Abstract
On May 24, 2019, the Food and Drug Administration approved ruxolitinib for steroid‐refractory acute graft‐versus‐host disease (SR‐aGVHD) in adult and pediatric patients 12 years and older. Approval was based on Study INCB 18424‐271 (REACH‐1; NCT02953678), an open‐label, single‐arm, multicenter trial that included 49 patients with grades 2–4 SR‐aGVHD occurring after allogeneic hematopoietic stem cell transplantation. Ruxolitinib was administered at 5 mg twice daily, with dose increases to 10 mg twice daily permitted after 3 days in the absence of toxicity. The Day‐28 overall response rate was 57.1% (95% confidence interval [CI]: 42.2–71.2). The median duration of response was 0.5 months (95% CI: 0.3–2.7), and the median time from Day‐28 response to either death or need for new therapy for acute GVHD was 5.7 months (95% CI: 2.2 to not estimable). Common adverse reactions included anemia, thrombocytopenia, neutropenia, infections, edema, bleeding, and elevated transaminases. Ruxolitinib is the first drug approved for treatment of SR‐aGVHD.
Implications for Practice
Ruxolitinib is the first Food and Drug Administration–approved treatment for steroid‐refractory acute graft‐versus‐host disease in adult and pediatric patients 12 years and older. Its approval provides a treatment option for the 60% of those patients who do not respond to steroid therapy.
Keywords: Ruxolitinib, Graft‐versus‐host disease, Hematopoietic stem cell transplantation
Short abstract
Ruxolitinib is the first FDA‐approved treatment for steroid‐refractory acute graft‐versus‐host disease. This article provides a summary of the FDA review of ruxolitinib, based on the REACH‐1 Study.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is frequently the only curative option for patients with hematological malignancies, but only about 8,000 patients undergo allogeneic HSCT each year 1. More common use of allogeneic HSCT is limited by the potentially severe or fatal complications, including acute graft‐versus‐host disease (aGVHD); for patients who develop grades 3–4 aGVHD, the Day‐100 mortality is 35% 2. aGVHD arises when donor T cells recognize alloantigens on recipient antigen‐presenting cells. This interaction results in T‐cell activation and secretion of inflammatory cytokines, which trigger a cascade that ultimately results in cell‐mediated and cytokine‐mediated damage to the target organs 3. The major clinical manifestations of aGVHD include skin rash, elevated bilirubin, and enteritis with nausea and diarrhea.
In view of the inflammatory nature of the disorder, a corticosteroid such as methylprednisolone (MP) is the standard first‐line treatment for grades 2–4 aGVHD 4. Approximately 60% of patients with aGVHD, estimated to be up to 2,000 patients annually, do not respond to first‐line treatment or recur after a response and are considered steroid‐refractory (SR) 5, 6. Patients with SR‐aGVHD are generally treated by off‐label use of any of numerous immunosuppressive drugs with varying response rates, but no optimal second‐line treatment has been identified 4, and until recently, there was no drug approved as second‐line treatment of aGVHD.
Janus kinases (JAKs) are intracellular tyrosine kinases that transmit cytokine receptor signaling in concert with a signal transducer and activator of transcription (STAT). The JAK‐STAT pathway is integral to the effects of inflammatory cytokines on the immune system 7. Ruxolitinib (Jakafi, Incyte Corp., Wilmington, DE; Table 1) is an inhibitor of JAK1 and JAK2. In vitro, T cells exposed to ruxolitinib had reduced proliferation and cytokine production in response to alloantigen 8, and in vivo treatment with ruxolitinib reduced GVHD severity in treatment‐naive and steroid‐refractory major histocompatibility complex–mismatched murine models 9. In published case series and retrospective reviews, complete response (CR) rates of 8%–46% and overall response rates (ORR) of 38%–82% were reported for adult and pediatric patients with aGVHD refractory to steroids alone or in addition to other immunosuppressive drugs 8, 10, 11, 12, 13, 14, 15. Herein we provide a summary of the Food and Drug Administration's (FDA's) review of ruxolitinib for treatment of SR‐aGVHD based on the first prospective trial, the REACH‐1 Study.
Table 1.
Ruxolitinib background information
| Structure |
|
| Mechanism of action |
|
| Prior approvals |
|
| New indication | Treatment of steroid‐refractory acute GVHD in adult and pediatric patients 12 years and older. |
| Pharmacokinetics |
|
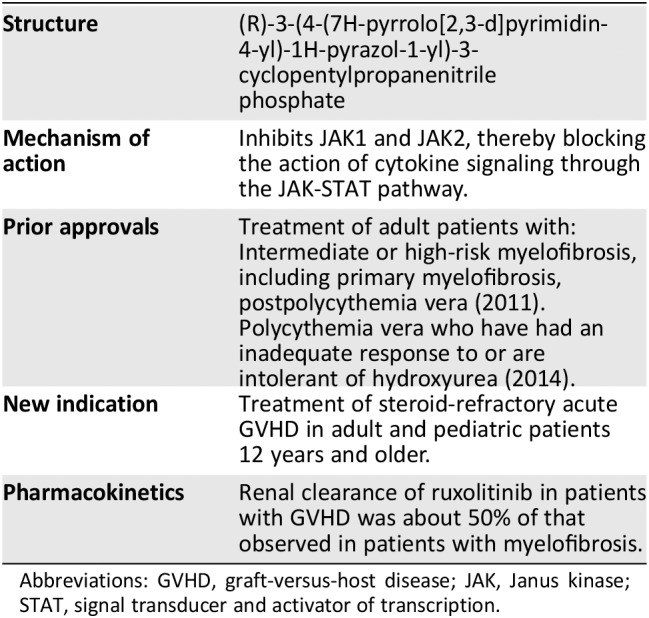
Abbreviations: GVHD, graft‐versus‐host disease; JAK, Janus kinase; STAT, signal transducer and activator of transcription.
Trial Design
Study 271 (REACH‐1 Study; INCB 18424‐271; NCT02953678) was an open‐label, single‐arm, multicenter study of ruxolitinib for treatment of patients 12 years of age or older with grades 2–4 SR‐aGVHD (Mount Sinai Acute GVHD International Consortium [MAGIC] criteria) 16 occurring after allogeneic hematopoietic stem cell transplantation. Patients were to be excluded if they had received more than one systemic treatment in addition to corticosteroids for treatment of aGVHD.
Study treatment consisted of ruxolitinib 5 mg orally twice daily (escalating to 10 mg orally twice daily on day 3 in the absence of ≥50% decrease in platelet count and/or absolute neutrophil count relative to Day 1 or other toxicity) in combination with MP 2.0 mg/kg/day equivalent. Corticosteroids were to be tapered as tolerated (reduction to 0.2 mg/kg/day by day 28 recommended). Ruxolitinib was to be continued to study day 180 and tapered thereafter provided the patient was in CR or very good partial response (VGPR) and corticosteroids had been discontinued for at least 8 weeks. Routine GVHD prophylaxis in use at baseline was continued on study.
The primary endpoint of the study was Day‐28 ORR, including CR, VGPR, or partial response (PR). The statistical analysis plan prespecified that a positive result is concluded if the lower limit of the 95% confidence interval (CI) of the ORR is above the prespecified threshold of 40%; the accrual target was 70 patients. There was one planned futility analysis when 35 patients (50% of the accrual target) completed the Day‐28 visit. The results of the futility analysis did not trigger the rule, and the study was continued. The final analysis of ORR was to be performed when enrollment target was met and all subjects completed the Day‐28 response assessments or discontinued earlier. Key secondary endpoints were duration of response (DOR), nonrelapse mortality, and overall survival. Seventy‐one patients were enrolled. The Day‐28 ORR in the primary analysis of the 71 patients was reported as 54.9% (95% CI: 42.7–66.8); the lower limit of the 95% CI exceeded 40%, so the study was concluded to be positive.
For the purposes of establishing efficacy in the intended population of patients with SR‐aGVHD, FDA's analysis included only patients who (a) progressed after 3 days of treatment with MP 2 mg/kg/day equivalent, (b) did not improve after 7 days of treatment with MP 2 mg/kg/day equivalent, (c) progressed to a new organ after treatment with MP 1 mg/kg/day equivalent for skin and upper gastrointestinal (GI) GVHD, or (d) recurred during or after a steroid taper. Additionally, patients were excluded if they had received a systemic treatment other than corticosteroids for aGVHD. Using these parameters, the final population for FDA's efficacy analysis included 49 patients.
For the assessment of response, FDA used an algorithmic approach to derive the organ staging according to Harris et al. 16 based on serial recordings of total bilirubin, stool output episodes or volume, presence of grossly bloody stool, severe abdominal pain, skin rash percentage, presence of erythroderma with bullae or desquamation, presence of persistent nausea, vomiting or anorexia, and additional explanatory comments. The response at Day 28 (±2 days) was determined according to the definitions in supplemental online Table 1.
Additional follow‐up through at least Day 180 was needed to establish durability of the responses. After the Day‐28 assessment, additional evaluations were to be performed weekly for 4 weeks and every 28 days thereafter, including Days 100, 180, and 365. The duration of response was calculated from the Day‐28 response to the day of progression, new systemic therapy for aGVHD, or death from any cause. FDA also calculated an alternate measure of durability of response based on the interval from the Day‐28 response to the day of new therapy or death from any cause. Progression was defined as worsening by one stage in any organ without improvement in other organs in comparison with the prior response assessment; new therapy was defined as new systemic treatment for aGVHD or an increase in corticosteroids to MP 2 mg/kg (±10%) equivalent or more.
Efficacy
There were 49 patients with aGVHD refractory to steroids alone in Study 271. Table 2 shows the demographics of the efficacy analysis population, and Table 3 shows the baseline information about aGVHD in these patients. The efficacy analysis population was composed largely of adults with grades 3–4 (73%) GVHD; most had undergone transplantation using peripheral blood stem cells (80%) from a matched related or unrelated donor (76%). The cohort had a median MAGIC biomarker score 17 of 0.47, and 83% were in the MAGIC biomarker high‐risk group 18. The median duration of prior corticosteroid exposure at baseline was 15 days (range: 3–106 days).
Table 2.
Demographics of the efficacy and safety populations
| Demographics | Primary efficacy population (n = 49) | Safety analysis population (n = 71) |
|---|---|---|
| Median age (range), years | 57 (18–72) | 58 (18–73) |
| Age group, years | ||
| <65 | 43 (88%) | 58 (82%) |
| ≥65 | 6 (12%) | 13 (18%) |
| Sex | ||
| Male | 23 (47%) | 35 (49%) |
| Female | 26 (53%) | 36 (51%) |
| Race | ||
| White | 45 (92%) | 66 (93%) |
| Black | 2 (4%) | 3 (4%) |
| Asian | 2 (4%) | 2 (3%) |
| Ethnicity | ||
| Hispanic/Latino | 7 (14%) | 9 (13%) |
| Not Hispanic/Latino | 41 (84%) | 60 (85%) |
| Not reported | 1 (2%) | 2 (3%) |
| Diagnosis | ||
| Acute leukemia/MDS | 32 (65%) | 48 (68%) |
| Lymphoma/CLL | 7 (14%) | 12 (17%) |
| Other | 10 (20%) | 11 (15%) |
| Donor | ||
| Matched related | 17 (35%) | 23 (32%) |
| Matched unrelated | 20 (41%) | 32 (45%) |
| Mismatched unrelated | 6 (12%) | 9 (13%) |
| Haploidentical | 5 (10%) | 6 (8%) |
| Mismatched cord blood | 1 (2%) | 1 (1%) |
| Stem cell type | ||
| Apheresis | 39 (80%) | 57 (80%) |
| Marrow | 9 (18%) | 13 (18%) |
| Cord blood | 1 (2%) | 1 (1%) |
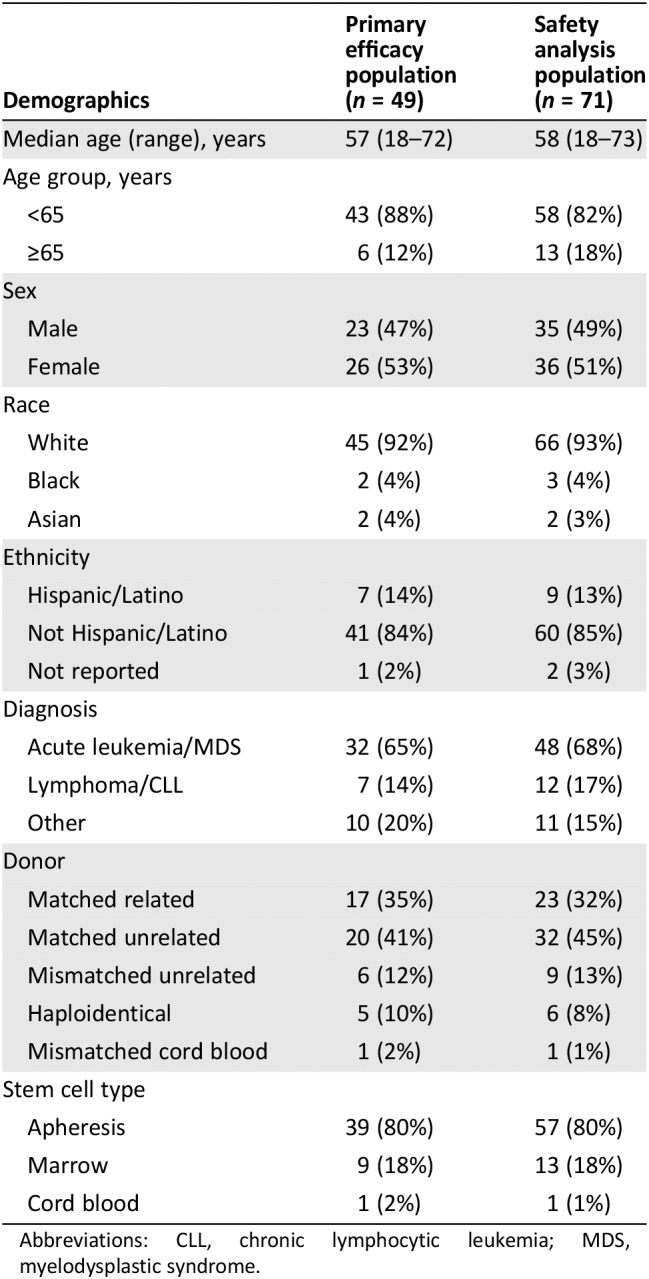
Abbreviations: CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome.
Table 3.
GVHD characteristics of the efficacy and safety populations
| Characteristics | Primary efficacy population (n = 49) | Safety analysis population (n = 71) |
|---|---|---|
| Prior GVHD treatment | ||
| Failed steroids alone | 49 (100%) | 49 (69%) |
| Failed steroids + other(s) | 0 | 12 (17%) |
| Undertreated | 0 | 10 (14%) |
| Baseline GVHD grade | ||
| Grade 2 | 13 (27%) | 22 (31%) |
| Grade 3 | 27 (55%) | 33 (46%) |
| Grade 4 | 9 (18%) | 16 (23%) |
| Visceral GVHD | ||
| Yes | 41 (84%) | 58 (82%) |
| GVHD onset before HSCT Day 100 | ||
| Yes | 34 (69%) | 52 (73%) |
| MB risk groupa | ||
| Low | 8 (17%) | 12 (17%) |
| High | 39 (83%) | 57 (83%) |
| Median MB score (range) | 0.47 (0.10–0.92) | 0.46 (0.10–0.96) |
| Median log10ST2 (range), ng/mL | 5.52 (4.74–6.11) | 5.50 (4.74–6.33) |
| Median time from HSCT to ruxolitinib (range), days | 66 (25–298) | 74 (25–357) |

Biomarker data available for 47 patients in the efficacy population and 69 patients in the safety population.
Abbreviations: GVHD, graft‐versus‐host disease; HSCT, hematopoietic stem cell transplantation; MB, MAGIC biomarker 17; ST2, suppressor of tumorigenicity 2.
With regard to treatment, 48 (98%) patients started ruxolitinib at 5 mg twice daily (1 started at 5 mg daily), and 28 (57%) increased the ruxolitinib dose to 10 mg twice daily by study day 7. Additional poststudy treatments with other immunosuppressive drugs or extracorporeal photopheresis were reported for 18 (37%) patients.
The FDA‐adjudicated Day‐28 ORR was 57.1% (95% CI: 42.2–71.2; Table 4); more than half of the responses were a CR. The median follow‐up for responders was 5.2 months (range, 1.1–14.4 months). For the 28 responders, the median duration of response was 0.5 months (95% CI: 0.3–2.7 months), and the median time to either death or new therapy was 5.7 months (95% CI: 2.2 to not estimable [NE]).
Table 4.
Response analysis
| Efficacy outcome | Primary efficacy population (n = 49) |
|---|---|
| Overall responsea | 28 (57.1%) |
| (95% CI) | (42.2%–71.2%) |
| Complete response | 15 (30.6%) |
| Very good partial response | 2 (4.1%) |
| Partial response | 11 (22.4%) |
|
Median duration of response (95% CI), days |
16 (9–83) |
| Median time to death or new therapy (95% CI), days | 173 (66–NE) |
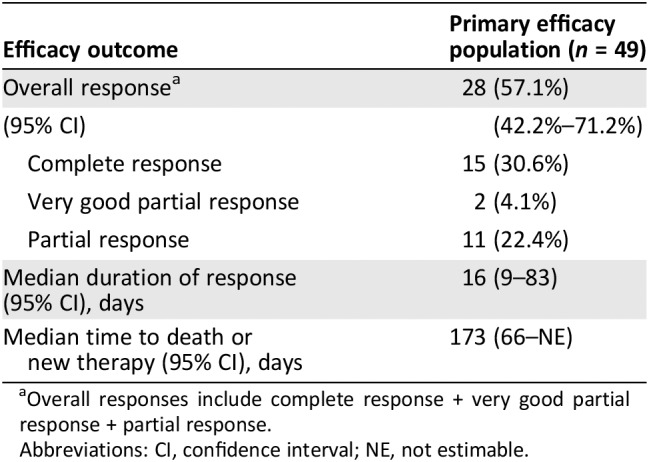
Overall responses include complete response + very good partial response + partial response.
Abbreviations: CI, confidence interval; NE, not estimable.
The Day‐28 ORR was largely consistent across demographic subpopulations (supplemental online Table 2). By baseline aGVHD characteristics, there were substantial differences in ORR and CR by baseline GVHD grade, baseline serum suppressor of tumorigenicity 2 (ST2) concentration, and baseline MAGIC biomarker score (supplemental online Table 2). There were 24 patients with stages 3–4 lower GI GVHD at baseline; of these, 10 (42%) achieved Day‐28 ORR.
Mortality was reported for 24 (49%) patients. The median overall survival was 10.9 months (95% CI: 3.0–NE). The cumulative incidence rate of nonrelapse mortality at month 6 was 46.9% (95% CI: 32.5–61.7). The follow‐up was not sufficient to provide an accurate estimate of the rate of occurrence of chronic GVHD.
Safety
The safety population included all 71 patients treated on Study 271. The demographics and baseline GVHD characteristics of the safety population are shown in Tables 2 and 3, respectively. The median duration of treatment with ruxolitinib was 46 days (range, 4–382 days). At the end of treatment, the dose of ruxolitinib was 10 mg twice daily for 20 (28%) patients, 5 mg twice daily for 20 (28%) patients, and 5 mg daily for 31 (44%) patients.
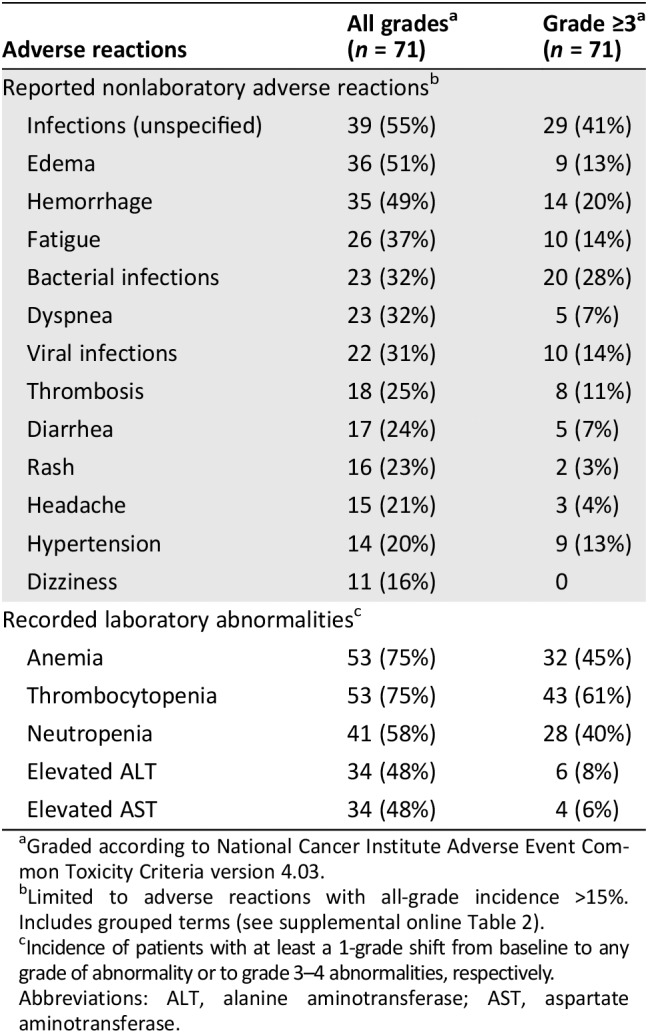
FDA adjudicated the root cause of death as relapse for any patient who died after relapse on study, as GVHD for any patient who died with active GVHD, and as infection for any patient who died of infection without active GVHD. Within 30 days of the last dose of ruxolitinib, 21 (30%) patients died of GVHD, 2 (3%) died of infection, none died of relapse, and none died of an adverse reaction to ruxolitinib. An adverse reaction resulting in treatment discontinuation occurred in 31% of patients. The most common adverse reaction leading to treatment discontinuation was infection (10%). The most common (≥10%) adverse reactions leading to dose interruption or dose reduction were infection, thrombocytopenia, and neutropenia.
The adverse events of special interest included infections, bleeding, thrombosis, relapse, graft failure, and post‐transplant lymphoproliferative disorder (PTLD). An infection of any type was reported in 78% of patients, and the infection was grades 3–5 in 62%. The most common infections were sepsis (25%), cytomegaloviral infections (20%), lower respiratory tract infections (13%), urinary tract infections (11%), and staphylococcal infections (10%). Supplemental online Table 4 shows the range of infections reported. A hemorrhage event was reported in 49% of patients, and the event was grades 3–5 in 20%. The sites with the highest incidences of bleeding were the gastrointestinal tract (25%), urinary tract (15%), and upper respiratory tract (e.g., epistaxis; 11%). A thrombosis event was reported in 27% of patients, and the event was grades 3–5 in 13%; the most common such event was deep vein thrombosis (6%). Two (3%) patients had relapse of the prior malignancy, one (1%) had secondary graft failure, and none developed a PTLD. Table 5 shows the common adverse reactions of ruxolitinib in the safety population.
Table 5.
Adverse reactions in the safety population
| Adverse reactions | All gradesa (n = 71) | Grade ≥3a (n = 71) |
|---|---|---|
| Reported nonlaboratory adverse reactionsb | ||
| Infections (unspecified) | 39 (55%) | 29 (41%) |
| Edema | 36 (51%) | 9 (13%) |
| Hemorrhage | 35 (49%) | 14 (20%) |
| Fatigue | 26 (37%) | 10 (14%) |
| Bacterial infections | 23 (32%) | 20 (28%) |
| Dyspnea | 23 (32%) | 5 (7%) |
| Viral infections | 22 (31%) | 10 (14%) |
| Thrombosis | 18 (25%) | 8 (11%) |
| Diarrhea | 17 (24%) | 5 (7%) |
| Rash | 16 (23%) | 2 (3%) |
| Headache | 15 (21%) | 3 (4%) |
| Hypertension | 14 (20%) | 9 (13%) |
| Dizziness | 11 (16%) | 0 |
| Recorded laboratory abnormalitiesc | ||
| Anemia | 53 (75%) | 32 (45%) |
| Thrombocytopenia | 53 (75%) | 43 (61%) |
| Neutropenia | 41 (58%) | 28 (40%) |
| Elevated ALT | 34 (48%) | 6 (8%) |
| Elevated AST | 34 (48%) | 4 (6%) |
Graded according to National Cancer Institute Adverse Event Common Toxicity Criteria version 4.03.
Limited to adverse reactions with all‐grade incidence >15%. Includes grouped terms (see supplemental online Table 2).
Incidence of patients with at least a 1‐grade shift from baseline to any grade of abnormality or to grade 3–4 abnormalities, respectively.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 5 also shows laboratory tests considered adverse reactions with at least a 1‐grade shift from baseline. Anemia, thrombocytopenia, and neutropenia were the most common laboratory tests that worsened to grades 3–4 on treatment. These cytopenias were transient and largely resolved with dose modification. Transaminases also were elevated in a substantial proportion of patients, but few were grades 3–4. Additionally, hypertriglyceridemia was reported as an adverse reaction in 11%.
Clinical Pharmacology
Study 271 used a starting dose of ruxolitinib of 5 mg twice daily (Table 6), a dose that was reported to have clinical activity in retrospective case series for treatment of patients with refractory aGVHD 8, 10. This dose is lower than that recommended for treatment of myelofibrosis in the absence of severe thrombocytopenia or for polycythemia vera. The ruxolitinib starting dose of 5 mg twice daily was supported by efficacy and safety in Study 271 in patients with refractory aGVHD. The appropriateness of the dose in patients with aGVHD was also confirmed by an approximately 50% lower ruxolitinib clearance (CL/F; 11.9 L/hour) compared with that in patients with myelofibrosis.
Table 6.
Recommended ruxolitinib starting dose for patients with steroid‐refractory acute GVHD
| Setting | Ruxolitinib dose |
|---|---|
| Usual starting dose | 5 mg twice daily |
| Modifications for use with strong CYP3A4 inhibitors | |
| With ketoconazole | 5 mg daily |
| With itraconazole | 5 mg twice daily and monitor more frequently for toxicities |
| With other strong CYP3A4 inhibitorsa | 5 mg twice daily |
| Modification for renal impairment | |
| Moderate (CLcr 30–59 mL/minute) or severe (CLcr 15–29 mL/minute) | 5 mg daily |
| ESRD (CLcr <15 mL/minute) on dialysis | 5 mg daily after the dialysis session |
| ESRD not requiring dialysis | Avoid |
| Modification for hepatic disease | |
| Stage 3–4 liver GVHD | Monitor for toxicity more frequently and consider starting at 5 mg daily |

Adapted from the U.S. Prescribing Information dated May 2019.
Including fluconazole.
Abbreviations: CLcr, creatinine clearance; ESRD, end‐stage renal disease; GVHD, graft‐versus‐host disease.
Ruxolitinib is metabolized by CYP3A4 and to a lesser extent by CYP2C9. Patients with aGVHD are treated commonly with antifungal drugs that are strong and moderate CYP3A inhibitors, which can increase ruxolitinib plasma concentrations. The coadministration of ketoconazole, a strong CYP3A inhibitor, increased ruxolitinib exposure (area under the curve) by 91% in a drug–drug interaction study in healthy subjects. Dose reduction to 5 mg once daily is recommended when ruxolitinib is coadministered with ketoconazole in patients with aGVHD (Table 6). More frequent monitoring for toxicity and ruxolitinib dose adjustment, if necessary, are proposed when ruxolitinib is coadministered with itraconazole, a strong CYP3A inhibitor. No dose adjustment is recommended for ruxolitinib with other CYP3A inhibitors, including fluconazole.
Ruxolitinib and its metabolites are excreted in urine (74%) and feces (22%). No clinically relevant effects of hepatic impairment on ruxolitinib pharmacokinetics were observed in patients with aGVHD. Consequently, no dose adjustment is recommended with any hepatic impairment based on National Cancer Institute criteria 19 in patients with aGVHD. More frequent monitoring of blood counts for toxicity and dose adjustment to 5 mg once daily may be considered for stage 3 or 4 liver involvement (Table 6). Total exposure of ruxolitinib and its active metabolites increased by 1.5‐ to 1.9‐fold in subjects with moderate to severe renal impairment (creatinine clearance (CLcr) 15–59 mL/minute) and by 1.6‐fold in subjects with end‐stage renal disease (ESRD; CLcr <15 mL/minute) after dialysis, compared with those in subjects with normal renal function (CLcr ≥90 mL/min) in a clinical pharmacokinetic study. Dose adjustment to 5 mg once daily is recommended for patients with aGVHD and moderate (CLcr 30–59 mL/minute) to severe (CLcr 15–29 mL/minute) renal impairment, and 5 mg after a dialysis session for patients with ESRD (Table 6). Use of ruxolitinib should be avoided in patients with ESRD not on dialysis.
Regulatory Insights
Ruxolitinib is the first drug to be approved for treatment of SR‐aGVHD. FDA frequently requires a randomized trial to support traditional approval. In this case, however, where the disease is life‐threatening, there are no approved therapies and no optimal therapy identified, the efficacy endpoint is objective, the activity of the drug is established in other diseases, and there is a substantial safety database, FDA accepted the results of Study 271, a single‐arm trial, as the sole basis of efficacy in this application (Table 7). The Day‐28 ORR of 57.1% with a lower 95% confidence interval bound excluding 40% was considered a clinically meaningful response rate for ruxolitinib in patients with SR‐aGVHD.
Table 7.
Food and Drug Administration benefit‐risk assessment
| Dimension | Evidence and uncertainties | Conclusions and reasons |
|---|---|---|
| Analysis of condition |
|
Patients with steroid‐refractory acute GVHD have a poor prognosis. |
| Current treatment options |
|
There is a need for therapies for patients with steroid‐refractory acute GVHD. |
| Benefit |
|
The magnitude of ORR and durability of response to treatment demonstrates that ruxolitinib is active in this disease. |
| Risks and risk management |
|
The major potential risks of cytopenias, infections, and hemorrhage can be mitigated through labeling. |
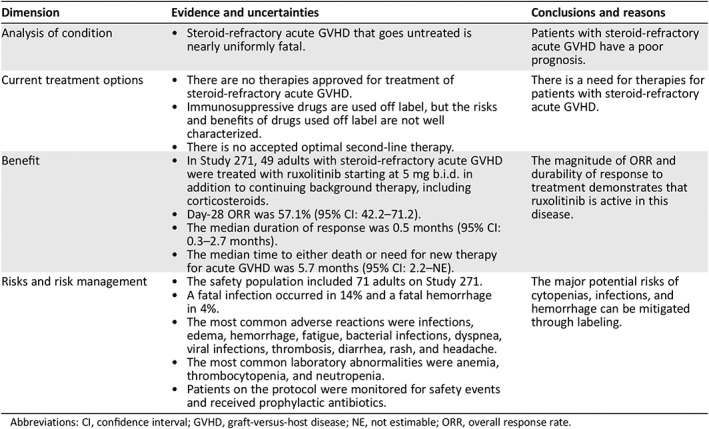
Abbreviations: CI, confidence interval; GVHD, graft‐versus‐host disease; NE, not estimable; ORR, overall response rate.
The literature is somewhat confusing with regard to the criteria that describe the steroid‐refractory population of patients with aGVHD; in many cases, the term as used encompassed patients who failed steroid treatment at any time whether or not they received additional treatments for aGVHD, as was interpreted by some investigators on Study 271. FDA's efficacy analysis population included patients treated with ruxolitinib alone as second‐line therapy. There were 12 patients who received ruxolitinib as a third or later line of treatment of aGVHD in Study 271, but the number of patients in this cohort was too small to make firm conclusions about the efficacy of ruxolitinib as a later line of therapy.
The primary endpoint of Study 271 was Day‐28 ORR (CR + VGPR + PR). The definition of this endpoint and its acceptance as a clinical benefit was discussed at the open public workshop on Clinical Trial Endpoints for Acute Graft‐vs‐Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation held May 19, 2009 20, 21. The definition of CR used by FDA in the current analysis (supplemental online Table 1) differed slightly from the proposed consensus definition. In the latter, CR is defined as "resolution of all signs and symptoms of aGVHD," 22 whereas FDA used Stage 0 16 in all organs, which allows trivial residual abnormalities that otherwise might be scored as a VGPR. Because the primary endpoint is a composite that includes both CR and VGPR, the difference would not affect the overall conclusion regarding efficacy.
Durability of response is used to substantiate clinical benefit, but the consensus endpoint definitions for GVHD response did not address this outcome. The DOR, defined as the interval from response to progression or death, is used frequently for oncologic trials. In Study 271, the DOR was quite short (0.5 months). However, this definition of DOR does not take into account that GVHD may flare and resolve without additional systemic treatment. The additional measure of median time to either death or need for new therapy for aGVHD (without consideration of flares as progression) of 5.7 months is considered a meaningful representation of the durability of the response. Nonetheless, the ultimate goal of GVHD therapeutics should be resolution of the disease process such that flares do not occur, so both measures of durability of response are of interest for the evaluation of clinical benefit at this time.
For patients with SR‐aGVHD, the factors prognostic for response, nonrelapse mortality, or survival have variously been reported to include baseline GVHD grade, organs involved, degree of donor mismatch, age, and primary disease for which the patient was transplanted 23, 24, 25. Additionally, a combination of two serum biomarkers, ST2 and regenerating family member 3 alpha, was associated with GVHD‐related mortality when measured day 7 after HSCT, 26 at diagnosis of aGVHD, 27 or in patients who had not responded to 1 week of systemic treatment of aGVHD 18. In the subgroup analysis of Study 271 (supplemental online Table 2), the greatest differences in ORR or CR were distinguished by baseline GVHD grade, baseline serum ST2 concentration (using median as the cut point), and baseline MAGIC biomarker score 17. Although the differences in ORR or CR by these subcategorizations could be due to chance among multiple comparisons, they are also biologically plausible and add to the emerging literature on biomarkers for characterization of the prognosis of patients with SR‐aGVHD that will be useful in clinical trial design and analysis.
No unexpected adverse drug reactions were encountered in the patients with SR‐GVHD treated with ruxolitinib on Study 271. Major toxicities of ruxolitinib are the cytopenias and immunosuppression. Although these toxicities may be contributing factors in fatal infection and hemorrhage, with the high background rate of these events in patients with SR‐aGVHD independent of treatment 25, 28 and in the absence of a randomized control for comparison, it was not possible to clearly confirm a causal association. Hence, no deaths could be attributed solely to ruxolitinib. Close monitoring of blood counts is routine practice in this population, but the risk of infections noted warranted adding a recommendation for active surveillance and prophylactic antibiotics to the warning about infections in the U.S. Prescribing Information.
The Study 271 population did not include children. On the basis of the biology of GVHD and mechanism of action of ruxolitinib, the efficacy of ruxolitinib for pediatric patients with this indication can be extrapolated from the adult experience. The safety of ruxolitinib is described in labeling down to age 2 years. However, at the recommended dose for treatment of aGVHD, the lowest available formulation and strength of ruxolitinib (5 mg tablets) limits use to patients comparable in size to adults. Overall then, given the observed response rate and durability, and with the labeling modifications in place for safety concerns, the clinical benefit of ruxolitinib outweighs the risks for treatment of SR‐aGVHD in adult and pediatric patients ages 12 years and older (Table 7).
Author Contributions
Conception/design: Donna Przepiorka, Lola Luo, Sriram Subramaniam, Junshan Qiu, Ramadevi Gudi, Lea C. Cunningham, Lei Nie, Ruby Leong, Lian Ma, Christopher Sheth, Albert Deisseroth, Kirsten B. Goldberg, Gideon M. Blumenthal, Richard Pazdur
Provision of study material or patients: Donna Przepiorka, Lola Luo, Sriram Subramaniam, Junshan Qiu, Ramadevi Gudi, Lea C. Cunningham, Lei Nie, Ruby Leong, Lian Ma, Christopher Sheth, Albert Deisseroth, Kirsten B. Goldberg, Gideon M. Blumenthal, Richard Pazdur
Collection and/or assembly of data: Donna Przepiorka, Lola Luo, Sriram Subramaniam, Junshan Qiu, Ramadevi Gudi, Lea C. Cunningham, Lei Nie, Ruby Leong, Lian Ma, Christopher Sheth, Albert Deisseroth, Kirsten B. Goldberg, Gideon M. Blumenthal, Richard Pazdur
Data analysis and interpretation: Donna Przepiorka, Lola Luo, Sriram Subramaniam, Junshan Qiu, Ramadevi Gudi, Lea C. Cunningham, Lei Nie, Ruby Leong, Lian Ma, Christopher Sheth, Albert Deisseroth, Kirsten B. Goldberg, Gideon M. Blumenthal, Richard Pazdur
Manuscript writing: Donna Przepiorka, Lola Luo, Sriram Subramaniam, Junshan Qiu, Ramadevi Gudi, Lea C. Cunningham, Lei Nie, Ruby Leong, Lian Ma, Christopher Sheth, Albert Deisseroth, Kirsten B. Goldberg, Gideon M. Blumenthal, Richard Pazdur
Final approval of manuscript: Donna Przepiorka, Lola Luo, Sriram Subramaniam, Junshan Qiu, Ramadevi Gudi, Lea C. Cunningham, Lei Nie, Ruby Leong, Lian Ma, Christopher Sheth, Albert Deisseroth, Kirsten B. Goldberg, Gideon M. Blumenthal, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Acknowledgments
We thank Suria Yesmin, M.S., and Rosa J. Lee‐Alonzo, Pharm.D., for expert review management.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. D'Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2018. Available at https://www.cibmtr.org. Accessed July 31, 2019.
- 2. Khoury HJ, Wang T, Hemmer MT et al. Improved survival after acute graft‐versus‐host disease diagnosis in the modern era. Haematologica 2017;102:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reddy P, Ferrara JL. Immunobiology of acute graft‐versus‐host disease. Blood Rev 2003;17:187–194. [DOI] [PubMed] [Google Scholar]
- 4. Martin PJ, Rizzo JD, Wingard JR et al. First‐ and second‐line systemic treatment of acute graft‐versus‐host disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2012;18:1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deeg HJ. How I treat refractory acute GVHD. Blood 2007;109:4119–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jagasia M, Arora M, Flowers ME et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012;119:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of JAK‐STAT signaling in the immune system. Nat Immunol 2017;18:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spoerl S, Mathew NR, Bscheider M et al. Activity of therapeutic JAK 1/2 blockade in graft‐versus‐host disease. Blood 2014;123:3832–3842. [DOI] [PubMed] [Google Scholar]
- 9. Juvekar A, Ruggeri B, Condon S et al. Ruxolitinib, a JAK1/JAK2 selective inhibitor is highly efficacious in corticosteroid untreated and refractory MHC‐mismatched mouse model of acute GvHD. Blood 2018;132:4523. [Google Scholar]
- 10. Zeiser R, Burchert A, Lengerke C et al. Ruxolitinib in corticosteroid‐refractory graft‐versus‐host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015;29:2062–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maffini E, Giaccone L, Festuccia M et al. Ruxolitinib in steroid refractory graft‐vs.‐host disease: A case report. J Hematol Oncol 2016;9:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khandelwal P, Teusink‐Cross A, Davies SM et al. Ruxolitinib as salvage therapy in steroid‐refractory acute graft‐versus‐host disease in pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant 2017;23:1122–1127. [DOI] [PubMed] [Google Scholar]
- 13. Sarmiento Maldonado M, Ramirez Villanueva P, Cortes‐Monroy PB et al. Compassionate use of ruxolitinib in acute and chronic graft versus host disease refractory both to corticosteroids and extracorporeal photopheresis. Exp Hematol Oncol 2017;6:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez Vicent M, Molina B, Gonzalez de Pablo J et al. Ruxolitinib treatment for steroid refractory acute and chronic graft vs host disease in children: Clinical and immunological results. Am J Hematol 2019;94:319–326. [DOI] [PubMed] [Google Scholar]
- 15. Toama W, Flata MA, Pusic I et al. Ruxolitinib for steroid‐refractory acute graft‐versus‐host disease. Biol Blood Marrow Transplant 2019;25:S257. [Google Scholar]
- 16. Harris AC, Young R, Devine S et al. International, multicenter standardization of acute graft‐versus‐host disease clinical data collection: A report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 2016;22:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartwell MJ, Ozber U, Holler E et al. Corrigendum: An early‐biomarker algorithm predicts lethal graft‐versus‐host disease and survival. JCI Insight 2018;3:e124015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Major‐Monfried H, Renteria AS, Pawarode A et al. MAGIC biomarkers predict long‐term outcomes for steroid‐resistant acute GVHD. Blood 2018;131:2846–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel H, Egorin MJ, Remick SC et al. Comparison of Child‐Pugh (CP) criteria and NCI organ dysfunction working group (NCI‐ODWG) criteria for hepatic dysfunction (HD): Implications for chemotherapy dosing. J Clin Oncol 2004;22(suppl 14):6051a. [Google Scholar]
- 20. Center for International Blood and Marrow Transplant Research . Acute Graft‐versus‐Host Disease (GVHD) Workshop. Available at https://www.cibmtr.org/Meetings/Materials/GVHDworkshop/Pages/index.aspx. Accessed July 31, 2019.
- 21. Pavletic SZ. Response as an endpoint in treatment trials for acute GVHD. Bone Marrow Transplant 2012;47:161–163. [DOI] [PubMed] [Google Scholar]
- 22. Martin PJ, Bachier CR, Klingemann HG et al. Endpoints for clinical trials testing treatment of acute graft‐versus‐host disease: A joint statement. Biol Blood Marrow Transplant 2009;15:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacMillan ML, Weisdorf DJ, Davies SM et al. Early antithymocyte globulin therapy improves survival in patients with steroid‐resistant acute graft‐versus‐host disease. Biol Blood Marrow Transplant 2002;8:40–46. [DOI] [PubMed] [Google Scholar]
- 24. MacMillan ML, Couriel D, Weisdorf DJ et al. A phase 2/3 multicenter randomized clinical trial of ABX‐CBL versus ATG as secondary therapy for steroid‐resistant acute graft‐versus‐host disease. Blood 2007;109:2657–2662. [DOI] [PubMed] [Google Scholar]
- 25. Socie G, Vigouroux S, Yakoub‐Agha I et al. A phase 3 randomized trial comparing inolimomab vs usual care in steroid‐resistant acute GVHD. Blood 2017;129:643–649. [DOI] [PubMed] [Google Scholar]
- 26. Hartwell MJ, Ozbek U, Holler E et al. An early‐biomarker algorithm predicts lethal graft‐versus‐host disease and survival. JCI Insight 2017;2:e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Etra A, Gergoudis S, Morales G et al. Comparison of GVHD biomarker algorithms for predicting lethal GVHD and non‐relapse mortality. Biol Blood Marrow Transplant 2019;25:553–554. [Google Scholar]
- 28. Garcia‐Cedenas I, Rivera I, Martino R et al. Patterns of infection and infection‐related mortality in patients with steroid‐refractory acute graft versus host disease. Bone Marrow Transplant 2017;52:107–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables


