a checkpoint agonist makes it a counterintuitive choice; however, it was recently used successfully to treat severe steroid‐resistant immunotherapy‐induced myocarditis 46. Physical therapy is a crucial component of therapy. Management requires collaboration between oncologists, rheumatologists, and dermatologists; discontinuation of the checkpoint inhibitor seems necessary.
Multiple studies show that higher baseline eosinophil counts are predictive of better overall survival in melanoma patients treated with PD‐1 blockade, CTLA‐4 blockade, or a combination. An increase in eosinophil count during treatment with ipilimumab in melanoma patients has also been associated with an improved clinical response. Conversely, high eosinophil counts are associated with toxicities.
Conclusion
Eosinophilic fasciitis should be considered in CPI‐treated patients presenting with myalgias, edema, and/or skin tightening. A high peripheral absolute eosinophil count is not necessary for diagnosis. Ideally, a full‐thickness skin biopsy is the best way to make an accurate diagnosis; however, this can sometimes be difficult to obtain, or there may be concern about poor wound healing. In this scenario, MRI findings can be useful when combined with the appropriate clinical scenario, as outlined by the Pinal‐Fernandez criteria 7. A high index of suspicion is necessary because if recognized early, morbidity can be limited by early intervention. Untreated, EF can lead to profound joint contractures and functional disability. Collaboration among the patient's oncologist, dermatologist, and rheumatologist is vital. Discontinuation of CPI therapy may be necessary, and systemic steroids should be initiated promptly. If immunomodulator therapy beyond steroids is needed, methotrexate has been demonstrated to be effective in de novo EF, although the response in our series of four patients has been less impressive. Physical therapy should be encouraged, especially in patients with compromised mobility.
Author Contributions
Conception/design: Karmela Kim Chan
Provision of study material or patients: Karmela Kim Chan, Cynthia Magro, Alexander Shoushtari, Charles Rudin, Cecilia Lezcano, John Carrino, Michael A. Postow, Arlyn Apollo, Mario E. Lacouture, Anne R. Bass
Collection and/or assembly of data: Karmela Kim Chan, Veronica Rotemberg, Anthony Rossi, David Fernandez, Mario E. Lacouture
Data analysis and interpretation: Karmela Kim Chan, Cynthia Magro, Alexander Shoushtari, Charles Rudin, Cecilia Lezcano, John Carrino, Michael A. Postow, Anne R. Bass
Manuscript writing: Karmela Kim Chan, Cynthia Magro, Michael A. Postow, Mario E. Lacouture, Anne R. Bass
Final approval of manuscript: Karmela Kim Chan, Cynthia Magro, Alexander Shoushtari, Charles Rudin, Veronica Rotemberg, Anthony Rossi, Cecilia Lezcano, John Carrino, David Fernandez, Michael A. Postow, Arlyn Apollo, Mario E. Lacouture, Anne R. Bass
Disclosures
Alexander Shoushtari: Bristol‐Myers Squibb, Immunocore, Castle Biosciences (C/A), Bristol‐Myers Squibb, Immunocore, Xcovery (RF); John Carrino: Pfizer, Covera Health, Simplify Medical, Image Biopsy, Image Analysis Group (C/A); Michael A. Postow: Bristol‐Myers Squibb, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro (C/A), Bristol‐Myers Squibb, Merck (H), RGenix, Infinity, Bristol‐Myers Squibb, Merck, Array BioPharma, Novartis, AstraZeneca (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 3. Cappelli LC, Gutierrez AK, Bingham CO et al. Rheumatic and musculoskeletal immune‐related adverse events due to immune checkpoint inhibitors: A systematic review of the literature. Arthritis Care Res (Hoboken) 2017;69:1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lakhanpal S, Ginsburg WW, Michet CJ et al. Eosinophilic fasciitis: Clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum 1988;17:221–231. [DOI] [PubMed] [Google Scholar]
- 5. Lebeaux D, Sène D. Eosinophilic fasciitis (Shulman disease). Best Pract Res Clin Rheumatol 2012;26:449–458. [DOI] [PubMed] [Google Scholar]
- 6. Fett N, Arthur M. Eosinophilic fasciitis: Current concepts. Clin Dermatol 2018;36:487–497. [DOI] [PubMed] [Google Scholar]
- 7. Pinal‐Fernandez I, Selva‐O'Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev 2014;13:379–382. [DOI] [PubMed] [Google Scholar]
- 8. Khoja L, Maurice C, Chappell M et al. Cancer immunology miniatures eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res 2016;4:175–178. [DOI] [PubMed] [Google Scholar]
- 9. Lidar M, Giat E, Garelick D et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018;17:284–289. [DOI] [PubMed] [Google Scholar]
- 10. Andrés‐Lencina JJ, Burillo‐Martínez S, Aragón‐Miguel R et al. Eosinophilic fasciitis and lichen sclerosus in a patient treated with nivolumab. Australas J Dermatol 2018;59:e302–e304. [DOI] [PubMed] [Google Scholar]
- 11. Le Tallec E, Ricordel C, Triquet L et al. An original case of an association of eosinophilic fasciitis with cholangitis induced by nivolumab. J Thorac Oncol 2019;14:e13–e15. [DOI] [PubMed] [Google Scholar]
- 12. Toussaint F, Hammon M, Erdmann M et al. Checkpoint inhibitor‐induced eosinophilic fasciitis following high eosinophilia associated with complete response. Rheumatology (Oxford) 2019;58:1875–1877. [DOI] [PubMed] [Google Scholar]
- 13. Rischin A, Brady B, McLean C et al. Immune checkpoint inhibitor‐induced lymphocytic fasciitis. Intern Med J 2018;48:1550–1552. [DOI] [PubMed] [Google Scholar]
- 14. Parker MJ, Roberts ME, Lorigan PC et al. Autoimmune fasciitis triggered by the anti‐programmed cell death‐1 monoclonal antibody nivolumab. BMJ Case Rep 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daoussis D, Kraniotis P, Liossis SN et al. Immune checkpoint inhibitor‐induced myo‐fasciitis. Rheumatology (Oxford) 2017;56:2161. [DOI] [PubMed] [Google Scholar]
- 16. Narváez J, Juarez‐López P, LLuch J et al. Rheumatic immune‐related adverse events in patients on anti‐PD‐1 inhibitors: Fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev 2018;17:1040–1045. [DOI] [PubMed] [Google Scholar]
- 17. Bronstein Y, Ng CS, Hwu P et al. Radiologic manifestations of immune‐related adverse events in patients with metastatic melanoma undergoing anti–CTLA‐4 antibody therapy. Am J Roentgenol 2011;197:W992–W1000. [DOI] [PubMed] [Google Scholar]
- 18. Igusa T, Hummers LK, Visvanathan K et al. Autoantibodies and scleroderma phenotype define subgroups at high‐risk and low‐risk for cancer. Ann Rheum Dis 2018;77:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manger B, Schett G. Paraneoplastic syndromes in rheumatology. Nat Rev Rheumatol 2014;10:662–670. [DOI] [PubMed] [Google Scholar]
- 20. Haddad H, Sundaram S, Magro C et al. Eosinophilic fasciitis as a paraneoplastic syndrome, a case report and review of the literature. Hematol Oncol Stem Cell Ther 2014;7:90–92. [DOI] [PubMed] [Google Scholar]
- 21. Ferreli C, Gasparini G, Parodi A et al. Cutaneous manifestations of scleroderma and scleroderma‐like disorders: A comprehensive review. Clin Rev Allergy Immunol 2017;53:306–336. [DOI] [PubMed] [Google Scholar]
- 22. Mazori DR, Femia AN, Vleugels RA. Eosinophilic fasciitis: An updated review on diagnosis and treatment. Curr Rheumatol Rep 2017;19:74. [DOI] [PubMed] [Google Scholar]
- 23. Toquet C, Hamidou MA, Renaudin K et al. In situ immunophenotype of the inflammatory infiltrate in eosinophilic fasciitis. J Rheumatol 2003;30:1811–1815. [PubMed] [Google Scholar]
- 24. Moreira A, Loquai C, Pföhler C et al. Myositis and neuromuscular side‐effects induced by immune checkpoint inhibitors. Eur J Cancer 2019;106:12–23. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki S. Immune checkpoint inhibitors and neuromuscular adverse events [in Japanese]. Brain Nerve 2018;70:461–466. [DOI] [PubMed] [Google Scholar]
- 26. Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. John S, Antonia SJ, Rose TA et al. Progressive hypoventilation due to mixed CD8+ and CD4+ lymphocytic polymyositis following tremelimumab ‐ durvalumab treatment. J Immunother Cancer 2017;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. French LE, Shapiro M, Junkins‐Hopkins JM et al. Eosinophilic fasciitis and eosinophilic cellulitis in a patient with abnormal circulating clonal T cells: Increased production of interleukin 5 and inhibition by interferon alfa. J Am Acad Dermatol 2003;49:1170–1174. [DOI] [PubMed] [Google Scholar]
- 29. Simon SCS, Utikal J, Umansky V. Opposing roles of eosinophils in cancer. Cancer Immunol Immunother 2019;68:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weller PF, Spencer LA. Functions of tissue‐resident eosinophils. Nat Rev Immunol 2017;17:746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dulos J, Carven GJ, van Boxtel SJ et al. PD‐1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother 2012;35:169–178. [DOI] [PubMed] [Google Scholar]
- 32. Fulkerson PC, Rothenberg ME. Eosinophil development, disease involvement, and therapeutic suppression. Adv Immunol 2018;138:1–34. [DOI] [PubMed] [Google Scholar]
- 33. Hollande C, Boussier J, Ziai J et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL‐33‐dependent eosinophil‐mediated control of tumor growth. Nat Immunol 2019;20:257–264. [DOI] [PubMed] [Google Scholar]
- 34. Martens A, Wistuba‐Hamprecht K, Geukes Foppen M et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 2016;22:2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weide B, Martens A, Hassel JC et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016;22:5487–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heppt MV, Heinzerling L, Kähler KC et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death‐1 or combined PD‐1/cytotoxic T‐lymphocyte antigen‐4 inhibition. Eur J Cancer 2017;82:56–65. [DOI] [PubMed] [Google Scholar]
- 37. Delyon J, Mateus C, Lefeuvre D et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: An early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 2013;24:1697–1703. [DOI] [PubMed] [Google Scholar]
- 38. Gebhardt C, Sevko A, Jiang H et al. Personalized medicine and imaging myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res 2015;21:5453–5459. [DOI] [PubMed] [Google Scholar]
- 39. Schindler K, Harmankaya K, Kuk D et al. Correlation of absolute and relative eosinophil counts with immune‐related adverse events in melanoma patients treated with ipilimumab. J Clin Oncol 2014;32(suppl 15):9096a. [Google Scholar]
- 40. Phillips GS, Wu J, Hellmann MD et al. Treatment outcomes of immune‐related cutaneous adverse events. J Clin Oncol 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wright NA, Mazori DR, Patel M et al. Epidemiology and treatment of eosinophilic fasciitis: An analysis of 63 patients from 3 tertiary care centers. JAMA Dermatol 2016;152:97. [DOI] [PubMed] [Google Scholar]
- 43. Lebeaux D, Frances C, Barete S et al. Eosinophilic fasciitis (Shulman disease): New insights into the therapeutic management from a series of 34 patients. Rheumatology (Oxford) 2012;51:557–561. [DOI] [PubMed] [Google Scholar]
- 44. Mertens JS, Zweers MC, Kievit W et al. High‐dose intravenous pulse methotrexate in patients with eosinophilic fasciitis. JAMA Dermatol 2016;152:1262–1265. [DOI] [PubMed] [Google Scholar]
- 45. Kim SR, Charos A, Damsky W et al. Treatment of generalized deep morphea and eosinophilic fasciitis with the Janus kinase inhibitor tofacitinib. JAAD Case Rep 2018;4:443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salem JE, Allenbach Y, Vozy A et al. Abatacept for severe immune checkpoint inhibitor‐associated myocarditis. N Engl J Med 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]


