Summary
The lung is a unique organ that must protect against inhaled pathogens and toxins, without mounting a disproportionate response against harmless particulate matter and without compromising its vital function. Tissue‐resident immune cells within the lung provide local immunity and protection from infection but are also responsible for causing disease when dysregulated. There is a growing appreciation of the importance of tissue‐resident memory T cells to lung immunity, but non‐recirculating, tissue‐resident, innate immune cells also exist. These cells provide the first line of defence against pulmonary infection and are essential for co‐ordinating the subsequent adaptive response. In this review, we discuss the main lung‐resident innate immune subsets and their functions in common pulmonary diseases, such as influenza, bacterial pneumonia, asthma and inflammatory disorders.
Keywords: innate, lung, tissue‐resident
This review discusses the main tissue‐resident innate immune populations found in the lung and describes their involvement in protection from infectious and non‐infectious disease.
Abbreviations
- AMs
alveolar macrophages
- cDCs
conventional dendritic cells
- COPD
chronic obstructive pulmonary disease
- DCs
dendritic cells
- IFN,
interferon
- IL,
interleukin
- ILCs
innate lymphoid cells
- IMs
interstitial macrophages
- MARCO
macrophage receptor with collagenous structure
- NK
natural killer
- NSCLC
non‐small‐cell lung cancer
- pDCs
plasmacytoid dendritic cells
- Th2,
T helper type 2
- Treg cells
regulatory T cells
- Trm cells
tissue‐resident T cells
Introduction
Human lungs are remarkable organs, which consume up to 11 500 l of air each day. This air contains small particulate matter such as dust, smoke, dirt, pollen and aerosols that the lung must tolerate, in addition to the mechanical forces of spontaneous respiration,1 while at the same time providing protection from inhaled viruses, bacteria and other respiratory pathogens. Clearly, proper lung function is essential for good health, and so immune cells in the lung must strike a delicate balance between immunity and tolerance. Studies within the last decade have shown that this relies on a complex network of non‐recirculating immune subsets that reside within lung tissue.
Tissue‐resident cells span across innate and adaptive immunity and provide localized, tissue‐specific immune protection. Many studies have focused on tissue‐resident T cells (Trm cells) within lung tissues, and these adaptive immune cells have been shown to play an important part in host defence against a variety of pulmonary infections.2, 3, 4 In addition, recent work suggests that tissue‐resident B cells also form an important part of the adaptive immune defence in the lung.5 However, it is important to remember that innate immune cells can also be tissue‐resident and are vital in providing the first line of defence against inhaled allergens and pathogens.6 Indeed, alveolar macrophages have long been recognized as a unique and non‐recirculating subset, which establishes during early development,7 and might be considered the prototypic lung‐resident immune subset. In this review, we focus on lung‐resident innate immune subsets of haematopoietic origin that are long‐lived and often maintained long‐term through self‐renewal. Other innate immune cells play vital roles in lung immunity, including eosinophils and neutrophils, and may be morphologically and phenotypically distinct from cells in the circulation.8, 9 However, as these cells are very short lived (1–2 days) and must be continually replenished from blood, they will not be discussed in this review.10, 11
Macrophages
In mice, lung development starts as early as 9 days after conception, but lungs remain sterile until birth.1 Within the developing embryo, lineage tracing, parabiosis and fate‐mapping experiments show that lung‐resident macrophages arise through at least two distinct developmental programmes.12, 13 In the first stage, primitive macrophages develop from the fetal yolk sac, bypassing the formation of monocyte intermediates.13, 14 These primitive macrophages predominantly give rise to microglia in the brain, but also go on to seed a variety of tissues, including the lung, where they contribute to a fraction of the tissue‐resident macrophage population.13 The majority of tissue‐resident macrophage populations, however, are derived from fetal monocyte precursors that colonize the liver during development.13 Following the first breath, alveologenesis begins and these macrophages differentiate into long‐lived alveolar macrophages (AMs)1, 15 and interstitial macrophages (IMs).12 At steady‐state, the majority of lung‐resident macrophages are AMs, residing within the alveolar space, whereas a smaller population of IMs reside with the lung parenchyma.16
Alveolar macrophages
Alveolar macrophages are adaptable cells that can adopt different functions depending on their microenvironment and differentiation state, but they are generally considered anti‐inflammatory (Fig. 1). Their primary location within the alveolar lumen means that they are continuously exposed to environmental stimulants; and their historical name of ‘dust cells’, reflects their role in phagocytosis of particulate matter, dying cells and cellular debris. The physical removal of this material is itself essential to limiting lung inflammation, and in order to prevent overt inflammatory responses to this process, under homeostatic conditions AMs are largely kept in a quiescent state.17, 18, 19 Over the last decade, fate‐mapping, parabiosis and adoptive transfer experiments have established that AM are a largely self‐renewing population that does not rely on replenishment from the bone marrow.15, 20 Hashimoto et al. developed three different fate‐mapping models, all of which showed that lung‐resident macrophages originate independently from monocytes and haematopoietic precursors. These findings are supported by parabiotic mouse experiments in which lung macrophages display negligible chimerism after 1 year, despite proliferation occurring. Furthermore, when host macrophages are depleted by a diphtheria‐toxin or Toxoplasma infection, repopulation occurs primarily through local proliferation and is largely independent of circulating monocytes.20 New AMs can develop from recruited blood monocytes, which then persist long term and gradually transition to more closely resemble tissue‐resident AMs.21 Hence, irrespective of their origin, AMs appear to be a truly unique lung‐resident population.
Figure 1.
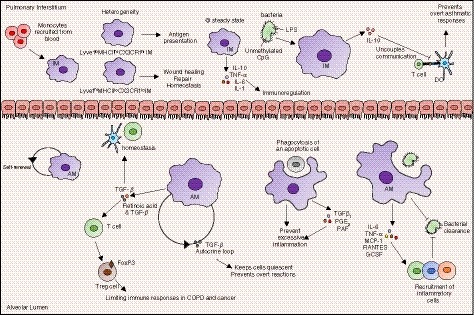
Tissue‐resident macrophages persist within lung tissues where they provide protection from inhaled pathogens and allergens but simultaneously prevent overt responses to harmless particulate matter. Interstitial macrophages (IM), which populate the lung interstitium, primarily derive from recruited blood monocytes, although a portion may be resident. At least two distinct subsets can be described, with respective roles in homeostasis or antigen presentation. IMs produce immunoregulatory cytokines both at steady state, and after exposure to environmental stimuli, which allows them to modulate inflammation. Alveolar macrophages (AM) are a self‐renewing population of resident macrophages within the alveolar space that do not rely on replenishment from the bone marrow. Primarily anti‐inflammatory cells, they mediate inflammation and promote homeostasis through phagocytosis of apoptotic cells before lysis and the production of anti‐inflammatory cytokines such as transforming growth factor‐β. AMs also provide protection from inhaled pathogens through phagocytosis and the recruitment of inflammatory cells which clear infections.
In the absence of inflammation, AMs are critical for maintaining immune homeostasis of other alveolar subsets, including alveolar epithelial cells, dendritic cells and T cells,16, 22, 23, 24 through the production of anti‐inflammatory molecules including transforming growth factor‐β (TGF‐β). This molecule has broad anti‐inflammatory effects and also limits AM activation through an autocrine loop.17 In addition, together with retinoic acid, TGF‐β from AMs is responsible for converting naive or activated T cells into FoxP3+‐expressing regulatory T (Treg) cells,25 which are important for limiting immune responses in many pulmonary diseases. AMs can also directly mediate suppressive immunity through continual contact‐mediated crosstalk with the lung epithelium26 and through secretion of vesicles that suppress cytokine secretion in these cells.27
Under inflammatory conditions however, the immunosuppressive signals in AMs can be overridden, through a variety of pathogen‐associated molecular pattern receptors.16 Their position in the alveolar space makes them ideally suited as a first line of defence against many bacterial and fungal pathogens; and their phagocytic capacity is generally increased during infection.28, 29 The importance of this process is suggested by the link between the reduced phagocytic capacity of the AMs of patients with chronic obstructive pulmonary disease (COPD), and their failure to clear lung infections.30 This is associated with decreased expression of phagocytic molecules such as the mannose receptor, and may be restored by treatment with azithromycin, which increases the phagocytic capacity of AMs,31 and may improve patient outcomes.32 In mice, other phagocytic receptors, such as the macrophage receptor with collagenous structure (MARCO), are important for clearance of respiratory pathogens including Streptococcus pneumoniae and Mycobacterium tuberculosis.33, 34 In humans, MARCO polymorphisms that reduce phagocytic capacity in monocyte‐derived macrophages are linked to tuberculosis susceptibility,33 and lower MARCO expression on AMs from diabetic mice reduces their ability to phagocytose M. tuberculosis.35 In addition to phagocytosis, AMs secrete numerous cytokines and chemokines, including interleukin‐6 (IL‐6), tumour necrosis factor‐α, monocyte chemoattractant protein 1, RANTES and granulocyte colony‐stimulating factor, which recruit other inflammatory cells.36, 37, 38
During infection, AMs still play an important role in limiting inflammation, which could otherwise be fatal. Critically, the phagocytosis of apoptotic cells by AM, before lysis, prevents the release of their inflammatory intracellular contents and is accompanied by the production of anti‐inflammatory cytokines such as TGF‐β 1, prostaglandin‐E2 and platelet‐activating factor. Defective AM phagocytosis is associated with increased inflammation, as observed in children with poorly controlled asthma.39 AMs can also mediate Janus kinase–signal transducer and activator of transcription signalling via the secretions of SOCS proteins 1 and 3, which inhibit signal transducer and activator of transcription activation.27 Indeed, the anti‐inflammatory properties of AMs during infection have been exploited by several pulmonary pathogens to enhance their persistence, as has been reviewed extensively elsewhere.40 In addition, AMs play an important role in promoting tissue repair after resolution of infection through a variety of mechanisms.41
Interstitial macrophages
Macrophages located within the lung tissue parenchyma (IMs) have previously been considered an interim state between recruited macrophages and true tissue‐resident AMs.41, 42 However, recent studies now show that stable IM populations exist in the lung and have important immunoregulatory properties,43, 44 although the origin and ontogeny of these cells is complex and less well documented than that of AMs. Runx1 lineage tracing experiments show that primitive IMs initially derive from the fetal yolk sac during embryogenesis, followed by a second wave of definitive IMs arising from bone‐marrow precursors. A small portion of these IMs persist through to adulthood and are localized in the perivascular mesothelium. However, mouse parabiosis experiments suggest that this pool is mostly maintained by circulating monocyte precursors.12 Adoptive transfer experiments demonstrate continual monocyte migration into lung tissue regardless of inflammation,45 and transcriptomic analyses confirm the expression of genes which repress self‐renewal (Maf and Mafb), as well as monocyte‐related genes CD14, CD163 and Csfr1.46 Hence, in contrast to AMs, IMs appear to rely on replenishment by blood monocytes at steady state.
Recent work suggests that multiple IM subsets exist in the lung that differ in their location, function and longevity. Gibbings et al.46 described three resident IM populations within the murine lung parenchyma, although their interrelatedness and roles in immunoregulation or disease were not clear. Subsequently, Chakarov et al. confirmed two genetically and phenotypically distinct monocyte‐derived IM populations in mice, distinguished by their expression of MHCII and CX3CR1 (Lyve1lo MHCIIhi CX3CR1hi and Lyve1hi MHCIIlo CX3CR1lo). Fate‐mapping experiments show that these cells derived from independent lineages, and differed in their function and localization within the lung.47 Importantly, similar populations were identified in human lung‐tissue samples by transcriptomics. These distinct lung IMs have been independently confirmed by single‐cell transcriptomics and can be further distinguished by expression of the mannose receptor CD206.48 IMs expressing CD206+ (MHCIIlo CX3CR1lo), are peribronchial, involved in immunoregulation, wound healing and repair, and are self‐sustaining over an extended period. The CD206− MHCIIhi CX3CR1lo IMs, on the other hand, are involved in antigen presentation, associate with the alveolar interstitium, and, although also long lived in the lung, appear to be continually replenished for extravasated blood monocytes.48 More work is needed to understand the different IM populations present in the lung, which may be further complicated by the fact that IMs appear to be highly plastic and to adapt their phenotype and function in response to the unique disease environment.49
Irrespective of their origin, IMs play a key role in immunoregulation within the lung, and they are important sources of immunoregulatory cytokines at steady state.43, 50, 51, 52 IMs express IL‐10 both constitutively47 and after exposure to environmental stimuli, such as unmethylated CpG DNA and lipopolysaccharide from bacteria43, 44, 49 (Fig. 1). Indeed, this latter property may be central to the reduced risk of asthma development in microbe‐rich environments.44 IM‐secreted IL‐10 has also been implicated in limiting T helper type 2 (Th2) allergic inflammation43 and neutrophilic asthma.51 Aside from their production of immunoregulatory cytokines, lung Lyve1hi MCHIIlo CX3CR1lo IMs express genes linked with wound healing and repair. Depletion of this subset was associated with increased fibrosis, highlighting an important antifibrotic role, probably by preventing excessive immune cell infiltration.47 Although not as phagocytic as AM, a number of studies have demonstrated the ability of IMs to phagocytose small particles,43, 53 which is enhanced upon exposure to lipopolysaccharide along with their chemotaxis and ability to produce reactive oxygen species.50 The surface expression of MHC‐II in mice44, 46, 47, 51 and HLA‐DR in humans52 by IM also suggests a role in antigen presentation; and in co‐culture experiments, IMs possess superior antigen‐presentation capacities compared with AMs, and drive both T‐cell proliferation and Treg cell differentiation.47
Overall, our understanding of IM is not as advanced as that of AM, and more work is needed to unpick this complex and highly heterogeneous tissue cell type, particularly the importance of the tissue‐resident fraction.
Innate lymphoid cells
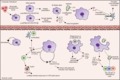
Innate lymphoid cells (ILCs) are another innate immune subset that is important for maintaining tissue homeostasis within the lung (Fig. 2). These diverse lymphoid cells share many functional characteristics of their T‐cell counterparts, but lack antigen‐specific receptors and respond primarily to locally secreted cytokines. ILCs mediate protective immunity from pathogens and parasites and promote tissue repair and homeostasis following infections. However, when their functions become dysregulated, ILCs may also play roles in pathogenesis.54, 55, 56 Based on functional characteristics, ILCs have been designated into three subsets (ILC1s, ILC2s and ILC3s), which are roughly analogous to Th1, Th2 and Th17/22 cells.57 However, ILCs remain somewhat plastic and can alter their phenotype and function in response to signals from their surrounding tissue microenvironments.58, 59 Importantly, although ILCs do circulate in peripheral blood, they are considered to have an extreme ‘sedentary’ lifestyle, and are maintained by self‐renewal in broadly different tissue microenvironments and physiological settings; consistent with their proposed roles as sentinels and local keepers of tissue function.60, 61 Most ILC pathways and functions have been elucidated in the mouse model; however, two studies have characterized pulmonary ILCs in humans.62, 63
Figure 2.

Innate lymphoid cells (ILCs) and natural killer (NK) cells are important tissue‐resident cells and mediators of homeostasis within the lung. NK cells are recruited during infection, although potentially resident populations have been described. NK cells are cytolytic cells that produce granzymes that mediate killing of infected macrophages. NK cells themselves are susceptible to direct infection by influenza but remain important producers of interferon‐γ (IFN‐γ), which contributes to inflammation. ILC1s are non‐cytotoxic counterparts of NK cells. That produce IFN‐γ and are important mediators of inflammation and viral clearance. Dendritic cell (DC) ‐derived interleukin‐12 (IL‐12) and IL‐18 can transition ILC2s into ‘ILC1‐like’ cells which may play a pathological role in chronic obstructive pulmonary disease. ILC2s produce type 2 cytokines IL‐5 and IL‐13, which are important in allergic responses and helminth clearance in the lung. They also facilitate tissue repair through their production of amphiregulin. ILC3s are the most abundant ILC subset in the human lung. IL‐22 producing ILC3s are important for bacterial clearance and tissue repair, while IL‐17 from ILC3s contributes to inflammation but may also have an important role in protection from extracellular bacteria and fungi.
ILC1s
ILC1s and cytotoxic natural killer (NK) cells have shared developmental pathways and are considered to fall under the same immunological lineage 57; however, as NK cells have been long described and ILC1s are a relatively new player, here we will review cytotoxic ILC1s (‘NK cells’) and non‐cytotoxic ILC1s (referred to as ‘ILC1s’) separately. Despite only representing a minor fraction of total ILCs in the lung,58, 64 there is evidence that ILC1s play a role in immunosurveillance and infection control. In mice infected with H1N1 influenza virus, ILC1s become activated and produce interferon‐γ (IFN‐γ) and tumour necrosis factor‐α as early as day 3, suggesting a role in initiating the early response to infection;65 and transfer of ILC1s to lymphocyte‐deficient mice (Rag2−/− γC−/−), reduces viral titres in the lung. The concept of ILC1s as important early producers of IFN‐γ in tissue is supported by a recent study showing depletion of ILC1s from T‐cell‐deficient mice increased titres of another respiratory virus, Sendai virus in the lung following nasal challenge. Of all the known IFN‐γ‐producing lymphocyte subsets in the lung, including NK cells and Trm CD8s, only ILC1s, were found to produce significant amounts of this cytokine early in infection with Sendai virus or PR8 influenza.66 In both studies, ILC1s are probably responding to IL‐12 or IL‐18 produced by lung‐resident dendritic cell (DC) subsets. In addition, these cytokines have been shown to drive transition of ILC2 into ‘ILC1‐like’ during influenza infection, and other canonical triggers of COPD including bacterial infection and cigarette smoke.59 Interestingly, the frequency of ‘ILC1‐like’ cells is increased in COPD patients and inversely correlates with disease severity and lung function, suggesting a pathological role for these cells in human COPD. Finally, IFN‐γ‐producing ILCs, either from the ILC1 lineage or transitional ILC2 subsets, also play a role in shaping vaccine responses in mice,67 suggesting that this subset may be leveraged during vaccination. The precise mechanisms are unclear, but ILC1s may mediate crosstalk between DCs and CD8+ T cells, as co‐culturing with ILC1 leads to DC maturation/activation and increased IFN‐γ production by T cells during in vitro influenza challenge.65
ILC2s
As the name might suggest, ILC2s phenotypically and functionally mirror Th2 cells, and produce type 2 cytokines IL‐13, IL‐5 and IL‐4 in response to IL‐25, IL‐33 and thymic stromal lymphopoeitin (TSLP).57 Consequently, like their Th2 counterparts, they are important in allergic responses, asthma and the clearance of helminth infections from the lung. ILC2s in the lung constitutively express IL‐5 and are induced to secrete IL‐13 under inflammatory conditions, resulting in eotaxin production and thereby controlling local eosinophil accumulation,68 a key immune subset in allergy, asthma and response to multicellular pathogens. In the mouse model of allergic asthma, expansion of ILC2 numbers leads to increased IL‐5 and IL‐13 in the lung, which in turn exacerbates allergic inflammation and smooth muscle tissue hyperreactivity,69 and mucus hyperproduction.70 Although Th2 cells produce the same cytokines, ILC2s appear to be important, as Rag2−/− γ −/− mice experience reduced allergic inflammation compared with Rag2−/− mice (which retain ILCs).71 Indeed, as with ILC1s, there is probably important crosstalk between ILC2s and other immune subsets during airway inflammation. The Th2 response to inhaled allergens, for example, is enhanced by the presence of ILC2s.71 Unsurprisingly, data from humans are more sparse, but ILC2s are enriched in sputum from patients with asthma,72 and have been shown to produce large amounts of type 2 cytokines.73 Moreover, the frequency of ILC2s in bronchoalveolar lavage fluid was found to correlate inversely with lung function.74
The role of ILC2s in the immune response to multicellular parasites is best studied in Nippostrongylus brasiliensis infection.55 In response to this parasite, secretion of IL‐25 and IL‐33 by epithelial cells triggers a robust expansion of lung ILC2s and production of IL‐13; which alone is sufficient to mediate worm clearance.75 More recently, it was found that IL‐33 specifically up‐regulates OX40L on ILC2s, which is essential for licensing the required Th2 and Treg response.76 Ablation of OX40L on ILC2s alone prevented the development of effective Th2 immunity and pathogen clearance.
As mentioned, ILC2s are important mediators of lung tissue repair after infection. Monticelli et al. 55 were the first to demonstrate this in a mouse model of influenza in which depletion of ILC2s was associated with poorer lung function and failure to restore epithelial integrity. The tissue restorative functions of ILC2s were attributed to amphiregulin and its production by ILC2s was found to be increased in the lungs of mice with helminth infection.77 ILC2s may also limit tissue damage through secretion of IL‐9, which protects against endothelial cell death, limits lung inflammation during sepsis,78 and stimulates ILC2s to secrete IL‐13 and IL‐5 in an autocrine loop.79 ILC2s are the main secretors of IL‐9 in the lung, and IL‐9 receptor expression on ILC2s is required for their engagement in tissue repair following lung damage, including secretion of amphiregulin.77 However, the role of ILC2s in lung tissue repair is clearly context dependent, because ILC2 secretion of IL‐13 can directly reduce bronchial epithelial barrier integrity by impairing tight junction formation.80
ILC3s
ILC3s are defined by their expression of the retinoic acid receptor‐related orphan receptor γt transcription factor (RORγt), and consequently are functionally similar to Th17/Th22 cells. They appear to be the most abundant ILC group in the human lung,62 but studies highlighting the role of ILC3s in the lung are limited. However, because they rapidly secrete both IL‐17 and IL‐22, key molecules in pulmonary immunity, they are likely to be important to lung health.81 ILC3s are in fact the major producers of IL‐22 in the lung,82, 83 and consequently are probably important for tissue repair following viral infection. Genetic IL‐22 knockout mice, for example, had impaired lung epithelial regeneration following influenza infection, which could be restored through adoptive transfer of ‘ILC3‐like’ CD3− NCR1+ NK1.1+ cells.84 IL‐22‐producing ILC3s are also important for clearance of bacterial infections, such as S. pneumoniae. Indeed, boosting of IL‐22 production by exogenous administration of flagellin allowed mice to clear an otherwise lethal infection.82 Both IL‐22 and IL‐17 are important in protecting mice from hypervirulent strains of M tuberculosis,85, 86 and IL‐22 from CD3− CD56+ ILC3‐like cells inhibits M. tuberculosis growth through enhanced phagolysosomal fusion.87 Consistent with this, we recently showed that ILC3s play an important role in the immune response to M. tuberculosis infection. As with many of the examples cited above, timing is probably a key factor in their importance, as ILCs are the earliest responders to infection and appear to help orchestrate the subsequent immune response.88 Various ILC3 knock‐out mouse models display reduced control of early bacteraemia, which could be restored by adoptive transfer of purified ILCs.
Interleukin‐17 production by ILC3s may also play an important role in protection from extracellular bacteria and fungi.81 In mice infected with Klebsiella pneumoniae, IL‐17‐secreting ILC3s were essential for bacterial clearance,89 and ILC3‐derived IL‐17 is required for survival in mice infected with Pseudomonas aeruginosa, a pulmonary pathogen commonly associated with cystic fibrosis and COPD.90 ILC3s can also drive inflammatory responses, as IL‐17+ ILC3s mediate airway hyperresponsiveness in both obesity‐linked asthma91 and allergic asthma induced by house dust mite challenge.92 In humans with severe asthma, populations of IL17+ ILC3s are enriched in bronchoalveolar lavage samples91 and ILC3 gene signatures are enriched in nasal brushings from patients with adult‐onset asthma.93
NK cells
NK cells are classified as members of the ILC1 family based on shared transcription factor requirements and IFN‐γ production, but perform important cytolytic functions through their secretion of perforin and granzyme B.94 Initial mapping of NK cells from healthy human tissues demonstrates that the lung contains several distinct populations based on CD56 and NKp46 expression levels.95 Later, Marquardt et al. described lung‐resident NK cells in humans as hyperdifferentiated with a CD56dim CD16+ phenotype and hyporesponsive to target cell stimulation.96, 97 Interestingly, most of these NK cells lack the tissue‐resident marker CD69, which might suggest that they are predominantly recirculating rather than resident within the lung. Indeed, detailed mouse parabiotic experiments found that, unlike other ILC subsets in the lung, NK cells readily recirculate.60 However, other studies have found evidence of a functional and potentially resident NK subset in human lung explants that are CD49a+ CD103+ CD69+ and present in parenchyma, and that degranulate in response to viral infection.98 Additionally, blocking recruitment of circulating NK cells into the lung has no effect on the ability of this cell type to control tumour growth, suggesting both the existence and importance of lung‐resident NK cells.99 Lung NK cells from influenza‐naive Indian rhesus macaques infected with seasonal H1N1 increase early in infection, and up‐regulate CD107a and IFN‐γ.100 Similarly, populations of CD107a+ IFN‐γ + NK cells were also increased in lungs of influenza‐infected mice.101 As with ILC1s, in this setting NKs are important early producers of IFN‐γ that limit initial disease severity, although they are ultimately not required for viral clearance.102 Indeed, NK cells have also been observed to exacerbate pathology during high‐dose influenza infection,103 and may themselves be susceptible to influenza infection, causing reduced cytotoxicity and production of pro‐inflammatory cytokines.104 In humans, lung‐associated NK cells respond to influenza A infection by up‐regulating degranulation/cytotoxic activation marker CD107a and produce granzyme B and IFN‐γ, which mediates killing of infected macrophages.98
Like ILC1s, lung NK cells may contribute to chronic inflammatory disorders, such as COPD and asthma through production of inflammatory cytokines. CD56+ CD16+ NK cells isolated from the airways of COPD patients have increased natural cytotoxicity in comparison to those from controls,93 and demonstrate increased killing of autologous lung cells in co‐culture experiments.105 Recently, Finch et al. 106 showed increased killing of lung epithelial cells by lung NK cells, but not blood NK cells, from patients with COPD was due to DC–NK cell interactions and IL‐15a transpriming. In individuals with asthma, NKs cells in bronchoalveolar lavage fluid are skewed to a cytolytic phenotype and express higher levels of granzyme A.107 However, the importance of lung‐resident NK cells, compared with recruited NKs overall, remains unclear.
Dendritic cells
Dendritic cells are professional antigen‐presenting cells, whose primary role in the respiratory tract is to sample inhaled pathogens before migration to lymph nodes where they present processed peptides to antigen‐specific T cells (Fig. 3). DCs must be continually replaced by new recruits from the bone marrow and are not a self‐replenishing tissue‐resident population in the strictest sense. However experiments with parabiosis and cell tracking demonstrate that the turnover of lung DCs is considerably lower than that of other non‐lymphoid tissues16, 108; and lung DCs have been observed presenting antigen for up to 8 weeks after exposure.109 In addition, there is evidence of bone‐marrow‐derived preDCs in the lung, which give rise to CD103+ DCs in other non‐lymphoid tissues, although not specifically shown in the lung.108 Given the longevity and the potential existence of DC precursors, it is probably helpful to think of lung DCs as distinctive subsets, even if they do not display the same ‘extreme sedentary’ lifestyle as lung ILCs or macrophages.
Figure 3.
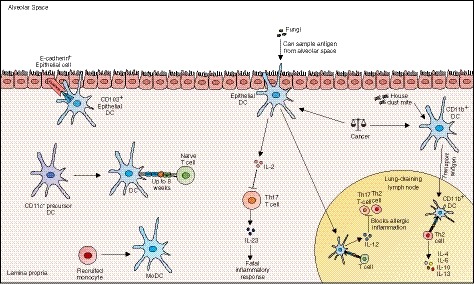
Dendritic cells (DCs) are professional antigen‐presenting cells that sample and present antigen to T cells within the lamina propria and associated lymph nodes. Although lung DCs may not be tissue‐resident in the strictest sense (because they do not appear to self‐renew), they can arise from precursor cells and persist in the lung for up to 8 weeks. DCs of the lung are heterogeneous and different subsets have different immunogenic functions. Epithelial DCs express CD103 and sample antigen from the alveolar space. These cells limit allergic inflammation through interleukin‐12 (IL‐12) and prevent fatal inflammatory responses via IL‐2. CD11b+ DCs traffic antigen to the lymph node where they coordinate T helper type 2 responses to house dust mite challenge. In cancer, the balance between CD103+ DCs and CD11b+ DCs is important and is being exploited in novel treatment strategies.
Although the heterogeneity of DCs in the lung is still being uncovered, they broadly fit into three subsets: plasmacytoid DCs (pDCs) and myeloid DCs, which are further subdivided into cDC1 and cDC2, which all develop under the control of key transcription factors (an detailed review is given in ref. 110). The cDC1s closely associate with the airway epithelium and are distinguished by expression of CD103, whereas cDC2s lack CD103 but express CD11b and, like pDCs, are mainly found in the lung interstitium.111 The cDC1s are specialized to sample antigen from the alveolar space, but all DC subsets, including pDCs, are able to transport antigen to draining lymph nodes.112, 113 In addition, DCs can rapidly mature from circulating monocytes recruited during injury and inflammation, and distinguishing monocyte‐derived DCs and tissue‐resident DCs can be a challenge, particularly in humans.16, 114 It is also important to note that the nomenclature can be confusing because definitions have varied over time, and the terms cDC and myeloid DC are used to describe the same cells.
Data from experimental mouse models with house dust mite (HDM) challenge demonstrate the complex roles that DCs play in lung inflammation, and the importance of different DC subsets in determining the subsequent T‐cell response. CD11b‐expressing cDC2s, for example, readily take up allergens and are responsible for generating robust Th2 and Th17 responses to HDM challenge,115, 116, 117 through co‐stimulatory molecules and secretion of cytokines and chemokines. CD103+ cDC1s in contrast, are important for limiting allergic inflammation in the context of chronic HDM exposure, by regulating both Th2 and Th17 immune responses via production of IL‐12.118 This subset is also able to induce Treg cells in response HDM via retinoic acid signalling.119 The pDCs may also play a tolerogenic role in allergic lung inflammation, at least in part through up‐regulation of the T‐cell inhibitory ligand PD‐L1.120 However, more recently, pDCs have been implicated in driving both allergen‐ and virus‐induced asthma in both animal models and humans through potentiation of the Th2 response.121 In humans, both cDC1s and cDC2s are reported to be expanded in the lungs of individuals with asthma following allergen challenge, and promote Th2 and Th9 responses, although this is not always consistent.122, 123, 124, 125 Interestingly, asthma in humans has been linked to a lack of exposure to environmental bacteria such as Helicobacter pylori, and sensitization of mice with H. pylori extract prevents subsequent allergic inflammation. Importantly, CD103+ cells accumulate during sensitization and are strictly required for a protective effect,126 suggesting a potentially similar role of these cells in humans.
The distinct functional roles of lung DC subsets can also be seen in the context of infections, such as influenza, where CD103+ cDCs are responsible for generating effector CD8 T cells in the lung,127 while CD11b+ cDCs establish long‐lived memory subsets.128 Lung pDCs, which are generally considered poor at priming T cells, play an important role in the antiviral response by producing copious amounts of type I interferon.129 CD103+ DCs alone appear to orchestrate the appropriate Th17 response to fungal infection through production of IL‐2 and IL‐23.130
Another important aspect of lung health for which DC appear to play a central role is that of cancer. DCs take up necrotic and apoptotic tumour fragments to present to antigen‐specific cytotoxic and helper T cells, and are therefore important providers of protective anti‐cancer immunity.131, 132 Cell‐tracking experiments in murine melanoma tumour models show that CD103+ DCs are the only intra‐tumoral myeloid cell type able to transport and present intact antigen to tumour‐specific CD8+ T cells and their activation was protective against tumour re‐challenge.133 However, these cells are rare and transcriptomic analysis of lung cancers and peritumoral tissues from mice demonstrate that the majority of infiltrating myeloid cells are actually CD11b+ DCs. These cells strongly up‐regulate expression of PD‐L1 and are associated with cancer growth, suggesting that lung tumours can actually exploit DC flexibility to promote an environment supportive of cancer progression.130, 131 The importance of DCs in lung cancer is demonstrated by on‐going efforts to harness these cells for therapeutic purposes. In a phase III control trial, non‐small‐cell lung cancer (NSCLC) patients receiving immunotherapy (consisting of adoptive transfer of autologous DCs and activated T cells from the patients’ own lymph nodes) in addition to chemotherapy, had increased survival rates in comparison to those receiving chemotherapy alone.134 In a more recent phase I trial, NSCLC patients receiving autologous genetically modified DCs (overexpressing lymphoid‐tissue organizing chemokine CCL21) had better anti‐tumour immunity, CD8 T‐cell infiltration and increased tumour PD‐L1 expression.135 Recently in mice, adoptive transfer of CD1d+ DCs led to T‐cell activation and cytotoxicity, and reduced tumour growth. In humans, CD1d expression has also been associated with better clinical outcomes.136 Hence, DC‐based immunotherapy is a promising new strategy against lung cancer; however, the interactions between lung tumours and DCs are complex and the safe and effective manipulation of this system will require extensive understanding.
Concluding remarks
The unique environment of the lung is protected through complex immune interactions. Cells of the innate immune system provide the first lines of defence against inhaled pathogens and toxins through direct mechanisms and orchestrate the adaptive immune system through crosstalk. Innate immune cells respond to non‐antigen‐specific signals from their surrounding environment, allowing them to act rapidly in the presence of a threat. However, these prompt responses come at a cost, as they sometimes mount disproportionate responses against harmless particles – resulting in excessive inflammation, fibrosis and even tissue damage. Innate immune cells are often described as a double‐edged sword, necessary for host defence, but also drivers of pathogenesis, their exact role is often dependent on context. In this sense, understanding the importance of the tissue‐resident innate immune subset is key and may provide unique opportunities for beneficial modulation of the host immune response. Animal models are valuable tools but are not always translatable to humans, and human studies have often focused on the peripheral blood. Growing efforts to study lung‐resident immune populations in humans are providing essential evidence as to how the immune system functions within the lung tissue environment and will be key to developing novel treatment and disease prevention strategies in future.
Funding
AL is supported by the WT (210662/Z/18/Z).
Disclosure
The authors have no conflict of interest to report.
References
- 1. Saluzzo S, Gorki A‐D, Rana BMJ, Martins R, Scanlon S, Starkl P et al First‐breath‐induced type 2 pathways shape the lung immune environment. Cell Rep 2017; 18:1893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suarez‐Ramirez JE, Chandiran K, Brocke S, Cauley LS. Immunity to respiratory infection is reinforced through early proliferation of lymphoid TRM cells and prompt arrival of effector CD8 T cells in the lungs. Front Immunol 2019; 10:1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol 2018; 11:249–56. [DOI] [PubMed] [Google Scholar]
- 4. Benoun JM, Peres NG, Wang N, Pham OH, Rudisill VL, Fogassy ZN et al Optimal protection against Salmonella infection requires noncirculating memory. Proc Natl Acad Sci USA 2018; 115:10416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allie SR, Bradley JE, Mudunuru U, Schultz MD, Graf BA, Lund FE et al The establishment of resident memory B cells in the lung requires local antigen encounter. Nature Immunol 2019; 20:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chun Chou MOL. Tissue‐resident lymphocytes across innate and adaptive lineages. Front Immunol 2018; 9:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Furth R. Origin and kinetics of mononuclear phagocytes. Ann NY Acad Sci 1976; 278:161–75. [DOI] [PubMed] [Google Scholar]
- 8. Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D et al Lung‐resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016; 126:3279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barletta KE, Cagnina RE, Wallace KL, Ramos SI, Mehrad B, Linden J. Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J Immunol Methods 2012; 375:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlens J, Wahl B, Ballmaier M, Bulfone‐Paus S, Förster R, Pabst O. Common γ‐chain‐dependent signals confer selective survival of eosinophils in the murine small intestine. J Immunol 2009; 183:5600–7. [DOI] [PubMed] [Google Scholar]
- 11. Yipp BG, Kim JH, Lima R, Zbytnuik LD, Petri B, Swanlund N et al The lung is a host defense niche for immediate neutrophil‐mediated vascular protection. Sci Immunol 2017; 2 pii: eaam8929. doi: 10.1126/sciimmunol.aam8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan SYS, Krasnow MA. Developmental origin of lung macrophage diversity. Development 2016; 143:1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P et al C‐Myb+ erythro‐myeloid progenitor‐derived fetal monocytes give rise to adult tissue‐resident macrophages. Immunity 2015; 42:665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi K, Yamamura F, Naito M. Differentiation maturation and proliferation of macrophages in the mouse yolk Sac: a light‐microscopic, enzyme‐cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol 1989; 45:87–96. [DOI] [PubMed] [Google Scholar]
- 15. Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S et al Alveolar macrophages develop from fetal monocytes that differentiate into long‐lived cells in the first week of life via GM‐CSF. J Exp Med 2013; 210:1977–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kopf M, Schneider C, Nobs SP. The development and function of lung‐resident macrophages and dendritic cells. Nat Immunol 2014; 16:36–44. [DOI] [PubMed] [Google Scholar]
- 17. Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B et al The cytokine TGF‐β promotes the development and homeostasis of alveolar macrophages. Immunity 2017; 47:903–4. [DOI] [PubMed] [Google Scholar]
- 18. Gardai SJ, Xiao Y‐Q, Dickinson M, Nick JA, Voelker DR, Greene KE et al By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003; 115:13–23. [DOI] [PubMed] [Google Scholar]
- 19. Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L et al A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol 2008; 9:1074–83. [DOI] [PubMed] [Google Scholar]
- 20. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M et al Tissue‐resident macrophages self‐maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013; 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Misharin AV, Morales‐Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie‐Pimentel AC et al Monocyte‐derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 2017; 214:2387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strickland D, Kees UR, Holt PG. Regulation of T‐cell activation in the lung: isolated lung T cells exhibit surface phenotypic characteristics of recent activation including down‐modulated T‐cell receptors, but are locked into the G0/G1 phase of the cell cycle. Immunology 1996; 87:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G et al Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 1993; 177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 1989; 170:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF et al Lung‐resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med 2013; 210:775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS et al Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 2014; 506:503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bourdonnay E, Zasłona Z, Penke LRK, Speth JM, Schneider DJ, Przybranowski S et al Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med 2015; 212:729–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldstein E, Lippert W, Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest 1974; 54:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Green GM, Kass EH. The, role of, the alveolar macrophage in the clearance of bacteria from the lung. J Exp Med 1964; 119:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jubrail J, Kurian N, Niedergang F. Macrophage phagocytosis cracking the defect code in COPD. Biomed J 2017; 40:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hodge S, Hodge G, Jersmann H, Matthews G, Ahern J, Holmes M et al Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 178:139–48. [DOI] [PubMed] [Google Scholar]
- 32. Vermeersch K, Gabrovska M, Aumann J, Demedts IK, Corhay J‐L, Marchand E et al Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalization (BACE). A multicenter, randomized. Double‐Blind, Placebo‐controlled Trial. Am J Respir Crit Care Med 2019; 200:857–68. [DOI] [PubMed] [Google Scholar]
- 33. Thuong NTT, Tram TTB, Dinh TD, Thai PVK, Heemskerk D, Bang ND et al MARCO variants are associated with phagocytosis, pulmonary tuberculosis susceptibility and Beijing lineage. Genes Immun 2016; 17:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K et al The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 2004; 200:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez N, Ketheesan N, West K, Vallerskog T, Kornfeld H. Impaired recognition of Mycobacterium tuberculosis by alveolar macrophages from diabetic mice. J Infect Dis 2016; 214:1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin‐1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol 2006; 176:3717–24. [DOI] [PubMed] [Google Scholar]
- 37. Dubourdeau M, Athman R, Balloy V, Huerre M, Chignard M, Philpott DJ et al Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J Immunol 2006; 177:3994–4001. [DOI] [PubMed] [Google Scholar]
- 38. Zhang H‐J, Qu J‐M, Shao C‐Z, Zhang J, He L‐X, Yuan Z‐H. Aspergillus fumigatus conidia upregulates NOD2 protein expression both in vitro and in vivo . Acta Pharmacol Sin 2008; 29:1202–8. [DOI] [PubMed] [Google Scholar]
- 39. Fitzpatrick AM, Holguin F, Teague WG, Brown LAS. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol 2008; 121: 1372–8.e1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen‐driven escape mechanism? Front Immunol 2014; 5:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol 2007; 179:3488–94. [DOI] [PubMed] [Google Scholar]
- 42. Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol 2014; 192:2821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E et al Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest 2009; 119:3723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C et al Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity 2017; 46:457–73. [DOI] [PubMed] [Google Scholar]
- 45. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006; 7:311–7. [DOI] [PubMed] [Google Scholar]
- 46. Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T et al Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol 2017; 57:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J et al Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019; 363 pii: eaau0964. doi: 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- 48. Schyns J, Bai Q, Ruscitti C, Radermecker C, Schepper S, Chakarov S et al Non‐classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat Commun 2019; 10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schyns J, Bureau F, Marichal T. Lung interstitial macrophages: past, present, and future. J Immunol Res 2018; 2018:5160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wizemann TM, Laskin DL. Enhanced phagocytosis, chemotaxis, and production of reactive oxygen intermediates by interstitial lung macrophages following acute endotoxemia. Am J Respir Cell Mol Biol 1994; 11:358–65. [DOI] [PubMed] [Google Scholar]
- 51. Kawano H, Kayama H, Nakama T, Hashimoto T, Umemoto E, Takeda K. IL‐10‐producing lung interstitial macrophages prevent neutrophilic asthma. Int Immunol 2016; 28:489–501. [DOI] [PubMed] [Google Scholar]
- 52. Hoppstädter J, Diesel B, Zarbock R, Breinig T, Monz D, Koch M et al Differential cell reaction upon Toll‐like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir Res 2010; 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fathi M, Johansson A, Lundborg M, Orre L, Sköld CM, Camner P. Functional and morphological differences between human alveolar and interstitial macrophages. Exp Mol Pathol 2001; 70:77–82. [DOI] [PubMed] [Google Scholar]
- 54. Satoh‐Takayama N, Vosshenrich CAJ, Lesjean‐Pottier S, Sawa S, Lochner M, Rattis F et al Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008; 29:958–70. [DOI] [PubMed] [Google Scholar]
- 55. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA et al Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ et al Innate lymphoid cells drive interleukin‐23‐dependent innate intestinal pathology. Nature 2010; 464:1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol 2013; 13:145–9. [DOI] [PubMed] [Google Scholar]
- 58. Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K et al IL‐1β, IL‐4 and IL‐12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol 2016; 17:636–45. [DOI] [PubMed] [Google Scholar]
- 59. Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L et al Erratum: Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol 2016; 17:1005. [DOI] [PubMed] [Google Scholar]
- 60. Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015; 350:981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheng H, Jin C, Wu J, Zhu S, Liu Y‐J, Chen J. Erratum to: Guards at the gate: physiological and pathological roles of tissue‐resident innate lymphoid cells in the lung. Protein Cell 2017; 8:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grove KC, Provoost S, Verhamme FM, Bracke KR, Joos GF, De Maes T. Characterization and quantification of innate lymphoid cell subsets in human lung. PLoS ONE 2016; 11:e0145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Simoni Y, Fehlings M, Kloverpris HN, McGovern N, Koo S‐L, Loh CY et al Human innate lymphoid cell subsets possess tissue‐type based heterogeneity in phenotype and frequency. Immunity 2017; 46:148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stehle C, Hernández DC, Romagnani C. Innate lymphoid cells in lung infection and immunity. Immunol Rev 2018; 286:102–19. [DOI] [PubMed] [Google Scholar]
- 65. Vashist N, Trittel S, Ebensen T, Chambers BJ, Guzmán CA, Riese P. Influenza‐activated ILC1s contribute to antiviral immunity partially influenced by differential GITR expression. Front Immunol 2018; 9:75–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weizman O‐E, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C et al ILC1 confer early host protection at initial sites of viral infection. Cell 2017; 171:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Z, Jackson RJ, Ranasinghe C. Vaccination route can significantly alter the innate lymphoid cell subsets: a feedback between IL‐13 and IFN‐γ . npj Vaccines 2019; 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB et al Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013; 502:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wolterink R, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y Klein Pulmonary innate lymphoid cells are major producers of IL‐5 and IL‐13 in murine models of allergic asthma. Eur J Immunol 2018; 42:1106–16. [DOI] [PubMed] [Google Scholar]
- 70. Chang Y‐J, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE et al Innate lymphoid cells mediate influenza‐induced airway hyper‐reactivity independently of adaptive immunity. Nat Immunol 2011; 12:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Halim TYF, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic‐acid‐receptor‐related orphan nuclear receptor α is required for natural helper cell development and allergic inflammation. Immunity 2012; 37:463–74. [DOI] [PubMed] [Google Scholar]
- 72. Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol 2016; 137:624–6. [DOI] [PubMed] [Google Scholar]
- 73. Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria J‐P, O'Byrne PM et al Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol 2016; 137:75–8. [DOI] [PubMed] [Google Scholar]
- 74. Liu T, Wu J, Zhao J, Wang J, Zhang Y, Liu L et al Type 2 innate lymphoid cells: a novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med 2015; 109:1391–6. [DOI] [PubMed] [Google Scholar]
- 75. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA et al Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature 2010; 464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE et al Tissue‐restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on Group 2 innate lymphoid cells. Immunity 2018; 48:1195–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Turner J‐E, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld J‐C et al IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med 2013; 210:2951–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lai D, Tang J, Chen L, Fan EK, Scott MJ, Li Y et al Group 2 innate lymphoid cells protect lung endothelial cells from pyroptosis in sepsis. Cell Death Dis 2018; 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang H‐E, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4‐IL‐9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol 2016; 9:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Rückert B et al Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol 2017; 139:93–103. [DOI] [PubMed] [Google Scholar]
- 81. Gurczynski SJ, Moore BB. IL‐17 in the lung: the good, the bad, and the ugly. Am Cell Mol Physiol 2017; 314:L6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Van Maele L, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E et al Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis 2014; 210:493–503. [DOI] [PubMed] [Google Scholar]
- 83. Guo H, Topham DJ. Interleukin‐22 (IL‐22) production by pulmonary Natural Killer cells and the potential role of IL‐22 during primary influenza virus infection. J Virol 2010; 84:7750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL‐22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol 2013; 6:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko A et al Unexpected role for IL‐17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 Infection. PLoS Pathog 2014; 10:e1004099–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Treerat P, Prince O, Cruz‐Lagunas A, Muñoz‐Torrico M, Salazar‐Lezama MA, Selman M et al Novel role for IL‐22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol 2017; 10:1069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LVM et al IL‐22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 2009; 183:6639–45. [DOI] [PubMed] [Google Scholar]
- 88. Ardain A, Domingo‐Gonzalez R, Das S, Kazer SW, Howard NC, Singh A et al Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature, 2019: 570:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG. Innate lymphocyte/Ly6Chi monocyte crosstalk promotes Klebsiella pneumoniae Clearance. Cell 2016; 165:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bayes HK, Ritchie ND, Evans TJ, McCormick BA. Interleukin‐17 is required for control of chronic lung infection caused by Pseudomonas aeruginosa . Infect Immun 2016; 84:3507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim HY, Lee HJ, Chang Y‐J, Pichavant M, Shore SA, Fitzgerald KA et al Interleukin‐17–producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity‐associated airway hyperreactivity. Nat Med 2014; 20:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Everaere L, Ait‐Yahia S, Molendi‐Coste O, Vorng H, Quemener S, LeVu P et al Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J Allergy Clin Immunol 2016; 138:1309–11. [DOI] [PubMed] [Google Scholar]
- 93. Hekking P‐P, Loza MJ, Pavlidis S, de Meulder B, Lefaudeux D, Baribaud F et al Pathway discovery using transcriptomic profiles in adult‐onset severe asthma. J Allergy Clin Immunol 2018; 141:1280–90. [DOI] [PubMed] [Google Scholar]
- 94. Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K et al Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013; 14:221–9. [DOI] [PubMed] [Google Scholar]
- 95. Tomasello E, Yessaad N, Gregoire E, Hudspeth K, Luci C, Mavilio D et al Mapping of NKp46+ cells in healthy human lymphoid and non‐lymphoid tissues. Front Immunol 2012; 3: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wissinger E, Goulding J, Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol 2009; 21:147–55. [DOI] [PubMed] [Google Scholar]
- 97. Marquardt N, Kekäläinen E, Chen P, Kvedaraite E, Wilson JN, Ivarsson MA et al Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69−CD56dim cells. J Allergy Clin Immunol 2017; 139:1321–4. [DOI] [PubMed] [Google Scholar]
- 98. Cooper GE, Ostridge K, Khakoo SI, Wilkinson TMA, Staples KJ. Human CD49a+ lung natural killer cell cytotoxicity in response to influenza a virus. Front Immunol 2018; 9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yamamoto Y, Miyazato K, Takahashi K, Yoshimura N, Tahara H, Hayakawa Y. Lung‐resident natural killer cells control pulmonary tumor growth in mice. Cancer Sci 2018; 109:2670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody‐dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol 2013; 87:5512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang J, Li F, Zheng M, Sun R, Wei H, Tian Z. Lung natural killer cells in mice: phenotype and response to respiratory infection. Immunology 2012; 137:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abboud G, Tahiliani V, Desai P, Varkoly K, Driver J, Hutchinson TE et al Natural killer cells and innate interferon gamma participate in the host defense against respiratory vaccinia virus infection. J Virol 2016; 90:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhou G, Juang SWW, Kane KP. NK cells exacerbate the pathology of influenza virus infection in mice. Eur J Immunol 2013; 43:929–38. [DOI] [PubMed] [Google Scholar]
- 104. Guo H, Kumar P, Moran TM, Garcia‐Sastre A, Zhou Y, Malarkannan S. The functional impairment of natural killer cells during influenza virus infection. Immunol Cell Biol 2009; 87:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Freeman CM, Stolberg VR, Crudgington S, Martinez FJ, Han MK, Chensue SW et al Human CD56+ cytotoxic lung lymphocytes kill autologous lung cells in chronic obstructive pulmonary disease. PLoS ONE 2014; 9:e103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Finch DK, Stolberg VR, Ferguson J, Alikaj H, Kady MR, Richmond BW et al Dendritic cells drive natural killer cytotoxicity in chronic obstructive pulmonary disease via IL‐15Rα . Am J Respir Crit Care Med 2018; 198:1140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Duvall MG, Barnig C, Cernadas M, Ricklefs I, Krishnamoorthy N, Grossman NL et al Natural killer cell‐mediated inflammation resolution is disabled in severe asthma. Sci Immunol 2017; 2:eaam5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D et al The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 2009; 206:3115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O’Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity 2002; 16:271–83. [DOI] [PubMed] [Google Scholar]
- 110. Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology 2018; 154:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vroman H, Hendriks RW, Kool M. Dendritic cell subsets in asthma: impaired tolerance or exaggerated inflammation? Front Immunol 2017; 8:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hemann EA, Sjaastad LE, Langlois RA, Legge KL. Plasmacytoid dendritic cells require direct infection to sustain the pulmonary influenza cd8 response. J Virol 2016; 90:2830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kohli K, Janssen A, Förster R. Plasmacytoid dendritic cells induce tolerance predominantly by cargoing antigen to lymph nodes. Eur J Immunol 2016; 46:2659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T et al Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung‐draining lymph nodes. Am J Respir Crit Care Med 2016; 193:614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhou Q, Ho AWS, Schlitzer A, Tang Y, Wong KHS, Wong FHS et al GM‐CSF‐licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis . J Immunol 2014; 193:496–509. [DOI] [PubMed] [Google Scholar]
- 116. Mesnil C, Sabatel CM, Marichal T, Toussaint M, Cataldo D, Drion P‐V et al Resident CD11b+Ly6C– lung dendritic cells are responsible for allergic airway sensitization to house dust mite in mice. PLoS ONE 2012; 7:e53242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco‐Madeira F, Toussaint W et al Conventional and monocyte‐derived CD11b+ dendritic cells initiate and maintain T helper 2 cell‐mediated immunity to house dust mite allergen. Immunity 2013; 38:322–35. [DOI] [PubMed] [Google Scholar]
- 118. Conejero L, Khouili SC, Martínez‐Cano S, Izquierdo HM, Brandi P, Sancho D. Lung CD103+ dendritic cells restrain allergic airway inflammation through IL‐12 production. JCI Insight 2017;2 pii: 90420. doi: 10.1172/jci.insight.90420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Khare A, Krishnamoorthy N, Oriss TB, Fei M, Ray P, Ray A. Cutting edge: inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. J Immunol 2013; 191:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kool M, van Nimwegen M, Willart MAM, Muskens F, Boon L, Smit JJ et al An anti‐inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol 2009; 183:1074–82. [DOI] [PubMed] [Google Scholar]
- 121. Chairakaki A‐D, Saridaki M‐I, Pyrillou K, Mouratis M‐A, Koltsida O, Walton RP et al Plasmacytoid dendritic cells drive acute asthma exacerbations. J Allergy Clin Immunol 2018; 142:542–556. e12. [DOI] [PubMed] [Google Scholar]
- 122. Dua B, Tang W, Watson R, Gauvreau G, O'Byrne PM. Myeloid dendritic cells type 2 after allergen inhalation in asthmatic subjects. Clin Exp Allergy 2014; 44:921–9. [DOI] [PubMed] [Google Scholar]
- 123. Yerkovich ST, Roponen M, Smith ME, McKenna K, Bosco A, Subrata LS et al Allergen‐enhanced thrombomodulin (blood dendritic cell antigen 3, CD141) expression on dendritic cells is associated with a TH2‐skewed immune response. J Allergy Clin Immunol 2009; 123:209–216. e4. [DOI] [PubMed] [Google Scholar]
- 124. El‐Gammal A, Oliveria J‐P, Howie K, Watson R, Mitchell P, Chen R et al Allergen‐induced changes in bone marrow and airway dendritic cells in subjects with asthma. Am J Respir Crit Care Med 2016; 194:169–77. [DOI] [PubMed] [Google Scholar]
- 125. Froidure A, Shen C, Gras D, Van Snick J, Chanez P, Pilette C. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin‐mediated induction of Th2 and Th9 responses. Allergy 2014; 69:1068–76. [DOI] [PubMed] [Google Scholar]
- 126. Engler DB, Reuter S, van Wijck Y, Urban S, Kyburz A, Maxeiner J et al Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3‐dependent dendritic cells and IL‐10. Proc Natl Acad Sci USA 2014; 111:11810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hemann EA, Green R, Turnbull JB, Langlois RA, Savan R, Gale M. Interferon‐λ modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat Immunol 2019; 20:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8+ T cell differentiation by a CD24‐dependent mechanism. Immunity 2014; 40:400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rahmatpanah F, Agrawal S, Jaiswal N, Nguyen HM, McClelland M, Agrawal A. Airway epithelial cells prime plasmacytoid dendritic cells to respond to pathogens via secretion of growth factors. Mucosal Immunol 2019; 12:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zelante T, Wong AYW, Ping TJ, Chen J, Sumatoh HR, Viganò E et al CD103+ dendritic cells control Th17 cell function in the lung. Cell Rep 2015; 12:1789–801. [DOI] [PubMed] [Google Scholar]
- 131. Pyfferoen L, Brabants E, Everaert C, De Cabooter N, Heyns K, Deswarte K et al The transcriptome of lung tumor‐infiltrating dendritic cells reveals a tumor‐supporting phenotype and a microRNA signature with negative impact on clinical outcome. Oncoimmunology 2017; 6:e1253655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang JB, Huang X, Li FR. Impaired dendritic cell functions in lung cancer : a review of recent advances and future perspectives. Cancer Commun 2019; 39:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S et al Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD‐L1 and BRAF Inhibition. Immunity 2016; 44:924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kimura H, Matsui Y, Ishikawa A, Nakajima T, Yoshino M, Sakairi Y. Randomized controlled phase III trial of adjuvant chemo‐immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol Immunother 2015; 64:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lee JM, Lee M‐H, Garon E, Goldman JW, Salehi‐Rad R, Baratelli FE et al Phase I trial of intratumoral injection of CCL21 gene‐modified dendritic cells in lung cancer elicits tumor‐specific immune responses and CD8+ T‐cell infiltration. Clin Cancer Res 2017; 23:4556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li Y, Zhao C, Liu J, Lu Z, Lu M, Gu J et al CD1d highly expressed on DCs reduces lung tumor burden by enhancing antitumor immunity. Oncol Rep 2019; 41:2679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


