Abstract
Background
The loss of muscle mass, known as sarcopenia, is a natural process of aging that is associated with adverse health outcomes regardless of age. Because cancer is a disease of aging, interest in sarcopenia and its potential impact in multiple cancer populations has increased significantly. Bioelectrical impedance analysis (BIA) is a guideline‐accepted method for sarcopenia detection. This systematic review assesses the literature pertaining to BIA use in the detection of sarcopenia in adults with cancer.
Materials and Methods
In this systematic review, a search of the literature for randomized controlled trials and observational studies was conducted using MEDLINE, Cochrane CENTRAL, and EMBASE, through July 15, 2019. The study is registered at Prospero (CRD 42019130707). For study inclusion, patients had to be aged 18 years or older and diagnosed with solid or hematological neoplasia, and BIA had to be used to detect sarcopenia.
Results
Through our search strategy, 5,045 articles were identified, of which 24 studies were selected for inclusion in the review (total number of 3,607 patients). In five studies, BIA was rated comparable to axial computed tomography (CT) scan, calf circumference, or grip strength for sarcopenia screening. In 14 studies, BIA‐identified sarcopenia was associated with adverse clinical outcomes.
Conclusion
BIA is an accurate method for detecting sarcopenia in adults with cancer prior to treatment and is a viable alternative to CT, dual‐energy x‐ray absorptiometry, and magnetic resonance imaging in oncology clinical practice.
Implications for Practice
Bioelectrical impedance analysis (BIA) is an attractive method for identifying sarcopenic patients in clinical practice because it provides an affordable, noninvasive test that can be completed within a few minutes during a clinic visit. BIA does not require highly skilled personnel, and results are immediately available. This systematic review summarizes the literature pertaining to BIA assessment of sarcopenia in adults with cancer, with a focus on its use in diverse cancer populations.
Keywords: Bioimpedance electrical analysis, Sarcopenia, Cancer, Pretreatment
Short abstract
Sarcopenia is a natural process of aging that is associated with adverse health outcomes. Because cancer is a disease of aging, interest in sarcopenia and its potential impact in multiple cancer populations has increased. Bioelectrical impedance analysis (BIA) is a guideline‐accepted method for sarcopenia screening. This systematic review assesses the literature pertaining to BIA use in the detection of sarcopenia in adults with cancer.
Introduction
Sarcopenia was first identified in older persons as a natural process of loss of muscle mass associated with aging 1. As understanding of sarcopenia has evolved, so has the definition of sarcopenia as evidenced by the European Working Group on Sarcopenia in Older People (EWGSOP) 2 description of sarcopenia as a “syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death.” Studies have shown that sarcopenic adults have an 87% increased risk for overall mortality compared with nonsarcopenic adults (hazard ratio = 1.87; 95% confidence interval = 1.61–2.18; p = .03) 3. Because cancer is largely a disease of aging, interest in sarcopenia and its potential impact in multiple cancer populations has increased significantly over the past decade 4, 5. A recent systematic review that included 6,894 adults diagnosed with cancer found that sarcopenia was associated with postoperative complications, chemotherapy‐induced toxicity, and poor survival 6. Similarly, a recent meta‐analysis that included 7,843 patients found that sarcopenic patients with cancer had worse overall survival and disease‐free survival compared with nonsarcopenic patients 7.
Several methods can be used to screen for sarcopenia. Body mass index (BMI) has long been used to assess obesity 8 because it is readily available and commonly used in clinical practice to estimate nutritional status. However, BMI lacks sensitivity and specificity in body composition assessment 9, especially in its ability to detect sarcopenia 10. Imaging techniques, by contrast, yield more accurate estimates of sarcopenia 11. Computed tomography (CT) scans are frequently used in research studies, particularly in oncology populations, in light of their use in clinical diagnosis, with most assessments using cross‐sectional images at the level of the third lumbar vertebra (L3) 2, 12. Magnetic resonance imaging (MRI) 13, 14 is another precise method that can easily differentiate body structures and provide accurate measures of body composition. Dual‐energy x‐ray absorptiometry (DXA) scans are generally associated with bone density analysis but can also provide accurate assessments of other lean body mass 15. However, DXA does not assess skeletal muscle mass.
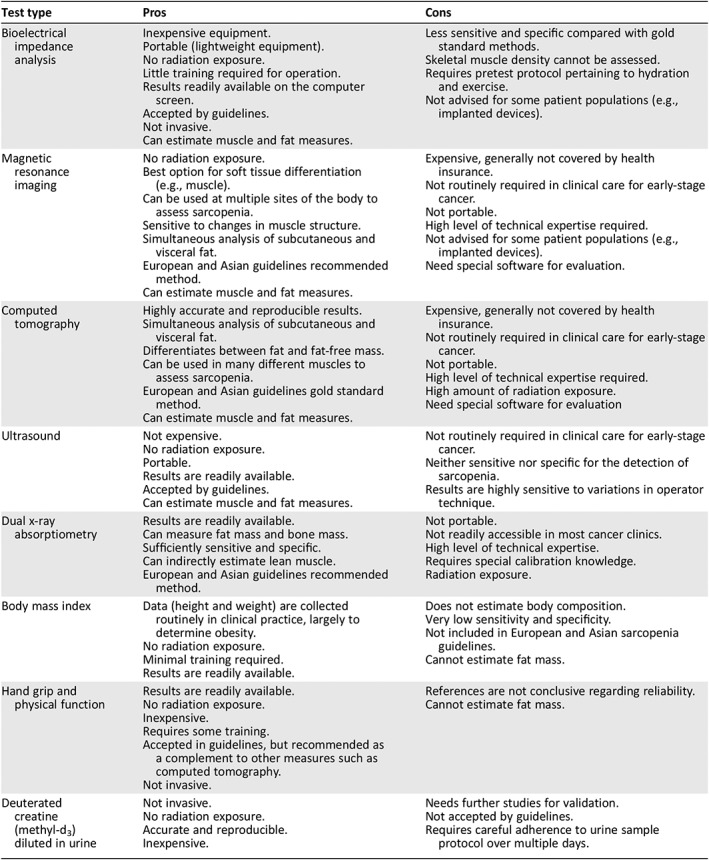
Other methods include bioelectrical impedance analysis (BIA), which is a guideline‐accepted (Asian and European guidelines) method for objective assessment of body composition 2, 12. Hand grip strength and assessment of physical function are part of current sarcopenia guidelines and should be used in combination with other methods (e.g., CT scan, BIA, MRI) to increase the accuracy of body composition assessments 11. The SARC‐F is a validated patient‐reported measure of physical function that inquires about five areas: strength, assistance in walking, rising from a chair, climbing stairs, and falls 16. The pros and cons of these methods are summarized in Table 1.
Table 1.
Methods of body composition assessment
| Test type | Pros | Cons |
|---|---|---|
| Bioelectrical impedance analysis |
Inexpensive equipment. Portable (lightweight equipment). No radiation exposure. Little training required for operation. Results readily available on the computer screen. Accepted by guidelines. Not invasive. Can estimate muscle and fat measures. |
Less sensitive and specific compared with gold standard methods. Skeletal muscle density cannot be assessed. Requires pretest protocol pertaining to hydration and exercise. Not advised for some patient populations (e.g., implanted devices). |
| Magnetic resonance imaging |
No radiation exposure. Best option for soft tissue differentiation (e.g., muscle). Can be used at multiple sites of the body to assess sarcopenia. Sensitive to changes in muscle structure. Simultaneous analysis of subcutaneous and visceral fat. European and Asian guidelines recommended method. Can estimate muscle and fat measures. |
Expensive, generally not covered by health insurance. Not routinely required in clinical care for early‐stage cancer. Not portable. High level of technical expertise required. Not advised for some patient populations (e.g., implanted devices). Need special software for evaluation. |
| Computed tomography |
Highly accurate and reproducible results. Simultaneous analysis of subcutaneous and visceral fat. Differentiates between fat and fat‐free mass. Can be used in many different muscles to assess sarcopenia. European and Asian guidelines gold standard method. Can estimate muscle and fat measures. |
Expensive, generally not covered by health insurance. Not routinely required in clinical care for early‐stage cancer. Not portable. High level of technical expertise required. High amount of radiation exposure. Need special software for evaluation |
| Ultrasound |
Not expensive. No radiation exposure. Portable. Results are readily available. Accepted by guidelines. Can estimate muscle and fat measures. |
Not routinely required in clinical care for early‐stage cancer. Neither sensitive nor specific for the detection of sarcopenia. Results are highly sensitive to variations in operator technique. |
| Dual x‐ray absorptiometry |
Results are readily available. Can measure fat mass and bone mass. Sufficiently sensitive and specific. Can indirectly estimate lean muscle. European and Asian guidelines recommended method. |
Not portable. Not readily accessible in most cancer clinics. High level of technical expertise. Requires special calibration knowledge. Radiation exposure. |
| Body mass index |
Data (height and weight) are collected routinely in clinical practice, largely to determine obesity. No radiation exposure. Minimal training required. Results are readily available. |
Does not estimate body composition. Very low sensitivity and specificity. Not included in European and Asian sarcopenia guidelines. Cannot estimate fat mass. |
| Hand grip and physical function |
Results are readily available. No radiation exposure. Inexpensive. Requires some training. Accepted in guidelines, but recommended as a complement to other measures such as computed tomography. Not invasive. |
References are not conclusive regarding reliability. Cannot estimate fat mass. |
| Deuterated creatine (methyl‐d3) diluted in urine |
Not invasive. No radiation exposure. Accurate and reproducible. Inexpensive. |
Needs further studies for validation. Not accepted by guidelines. Requires careful adherence to urine sample protocol over multiple days. |
Bioelectrical impedance analysis is an especially attractive method for identifying sarcopenic patients in clinical practice because it is an affordable, noninvasive test that can be completed within a few minutes. It does not require highly skilled personnel, and results are immediately available. In this systematic review, we summarize the literature pertaining to BIA assessment of sarcopenia in adults with cancer, with a focus on its use in diverse cancer populations. Our analysis includes a review of the definitions of sarcopenia, BIA methodology (equipment and assessment), outcomes of interest, and overall quality of the research. Our specific interest is whether BIA measurements are associated with clinical outcomes.
Materials and Methods
Bioelectrical Impedance Analysis
BIA estimates body composition using equations that calculate the difference of electrical conductivity among different tissues (e.g., bones, adipose, muscle, cartilage) 17. Impedance (tissue resistance and reactance) is registered when a low‐voltage current is passed through the body 18. The major differences among BIA devices are prediction equations, the number of tactile electrodes, and the frequencies of alternating current 19. The accuracy of BIA depends primarily on the adequacy of tissue hydration. In supplemental online Appendix 1, we provide a basic illustration of the BIA test/examination. The patient can be either seated or lying down, with BIA stickers attached to the right hand and right foot. The patient's age and weight are entered into the BIA software. Body mass measures are available within seconds on the output screen.
Search Strategy
This systematic review is registered at PROSPERO (international database of prospectively registered systematic reviews) 20 (CRD 42019130707). With assistance from the University of North Carolina Health Sciences Library in the use of COVIDENCE systematic review software (Veritas Health Innovation, Melbourne, Australia), two reviewers (G.F.A. and K.A.N.) independently performed the search in several databases (PubMed/Medline, Cochrane Central Register for Clinical Trials and EMBASE) with a publication cutoff date of July 15, 2019. References from systematic reviews pertaining to BIA in patients with cancer were also searched for studies 6, 21, 22, 23. The search strategy (see supplemental online Appendix 2 for search terms) followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) framework 24, using combined terms and MeSH (Medical Subject Headings of the National Library of Medicine) descriptors, had the following criteria: (a) population: patients aged 18 years or older diagnosed with either solid or hematological neoplasia; (b) interest: BIA; (c) comparison or control: not applicable; (d) outcomes: primary outcome—use of BIA to detect sarcopenia (with defined cut‐points); secondary outcomes—association of sarcopenia with clinical outcomes; and (e) study design: observational or randomized controlled trial (including abstracts).
Two independent reviewers (G.F.A. and K.A.N.) selected the articles and extracted the data. Any discrepancy was resolved by consensus between the reviewers or after discussion with a third author (G.R.W.). The reviewers reviewed the title and abstract for all studies that were found through COVIDENCE. Full texts were evaluated when there was insufficient information in the title and abstract to make a decision about inclusion or exclusion. References in reviewed and excluded articles were examined to identify studies that may not have been identified through the primary search strategy. The search was not limited to the English language. A final list of studies for inclusion in the systematic review was generated.
Data Analysis
Extracted data included details regarding authors, year of publication, country of the study population, inclusion/exclusion criteria (patient characteristics), stage of cancer when the BIA measure was taken, and method of BIA measurement (e.g., equipment and assessment). Data were also extracted regarding the definition of sarcopenia (cut‐points) and clinical outcomes (e.g., mortality, postoperative complications, quality of life, etc.). The Newcastle‐Ottawa Scale (NOS) 25 for the assessment of cohort studies was used by two independent researchers (G.F.A. and G.R.W.) to assess the methodological quality of included studies.
Role of the Funding Source
There was no funding source for this study.
Results
A total of 5,045 articles were identified through the search strategy. Figure 1 presents the PRISMA 26 diagram. After duplicates were removed, the two primary reviewers screened titles and abstracts for 4,579 articles. For articles that remained after the initial screen, 65 full texts were reviewed for eligibility. Ultimately, 24 studies were selected for inclusion in the systematic review—18 articles 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47 and 6 abstracts 33, 44, 45, 46, 48, 53—with a total of 3,607 patients.
Figure 1.
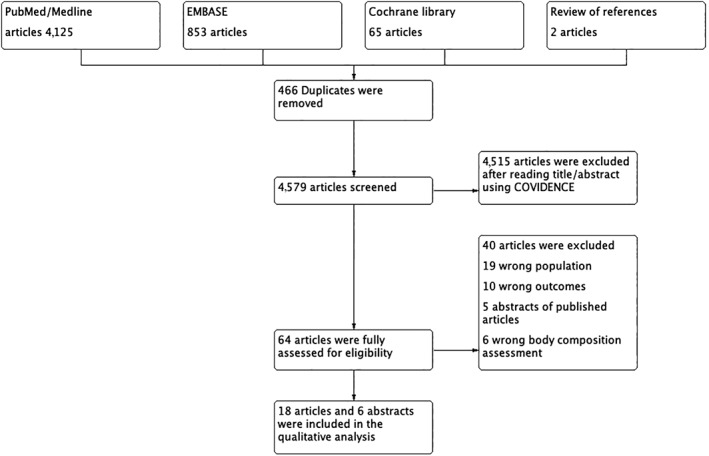
PRISMA search.
Table 2 provides an overview of included studies regarding number of study participants, cancer types, inclusion criteria, and stage of care when the BIA assessment was conducted. Cancer sites included gastric 31, 32, 33, 47, 48, esophageal 30, 33, 34, 35, 36, 37, 38, 39, lung 33, colorectal 44, 49, urothelial 33, 50, and multiple 28, 29, 40, 42, 43, 46, 51. BIA assessment was conducted before surgery in 12 studies 28, 30, 31, 32, 34, 37, 44, 45, 46, 47, 48, 49, before chemotherapy in 7 studies 29, 33, 38, 39, 40, 43, 50, and before both chemotherapy and surgery in 5 studies 19, 35, 36, 51, 52. In none of the studies, the BIA was conducted during or after chemotherapy or radiation, although in one study the patients were receiving palliative care 42.
Table 2.
Characteristics of included studies (n = 3,146 patients)
| Author [reference], year, country | n | Types of cancer | Inclusion and exclusion criteria | Stage of care |
|---|---|---|---|---|
| Perez Camargo 42, 2017, Mexico | 628 | Multiple solid (94%) and hematologic (6%) |
Inclusion: >18 years of age, not severely disabled, Karnofsky Performance Status >40. Exclusion: delirium, metallic prosthesis, cardiac pacemaker, any affliction preventing BIA. |
Palliative treatment |
| Oflazoglu 43, 2019, Turkey | 461 | Multiple |
Inclusion: >18 years of age, newly diagnosed with early‐stage or metastatic cancer; received no chemotherapy or radiotherapy prior to BIA assessment. Exclusion: deformities that made impossible to be tested for muscle strength |
Prechemotherapy or radiation, or surgery |
| Otten 27, 2019, Germany | 439 | Multiple |
Inclusion: >60 years of age, consecutive solid or hematologic tumor disease of any type or stage. Exclusion: currently in a drug or interventional trial, implanted device, neuromuscular diseases, hemiplegia, severe osteoarthritis. |
Prechemotherapy or radiation, or surgery or no treatment |
| Harter 28, 2017, Brazil | 60 | Multiple |
Inclusion: 18 years of age, diagnosed with cancer. Exclusion: edema of lower limbs, amputation of limb, inability to stay in dorsal decubitus or inability to walk, pacemaker, other limitations. |
Preoperative |
| Gonzalez 29, 2014, Brazil | 175 | Multiple |
Inclusion: >18 years of age, receiving chemotherapy for cancer treatment for the first time, able to rest quietly. Exclusion: pacemaker, clinically not able to receive chemotherapy, edema. |
Prechemotherapy |
| Harter 46, 2017, Brazil | 59 | Multiple | Inclusion: 18 years of age, diagnosed with cancer. | Preoperative |
| Fukuda 47, 2015, Japan | 99 | Gastric | Criteria: >65 years of age, underwent gastrectomy, had previous body composition analysis. | Preoperative |
| Sato 31, 2016, Japan | 293 | Gastric | Criteria: patients who underwent gastrectomy D2, pathologically confirmed adenocarcinoma, R0 or R1 was achieved, ECOG‐PS 0–2, no other primary cancer, BIA and handgrip test before surgery. | Preoperative |
| Tamura 32, 2019, Japan | 153 | Gastric |
Criteria: patients who underwent gastrectomy and had previous body composition analysis. Exclusion: patients with remnant gastric cancer, had subsequent R2 resection, no prior body composition analysis. |
Preoperative |
| Yamamoto 33, 2017, Japan | 90 | Gastric | Criteria: patients 65 years or older who were scheduled to undergo gastrectomy. | Preoperative |
| Blake 48, 2017, U.S. | 125 | Esophageal, gastric | Sample: consecutive patients with esophageal and gastric neoplasia. | Preoperative |
| Miyata 35, 2017, Japan | 94 | Esophageal | Criteria: patients with esophageal cancer stages Ib–IV without distant organ metastasis who underwent surgery and had previous body composition assessment. | Pre‐ and postneoadjuvant therapy |
| Matsunaga 34, 2019, Japan | 163 | Esophageal | Criteria: patients with thoracic esophageal cancer who were scheduled for esophagectomy and had preoperative body composition analysis. | Preoperative |
| Ota 36, 2019, Japan | 31 | Esophageal | Sample: consecutive patients who received neoadjuvant chemotherapy followed by surgery for esophageal cancer and had body composition analysis. | Preneoadjuvant chemotherapy |
| Ida 37, 2015, Japan | 138 | Esophageal | Sample: patients scheduled for esophagectomy. | Preoperative |
| Makiura 30, 2016, Japan | 104 | Esophageal |
Inclusion: patients with esophageal cancer scheduled for esophagectomy, prior body composition analysis. Exclusion: recurrent cancer, declined consent. |
Preoperative |
| Ishikawa 38, 2016, Japan | 33 | Esophageal |
Inclusion criteria: aged 20–80 years; ECOG‐PS of 2 or less; at least 6 months since the last chemotherapy or radiation therapy; and adequate hematologic, hepatic, renal, and cardiac function. Exclusion criteria: uncontrolled infection; severe gastrointestinal stenosis or bleeding; requirement for total parental nutrition; use of other nutritional supplements; poor control of diabetes or the use of insulin treatment. |
Prechemotherapy or chemoradiation |
| Collins 39, 2017, U.K. | 62 | Lung | Inclusion: high suspicion for non‐small cell lung cancer, ECOG‐PS 0–2, no physical or neurological impediments precluding the ability to complete study assessments, no implanted cardiac devices. | Prechemotherapy |
| Miyake 50, 2018, Japan | 35 | Urothelial | Criteria: bladder, renal pelvis, or ureter cancer stages II–IV, before chemotherapy. | Prechemotherapy |
| Dos Reis 45, 2018, Brazil | 40 | Kidney, bladder | Sample: patients with bladder or kidney cancer, age 20 years or more. | Preoperative |
| Hopanci‐ Bicakli 40, 2019, Turkey | 153 | Multiple (colorectal, stomach, pancreas). |
Inclusion: geriatric patients (≥65 years of age) with diagnosis of gastrointestinal cancer (colorectal 51.6%, stomach 26.8%, pancreas 11.8%). Exclusion: <65 years, ascites, edema, inability to stand, hearing and perception problems. |
Prechemotherapy |
| Gibson 49, 2017, U.S. | 43 | Colorectal | Sample: patients with colorectal cancer who were losing weight and planned surgery. | Preoperative |
| Barbosa 44, 2018, Brazil | 73 | Colorectal | Sample: patients with colorectal cancer. | Preoperative |
| Kamiya 53, 2018, Japan | 56 | Hematologic | Inclusion: aged ≥60 years with hematologic malignancies diagnosed. | Prechemotherapy |
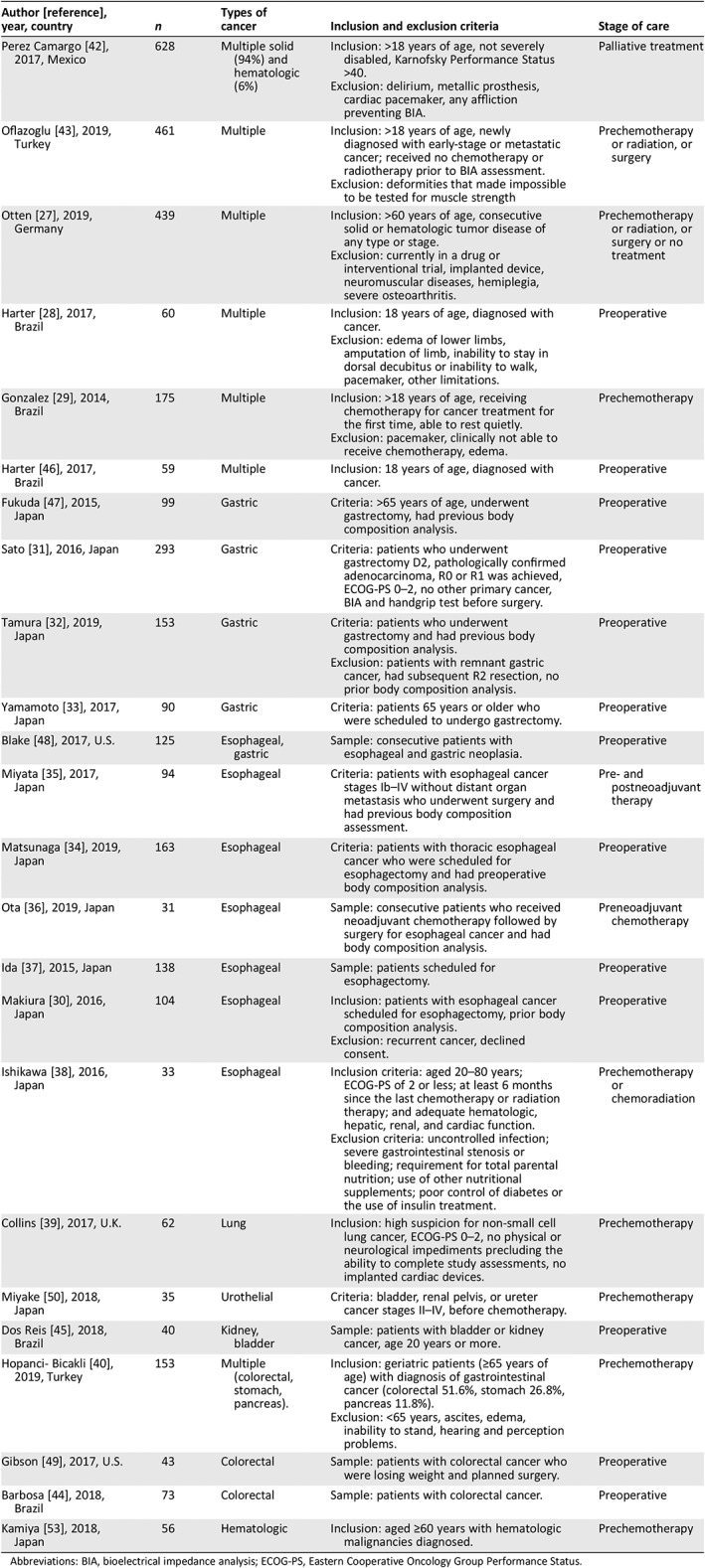
Abbreviations: BIA, bioelectrical impedance analysis; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status.
Table 3 provides an overview of the definition of sarcopenia that was used, BIA equipment and body composition measures, percentage of the sample that was identified as sarcopenic, mean age of the study sample, and association of sarcopenia with clinical outcomes. Three studies defined sarcopenia using skeletal muscle mass (SMM) 34, 35, 52, and 16 used muscle mass index (MMI), which is obtained by dividing the SMM by the square of the patient's height 28, 30, 31, 32, 36, 38, 43, 44, 45, 46, 47, 48, 49, 50, 51, 53. Three studies used the fat‐free mass index, which reflects masses of skeletal muscle, organs, bone, and connective tissue and is an indicator of resting energy expenditure 29, 31, 49. Cut‐points for sarcopenia varied among the studies, with the vast majority using cut‐points from either the European 2 or Asian 12 consensus 27, 28, 30, 33, 36, 38, 39, 40, 42, 43, 44, 45, 46, 47, 50, 53 and the remainder using other cut‐points such as quartile of muscle mass or one standard deviation below the mean of the cohort 29, 31, 32, 34, 35, 37, 48, 49.
Table 3.
Sarcopenia assessment and clinical outcomes (n = 3,146 patients)
| Author [reference], year, country | n | Sarcopenia definition [reference] | Use of hand grip and/or physical performance | BIA equipment | Percent sarcopenia | Associations of sarcopenia with clinical outcomes | Age, years |
|---|---|---|---|---|---|---|---|
| Perez Camargo 42, 2017, Mexico | 628 |
SMI: Men <8.87 kg/m2 Women <6.42 kg/m2 71 |
No | InBody 720 (Biospace, Tokyo, Japan) Multi‐frequency | 46% | Sarcopenic patients had worse survival (HR 1.4; 95% CI 1.1–1.8; p = .001). Also, more fatigue (p < .0001), pain (p = .008), insomnia (p = .032), and constipation (p = .041). |
Median: 57 Range: 19–89 |
| Otten 27, 2019, Germany | 439 |
SMI: Men <10.75 kg/m2 Women <6.75 kg/m2 2 |
No | Nutriguard M (Data‐Input GmbH) Multi‐frequency | 27.1% |
Observational: Sarcopenia was associated with lower 1‐year overall mortality (HR 1.53; 95% CI 1.034–2.25; p < .05). Sarcopenia was nearly as predictive of 1‐year mortality from advanced disease. |
Mean: 69.6 SD: 6.2 |
| Oflazoglu 43, 2019, Turkey | 461 |
SMI: Men <10.76 kg/m2 Women <6.76 kg/m2 2 |
Yes |
TANITA SC 330 (Tanita Corp., Japan) Single frequency |
16.7% |
Observational: Identified the prevalence of sarcopenia in newly diagnosed patients with cancer. Sarcopenia was more prevalent in patients who were older (p = .03) and who had a higher ECOG score (p = .02). |
Mean: 58.2 SD: (11.1) |
| Harter 28, 2017, Brazil | 60 |
SMI: Men <7.0 kg/m2 Women <6.0 kg/m2 2 |
Yes |
Quantum II (RJL Systems) Multi‐frequency |
18.6% | Observational: No difference in sarcopenic patients with regard to severe postoperative (grade 3, 4, 5) complications (18.2% vs. 12.5%, p = .635). |
18–39 (18%) 40–59 (36%) >60 (45%) |
| Gonzalez 29, 2014, Brazil | 175 |
FFMI (kg/m2) 72 Men <17.4 kg/m2 Women <15.0 kg/m2 |
No |
Quantum 101 (RJL Systems) Single‐frequency |
10.2% |
Observational: Patients with cancer with high mortality risk (HR 6.39; 95% CI 3.54–11.54) can be identified through body composition assessment, even with adjustment for possible confounding variables (HR 4.35; 95% CI 2.11–8.99; p < .0001). Sarcopenia was related with shorter median survival (p < .001). Over 6 months, 50% of sarcopenic patients had died. |
Mean: 56.9 SD: (12.8) |
| Harter 46, 2017, Brazil | 59 |
SMI: Men <7.0 kg/m2 Women <6.0 kg/m2 2 |
Yes |
Quantum II (RJL Systems) Multi‐frequency |
17% | Observational: Physical activity was decreased in all the sarcopenic patients, and nutritional and functional status was impaired. | Not shared |
| Fukuda 47, 2015, Japan | 99 |
SMI: Men <10.75 kg/m2 Women <6.75 kg/m2 2 |
Yes |
InBody 720 (Biospace, Tokyo, Japan) Multi‐frequency |
21.2% |
Observational: Preoperative sarcopenia is a risk factor for severe postoperative complications (4.76; 95% CI 1.03–24.3; p = .046) in patients with cancer undergoing gastrectomy. No difference in overall complications. |
Median: 76 Range: 66–91 |
| Sato 31, 2016, Japan | 293 |
FFMI: Each cut‐point was determined as the gender‐specific lowest 20% of the distribution for each measure based on previously published studies 73, 74 Men <17 kg/m2 Women <14.8 kg/m2 |
Yes |
MC‐180 (Tanita, Tokyo, Japan) Multi‐frequency |
19.7% | Observational: Sarcopenia was not a risk factor for mortality (OR 1.048; p = .99). Hand grip strength (OR 2.254; p = .044) was a risk factor for morbidity in patients with gastric cancer. |
Median: 66 Range: 33–85 |
| Tamura 32, 2019, Japan | 153 |
SMI: Sarcopenia was defined as SMI value of one standard deviation below the gender‐specific mean in the group 75 Men <15.44 kg/m2 Women <13.33 kg/m2 |
No | InBody 3.0 (Biospace, Tokyo, Japan) Multi‐frequency | 15.7% | Observational: Sarcopenic patients had more grade 2 or more general complications (37.5% vs. 16.3%; p = .024), higher laparotomy rate (70.8 % vs. 44.2%; p = .015) and infection complications (29.2% vs. 10%; p = .021) than nonsarcopenic. |
Median sarcopenia: 74 Range: 38–83 Nonsarcopenia: 68 Range: 32–83 |
| Yamamoto 33, 2017, Japan | 90 |
SMI: Men <10.75 kg/m2 Women <6.75 kg/m2 2 |
Yes | Not shared | 24.4% | Interventional: Sarcopenic patients submitted to 3 weeks of exercise and modified diet had increased lean muscle mass (p = .02) and no changes in surgical complications, e.g., pneumonia, leakage, fistula, abscess, bleeding, heart failure, and severe grade > 3 complications. |
Mean: 75 SD: 5 |
| Blake 33, 2017, USA | 125 |
SMI The last quartile of the group |
No |
Maltron Bioscan 920 Multi‐frequency |
25% | Observational: Sarcopenic patients had worse overall survival (HR 1.072; 95% CI 1.01–1.13; p = .009). Sarcopenia was also associated with preoperative anaerobic threshold (R = .277), postoperative morbidity and mortality (p = .005 and p = .031). |
Median: 66 Range: 24–86 |
| Miyata 35, 2017, Japan | 94 |
SMM: <90% lower limit SMM given by the BIA |
No | InBody 720 (Biospace, Tokyo, Japan) Multi‐frequency | 46.8% | Observational: Sarcopenia at baseline is not associated with toxicity (p > .05). Patients who had a larger relative decrease in SMM had higher toxicity (p = .013). |
Mean: 64.7 SD: 8.8 |
| Matsunaga 34, 2019, Japan | 163 | SMM: <90% lower limit | No |
InBody 720 (Biospace, Tokyo, Japan) Multi‐frequency |
50.3% | Observational: Sarcopenia is prognostic for overall survival and is associated with systemic inflammatory response. Sarcopenic patients had higher inflammatory markers; CRP/albumin ratio (p = .046), mGPS (p = .041), and platelet count (p = .016). Sarcopenic patients had worse 2‐year OS (73.1% vs. 85.1%; p = .025) and poor 2‐year RFS (62.8% vs. 76.3%; p = .065). |
Mean: 64.7 SD: 8 |
| Ota 36, 2019, Japan | 31 |
SMI: Men <7 kg/m2 Women <5.7 kg/m2 12 |
No |
InBody 720 (Biospace, Tokyo, Japan) Multi‐frequency |
51.6% |
Observational: Sarcopenia is an independent predictor of poor pathological response (p = .038) but not therapeutic response (p = .103). In multivariate analysis, sarcopenia was an independent risk factor for poor therapeutic response (OR 8.02; 95% CI 1.12–165.41; p = .037). Toxicities were not different between both groups. |
Median: 66 Range: 41–75 |
| Ida 37, 2015, Japan | 138 | SMM: <90% lower limit | No | InBody 720 (Biospace, Tokyo, Japan) Multi‐frequency | 44.2% | Observational: Sarcopenic patients had more pulmonary complications after esophagectomy compared with nonsarcopenic patients (15.2% vs. 6.5%; p < .01). |
Mean sarcopenic: 67.8 SD: 1 Nonsarcopenic: 63.1 SD: 0.9 |
| Makiura 30, 2016, Japan | 104 |
SMI: Men <7 kg/m2 Women <5.7 kg/m2 12 |
Yes |
DF‐860 (Yamato, Hyogo, Japan) Multi‐frequency |
27.9% | Observational: Sarcopenia is associated with more pulmonary complications (37.9% vs. 17.3%; p = .04). Also, greater blood loss (p < .001) and longer hospitalizations (p < .001). No differences with other complications or perioperative functional changes. |
Median sarcopenic: 69 Range: 66–76 Nonsarcopenic: 64 61–71 |
| Ishikawa 38, 2016, Japan | 33 |
SMI: Men <7.0 kg/m2 Women <5.7 kg/m2 2 |
No | InBody 720 (Biospace Tokyo, Japan)Multi‐frequency | 60.6% |
Intervention: Elental nutrition can counteract sarcopenia development during chemoradiotherapy for esophageal cancer (p = .007). It did not affect chemotoxicity. |
Median: 68 Range: 50–76 |
| Collins 39, 2017, U.K. | 62 |
SMI: Men <7.26 kg/m2 Women <5.45 kg/m2 |
No |
Tanita BC‐418 Single frequency |
19.4% | Observational: No difference in sarcopenic patients regarding completion of chemotherapy or adverse events. BIA overestimates muscle mass compared with DXA scan. |
Mean: 68.2 SD: 9.6 |
| Miyake 50, 2017, Japan | 35 |
SMI: Men <7.0 kg/m2 Women <6.0 kg/m2 68 |
No | InBody S20 (Biospace, Tokyo, Japan) Multi‐frequency | 20% |
Observational: BIA has similar findings to CT scans for assessing sarcopenia in metastatic urothelial carcinoma. Sarcopenia was present in five patients using CT and in seven using BIA; three patients were sarcopenic according to both methods. |
Median: 73 Range: 64–77 |
| Dos Reis 45, 2018, Brazil | 40 |
SMI: Men <10.75 kg/m2 Women <6.75 kg/m2 |
Yes | Not shared | 62.5% | Observational: Sarcopenic patients have no differences in health‐related quality of life. | Mean: 62 |
| Hopanci Bicakli 40, 2019, Turkey | 153 |
SMI: Men <10.75 kg/m2 Women <6.75 kg/m2 |
Yes |
Tanita MC 718 Multi‐frequency |
80% (30% sarcopenic obese) | Observational: BIA assessed sarcopenia correlates with calf circumference (r = .598, p < .001). |
Mean: 70.5 SD: 5.6 |
| Gibson 49, 2017, U.S. | 43 | FFMI: <1 SD from the young adult norm | No |
Bodystat 1500 Dual‐frequency |
18.6% | Observational: BIA has a high correlation with CT scan for screening sarcopenia (r = .711, p < .001); however, the incidence was higher with BIA. |
Mean: 69.5 SD: 12 |
| Barbosa 44, 2018, Brazil | 73 |
SMI: Men <10.75 kg/m2 Women <6.75 kg/m2 |
No | Not shared | 46% | Observational: Sarcopenic patients have no differences in health‐related quality of life. |
Mean: 63.9 SD: 11.3 |
| Kamyia 53, 2018, Japan | 56 |
SMI: Men <7.0 kg/m2 Women <5.7 kg/m2 2 |
Yes |
InBody 720 (Biospace, Tokyo, Japan) Multi‐frequency |
36% | Observational: Sarcopenic patients had significantly lower levels of serum dehydroepiandrosterone‐sulfate and a higher CCI score than patients who were not sarcopenic. More sarcopenic patients failed to complete the treatment planned as compared with nonsarcopenic patients (p = .001). |
Median: 77 Range: 60–93 |
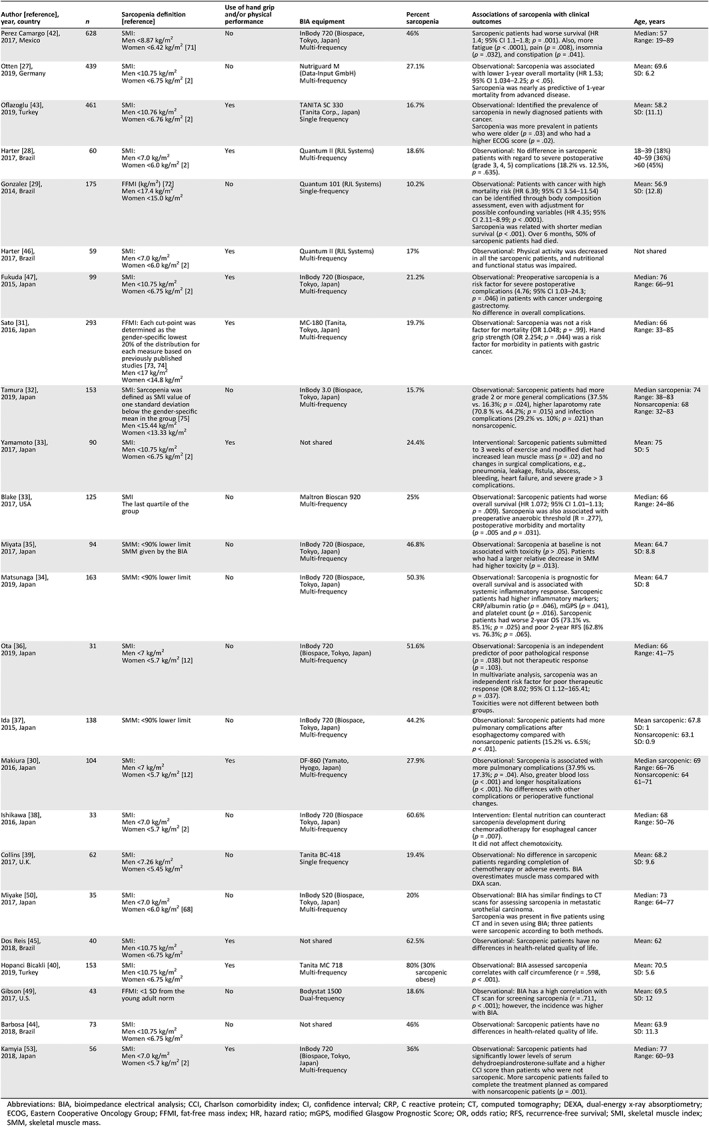
Abbreviations: BIA, bioimpedance electrical analysis; CCI, Charlson comorbidity index; CI, confidence interval; CRP, C reactive protein; CT, computed tomography; DEXA, dual‐energy x‐ray absorptiometry; ECOG, Eastern Cooperative Oncology Group; FFMI, fat‐free mass index; HR, hazard ratio; mGPS, modified Glasgow Prognostic Score; OR, odds ratio; RFS, recurrence‐free survival; SMI, skeletal muscle index; SMM, skeletal muscle mass.
The mean prevalence of sarcopenia for the overall sample was 32.9% (range 10%–80%). The mean prevalence of sarcopenia differed by cancer site and was 21.2% (range 16%–25%) for gastric, 44% (range 25%–61%) esophageal, 19% lung (one study), 41% (range 20%–63%) urothelial, 32% (range 19%–46%) colorectal, 36% hematological, and 30.8% (range 10%–80%) for multiple cancer types.
Five studies assessed the accuracy of BIA compared with other methods for assessing sarcopenia. In patients with metastatic urothelial carcinoma and colorectal carcinoma, BIA was deemed comparable to axial L3 CT scan imaging in estimating sarcopenia 50. BIA had high correlation with calf circumference and grip strength in patients with gastrointestinal neoplasia 31, 40. BIA overestimated muscle mass when compared with DXA scan in patients with lung cancer 39. Ten studies used either hand grip or physical performance analysis to complement the BIA‐based diagnosis of sarcopenia 28, 30, 31, 33, 40, 43, 45, 46, 47, 53.
In 13 studies, BIA‐defined sarcopenia was associated with adverse clinical outcomes in adults with cancer 29, 30, 32, 34, 36, 37, 39, 45, 47, 48, 51, 53. Sarcopenia was associated with worse overall survival in five studies 27, 34, 42, 48, 51 and severe surgical complications (e.g., infection, hematological) in four studies 30, 32, 37, 47. Other outcomes included worse pathological response in patients with esophageal cancer treated with chemotherapy 36, worse inflammatory response in patients with esophageal cancer treated with surgery 34 (which may preclude worse surgical response, e.g., more infections, higher reoperation rates), and higher patient‐reported pain, fatigue, insomnia, and constipation 42. BIA‐defined sarcopenic patients at cancer diagnosis also had worse nutritional status and functional status 46. In six studies, sarcopenic and nonsarcopenic patients had similar outcomes for health related quality of life 44, 45, completion of lung cancer chemotherapy 39, and presence of severe postoperative complications 28, 31, 35 (e.g., hematological, infection, or further surgery). In four studies, clinical outcomes associated with sarcopenia were not assessed 40, 43, 49, 50.
Two studies were interventional. In one trial, BIA was used to diagnose sarcopenia for an intervention testing the use of Elental to preserve lean body mass during chemotherapy (chemoradiation) in patients with esophageal cancer 38. This trial showed that Elental is effective in counteracting sarcopenia in patients who undergo chemotherapy. In the other intervention study, preoperative exercise and nutritional support were tested for their potential to reduce sarcopenia and improve postoperative outcomes in patients with gastric cancer, with promising results 33.
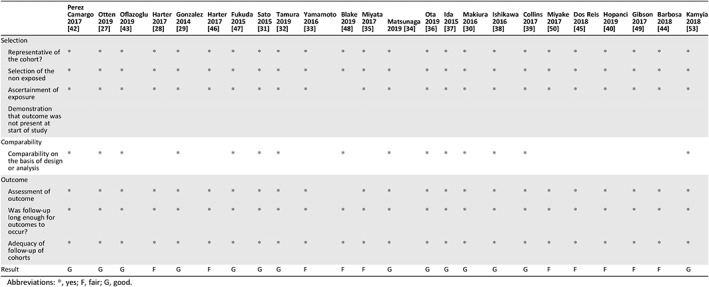
Table 4 summarizes results from the NOS for quality assessment of the studies included in our analysis. Fifteen studies were classified as good 27, 29, 30, 31, 32, 34, 36, 37, 38, 39, 41, 42, 43, 47, 53 with regard to selection (representativeness of the cohort, selection of nonexposed, ascertainment of exposure, and demonstration that the outcome was not present at the start of the study), comparability (design and analysis), and outcomes (assessment of outcomes, adequacy of follow‐up of cohorts). Ten studies were classified as fair 28, 33, 35, 40, 44, 45, 46, 48, 49, 50, primarily because of inadequate comparability.
Table 4.
Newcastle‐Ottawa Scale for the assessment of cohort studies
| Perez Camargo 2017 42 | Otten 2019 27 | Oflazoglu 2019 43 | Harter 2017 28 | Gonzalez 2014 29 | Harter 2017 46 | Fukuda 2015 47 | Sato 2015 31 | Tamura 2019 32 | Yamamoto 2016 33 | Blake 2019 48 | Miyata 2017 35 | Matsunaga 2019 34 | Ota 2019 36 | Ida 2015 37 | Makiura 2016 30 | Ishikawa 2016 38 | Collins 2017 39 | Miyake 2017 50 | Dos Reis 2018 45 | Hopanci 2019 40 | Gibson 2017 49 | Barbosa 2018 44 | Kamyia 2018 53 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | ||||||||||||||||||||||||
| Representative of the cohort? | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Selection of the non exposed | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Ascertainment of exposure | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| Demonstration that outcome was not present at start of study | ||||||||||||||||||||||||
| Comparability | ||||||||||||||||||||||||
| Comparability on the basis of design or analysis | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Outcome | ||||||||||||||||||||||||
| Assessment of outcome | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| Was follow‐up long enough for outcomes to occur? | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Adequacy of follow‐up of cohorts | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Result | G | G | G | F | G | F | G | G | G | F | F | F | G | G | G | G | G | G | F | F | F | F | F | G |
Abbreviations: *, yes; F, fair; G, good.
Discussion
Over the past decade, interest in sarcopenia in patients with cancer has grown considerably because of its potential as a predictor of adverse clinical outcomes 54, 55, 56, 57, 58. Yet, despite growing evidence that sarcopenia is prognostic for numerous outcomes (e.g., chemotherapy toxicity, overall survival), sarcopenia is rarely assessed in routine clinical practice because of perceived logistical difficulties and costs. Our systematic review suggests BIA as an accurate and practical option for sarcopenia assessment in clinical practice because it is inexpensive, noninvasive, and portable, does not expose patients to radiation, does not require extensive training for operation, and provides readily available data for detecting sarcopenia prior to cancer treatment. BIA is accepted as an appropriate method for assessing sarcopenia by European 2 and Asian 12 guidelines as well as the international cachexia consensus 59, 60 and has high validity in well‐hydrated and nonobese populations 12, 61. Our review shows that BIA is accurate and useful for evaluating low lean muscle mass for the diagnosis of sarcopenia and that BIA‐identified sarcopenia is relevant to outcomes in multiple cancers populations.
The large number of studies included in our analysis (which is comparable to the number of studies included in other systematic reviews pertaining to different sarcopenia‐detecting techniques) 7 shows that BIA is feasible for evaluating low lean muscle mass for the diagnosis of sarcopenia prior to treatment in adults with cancer. Two studies showed that BIA was comparable to gold standard methods (CT, MRI) in detecting sarcopenia, which is why BIA has been accepted as a reliable method in European and Asian sarcopenia guidelines 2, 12 as well as the international cachexia consensus 59, 60. This finding is particularly important when gold standard imaging methods are not readily available or clinically indicated. For example, patients whose cancer does not require CT or MRI at the time of diagnosis (e.g., early breast cancer, early prostate cancer) could benefit from the use of BIA to assess sarcopenia. It is important to note that our review did not identify any studies in which BIA was conducted during or after chemotherapy or radiation. This is because patients can become severely dehydrated during treatment, which, in turn, affects the accuracy of the BIA measure 62.
In our review, we report that BIA‐assessed sarcopenic patients who received chemotherapy had longer hospitalizations, increased hematological toxicity, more infections, more grade 3–4 complications, and worse survival. When surgery was the primary treatment, short‐term complications were more frequent and particularly concerning in sarcopenic patients. Our review found that BIA‐diagnosed sarcopenic patients (especially those with gastrointestinal cancers) have more pulmonary complications, greater blood loss, longer hospitalizations, and more complications in general. Our findings are similar to those of previous meta‐analyses of patients with esophageal and gastric cancer undergoing surgery 22, 23. Even in the palliative care setting, BIA‐assessed sarcopenia is prognostic for survival, fatigue, constipation, and insomnia. The prognostic value of BIA data supports the importance of muscle mass assessment even when anticancer therapy is not indicated (e.g., palliative care) 42, 63.
The limitations of our study reflect the drawbacks of using BIA to identify sarcopenia. Different scales, different BIA equipment, different equations and cut‐points for determining sarcopenia, and different types of cancers precluded meta‐analysis of our data. Furthermore, a majority of patients included in our review were older, which may have influenced their hydration status and BMI at the time of BIA measurement. This is a common concern for the use of BIA and is especially relevant in cancers that cause severe weight loss and hydration changes, such as gastrointestinal and head and neck cancers. Interestingly, gastrointestinal cancers were the most common type of cancer in our review, which confirms that even in these patient populations, BIA can be effective when appropriate protocols are followed. It would also be informative to conduct BIA measures in a younger patient population to see if outcomes remain similar to the older patients included in our review. Another concern is that some studies have shown that BIA should be used with caution in morbidly obese patients (BMI > 35 kg/m2), as it may underestimate body fat and overestimate fat free mass 64.
We also note that not all societies accept BIA as a valuable method for lean mass estimation. Recently, the Society of Sarcopenia Cachexia and Wasting Disorders (SCWD) recommended that physicians should make a formal diagnosis of sarcopenia using grip strength or chair stand and—if possible—a measurement of fat‐free mass. SCWD recommends appendicular skeletal muscle per height squared estimated by DXA, but they also recognize that CT, BIA, ultrasound, or creatine dilution techniques may become as good or more accurate for estimating muscle mass in the future 65. Also, BIA was not strongly endorsed in the latest EWGSOP report for measuring muscle mass; however, it was recognized that BIA's portability, affordability, and availability make this measure a feasible tool for estimating muscle mass in many care settings 2. We also note that Newcastle Ottawa Scale analysis rated 38% of the studies as only fair quality, which indicates higher risk for bias. Despite the wide spectrum of cancers included in our review, some types of cancer such as melanoma, pancreatic, and breast 56, 66, 67 did not have BIA studies for sarcopenia. Finally, there was a high degree of heterogeneity among the included studies.
BIA‐based estimates of sarcopenia can be influenced by the patient's ethnicity (note the differences in European and Asian guidelines pertaining to the use of BIA for sarcopenia detection), medical condition and comorbidities, hydration, exercise history, and food intake 68. These factors can influence the accuracy of BIA‐determined sarcopenia among patients, even within the same patient at different time points of the day. It had been shown that multifrequency BIA (using a combination of low and high frequencies to calculate intercellular water, extracellular water, and total body water) has a greater accuracy than single‐frequency BIA (which generally uses a 50‐kHz current that passes through extracellular and intracellular fluids for the estimation of total body water), especially in obese and underweight patients 69, 70. These variables need to be considered in establishing optimal pretest preparation, selecting the equation to be used by the BIA software, and determining sarcopenia cut‐points for a specific patient population in order to reduce potential inaccuracies in BIA measurements. It is suggested that in clinical practice, patients should receive instructions pertaining to hydration, food consumption, and exercise prior to the BIA, such as not drinking excessive amounts of water or performing vigorous physical activity 2–3 hours before the assessment. This is not unlike instructions provided to patients prior to tests such as a routine blood pressure measurement.
The value of BIA would also be greatly enhanced by an international consensus on cut‐points for BIA‐assessed sarcopenia in different cancer populations. Also, further research is needed to see if BIA is useful or accurate for detecting low lean muscle mass for the diagnosis of sarcopenia in patients with cancers that were under‐represented in our review, such as breast cancer and melanoma, and in younger patients. In addition, further research is needed to assess the potential benefits of BIA‐generated data for treatment decisions and overall cancer care.
Conclusion
This review suggests that BIA‐assessed sarcopenia can be of prognostic value for multiple types of cancer. BIA is an affordable, portable, easy‐to‐use, effective method for detecting sarcopenia in adults with cancer before treatment and a viable alternative to CT, DXA scans, and MRIs in clinical practice. Imaging studies have higher accuracy compared with BIA and remain the gold standard, but their implementation complexity and cost reduce their applicability in oncologic clinical practice. Further research is needed to improve our understanding of how routine assessment of sarcopenia can lead to interventions that improve outcomes in patients with cancer.
Author Contributions
Conception/design: Gabriel F.P. Aleixo, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss, Claudio L. Battaglini, Grant R. Williams
Provision of study material or patients: Gabriel F.P. Aleixo, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss, Claudio L. Battaglini, Grant R. Williams
Collection and/or assembly of data: Gabriel F.P. Aleixo, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss, Claudio L. Battaglini, Grant R. Williams
Data analysis and interpretation: Gabriel F.P. Aleixo, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss, Claudio L. Battaglini, Grant R. Williams
Manuscript writing: Gabriel F.P. Aleixo, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss, Claudio L. Battaglini, Grant R. Williams
Final approval of manuscript: Gabriel F.P. Aleixo, Shlomit S. Shachar, Kirsten A. Nyrop, Hyman B. Muss, Claudio L. Battaglini, Grant R. Williams
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Appendices
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Delmonico MJ, Beck DT. The current understanding of sarcopenia: Emerging tools and interventional possibilities. Am J Lifestyle Med 2016;11:167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang SF, Lin PL. Systematic literature review and meta‐analysis of the association of sarcopenia with mortality. Worldviews Evid Based Nurs 2016;13:153–162. [DOI] [PubMed] [Google Scholar]
- 4. National Cancer Institute . Surveillance, Epidemiology, and End Results (SEER) Program. Cancer: SEER Stat Fact Sheets. 2017. Available at https://seer.cancer.gov/statfacts/. Accessed November 1, 2019. [Google Scholar]
- 5. Williams GR, Rier HN, McDonald A et al. Sarcopenia & aging in cancer. J Geriatr Oncol 2019;10:374–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pamoukdjian F, Bouillet T, Levy V et al: Prevalence and predictive value of pre‐therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr 2018;37:1101–1113. [DOI] [PubMed] [Google Scholar]
- 7. Shachar SS, Williams GR, Muss HB et al: Prognostic value of sarcopenia in adults with solid tumours: A meta‐analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . BMI Classification, 2016. Available at https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed November 1, 2019.
- 9. Fukuoka Y, Narita T, Fujita H et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig 2019;10:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shachar SS, Williams GR. The obesity paradox in cancer ‐ Moving beyond BMI ‐ Response. Cancer Epidemiol Biomarkers Prev 2017;26:981. [DOI] [PubMed] [Google Scholar]
- 11. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen LK, Liu LK, Woo J et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 13. Khan AI, Reiter DA, Sekhar A et al. MRI quantitation of abdominal skeletal muscle correlates with CT‐based analysis: Implications for sarcopenia measurement. Appl Physiol Nutr Metab 2019;44:814–819. [DOI] [PubMed] [Google Scholar]
- 14. Yang YX, Chong MS, Lim WS et al. Validity of estimating muscle and fat volume from a single MRI section in older adults with sarcopenia and sarcopenic obesity. Clin Radiol 2017;72:427.e9–e427.e14. [DOI] [PubMed] [Google Scholar]
- 15. Coin A, Sarti S, Ruggiero E et al. Prevalence of sarcopenia based on different diagnostic criteria using DEXA and appendicular skeletal muscle mass reference values in an Italian population aged 20 to 80. J Am Med Dir Assoc 2013;14:507–512. [DOI] [PubMed] [Google Scholar]
- 16. Malmstrom TK, Morley JE. SARC‐F: A simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–532. [DOI] [PubMed] [Google Scholar]
- 17. Gaba A, Kapus O, Cuberek R et al. Comparison of multi‐ and single‐frequency bioelectrical impedance analysis with dual‐energy X‐ray absorptiometry for assessment of body composition in post‐menopausal women: Effects of body mass index and accelerometer‐determined physical activity. J Hum Nutr Diet 2015;28:390–400. [DOI] [PubMed] [Google Scholar]
- 18. Kohli K, Corns R, Vinnakota K et al. A bioimpedance analysis of head‐and‐neck cancer patients undergoing radiotherapy. Curr Oncol 2018;25:e193–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raeder H, Kvaerner AS, Henriksen C et al. Validity of bioelectrical impedance analysis in estimation of fat‐free mass in colorectal cancer patients. Clin Nutr 2018;37:292–300. [DOI] [PubMed] [Google Scholar]
- 20. Centre for Reviews and Dissemination (CRD) . PROSPERO: Internation prospective register for systematic reviews. York, U.K.: University of York, 2019. [Google Scholar]
- 21. Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—A comprehensive review. Eur J Clin Nutr 2015;69:1290–1297. [DOI] [PubMed] [Google Scholar]
- 22. Haverkort EB, Reijven PL, Binnekade JM et al. Bioelectrical impedance analysis to estimate body composition in surgical and oncological patients: A systematic review. Eur J Clin Nutr 2015;69:3–13. [DOI] [PubMed] [Google Scholar]
- 23. Boshier PR, Heneghan R, Markar SR et al. Assessment of body composition and sarcopenia in patients with esophageal cancer: A systematic review and meta‐analysis. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 24. Schardt C, Adams MB, Owens T et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wells GA, Shea B, O'Connell D et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analysis. Ottawa, ON, Canada: Ottawa Hospital Research Institute, 2011. [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. J Clin Epidemiol 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 27. Otten L, Stobaus N, Franz K et al. Impact of sarcopenia on 1‐year mortality in older patients with cancer. Age Ageing 2019;48:413–418. [DOI] [PubMed] [Google Scholar]
- 28. Harter J, Orlandi SP, Gonzalez MC. Nutritional and functional factors as prognostic of surgical cancer patients. Support Care Cancer 2017;25:2525–2530. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez MC, Pastore CA, Orlandi SP et al. Obesity paradox in cancer: New insights provided by body composition. Am J Clin Nutr 2014;99:999–1005. [DOI] [PubMed] [Google Scholar]
- 30. Makiura D, Ono R, Inoue J et al. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: A retrospective cohort study. J Geriatr Oncol 2016;7:430–436. [DOI] [PubMed] [Google Scholar]
- 31. Sato T, Aoyama T, Hayashi T et al. Impact of preoperative hand grip strength on morbidity following gastric cancer surgery. Gastric Cancer 2016;19:1008–1015. [DOI] [PubMed] [Google Scholar]
- 32. Tamura T, Sakurai K, Nambara M et al. Adverse effects of preoperative sarcopenia on postoperative complications of patients with gastric cancer. Anticancer Res 2019;39:987–992. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto K, Nagatsuma Y, Fukuda Y et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 2017;20:913–918. [DOI] [PubMed] [Google Scholar]
- 34. Matsunaga T, Miyata H, Sugimura K et al. Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res 2019;39:449–458. [DOI] [PubMed] [Google Scholar]
- 35. Miyata H, Sugimura K, Motoori M et al. Clinical assessment of sarcopenia and changes in body composition during neoadjuvant chemotherapy for esophageal cancer. Anticancer Res 2017;37:3053–3059. [DOI] [PubMed] [Google Scholar]
- 36. Ota T, Ishikawa T, Endo Y et al. Skeletal muscle mass as a predictor of the response to neo‐adjuvant chemotherapy in locally advanced esophageal cancer. Med Oncol 2019;36:15. [DOI] [PubMed] [Google Scholar]
- 37. Ida S, Watanabe M, Yoshida N et al. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol 2015;22:4432–4437. [DOI] [PubMed] [Google Scholar]
- 38. Ishikawa T, Yasuda T, Doi T et al. The amino acid‐rich elemental diet Elental® preserves lean body mass during chemo‐ or chemoradiotherapy for esophageal cancer. Oncol Rep 2016;36:1093–1100. [DOI] [PubMed] [Google Scholar]
- 39. Collins JT, Noble S, Chester J et al. The value of physical performance measurements alongside assessment of sarcopenia in predicting receipt and completion of planned treatment in non‐small cell lung cancer: An observational exploratory study. Support Care Cancer 2018;26:119–127. [DOI] [PubMed] [Google Scholar]
- 40. Hopanci Bicakli D, Cehreli R, Ozveren A et al. Evaluation of sarcopenia, sarcopenic obesity, and phase angle in geriatric gastrointestinal cancer patients: Before and after chemotherapy. Turk J Med Sci 2019;49:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akahori T, Sho M, Kinoshita S et al. Prognostic significance of muscle attenuation in pancreatic cancer patients treated with neoadjuvant chemoradiotherapy. World J Surg 2015;39:2975–2982. [DOI] [PubMed] [Google Scholar]
- 42. Perez Camargo DA, Allende Perez SR, Verastegui Aviles E et al. Assessment and impact of phase angle and sarcopenia in palliative cancer patients. Nutr Cancer 2017;69:1227–1233. [DOI] [PubMed] [Google Scholar]
- 43. Oflazoglu U, Alacacioglu A, Varol U et al. Prevalence and related factors of sarcopenia in newly diagnosed cancer patients. Support Care Cancer 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44. Barbosa MV, Dos Santos MP, Martucci RB. The impact of sarcopenia and frailty phenotype on health related quality of life of colorectal cancer patients. J Cachexia Sarcopenia Muscle 2018;9:1158–1159. [Google Scholar]
- 45. Dos Reis PF, Dos Santos MP, Martucci RB. Sarcopenia and frailty phenotype influence on quality of life of patients with bladder or kidney cancer. J Cachexia Sarcopenia Muscle 2018;9:1141–1142. [Google Scholar]
- 46. Härter J, Orlandi SP, Gonzalez MC. Phase angle and sarcopenia: Are they related? J Cachexia Sarcopenia Muscle 2017;8:1023. [Google Scholar]
- 47. Fukuda Y, Yamamoto K, Hirao M et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 2016;19:986–993. [DOI] [PubMed] [Google Scholar]
- 48. Blake P, Patel N, Brown C et al. Prognostic significance of body composition determined by bioelectrical impedance analysis (BIA) in upper gastrointestinal cancer surgery. Gastroenterology 2017;152:S1269. [Google Scholar]
- 49. Gibson DJ, Burden S, Todd C et al. Concurrent validity of fat free mass using computed tomography and bioelectrical impedance analysis in people with colorectal cancer (CRC) and weight loss. Clin Nutr 2014;33(suppl 1):S250–S251. [Google Scholar]
- 50. Miyake M, Owari T, Iwamoto T et al. Clinical utility of bioelectrical impedance analysis in patients with locoregional muscle invasive or metastatic urothelial carcinoma: A subanalysis of changes in body composition during neoadjuvant systemic chemotherapy. Support Care Cancer 2018;26:1077–1086. [DOI] [PubMed] [Google Scholar]
- 51. Otten L, Stobaus N, Franz K et al. Impact of sarcopenia on 1‐year mortality in older patients with cancer. Age Ageing 2019;48:413–418. [DOI] [PubMed] [Google Scholar]
- 52. Ida S, Watanabe M, Karashima R et al. Changes in body composition secondary to neoadjuvant chemotherapy for advanced esophageal cancer are related to the occurrence of postoperative complications after esophagectomy. Ann Surg Oncol 2014;21:3675–3679. [DOI] [PubMed] [Google Scholar]
- 53. Kamiya T, Mizuno K, Ogura S et al. A prospective observational study evaluating sarcopenia by using the bioelectrical impedance analysis in elderly patients with hematologic malignancies. Blood 2018;132. [Google Scholar]
- 54. Antoun S, Lanoy E, Iacovelli R et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013;119:3377–3384. [DOI] [PubMed] [Google Scholar]
- 55. Iritani S, Imai K, Takai K et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol 2015;50:323–332. [DOI] [PubMed] [Google Scholar]
- 56. Sabel MS, Lee J, Cai S et al. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011;18:3579–3585. [DOI] [PubMed] [Google Scholar]
- 57. Kazemi‐Bajestani SM, Mazurak VC, Baracos V. Computed tomography‐defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016;54:2–10. [DOI] [PubMed] [Google Scholar]
- 58. Rier HN, Jager A, Sleijfer S et al. The prevalence and prognostic value of low muscle mass in cancer patients: A review of the literature. The Oncologist 2016;21:1396–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blum D, Stene GB, Solheim TS et al. Validation of the Consensus‐Definition for Cancer Cachexia and evaluation of a classification model—A study based on data from an international multicentre project (EPCRC‐CSA). Ann Oncol 2014;25:1635–1642. [DOI] [PubMed] [Google Scholar]
- 60. Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 61. Earthman CP. Body composition tools for assessment of adult malnutrition at the bedside: A tutorial on research considerations and clinical applications. JPEN J Parenter Enteral Nutr 2015;39:787–822. [DOI] [PubMed] [Google Scholar]
- 62. Battaglini C, Naumann F, Groff D et al. Comparison of body composition assessment methods in breast cancer survivors. Oncol Nurs Forum 2011;38:E283–E290. [DOI] [PubMed] [Google Scholar]
- 63. Verlaan S, Van Ancum JM, Pierik VD et al. Muscle measures and nutritional status at hospital admission predict survival and independent living of older patients ‐ The EMPOWER Study. J Frailty Aging 2017;6:161–166. [DOI] [PubMed] [Google Scholar]
- 64. Coppini LZ, Waitzberg DL, Campos AC. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr Opin Clin Nutr Metab Care 2005;8:329–332. [DOI] [PubMed] [Google Scholar]
- 65. Bauer J, Morley JE, Schols A et al. Sarcopenia: A time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kurita Y, Kobayashi N, Tokuhisa M et al. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology 2019;19:127–135. [DOI] [PubMed] [Google Scholar]
- 67. Shachar SS, Deal AM, Weinberg M et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane‐based chemotherapy for early‐stage breast cancer. Clin Cancer Res 2017;23:3537–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gonzalez MC, Heymsfield SB: Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: What are we really estimating? J Cachexia Sarcopenia Muscle 2017;8:187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bedogni G, Malavolti M, Severi S et al. Accuracy of an eight‐point tactile‐electrode impedance method in the assessment of total body water. Eur J Clin Nutr 2002;56:1143–1148. [DOI] [PubMed] [Google Scholar]
- 70. Chumlea WC, Guo SS. Bioelectrical impedance and body composition: Present status and future directions. Nutr Rev 1994;52:123–131. [DOI] [PubMed] [Google Scholar]
- 71. Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community‐dwelling elderly people in Taiwan. J Am Geriatr Soc 2008;56:1710–1715. [DOI] [PubMed] [Google Scholar]
- 72. Kyle UG, Pirlich M, Lochs H et al. Increased length of hospital stay in underweight and overweight patients at hospital admission: A controlled population study. Clin Nutr 2005;24:133–142. [DOI] [PubMed] [Google Scholar]
- 73. Delmonico MJ, Harris TB, Lee JS et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 74. Newman AB, Kupelian V, Visser M et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 75. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Appendices


