ABSTRACT
Background
Functional mobility (FM) is a person's ability to move to accomplish activities of daily living; it bridges the concepts of mobility and functional ability. There is frequently a loss of FM in Parkinson's disease (PD). Several instruments have been used to assess this concept in PD; however, there is no consensus on which are the most appropriate.
Objective
We aimed to identify and critically appraise which measurement instruments have been used to assess FM.
Methods
A systematic review was conducted using the databases CENTRAL, MEDLINE, Embase, and PEDro from their inception to January 2019 to identify all observational and experimental studies conducted in PD or atypical parkinsonism that included an FM assessment. Two reviewers independently screened citations, extracted data, and assessed clinimetric properties.
Results
We included 95 studies that assessed FM in PD. Fifty‐five (57.9%) studies mentioned FM in the article, and 39 (41.1%) specified the measurement tools used to evaluate FM. FM was the primary outcome in 12 (12.6%) studies. The Timed Up and Go test was the most frequently used measurement tool. Only one study presented a definition of FM. Several overlapping terms were used, the most common being mobility.
Conclusion
Several studies reported the use of FM measurement tools in PD, though with frequent misconceptions, an inadequate context of use, or suboptimal assessment. We propose the establishment of the concept of FM applied to PD, followed by the adequate clinimetric validation of existing measurement tools to provide a comprehensive and reliable evaluation of FM in PD.
Keywords: Parkinson's disease, functional mobility, measurement instruments, systematic review, outcome measures
Functional mobility (FM) has been described as a person's physiological ability to move independently and safely in a variety of environments in order to accomplish functional activities or tasks and to participate in the activities of daily living at home, at work, and in the community (Fig. 1).1, 2 Although poorly defined, the concept of FM has been used in several recent research studies as a more global and illustrative outcome of patients’ health status in their environment.2, 3
Figure 1.
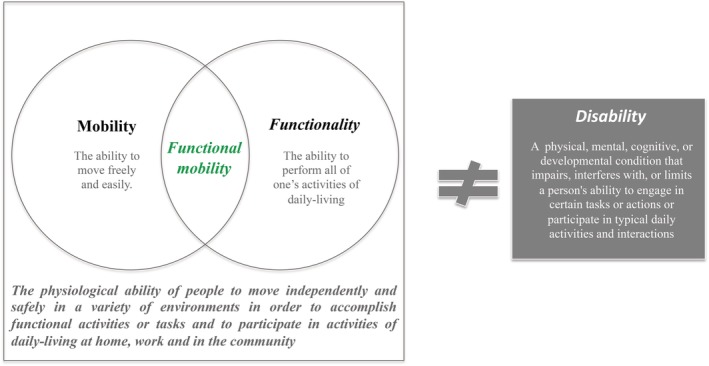
Definition of FM.2
Reduction in FM is common and has a multifactorial nature in Parkinson's disease (PD).2 Motor symptoms may contribute directly, through gait impairments, and indirectly because of bradykinesia, rigidity, and the presence of postural deformities (e.g., camptocormia or antecollis), which affect PD patients’ gait, balance, and transitions.2 Also, the inability to simultaneously perform a cognitive and a motor task, and the presence of orthostatic hypotension symptoms and fatigue complaints, seems also to play an important role.2 FM is associated with significant associated disability and loss of independence leading to immobility and institutionalization. Recognizing limitations in FM is important to better understand and address patients’ daily real‐life needs and monitoring them over time.4, 5
In spite of being loosely defined, several tests and rating scales have been used to assess FM in PD patients,3, 5 but there is no consensus on the most adequate tools for screening or for using as outcome measures to monitor change over time. This lack of consensus limits the interpretation of results from studies and hampers the evaluation of therapeutics and discussion among peers.
The present review aims to investigate which measurement tools have been used to evaluate FM in PD studies. Recommendations on which tools can be used and the need for modifications or replacements are made based on the results.
Methods
Defining the Concept
FM is not a concept defined in the International Classification of Functioning, Disability, and Health (ICF) and lacks a formal definition. To overcome this limitation, we adopted a definition previously used by Forhan and Gill1 in a study on obesity. To check the adequacy of our choice, we matched the adopted definition with those founded in a Medline/PubMed electronic open search, conducted to look for a formal definition of FM (regardless of the research topics). We found six additional articles that defined FM.6, 7, 8, 9, 10, 11 Although few, and none presenting a formal definition of FM, all shared with the Forhan and Gill description, the idea that FM is a subject's ability to move in his or her environment, focused on gait, balance, and transfers, in order to accomplish functional tasks of everyday living (e.g., walking in a corridor at work, climbing stairs at home, getting up from bed, rising from a chair to answer the phone, standing, and bending to reach an object). Therefore, we assume this as the most suitable definition to be in the context of this systematic review.
Literature Search
We searched CENTRAL, MEDLINE, Embase, and PEDro from their inception to January 2019 using a predefined search strategy (Supporting Information Appendix 1) designed by the authors in conjunction with Cochrane's highly sensitive search strategy12 and previous reviews in PD.13 Being aware of the laxity of the definition, we also ran some open electronic searches, in order to minimize the number of studies not found in the formal electronic search. Reference lists from the identified articles were cross‐checked to identify any further potentially eligible studies.
Study Selection
We included any observational and experimental study conducted in PD patients or atypical parkinsonisms. For intervention or controlled studies, there were no restrictions regarding the type of intervention or control arms. Studies had to include an FM assessment and describe what measurement tools were used (mentioned in the abstract and/or in the article). In order to get a full picture of the measurement tools that have been and could potentially be used to measure FM, we also included studies for which the description of the outcome measures matched the predefined concept of FM, as per consensus of the current authors (i.e., to present one or a set of instruments that measured gait, transfer, and/or balance). Studies did not need to present a definition of FM to be included in this review.
We excluded reviews and studies written in languages other than English, French, Spanish, and Portuguese. Two authors (R.B.M., M.P.) independently screened abstracts obtained from the database search. The full texts of potentially relevant articles were retrieved for further assessment. Disagreements were resolved by consensus or by consultation with a third reviewer (G.S.D.).
Data Extraction
Four predefined domains of items were extracted: general information (title, year, and journal of publication, aim of the study, study design, population, sample size, and intervention and comparator, if applicable); concept of FM (presence of the concept of FM in the title and/or in the article, if a definition of FM was presented and if other terms were used as synonyms); FM outcome tools (if FM was the primary outcome measure, which instruments were used, and the time‐point measures); and feasibility of the instrument (completion time, number of required instruments, easy administration, interpretability, patients’ comprehensibility, length of the outcome measurement instrument, ease of standardization, and clinician's comprehensibility).
We divided studies into those that specifically used the concept of FM and those that, while not mentioning the concept of FM, used outcome measures that could fit the concept according to our best judgment. Within the studies using the concept of FM, we divided those that specified which measurement tools were used to measure FM from those that only mentioned evaluation of FM in the aims or conclusions of the study.
Two authors (R.B.M., M.P.) independently extracted data. Discrepancies were resolved through discussion or by consultation with a third reviewer (G.S.D.).
Assessment of Measurement Properties
Based on previous reviews, we divided the measurement tools into clinically based tests, patient‐reported outcomes, and gait quantification methods.14
Recommendations were based on the criteria previously used in other reviews.15, 16 These included: (1) use in the assessment of FM; (2) use in published studies by individuals other than the developers; and (3) a “successful” clinimetric test (i.e., to have demonstrated the reliability, validity, and sensitivity to change of the instrument).
Measurement tools were classified as recommended, suggested, or listed, respectively, based on the number of criteria met and the feasibility evaluation.17
The search for studies assessing the clinimetric properties of the included measurement tools was made based on previous research14 and on the references of each measurement tool presented in the included studies.
Statistical Analysis
The primary outcome was to identify the measurement instruments currently used to evaluate FM in people with PD. We summarized the publication characteristics using frequencies and percentages.
Results
The electronic and hand searches identified 2,463 citations. After screening titles and abstracts, 103 articles were deemed potentially eligible. Full‐text assessment for eligibility resulted in eight studies being excluded. Overall, the main reasons for exclusion were: inadequately defined outcome (n = 1,395) and inappropriate study population (n = 222; Supporting Information Appendix 2).
General Data
Of the 95 included articles, 63 (66.3%) were interventional studies and 94 (98.4%) were conducted in PD patients, with a sample median [range] size of 32 [1, 3,408]. According to the year of publication, the earliest study was published in 2003, being 2014 and 2015 the years with the highest number of included studies (n = 15 in each). All interventional studies evaluated nonpharmacological interventions.
Fifty‐five (57.9%) of the included studies specifically mentioned the concept of FM in the article, 39 (41.1%) specified the measurement tools used to evaluate FM, and in 12 (12.6%) FM was the primary outcome. Forty studies were deemed to have used the concept of FM according to the reviewers (Fig. 2).
Figure 2.
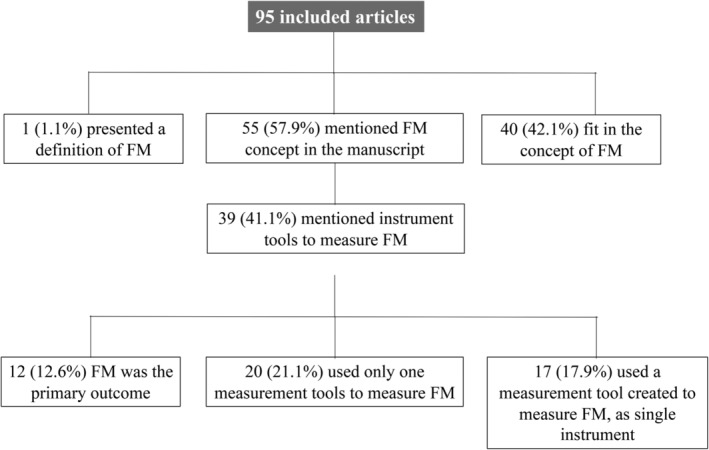
Number of included studies specifically mentioning the concept of FM and respective measurement tools in the article.
Studies Explicitly Using the Concept of FM
Of the 39 studies (41.1%) in which a measurement tool(s) was specified to evaluate FM, 34 (87.2%) were clinically based tests, six (15.4%) combined clinically based tests with gait quantification methods, one (2.6%) combined clinically based tests with patient‐reported outcomes, and one (2.6%) used only gait quantification methods.
The Timed Up and Go (TUG) test was the most frequently reported tool used as a single instrument (75% of studies; n = 15). The Short Physical Performance Battery (SPPB), the Five Times Sit‐to‐Stand test (FTSTS), the Modified Parkinson Activity Scale (mPAS), and the Dual‐Task TUG (TUG‐DT; cognitive) were also applied (Table 1). In those articles that used a combination of measurement tools to assess FM (n = 19; 48.7%), the most frequent associations were TUG with a: dual‐task test, balance test, gait assessment, and/or a transfer evaluation (Table 3). The association of the TUG test with a second gait, balance, or transfers test was the most used way (75%; n = 9) used to measure the primary outcome (n = 12; 30.8%), followed by the single TUG test (n = 2; 16.7%) and the single FTSTS test (8.3%; n = 1).
Table 1.
Measurement tools specifically used to measure FM and those used in studies that fit the FM concept
| Measurement Tools Specifically Used to Assess FM | Measurement Tools Not Specifically Used to Assess FM | ||||||
|---|---|---|---|---|---|---|---|
| One Only Instrument Tool | % (n) | As a Set of Outcomes Tools | As a Set of Outcomes Tools | % (n) | |||
| Timed Up and Go Test | 75% (15) | TUG with… | Timed Up and Go | 57.5% (23) | |||
| The Short Physical Performance Battery | 10% (2) | Dual‐task | Balance | Gait | Transfers | 6‐minute walk test | 30% (12) |
| Five Times Sit‐to‐Stand Test | 5% (1) | Cognitive | Berg | 10‐m walk test | 5× Sit to Stand test | Berg Balance Scale | 30% (12) |
| Modified Parkinson Activity Scale | 5% (1) | Manual | Mini‐BESTest | 6‐minute walk test | 360‐degree turn | 10‐m walk test | 22.5% (9) |
| Dual‐Task Timed Up and Go (cognitive) | 5% (1) | Functional reach | Dynamic gait index | Bed mobility test | Mini‐BESTest | 22.5% (9) | |
| UPDRS Part III | 22.5% (9) | ||||||
| Functional Reach Test | 17.5% (7) | ||||||
Table 3.
Feasibility characteristics of the most cited measurement tools
| Instruments | Completion Time (sec) | Required Equipment (n) | Easy Administration | Interpretability | Patient's Comprehension | Length of the Outcome Measurement Instrument | Ease of Standardization | Ease of Comprehensibility by Clinician |
|---|---|---|---|---|---|---|---|---|
| Timed Up and Go Test | 5 | 3 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| Dual‐Task Timed Up and Go | <5 | 4 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| Modified Parkinson Activity Scale | 10 to 15 | 8 | No | Yes | Adequate | Too long | Yes | Yes |
| Five Times Sit‐to‐Stand Test | <5 | 2 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| The Short Physical Performance Battery | 10 to 15 | 5 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| 10‐m walk test | 5 | 3 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| 6‐minute walk test | <10 | 4 | No | Yes | Adequate | Too long | Yes | Yes |
| 360 Degree Turn Test | <5 | 1 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| Berg Balance Scale | 10 to 20 | 6 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| Mini‐BESTest | 10 to 15 | 7 | Yes | Yes | Adequate | Adequate | Yes | Yes |
| Functional Reach Test | <5 | 3 | Yes | Yes | Difficult | Adequate | Yes | Yes |
| UPDRS Part III | <10 | 0 | Yes | Yes | Adequate | Too long | No | Yes |
Studies That Match the Concept of FM
Forty studies (42.1%) evaluated a set of outcomes including functional assessment of gait, balance, and transfers that we considered to match the concept of FM.
Of these 40 studies, 29 (72.5%) used clinically based tests as measurement tools, six (15%) used a combination of a clinically based and gait quantification method, and three (7.5%) a combination of a clinically based test and patient‐reported outcomes. One study (2.5%) only used gait quantification methods, and another study (2.5%) associated clinically based tests with gait quantification method analysis and patient‐reported outcomes.
Regarding clinically based tests, in four studies (10%), the TUG test was used as the only instrument. All other studies used a combination of measurement tools; the most used were the TUG test (57.5%; n = 23), the 6‐minute walk test (6MWT; 30%; n = 12), and the Berg Balance Scale (BBS; 30%; n = 12; Table 3).
Quality Assessment of Outcome Measurement Instruments
All measurement tools were administered to a PD population, with data on their use in clinical studies beyond the group that developed the instrument.14 Tables 2 and 3 summarize some of the characteristics of the most cited measurement instruments in the included studies. A more detailed description of the clinimetric properties (the previously published results of reliability, validity, and sensitivity to change of each instrument) and feasibility issues is presented below. The instruments have been divided according to whether they were used as a single instrument to measure FM or as part of a combination of instruments.
Table 2.
Characteristics and classification of the most cited measurement tools
| Instruments | Single Instrument to Measure FM | Created to Measure FM | Applied in PD to Measure FM |
Applied Beyond Original Developers |
Construct Assessed | Reliability | Validity | Sensitive to Change | Feasibility Issues | Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| Timed Up and Go Test | Yes | Yes | Yes | Yes | Functional mobility | Yes | Yes | Yes | No | Recommended |
| Dual‐Task Timed Up and Go | Yes | Yes | Yes | Yes | Functional mobility | No | No | No | No | Suggested |
| Modified Parkinson Activity Scale | Yes | Yes | Yes | Yes | Functional mobility | Yes | Yes | Yes | Yes | Suggested |
| Five Times Sit‐to‐Stand Test | Yes | No | Yes | Yes | Lower extremity strength | Yes | Yes | Yes | No | Listed |
| The Short Physical Performance Battery | Yes | No | Yes | Yes | Lower extremity physical performance status | Yes | No | No | No | Listed |
| 10‐m walk test | No | No | Yes | Yes | Walking speed | Yes | Yes | Yes | No | Listed |
| 6‐minute walk test | No | No | Yes | Yes | Physical capacity | Yes | Yes | Yes | Yes | Listed |
| 360 Degree Turn Test | No | No | No | Yes | Turning ability, freezing of gait | Yes | No | No | No | Listed |
| Berg Balance Scale | No | No | Yes | Yes | Functional standing Balance | Yes | Yes | Yes | No | Listed |
| Mini‐BESTest | No | No | Yes | Yes | Balance | Yes | Yes | Yes | No | Listed |
| Functional Reach Test | No | No | No | Yes | Static balance | Yes | Yes | Yes | Yes | Listed |
| UPDRS Part III | No | No | No | Yes | Motor performance | Yes | Yes | Yes | Yes | Listed |
A single instrument to measure FM
The TUG Test
Construct assessed: Functional mobility.
Test description: The participant is required to get up from a standard chair, to walk 3 m at a comfortable and safe pace, turn, and walk back to sit down on the chair.11, 14, 18, 19 The use of assistive devices is allowed.
Clinimetric properties: Planned comparisons using independent‐sample t tests were used to investigate changes in patients’ TUG scores in the off and on phases. Results showed differences across the stages of the medication, with a moderately strong correlation (r = 0.74; n = 12; P = 0.003) between off and on phase scores. Results demonstrate that TUG scores could be used to differentiate the performance of subjects with PD from controls and also to detect differences between the on and off phases of the medication cycle. No ceiling effects were found. Floor effects exist at scores of 10 to 15 seconds. The TUG test demonstrated adequate test‐retest and inter‐rater reliability in PD. Intraclass correlation coefficients (ICCs) were used to investigate the agreement between experienced and inexperienced raters in different phases of the levodopa cycle. Results showed a high degree of agreement across different conditions (ICCs between 0.87 and 0.99). Absolute minimal detectable change values in PD varied from 3.5 to 11 seconds, whereas relative changes >29.8% may reflect “true” change. Longer times to complete the test proved to be associated with an increased risk of falls.
Feasibility: An easy and quick test to administer. Limited to patients capable of walking (with or without assistive devices) and who are able to follow instructions. The safety training may interfere with TUG results given that patients take more time if focused on the use of safety strategies when getting up, turning, and sitting down.
The Dual‐Task TUG test
Construct assessed: Functional mobility in dual‐task conditions.
Test description: The participant is required to stand up from a chair, walk 3 m at a comfortable and safe speed, then turn and walk back to the chair and sit down.20, 21 In the TUG cognitive, while performing the test, the participant is asked to count backward by threes to a random number between 20 and 100. In the TUG manual, the participant is required to hold a cup filled with water during the test. The use of assistive devices is allowed.
Clinimetric properties: Unknown for PD patients. In healthy older adults, the TUG‐DT manual and cognitive strongly correlate with the Berg Balance Test (r = –0.72 and r = –0.66, respectively). Retest reliability is very good (TUG manual: rT1‐T2 = 0.97 and rT1‐T3 = 0.98; and TUG cognitive: rT1‐T2 = 0.98 and rT1‐T3 = 0.98). Intra‐rater reliability is very high with ICC values of 0.99 and 0.94 for the TUG manual and cognitive, respectively.
Feasibility: Quick and easy‐to‐apply tests to determine dual‐task interference in functional mobility and a predictive test to assess risk for falls. They may be more useful than TUG without dual‐task for evaluating intervention effects, given that the interference of safety strategies is minimized. Limited to patients who are capable of walking (with or without assistive devices), able to follow instructions, and not cognitively impaired.
The mPAS
Construct assessed: Functional mobility.
Test description: The mPAS includes 18 activities covering three functional mobility aspects: chair transfers (two items), gait akinesia (six items), and bed mobility (eight items).11, 22, 23 Raters evaluate the quality of the movement while patients perform the tasks.
Clinimetric properties: Specifically designed for the PD population. Based on 195 observations, the mPAS has no ceiling effect, good concurrent validity (0.64 with UPDRS motor scores and 0.79 with Visual Analogue Scale/Global Functioning), and good inter‐rater agreement with no differences between experts and nonexperts (P = 0.28).
Feasibility: It requires several accessories and space (e.g., a bed, a chair, sheets, and a blanket), which may hinder its use in daily practice.
The FTSTS
Construct assessed: Lower extremity strength.
Test description: Participants began the test seated in an armless chair with their arms folded across their chest and with their back against the chair.24, 25 The rater asks the participant to stand up and sit down five times as quickly as he/she can without the use of the upper limbs.
Clinimetric properties: The FTSTS significantly correlated (P < 0.01) with the Mini‐BESTest and the 6MWT. It is able to discriminate between fallers and nonfallers, with an area under the curve of 0.77. It has shown to have high inter‐rater and test‐retest reliability, with an ICC of 0.99 and 0.76, respectively.
Feasibility: The FTSTS requires a minimum of instrumentation and is a quick and objective measure to determine whether an individual with PD may be at risk for falling. The potential use of compensatory strategies in the sit‐to‐stand movement may impair the test's capacity for measuring disease progression. It does not provide detailed information on balance limitations during gait‐related activities and stationary balance. In people with PD, balance and bradykinesia seem to be the most important constructs influencing the results of the test.
The SPPB
Construct assessed: Lower extremity physical performance status.
Test description: A small battery including three components of daily activities: balance (ability to stand for 3 seconds with the feet together side by side, semitandem, and tandem), walking ability (two timed trials of 3 m walked at a fast pace), and transfers (time to rise from a chair five times).26, 27, 28, 29, 30 The SPPB utilizes an ordinal ranking system, from 0 to 12, where higher scores indicate better lower extremity function.
Clinimetric properties: Significantly correlates with disability measures (Older Americans Resource and Services Activities of Daily Living and Instrumental ADL subscale) and disease severity (H & Y, UPDRS‐II and ‐III, and total score). Although this test has been applied to PD patients, neither its relative and absolute reliability nor its responsiveness have been calculated. In community‐dwelling older populations and patients with chronic kidney disease, the SPPB has an excellent test‐retest reliability (ICC = 0.82 and 0.94, respectively). This battery also has good sensitivity to change in myocardial infarction, stroke, hip fracture, and congestive heart failure patients.
Feasibility: A practical measure rapid to administer and requiring minimal equipment. It has been found to be too easy for highly functioning patients.
Measurement Tools Used in Combination to Measure FM
10 Meter Walk Test
Construct assessed: Walking speed.
Test description: The participant is asked to walk a distance of 10 m at their self‐selected or maximal speed.14, 31, 32, 33 The time and number of steps needed to perform the task are recorded. Assistive devices are allowed.
Clinical properties: The test positively correlates with the 6MWT (gait endurance), has low‐to‐moderate correlation with the Mini‐BESTest (balance), and a low correlation with the UPDRS subscales (disease severity). The test has moderate‐to‐high test‐retest reliability in PD (ICCs, 0.75–0.98), with minimal detectable change (MDC) values of 0.18 and 0.25 m/s. Responsiveness was determined by significant differences after rehabilitation programs and DBS.
Feasibility: It is a frequently used test in PD clinical trials. It is easy to administer and useful for identifying changes in gait over time in mild to moderate PD. The presence of freezing of gait or postural instability may hinder the outcome.
6MWT
Construct assessed: Physical capacity.
Test description: Subjects are asked to cover as much ground as possible on a standardized walkway for 6 minutes.14, 31, 34 Assistive devices are allowed; patients are permitted to pause, if necessary.
Clinimetric properties: Its correlation with the UPDRS motor section is weak (it does not seem to be related with disease severity); however, it moderately to strongly correlates with the BBS, 10 Meter Walk Test, and TUG. The responsiveness of the 6MWT has been demonstrated in PD. The test has adequate test‐retest, inter‐rater reliability with ICCs ranging from 0.88 to 0.95. It seems to be a good predictor of a patient's ability to walk outside independently and safely, and useful for identifying improvements in gait endurance after treatment.
Feasibility: The major limitations of this test's use in clinical practice are the time and space needed. It can only be applied to patients with the capacity to walk (with or without assistive devices). Performance in PD may depend on the presence of freezing, balance, and bradykinesia. Learning effects may occur.
360 Degree Turn Test
Construct assessed: Turning ability, freezing of gait.
Test description: The participant is required to make quick 360‐degree turns, in both directions, while standing.35, 36, 37, 38 Time, number of steps, and presence of freezing episodes are recorded.
Clinimetric properties: The test has high test‐retest reliability as a functional test, with an ICC of 0.95. No further published data on reliability, validity, and responsiveness were found on the 360 Degree Turn Test as a measure of turning ability. However, a study aiming to evaluate reliability, validity, and responsiveness of the timed 360 Degree Turn Test in PD patients was registered in http://clinicaltrials.gov in July 2018 (http://clinicaltrials.gov Identifier: NCT03587168). As a measure of freezing of gait, it has high inter‐rater reliability (agreement, 97%; Cohen's kappa: 0.93).
Feasibility: Although an easy and quick test to evaluate the presence of freezing of gait, turning ability, and, indirectly, functionality, it is not a movement very frequent in daily life and does not provide much information on patients’ functional mobility. It is also limited to patients without postural instability.
BBS
Construct assessed: Functional standing balance.
Test description: The scale consists of 14 items, each scored from 0 to 4, to measure a subject's ability to maintain positions or movements of increasing difficulty by diminishing the base of support.14, 31, 39, 40 Tasks include sitting, standing, standing to a single‐leg stance, and positional changes.
Clinimetric properties: BBS score significantly correlates with indicators of motor functioning (UPDRS motor score: r = –0.58; P < 0.005), stage of disease (H & Y scale staging: r = –0.45; P < 0.005), and daily living capacity (Schwab and England ADL Scale rating: r = 0.55; P < 0.005). A ceiling effect has been reported. The ICCs for test‐retest reliability are above 0.90. A value for MDC has been calculated (MDC = 5).
Feasibility: The BBS is a relatively safe and simple to administer instrument. It may not be very useful in mild‐to‐moderate PD patients because of ceiling effects. It does not take into account the quality of movement and therefore may be less useful in PD, where motor control is a bigger contributor to poor balance than muscle weakness.
Mini‐BESTest
Construct assessed: Balance.
Test description: The Mini‐BESTest is a 14‐item tool to measure dynamic balance, which is associated with movement during transfers and gait, as well as external perturbations and cognitive dual‐task performance.14, 41, 42 It includes six domains: biomechanical constraints, verticality/stability limits, anticipatory postural adjustments, postural responses, sensory orientation, and stability in gait.
Clinimetric properties: The Mini‐BESTest has a strong relationship with the BESTest total score (r = 0.955) and a comparable ability to discriminate between fallers and nonfallers. It has a high inter‐rater and test‐retest reliability (ICC = 0.91 and 0.92, respectively). Information on minimal clinically important difference is available.
Feasibility: Although it requires equipment, it is feasible for use in clinical practice.
Functional Reach Test
Construct assessed: Static balance.
Test description: A ruler is mounted on the wall at shoulder height.14, 31, 43 The participant is required to reach forward the maximal distance beyond the arm's length, while maintaining a fixed base of support in the standing position.
Psychometric properties: Functional reach significantly correlates with the UPDRS (r = 0.69; P < 0.001) and H & Y (r = 0.71; P < 0.001). The test has a moderate (0.44–0.51) to strong (0.72–0.76) correlation with balance master items and reaching tasks. ICC values in test‐retest reliability were 0.84 for a 1‐day testing interval and 0.73 to 0.74 for 1 week. Responsiveness in PD has been demonstrated by significant differences in scores between exercise and control groups. MDC values range from 4 to 11.5 cm.
Feasibility: The Functional Reach Test is a practical balance tool used to evaluate the effect of interventions. It is limited to patients who can stand for 1 minute without support, and patients frequently need to be helped to correctly perform the required movement.
UPDRS Part III
Construct assessed: Motor performance.
Test description: A subsection of the most widely used clinical rating scale in PD to assess disease severity and progression and to determine treatment‐related benefits.4, 44, 45 Part III comprises 11 items, including ratings for tremor, slowness (bradykinesia), stiffness (rigidity), and balance. Punctuated from 0 to 4, with a higher score showing a higher level of disability.
Clinimetric properties: The UPDRS has adequate face validity, satisfactory construct validity, and is sensitive to changes in clinical status. It has excellent internal consistency throughout disease progression measured with the H & Y scale and adequate inter‐ and intrarater reliability.
Feasibility: Used in almost all PD clinical trials. It provides a comprehensive assessment, approaching several crucial constructs in PD that can be used across all patients regardless of severity, treatment, or age. Even in the revised version, the MDS‐UPDRS has no item, or set of items, that specifically measure functional mobility, and it is still very time‐consuming to use in everyday clinical practice.
Defining FM Concept
Of the 95 included studies, one defined the concept of FM and 55 (57.9%) mentioned the concept in the article. Among these, other concepts were used as synonyms for FM; the most used term was mobility (18.2%; n = 10). In the studies that did not overtly use the term FM, but for which we considered FM was assessed, the most used expressions were mobility (25%; n = 10) or mobility in association with functional activities/performance, motor function, gait‐related activity, or balance (25%; n = 10; Supporting Information Appendix 3).
Conclusion
The assessment of FM has been included in PD studies and has increased over the years. FM is an outcome that may best convey the patient's overall health status in his or her environment. FM incorporates a series of ill‐defined and loosely used concepts that are generally considered to assess motor function in the context of functional activities/performance. Several measurement tools have been used to measure FM, especially in association with TUG.
FM Measurement Instruments
Recommended and Suggested Measurement Tools
Among the reviewed instruments, only the TUG and mPAS were designed and are validated to measure FM in PD. The TUG‐DT, although an update of TUG and frequently used in PD clinical studies, has not been assessed clinimetrically. The TUG test is an easy and quick‐to‐apply test that is broadly used in PD. It is limited to subjects who have the ability to walk, follow instructions, and who do not suffer from severe freezing episodes. Although this test includes the three anchors of functional mobility (gait, balance, and transfers), and is considered a good predictor of FM, it is still a little distant from the reality of daily‐living activities, which hampers its ability to capture the patient's functional status in his or her environment.5, 14 This may explain the frequent association of TUG with one or more scales found in our results.
The mPAS is a scale specifically designed to evaluate PD that overcomes this limitation by assessing functional gait, balance, and transfers through different scenarios. Its major limitation is the number of accessories, space, and time needed to perform the test. The bed mobility items require a bed (large enough to turn to both sides), sheet, and a blanket, which may not be practical or feasible in all centers.14, 22
Listed Measurement Tools
The FTSTS and the SPPB, although used as single instruments to measure FM, are not validated to measure FM in PD. The FTSTS test assesses lower extremity strength asking the patient to stand up and sit five times, which is not representative of the FM concept. Although the SPPB can be considered to assess the three anchors of functional mobility (the FTSTS, one test of static balance [10 seconds with the feet together, in semitandem and full tandem], and a 3‐m walk), it uses very little functional and isolated tests, making its adequacy to measure FM, in our opinion, questionable. Compared with the SPPB, the TUG test seems more attractive given that it includes the anchors, in a simpler test, and, above all, in a sequential way, which makes it more functional and closer to the movements of daily life.
Potential Measurement Tools to Assess FM
One psychometric study46 has assessed, with positive results, a new scale to assess FM in PD: the Lindop Parkinson's Disease Mobility Assessment. This is a 10‐item rating scale that covers the same constructs as the mPAS in a simplified form. This scale was validated in 2009, but we did not find any studies that have used it to assess FM in PD. Nevertheless, it seems that it could be an alternative to the mPAS.
Although not validated for measuring FM in PD, the Mini‐BESTest seems worthy of being studied as an isolated tool to measure FM. Like the mPAS, the Mini‐BESTest assesses the three constructs of FM through different tasks, with the added value of including the TUG‐DT test, the assessment of gait in association with common tasks of daily living (e.g., changes in gait speed, walk with head turn, walk with pivotal turn, and step over obstacles), and the assessment of reactive postural control in four directions. It does not include the assessment of bed mobility.
Nine of the included studies (9.5%) used kinematic gait parameters to assess FM. Given that FM is a more global and illustrative outcome of patients’ health status, the use of technology‐based objective measures is very attractive. However, the most suitable parameters and instrument to this end need to be defined.
A 2016 study reviewed Instruments to Assess Posture, Gait, and Balance in Parkinson's Disease,14 a topic that overlaps largely with the aim of this review. However, there is an essential difference between these two reviews. Although posture, gait, and balance are crucial aspects of FM, the operationalization of this concept requires their simultaneous presence (along with transfers) during a task of daily living. The assessment of the three parameters, either separately or without carrying out a functional task, should not be considered an FM assessment.
The Concept of FM
Although frequently mentioned and increasingly used in clinical studies, the concept of FM is not included in the ICF.47 Only 1 of the 95 studies (1.1%) defined FM in the article.
In the absence of a universally accepted definition of FM, we adopted the Forhan and Gill1 definition, previously used in a study on obesity, after verifying its suitability through a match with other definitions found on an electronic search conducted in MEDLINE/PubMed to appraise for other operational definitions of FM. All the definitions share the anchor that FM is the subject's ability to move within a natural environment and to perform everyday tasks and the operationalization by the assessment of gait, balance, and transfers during the performance of a functional task. Frequently, the concept of mobility was used as a synonym of FM in the included studies. In order to verify what was understood by mobility, we reviewed its current ICF definition. According to this, mobility is defined as “moving by changing body position or location or by transferring from one place to another, by carrying, moving or manipulating objects, by walking, running or climbing, and by using various forms of transportation.”47 This is a broader concept than FM given that it is not restricted to actions conducted with the purpose of completing an activity of daily living, which is mandatory for FM. Although we acknowledge the absence of a universal definition for FM, we believe that the Forhan and Gill1 description, adopted in this review, is the most consensual definition of FM. Therefore, in the context of this review, we have defined FM as a domain of mobility, focused on a person's physiological ability to move independently and safely within a variety of environments in order to accomplish functional activities or tasks and to participate in the activities of daily living.1
Among the measurement tools assessed in this review on FM, the TUG test seems the most suitable for use in clinical practice and research, having been designed to evaluate FM and displaying strong clinimetric properties.
A limitation for establishing the most appropriate outcome tools is the absence of an established concept of FM and the misuse of several overlapping terms. We recommend the use of the Forhan and Gill1 as the most consensual and pragmatic operational definition of FM. Based on this, we suggest to validate the existing tools (e.g., the Mini‐BESTest) and potentially develop novel scales that measure FM in PD. We also highlight the need to study how FM behaves in the context of clinical trials, concretely its responsiveness to change in the assessment of pharmacological and nonpharmacological therapeutic interventions. The combination of various validated tools will possibly provide a more complete measurement of FM. The use of technology‐based objective measures is increasingly being used to asses PD patients, with the added value of tracking FM from the users’ daily routine, using a smartphone or a similar device, without the need of any explicit test. Although still very new and fragile, future studies should also explore these as potential outcome tools for measuring FM.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
R.B.M.: 1A, 1B, 1C, 2A, 2B, 3A
G.S.D.: 1B, 1C, 2A, 2C, 3B
M.P.: 1C, 3B
A.C.C.: 2C, 3B
J.A.: 1C, 3B
R.M.F.: 2C, 3B
T.M.: 2C, 3B
R.M.: 2C, 3B
J.J.F.: 1A, 1B, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that neither the approval of an institutional review board nor patient consent was required for this work.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: R.B.M. received a PhD grant from Fundação para a Ciência e a Tecnologia (FCT) (SFRH/BD/120773/2016 to RBM). R.M.F. is a member of the IDMC for clinical trials conducted by ablynx/novartis and reviral in pediatric bronchiolitis and a member of CEC for a clinical trial conducted by janssen in RSV prevention. T.M. has consulted for CHDI Foundation/Management, Sunovion, and Valeo Pharma; is a member of the advisory boards of AbbVie; and has received grants from JPND, Ontario Research Funding, CIHR, The Michael J. Fox Foundation, Parkinson Canada, PDF/PSG, and LesLois Foundation. J.J.F. has consulted for Ipsen, GlaxoSmithKline, Novartis, Teva, Lundbeck, Solvay, Abbott, BIAL, Merck‐Serono, and Merz; has received research grants from GlaxoSmithKline, Grunenthal, Teva, and Fundaço MSD; and has been employed by Laboratory of Clinical Pharmacology and Therapeutics of Lisbon.
Supporting information
Appendix 1. Search strategy for functional mobility in PD research: a systematic review.
Appendix 2. Flow diagram of study selection process.
Appendix 3. FM‐related concepts used in the included studies.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Forhan M, Gill S V. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metab 2013;27:129–137. [DOI] [PubMed] [Google Scholar]
- 2. Bouça‐Machado R, Maetzler W, Ferreira JJ. What is functional mobility applied to Parkinson's disease? J Parkinsons Dis 2018;8:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santos MP, Da Silva BA, Ovando AC, Ilha J, Swarowsky A. Comparison between two functional mobility scales for Parkinson's disease directly applied to physical therapy practice: cross‐cultural adaptation and measurement properties. Eur J Phys Rehabil Med 2017;53:664–675. [DOI] [PubMed] [Google Scholar]
- 4. Bhidayasiri R, Martinez‐Martin P. Clinical assessments in Parkinson's disease. Int Rev Neurobiol 2017;132:129–182. [DOI] [PubMed] [Google Scholar]
- 5. Verheyden G, Kampshoff CS, Burnett ME, et al. Psychometric properties of 3 functional mobility tests for people with Parkinson disease. Phys Ther 2014;94:230–239. [DOI] [PubMed] [Google Scholar]
- 6. Scott‐Roberts S, Purcell C. Understanding the functional mobility of adults with developmental coordination disorder (DCD) through the International Classification of Functioning (ICF). Curr Dev Disord Rep 2018;5:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adair B, Said CM, Rodda J, Morris ME. Psychometric properties of functional mobility tools in hereditary spastic paraplegia and other childhood neurological conditions. Dev Med Child Neurol 2012;54:596–605. [DOI] [PubMed] [Google Scholar]
- 8. Harvey A, Graham HK, Morris ME, Baker R, Wolfe R. The functional mobility scale: ability to detect change following single event multilevel surgery. Dev Med Child Neurol 2007;49:603–607. [DOI] [PubMed] [Google Scholar]
- 9. Lawrence H, Hills S, Kline N, Weems K, Doty A. Effectiveness of exercise on functional mobility in adults with cerebral palsy: a systematic review. Physiother Can 2016;68:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shumway‐Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community‐dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80:896–903. [PubMed] [Google Scholar]
- 11. Keus S, Munneke M, Graziano M, et al. European Physiotherapy Guideline for Parkinson's Disease [Internet]. 1st ed, Vol. 1, KNGF/ParkinsonNet. The Netherlands; 2014. 1–191 pp. Available from: http://www.fizioterapeitiem.lv/attachments/article/307/4_eu_guideline_parkinson_201412-development.pdf. Accessed on April 19; 2016.
- 12. Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions. Higgins JP, Green S, eds. The Cochrane Collaboration; Oxford, UK: The Cochrane Collaboration; 2006:1–265. [Google Scholar]
- 13. Leal Rato M, Duarte GS, Ferreira AN, et al. Nocebo response in Parkinson's disease: a systematic review and meta‐analysis. Park Relat Disord 2019;65:13–19. [DOI] [PubMed] [Google Scholar]
- 14. Bloem BR, Marinus J, Almeida Q, et al. Measurement instruments to assess posture, gait, and balance in Parkinson's disease: critique and recommendations. Mov Disord 2016;31:1342–1355. [DOI] [PubMed] [Google Scholar]
- 15. Elble R, Bain P, João Forjaz M, et al. Task force report: scales for screening and evaluating tremor: critique and recommendations. Mov Disord 2013;28:1793–1800. [DOI] [PubMed] [Google Scholar]
- 16. Antonini A, Martinez‐Martin P, Chaudhuri RK, et al. Wearing‐off scales in Parkinson's disease: critique and recommendations. Mov Disord 2011;26:2169–75. [DOI] [PubMed] [Google Scholar]
- 17. Prinsen CAC, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”—a practical guideline. Trials 2016;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up, & Go” Test in people with Parkinson disease. Phys Ther 2001;81:810–818. [DOI] [PubMed] [Google Scholar]
- 19. Podsiadlo, D ; Richardson S. The Timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 20. Campbell CM, Rowse JL, Ciol MA, Shumway‐Cook A. The effect of cognitive demand on timed up and go performance in older adults with and without Parkinson disease. Neurol Rep 2003;27:2–7. [Google Scholar]
- 21. Hofheinz M, Schusterschitz C. Dual task interference in estimating the risk of falls and measuring change: a comparative, psychometric study of four measurements. Clin Rehabil 2010;24:831–842. [DOI] [PubMed] [Google Scholar]
- 22. Keus SHJ, Nieuwboer A, Bloem BR, Borm GF, Munneke M. Clinimetric analyses of the Modified Parkinson Activity Scale. Parkinsonism Relat Disord 2009;15:263–269. [DOI] [PubMed] [Google Scholar]
- 23. Nieuwboer A, De Weerdt W, Dom R, Bogaerts K, Nuyens G. Development of an activity scale for individuals with advanced Parkinson disease: reliability and “on‐off” variability. Phys Ther 2000;80:1087–1096. [PubMed] [Google Scholar]
- 24. Mayhew T, Rothstein J. Measurement of muscle performance with instruments. Percept Mot Skills 1995;80:163–166.7624188 [Google Scholar]
- 25. Duncan RP, Leddy AL, Earhart GM. Five times sit‐to‐stand test performance in Parkinson's disease. Arch Phys Med Rehabil 2011;92:1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alfieri FM, Riberto M, Gatz LS, et al. Functional mobility and balance in community‐dwelling elderly submitted to multisensory versus strength exercises. Clin Interv Aging 2010;5:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all‐cause mortality: systematic review and meta‐analysis. BMC Med 2016;14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rikli RE, Jones CJ. Development and validation of criterion‐referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013;53:255–267. [DOI] [PubMed] [Google Scholar]
- 29. de Villar LOP, Martínez‐Olmos FJ, Junqué‐Jiménez A, et al. Test‐retest reliability and minimal detectable change scores for the short physical performance battery, one‐legged standing test and timed up and go test in patients undergoing hemodialysis. PLoS One 2018;13:e0201035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freire AN, Guerra RO, Alvarado B, Guralnik JM, Zunzunegui MV. Validity and reliability of the short physical performance battery in two diverse older adult populations in Quebec and Brazil. J Aging Health 2012;24:863–878. [DOI] [PubMed] [Google Scholar]
- 31. Steffen T, Seney M. Test‐retest reliability and minimal detectable change on balance and ambulation tests, the 36‐Item Short‐Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Phys Ther 2008;88:733–746. [DOI] [PubMed] [Google Scholar]
- 32. Combs SA, Diehl MD, Filip J, Long E. Short‐distance walking speed tests in people with Parkinson disease: reliability, responsiveness, and validity. Gait Posture 2014;39:784–788. [DOI] [PubMed] [Google Scholar]
- 33. Hassett L, de Bie RA, Moseley AM, Bosman JM, van Loo MA. Test–re‐test reliability of walking speed, step length and step width measurement after traumatic brain injury: a pilot study. Brain Inj 2004;18:1041–1048. [DOI] [PubMed] [Google Scholar]
- 34. Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 35. Schenkman M, Cutson TM, Kuchibhatla M, Chandler J, Pieper C. Reliability of impairment and physical performance measures for persons with Parkinson's disease. Phys Ther 2017;77:19–27. [DOI] [PubMed] [Google Scholar]
- 36. Suteerawattananon M, Protas EJ. Reliability of outcome measures in individuals with Parkinson's disease. Physiother Theory Pract 2000;16:211–218. [Google Scholar]
- 37. Ziegler K, Schroeteler F, Ceballos‐Baumann AO, Fietzek UM. A new rating instrument to assess festination and freezing gait in Parkinsonian patients. Mov Disord 2010;25:1012–1018. [DOI] [PubMed] [Google Scholar]
- 38. Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non‐freezer: clinical assessment of freezing of gait. Parkonsonism Relat Disord 2012;18:149–154. [DOI] [PubMed] [Google Scholar]
- 39. Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the Berg Balance Scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil 2005;86:789–792. [DOI] [PubMed] [Google Scholar]
- 40. Berg K, Wood‐Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Heal 2014;83(Suppl 2):S7–S11. [PubMed] [Google Scholar]
- 41. Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini‐BESTest. J Rehabil Med 2013;42:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leddy AL, Crowner BE, Earhart GM. Utility of the mini‐BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther 2011;35:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol 1990;45:192–197. [DOI] [PubMed] [Google Scholar]
- 44. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 45. Movement Disorder Society . The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–750. [DOI] [PubMed] [Google Scholar]
- 46. Santos MP, da Silva BA, Ovando AC, Ilha J, Swarowsky A. Comparison between two functional mobility scales for Parkinson's disease directly applied to physical therapy practice: cross‐cultural adaptation and measurement properties. Eur J Phys Rehabil Med 2017;53:664–675. [DOI] [PubMed] [Google Scholar]
- 47. World Health Organization . Towards a Common Language for Functioning, Disability and Health: The International Classification of Functioning, Disability and Health. International Classification [Internet]. 2002;1149:1–22. Available from: http://www.who.int/classifications/icf/training/icfbeginnersguide.pdf. Accessed on March 08; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Search strategy for functional mobility in PD research: a systematic review.
Appendix 2. Flow diagram of study selection process.
Appendix 3. FM‐related concepts used in the included studies.


