Abstract
Chimeric antigen receptor (CAR)–engineered T‐cell therapy is becoming one of the most promising approaches in the treatment of cancer. On June 28, 2018, the Committee for Advanced Therapies (CAT) and the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Kymriah for pediatric and young adult patients up to 25 years of age with B‐cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse after transplant, or in second or later relapse and for adult patients with relapsed or refractory diffuse large B‐cell lymphoma (DLBCL) after two or more lines of systemic therapy. Kymriah became one of the first European Union–approved CAR T therapies. The active substance of Kymriah is tisagenlecleucel, an autologous, immunocellular cancer therapy that involves reprogramming the patient's own T cells to identify and eliminate CD19‐expressing cells. This is achieved by addition of a transgene encoding a CAR. The benefit of Kymriah was its ability to achieve remission with a significant duration in patients with ALL and an objective response with a significant duration in patients with DLBCL. The most common hematological toxicity was cytopenia in both patients with ALL and those with DLBCL. Nonhematological side effects in patients with ALL were cytokine release syndrome (CRS), infections, secondary hypogammaglobulinemia due to B‐cell aplasia, pyrexia, and decreased appetite. The most common nonhematological side effects in patients with DLBCL were CRS, infections, pyrexia, diarrhea, nausea, hypotension, and fatigue. Kymriah also received an orphan designation on April 29, 2014, following a positive recommendation by the Committee for Orphan Medicinal Products (COMP). Maintenance of the orphan designation was recommended at the time of marketing authorization as the COMP considered the product was of significant benefit for patients with both conditions.
Implications for Practice
Chimeric antigen receptor (CAR)–engineered T‐cell therapy is becoming the most promising approach in cancer treatment, involving reprogramming the patient's own T cells with a CAR‐encoding transgene to identify and eliminate cancer‐specific surface antigen–expressing cells. On June 28, 2018, Kymriah became one of the first EMA approved CAR T therapies. CAR T technology seems highly promising for diseases with single genetic/protein alterations; however, for more complex diseases there will be challenges to target clonal variability within the tumor type or clonal evolution during disease progression. Products with a lesser toxicity profile or more risk‐minimization tools are also anticipated.
Keywords: Acute lymphoblastic leukemia; Diffuse large B‐cell lymphoma; Chimeric antigen receptor; Kymriah (Tisagenlecleucel, CTL019); Replication‐competent lentivirus; Cytokine release syndrome
Short abstract
The EMA summarizes the reasons behind marketing authorization of the medicinal product Kymriah, one of the first European Union‐approved CAR T therapies, for B cell acute lymphoblastic leukemia and diffuse large B cell lymphoma.
Introduction
Acute Lymphoblastic Leukemia
Treatment strategies for children and adolescents with acute lymphoblastic leukemia (ALL) have developed greatly over the past few decades, leading to a cure in up to 90% of cases 1, 2.
However, relapses occur and still present the most common cause of treatment failure in childhood ALL, occurring in about 15%–20% of patients. Survival of relapsed patients can be predicted by site of relapse, length of first complete remission, and immunophenotype of relapsed ALL 3. Standard salvage regimens for relapsed ALL are still mostly based on different combinations of the same agents used in frontline therapy in various doses and schedules 4, 5, 6.
Postrelapse prognosis and survival are poor, with estimated survival rates of 22% at 1 year and 7% at 5 years. Factors predicting a good outcome after salvage therapy were young age OS 12% in patients aged <20 years vs. 3% in patients aged >50 years; p < .001) and short duration of first remission (CR1; OS, 11% in patients with a CR1 of >2 years vs. 5% in patients with a CR1 of <2 years; p < .001) 7.
Diffuse Large B‐Cell Lymphoma
The frontline standard of care for patients with diffuse large B‐cell lymphoma (DLBCL) includes a combination of CHOP (cyclophosphamide, vincristine, doxorubicin, and prednisone) with rituximab (R‐CHOP) 8.
The addition of rituximab, a monoclonal antibody directed against CD20, to first‐line chemotherapy has improved the outcome of patients with DLBCL, resulting in a survival rate of about 75% at 6 years 9. However, 30%–50% of the patients do not have long‐term benefit from first‐line therapy (approximately 30% relapse and 20% have refractory disease) 10.
For patients who are deemed eligible for high‐dose chemotherapy and autologous stem cell transplant (HD‐ASCT) based on adequate performance status (defined by age and absence of major organ dysfunctions), clinical treatment guidelines for patients with relapsed/refractory (r/r) DLBCL recommend salvage therapy with platinum‐based chemotherapy regimens followed by HD‐ASCT. However, about half of patients with r/r DLBCL are not eligible for autologous stem cell transplant because of advanced age and/or comorbidities. Furthermore, among patients suitable for HD‐ASCT, only about half will have a response to salvage therapy that is sufficient for proceeding to HD‐ASCT 11, 12. In addition, of patients proceeding to HD‐ASCT, 60% will relapse after transplant. Clinical studies, palliative chemotherapy, and in rare cases a second HD‐ASCT or allogeneic stem cell transplant are some of the options available for these patients 8.
Kymriah
Kymriah (tisagenlecleucel, CTL019) is an autologous, immunocellular cancer therapy that involves reprogramming the patient's own T cells with a transgene encoding a chimeric antigen receptor (CAR) to identify and eliminate CD19‐expressing cells. The CAR is composed of a murine single‐chain antibody fragment that recognizes CD19 and is fused to intracellular signaling domains from 4‐1BB (CD137) and CD3‐ζ. The CD3‐ζ component is critical for initiating T‐cell activation and antitumor activity, whereas 4‐1BB enhances the expansion and persistence of tisagenlecleucel. Upon binding to CD19‐expressing cells, the CAR transmits a signal promoting T‐cell expansion and persistence of tisagenlecleucel (Fig. 1).
Figure 1.
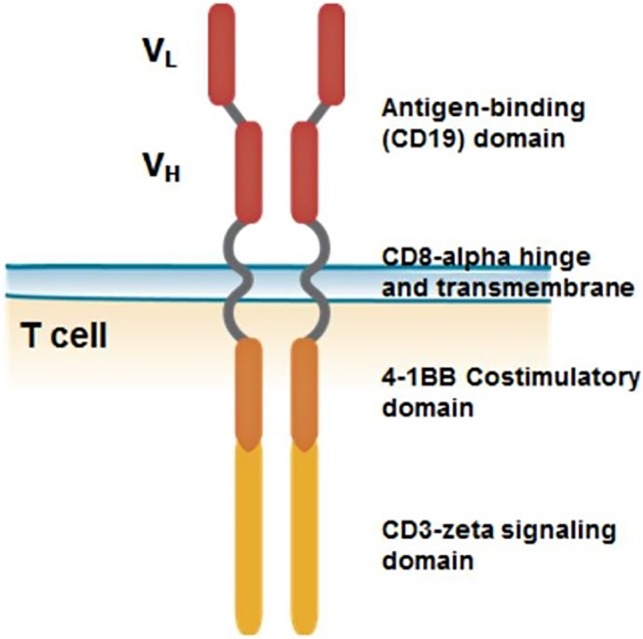
Structure of chimeric antigen receptor construct.
Abbreviations: VH, heavy chain variable region; VL, light chain variable region.
The tisagenlecleucel (murine) HIV‐1 vector is a replication‐defective, recombinant, third‐generation self‐inactivating lentiviral vector derived from the HIV‐1 lentiviral genome. It encodes a CAR against human CD19 expressed under the control of the human elongation factor 1α promoter (Fig. 2).
Figure 2.
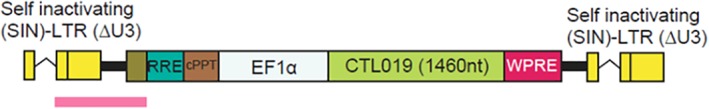
Genetic structure of the integrated vector.
Abbreviations: ΔU3, U3 region; cPPT, central polypurine tract; CTL019, tisagenlecleucel; EF1α, human elongation factor 1α; LTR, long terminal repeat; nt, nucleotides; RRE, Rev response elements; SIN, self‐inactivating; WPRE, woodchuck hepatitis virus posttranscriptional response element.
CTL019 tisagenlecleucel lentiviral vector is designed to minimize the likelihood of replication‐competent lentivirus (RCL) emergence, as packaging plasmids are provided in trans and split on to three different plasmids. Plasmid sequences have been optimized to have minimal homology with the parental HIV in order to minimize the chance of homologous recombination. Therefore, formation of RCL in patients is considered highly unlikely. Horizontal gene transfer of tisagenlecleucel lentiviral vector sequences could only happen upon generation of RCL in a significant number of patients, after administration of Kymriah, and RCL being shed into the environment. This would require co‐infection of CAR T cells together with retroviruses with sufficient sequence homologies for recombination to occur, which is highly improbable because the risk of RCL formation has been reduced to the minimum. Thus, the likelihood of the overall risk for the environment was also considered to be negligible.
Clinical Pharmacology
After the infusion of Kymriah into pediatric and young adult patients with r/r B‐cell ALL and r/r DLBCL, Kymriah typically exhibited an initial rapid expansion followed by a slower biexponential decline. In patients with ALL, tisagenlecleucel was present in the blood and bone marrow beyond 2 years, whereas in patients with DLBCL with complete response, tisagenlecleucel was detected for up to 2 years in peripheral blood and up to month 9 in bone marrow. There was no apparent relationship between cellular kinetic and dose or body weight. In the pediatric group, a high tumor burden at baseline resulted in higher expansion of CAR T cells. Tocilizumab (used for treatment of cytokine release syndrome [CRS]) was not shown to have an impact on the cellular kinetics of tisagenlecleucel, as the transgene continued to expand and persist following tocilizumab administration. Treatment‐induced anti‐murine CAR19 antibodies were shown in 34.6% of pediatric and young adult patients with ALL and in 5% of adult patients with DLBCL. However, preexisting and treatment‐induced antibodies were not associated with an impact on clinical response, nor did they have an impact on the expansion and persistence of tisagenlecleucel.
Dose Response Studies
For patients with ALL, the relationship between tisagenlecleucel dose and response (efficacy and safety) was explored. We observed an increasing trend in probability of response for doses between 0.1 × 108 and 1.0 × 108 total CAR‐positive T cells per kilogram for patients weighing >50 kg and between 0.2 × 106 and 1.5 × 106 CAR‐positive viable T cells per kilogram for patients weighing ≤50 kg. The dose had no significant impact on grade 3–4 CRS in general, although there was a slight trend for increased risk of grade 4 CRS with higher dose for patients weighing ≤50 kg.
The protocol‐specified dose range in the pivotal study of r/r DLBCL (C2201) was based on the experience in the University of Pennsylvania (UPCC13413) study in r/r lymphoma (18 patients with DLBCL, 8 patients with follicular lymphoma) 13, and preliminary clinical experiences also were taken into consideration 14. Some patients were given tisagenlecleucel even though the recommended dose of viable CAR T cells was not met, because other effective treatment options were not available. Patients who received doses below and above the target dose range had response rates similar to those of patients who received doses within the protocol‐specified dose.
A rapid loss of transgene and limited expansion were observed in patients with CD19‐positive relapsed disease compared with patients with sustained complete response (CR) or complete response with incomplete recovery (CRi). On the other hand, we noticed that patients with CD19‐negative relapse evaded CAR T‐cell–mediated recognition and clearance despite having persistent transgene.
Efficacy Data
For the ALL indication, efficacy results were based on the pivotal study B2202 (a phase II, single‐arm, multicenter trial to determine the efficacy and safety of Kymriah in pediatric patients with relapsed and refractory B‐cell ALL). An interim analysis was conducted of the first 50 patients infused with Kymriah who had completed 3 months from study day 1 infusion or had discontinued earlier (Table 1). The interim analysis was performed by testing the null hypothesis of overall response rate (ORR) within 3 months being less than or equal to 20% against the alternative hypothesis of ORR within 3 months being greater than 20% at an overall one‐sided 2.5% level of significance based on a previous clofarabine study response rate in the same population 15.
Table 1.
Study B2202: BOR and ORR at 3 months post–tisagenlecleucel infusion by independent review committee assessment FAS
| BOR | n (%) | p value |
|---|---|---|
| Interim efficacy analysis of first patients to receive tisagenlecleucel infusion (n = 50) | ||
| CR | 34 (68.0) | |
| CRi | 7 (14.0) | |
| No response or unknown | 9 (18.0) | |
| ORR (CR + CRi), n (%) [95% CI] | 41 (82.0) [68.6–91.4] | <.0001a |
| Full analysis set: April 25, 2017, cutoff (n = 75) | ||
| CR | 45 (60.0) | |
| CRi | 16 (21.3) | |
| No response | 6 (8.0) | |
| Unknown | 8 (10.7) | |
| ORR (CR + CRi), n (%) [95% CI] | 61 (81.3) [70.7–89.4] | <.0001b |
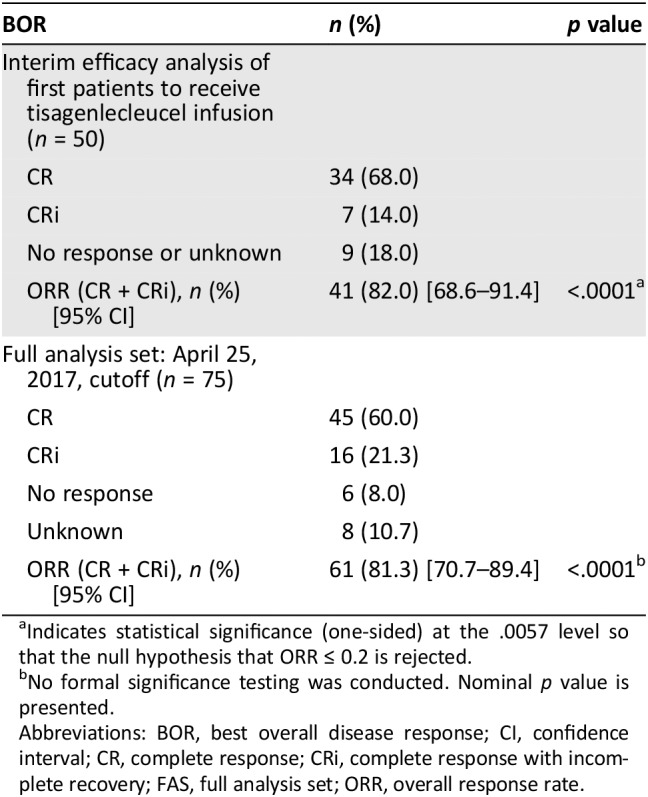
Indicates statistical significance (one‐sided) at the .0057 level so that the null hypothesis that ORR ≤ 0.2 is rejected.
No formal significance testing was conducted. Nominal p value is presented.
Abbreviations: BOR, best overall disease response; CI, confidence interval; CR, complete response; CRi, complete response with incomplete recovery; FAS, full analysis set; ORR, overall response rate.
The full analysis of the study showed a significant improved ORR: 61 of the 75 infused patients (81.3%) had a best overall disease response (BOR) of CR or CRi 16.
Regarding the secondary endpoints, the proportion of patients with BOR and minimal residual disease (MRD)–negative bone marrow (i.e., MRD <0.01%) at 3 months after Kymriah infusion was 61/75 (81.3%; 95% confidence interval [CI], 70.7–89.4). The majority of patients who had a CR or CRi after Kymriah treatment achieved a sustained response, and a median duration of response (DOR) per independent review committee assessment was not reached at the primary data cutoff (median follow‐up time of 7.5 months).
Patients in the B2202 study reported improvements in health‐related quality‐of‐life outcomes at 3 and 6 months among responders to therapy. Tisagenlecleucel infusion led to a decrease in the severity of problems as measured by the emotional, social, physical, and psychosocial health subscales as well as mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression as assessed via the EQ‐5D questionnaire 17. Thus, results indicate a meaningful improvement in patients responding to treatment.
For the DLBCL indication, the pivotal C2201 phase II study was an open‐label, multicenter, single‐arm study in adult patients with r/r DLBCL.
Of 147 patients enrolled, 99 patients received infusion with Kymriah; this was for the following reasons: five infusions were pending at the time of analysis, Kymriah could not be manufactured for 9 patients, there were 16 deaths prior to Kymriah infusion, physician decision in 12 patients, subject decision in 3 patients, adverse events in 2 patients, and protocol deviation in 1 patient. The majority of the patients in the C2201 study had relapsed or were refractory to either two or three prior therapies (75.7%). With the updated cutoff in the C2201 study, 165 patients were enrolled, 111 of whom were infused.
Among the 99 patients who received tisagenlecleucel infusion, 89 patients (90%) had received antineoplastic therapy after enrollment and prior to tisagenlecleucel infusion. The most frequently used (≥15% of patients) bridging therapies were rituximab (54.5%), gemcitabine (38.4%), dexamethasone (25.3%), etoposide (22.2%), cytarabine (19.2%), cisplatin (18.2%), and cyclophosphamide (15.2%). The median time from the end of the last antineoplastic therapy (prior to screening) to enrollment was longer in the long‐term responders (5.1 months) than in the nonresponders (2.8 months). The proportion of patients with lymphomas of double or triple hits in myc, bcl2, and bcl6 genes was also lowest in the long‐term responders. The manufacturing time in this study was longer than has been seen with other CAR T‐cell products. At the time of the primary analysis (data cut off [DCO], March 8, 2017; 99 patients infused), the median time from enrollment in the C2201 study to infusion was 54 days (range, 30–357 days), with a median time from screening to infusion of 119 days (range, 49–396 days). This considerable time span from screening and enrollment to infusion was a concern, especially as tisagenlecleucel is intended for the treatment of patients with an advanced disease expected to progress rapidly.
The pivotal study was designed to evaluate efficacy based on the infused patient population. However, for the efficacy outcomes, the results based on the intention‐to‐treat (ITT) population (all enrolled patients) was considered the most relevant analysis set from the point of view of the scientific assessment. The ORR was 33.9% (Table 2).
Table 2.
Study C2201: ORR and DOR results in the intention‐to‐treat population
| Primary endpoint | Enrolled patients (n = 165) |
|---|---|
| ORR (CR + PR), n (%) [95% CI] | 56 (33.9) [26.8–41.7] |
| CR, n (%) | 40 (24.2) |
| PR, n (%) | 16 (9.7) |
| Response at month 3 (n = 165) | |
| ORR, n (%) | 39 (23.6) |
| CR, n (%) | 33 (20.0) |
| Response at month 6 (n = 165) | |
| ORR, n (%) | 34 (20.6) |
| CR, n (%) | 30 (18.2) |
| DOR (n = 56) | |
| Median [95% CI], months | Not reached [10.0, NE] |
| Relapse‐free probability at 6 months, % | 66.7 |
| Relapse‐free probability at 12 months, % | 63.7 |
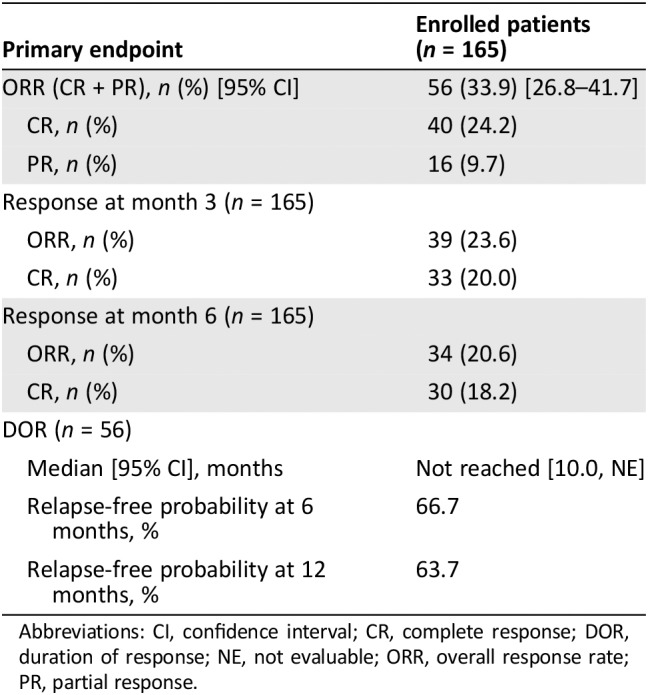
Abbreviations: CI, confidence interval; CR, complete response; DOR, duration of response; NE, not evaluable; ORR, overall response rate; PR, partial response.
Clinical Safety
ALL Indication and DLBCL Indication
Grade 3 or 4 adverse reactions were reported in 88% of patients with ALL. The most common grade 3 or 4 nonhematological adverse reaction was CRS (47%). The most common grade 3 and 4 hematological laboratory abnormalities were leucopenia (99%), neutropenia (95%), lymphopenia (95%), thrombocytopenia 77%), and anemia (53%).
Adverse reactions noted in patients with DLBCL were similar to those in the ALL population. Grade 3 or 4 CRS incidence was 22%, and infection incidence was 24%. In the DLBCL indication, adverse events were primarily observed within 8 weeks postinfusion; adverse events within 8 weeks and after 8 weeks postinfusion were reported in 84.8% and 28.2% of patients, respectively. No adverse reactions were reported after more than 1 year postinfusion.
Cytokine Release Syndrome
The most frequently reported drug reaction was CRS (ALL indication, 80.8% in the summary of clinical safety pool and DLBCL indication, 57.7%). In almost all cases, development of CRS occurred between 1 and 10 days (median onset, 3 days) after Kymriah infusion. The median time to resolution of CRS was 7 days. CRS was reversible in most cases and was managed with supportive care and as‐needed anti‐cytokine therapy. Approximately half of the patients with CRS required intensive care unit–level care (56.0%) at a median of 6 days after the infusion, where they remained for a median duration of 7 days.
CRS is managed solely based on clinical presentation and according to the CRS management algorithm. Anti–IL‐6–based therapy such as tocilizumab has been administered for moderate or severe CRS associated with Kymriah, and a minimum of four doses of tocilizumab must be on site and available for administration prior to Kymriah infusion. Corticosteroids may be administered in cases of life‐threatening emergencies. Patients with medically significant cardiac dysfunction should be managed by standards of critical care, and measures such as echocardiography should be considered. Tumor necrosis factor antagonists are not recommended for management of Kymriah‐associated CRS.
Neurological Events
Neurological events were of concern, observed in 38% of patients with ALL and in 21% of patients with DLBCL. Neurological events could be concurrent with cytokine release syndrome, following resolution of cytokine release syndrome, or in the absence of cytokine release syndrome. The majority of neurologic events occurred in the first 30 days after infusion. Neurological events occurring during the first 8 weeks postinfusion most commonly included agitation, encephalopathy, seizures, tremor, confusional state, delirium, irritability, and somnolence. Other manifestations included aphasia and speech disorder. The median time to onset of neurological events was 7 days in both B‐cell ALL and DLBCL. The median time to resolution was 7 days for B‐cell ALL and 12 days for DLBCL. The majority of neurological events resolved completely; however, 7% of patients with neurological events with the ALL indication and 5% with the DLBCL indication had not recovered at the time of data cutoff. Treatment with tocilizumab did not reverse the symptoms. No neurological events were suggested to be a contributing factor to any death. It was not clear if delayed or late neurological events (occurring >8 weeks postinfusion) had been observed. A recent publication on neurotoxicity associated with CAR T cells mentions CAR T‐cell–related encephalopathy, and a management guide is proposed 18.
Hypogammaglobulinemia
Hypogammaglobulinemia was seen in 45% of patients in the ALL indication and in 15% in the DLBCL indication. Secondary to the development of hypogammaglobulinemia, patients were more susceptible to infections. Infections were seen in 54.1% of adult patients with DLBCL. Immunoglobulin replacement therapy was given to 19.2% of those with hypogammaglobulinemia in the DLBCL indication. Prolonged depletion of normal B cells, or agammaglobulinemia, and infections are both categorized as identified risks.
Hematological Reactions
Cytopenias were seen in 36% of both patients with ALL and those with DLBCL and were observed within 28 days as well as several months post–tisagenlecleucel infusion.
Delayed toxicity of hematologic origin (such as myelodysplastic syndrome, aplastic anemia, and bone marrow failure) has been associated with prior treatment with chemotherapy and radiation and was observed in the tisagenlecleucel development program.
Other Safety Concerns
The insertion of lentiviral vector sequences throughout the genome has the potential to dysregulate local host cell gene expression, with a theoretical risk of insertional oncogenesis resulting from disruption of normal function of genes that control cell growth and a potential risk of development of secondary malignancies or recurrence of prior cancer. This has been categorized as a potential risk. Life‐long risk for development of secondary malignancies will be monitored. It was concluded that there is a negligible risk for the environment associated with the clinical use of Kymriah. RCL may be generated during tisagenlecleucel manufacturing or subsequently after introduction of vector‐transduced viable T cells into the patient. Generation of replication‐competent lentivirus has been categorized as a potential risk.
Benefit‐Risk Assessment
Results from the B2202 study demonstrated that a single infusion with tisagenlecleucel showed a significant increase of ORR in patients with aggressive relapsed or refractory ALL. Despite the limited follow‐up, results from time‐dependent endpoints such as DOR, event‐free survival, and OS provided support for sustained benefit of tisagenlecleucel. However, although the short‐term efficacy appeared to be promising, the long‐term response has yet to be established. Furthermore, issues regarding the uncontrolled nature of the study, such as selection and treatment bias, should be taken into account. For r/r DLBCL, the observed responses were modest in the ITT population. However, the activity could have potentially been higher with shorter manufacturing time, which was the objective of the Marketing Authorization Holder (MAH) in the postauthorization phase.
A major issue, identified during the assessment of the C2201 study for r/r DLBCL, was the prolonged time from leukapheresis and enrollment to infusion. The median time from enrollment to infusion (54 days) was considerably longer than the prespecified manufacturing time of 4–5 weeks, which again is longer than what would be expected based on the product's quality specification (approximately 3 weeks). It appeared that the prolonged median turnaround time was due to limited manufacturing capacity in the first part of the study. Therefore, the results observed in the ITT population in the study might likely represent a conservative estimate for the target patient population. On the other hand, the infused “modified ITT” population, though defined as the efficacy set for the efficacy endpoint analyses, was likely to add a further selection bias, as it was difficult to rule out the potential for overestimation of the treatment effect. Therefore, the ITT population was considered relevant for estimating the effect of the treatment strategy, regardless of whether patients were able to receive Kymriah, and representative based on reasonable assumptions and extrapolations.
Further data, including details of the manufacturing turnaround time (i.e., time from last relapse or confirmed refractory status, time from decision to treat, and time from leukapheresis to infusion), will be obtained from postauthorization studies. These include a prospective, observational study in patients with r/r DLBCL based on data from registry with efficacy outcome measures in line with the C2201 study and further follow‐up (24 months) for patients in the efficacy analysis set cohort and all infused patients from the C2201 study. The European Medicines Agency's Committee for Advanced Therapies (CAT) (whose main responsibility was to prepare a draft opinion on the advanced therapy medicinal product application submitted to the Committee for Medicinal Products for Human Use [CHMP], then to adopt the final opinion) also requested the company to submit the results of study CCTL019H2301, an open‐label, phase III study of Kymriah versus standard of care in adult patients with r/r aggressive B‐cell non‐Hodgkin lymphoma. The results are expected by 2025. Upon request from the CAT, a Scientific Advisory Group (SAG) Oncology meeting was convened on June 18, 2018, to address few issues regarding the DLBCL indications. The SAG acknowledged that there might be some selection bias in the pivotal trial. However, the ITT population was considered relevant and representative. Prior response to bridging chemotherapy did not seem to be of concern. Of note, the initial manufacturing problems and the delay in the infusion of Kymriah have to be taken into account, and one would expect less degree of progression prior to infusion on the basis of improvements in the manufacturing speed.
Based on the ITT analysis set, the complete response rate observed was somewhat in the range of what has been observed with other treatment modalities for third‐line therapy (CORAL studies: ORR ITT 40%, CR 28% 19). However, the duration of response was considered remarkable, with more than 60% of responders still responding at 13.9 months (DCO of December 8, 2017). Considering these observations together, based on the response rate and duration of response, and given the available treatment options, the SAG recommended by consensus that the clinical benefit was considered established despite the limitations for time‐dependent endpoints in single‐arm trials. Concerning OS, a number of suitable approaches have been explored based on matching. It was difficult to draw conclusions on the basis of the analyses presented, although they were informative, because important biases (including lead time bias) could not be excluded. Matching‐adjusted indirect comparison was used against the result of SCHOLAR‐1 study 20. Before matching, tisagenlecleucel was associated with significantly higher CR and ORR compared with salvage therapies in SCHOLAR‐1 (CR, 36.5% vs. 7.0%, p < .01; ORR, 47.6% vs. 26.0%, p < .01). After matching on the primary diagnosis (DLBCL or others), International Prognostic Index risk classification, and refractory category, the differences in CR and ORR remained significant (CR, 38.0% vs. 7.0%, p < .01; ORR, 47.4% vs. 26.0%, p < .01). CTL019 was associated with a 31.0% (95% CI, 19.1%–3.0%) higher CR rate and a 21.4% (95% CI, 8.8%–34.1%) higher ORR compared with salvage therapies. Furthermore, the SAG recommended that research on identification of biomarkers predictive of response should continue, with the aim to guide treatment decisions.
With regard to safety, long‐term data for Kymriah were missing, and hence the CAT requested a prospective registry (CTL019B2401) to collect real‐world safety and efficacy data in pediatric, young adult, and adult patients with hematological malignancies expressing CD19 who have received, or are due to receive, an autologous infusion of tisagenlecleucel for the treatment of an authorized indication. The primary objective is to evaluate safety as measured by type, frequency, and severity of adverse events (including secondary malignancies).
The COMP considered that the efficacy in the target patient population specified in the therapeutic indication granted offered a significant benefit in the treatment of these patients. Also based on the CAT and CHMP review of data on quality, safety, and efficacy, it was considered by majority decision that the risk‐benefit balance of Kymriah is favorable.
As per standard requirements, the CAT and CHMP conditions for approval included obligations for submission of periodic safety update reports via the European medicines agency web‐portal. The marketing authorization holder shall submit the first periodic safety update report within 6 months following authorization.
Additional risk‐minimization measures were required by CAT and CHMP and included the availability of tocilizumab and site qualification to minimize the CRS risks associated with Kymriah treatment; the MAH must ensure that hospitals and their associated centers that dispense Kymriah are specially qualified in accordance with the agreed‐upon control distribution program and ensure on‐site, immediate access to four doses of tocilizumab for each patient as CRS management medication prior to treating patients.
Conclusion
Although CAR T‐cell technology seems to be highly promising for diseases with single genetic/protein alterations, for more complex diseases there will be challenges to target the clonal variability within the tumor type or the clonal evolution during disease progression. We also await products with a lesser toxicity profile or more risk‐minimization tools.
Author Contributions
Collection and/or assembly of data: Sahra Ali, Rune Kjeken, Christiane Niederlaender, Greg Markey, Therese S. Saunders, Mona Opsata, Kristine Moltu, Bjørn Bremnes, Eirik Grønevik, Martine Muusse, Gro D. Håkonsen, Venke Skibeli, Maria Elisabeth Kalland, Ingrid Wang, Ingebjørg Buajordet, Ania Urbaniak, John Johnston, Khadija Rantell, Essam Kerwash, Martina Schuessler‐Lenz Tomas Salmonson, Jonas Bergh, Christian Gisselbrecht, Kyriaki Tzogani, Irene Papadouli, Francesco Pignatti
Data analysis and interpretation: Sahra Ali, Rune Kjeken, Christiane Niederlaender, Greg Markey, Therese S. Saunders, Mona Opsata, Kristine Moltu, Bjørn Bremnes, Eirik Grønevik, Martine Muusse, Gro D. Håkonsen, Venke Skibeli, Maria Elisabeth Kalland, Ingrid Wang, Ingebjørg Buajordet, Ania Urbaniak, John Johnston, Khadija Rantell, Essam Kerwash, Martina Schuessler‐Lenz Tomas Salmonson, Jonas Bergh, Christian Gisselbrecht, Kyriaki Tzogani, Irene Papadouli, Francesco Pignatti
Manuscript writing: Sahra Ali, Rune Kjeken, Christiane Niederlaender, Greg Markey, Therese S. Saunders, Mona Opsata, Kristine Moltu, Bjørn Bremnes, Eirik Grønevik, Martine Muusse, Gro D. Håkonsen, Venke Skibeli, Maria Elisabeth Kalland, Ingrid Wang, Ingebjørg Buajordet, Ania Urbaniak, John Johnston, Khadija Rantell, Essam Kerwash, Martina Schuessler‐Lenz Tomas Salmonson, Jonas Bergh, Christian Gisselbrecht, Kyriaki Tzogani, Irene Papadouli, Francesco Pignatti
Final approval of manuscript: Sahra Ali, Rune Kjeken, Christiane Niederlaender, Greg Markey, Therese S. Saunders, Mona Opsata, Kristine Moltu, Bjørn Bremnes, Eirik Grønevik, Martine Muusse, Gro D. Håkonsen, Venke Skibeli, Maria Elisabeth Kalland, Ingrid Wang, Ingebjørg Buajordet, Ania Urbaniak, John Johnston, Khadija Rantell, Essam Kerwash, Martina Schuessler‐Lenz Tomas Salmonson, Jonas Bergh, Christian Gisselbrecht, Kyriaki Tzogani, Irene Papadouli, Francesco Pignatti
Disclosures
The authors indicated no financial relationships.
Acknowledgments
The scientific assessment summarized in this report is based on important contributions from the rapporteur and co‐rapporteur assessment teams, CAT and CHMP members, and additional experts after the application for a marketing authorization from the company. This publication is a summary of the European Public Assessment Report (EPAR), the summary of product characteristics, and other product information. The EPAR is published on the EMA Web site (http://www.ema.europa.eu). For the most current information on this marketing authorization, please refer to the EMA Web site. The authors of this paper remain solely responsible for the opinions expressed in this publication.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Hunger SP, Lu X, Devidas M et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children's Oncology Group. J Clin Oncol 2012;30:1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Möricke A, Zimmermann M, Reiter A et al. Long‐term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL‐BFM study group from 1981 to 2000. Leukemia 2010;24:265–284. [DOI] [PubMed] [Google Scholar]
- 3. Locatelli F, Martin M, Bernardo ME et al. How I treat relapsed childhood acute lymphoblastic leukemia. Blood 2012;120:2807–2816. [DOI] [PubMed] [Google Scholar]
- 4. Tallen G, Ratei R, Mann G et al. Long‐term outcome in children with relapsed acute lymphoblastic leukemia after time‐point and site‐of‐relapse stratification and intensified short‐course multidrug chemotherapy: Results of trial ALL‐REZ BFM 90. J Clin Oncol 2010;28:2339–2347. [DOI] [PubMed] [Google Scholar]
- 5. Bailey LC, Lange BJ, Rheingold SR et al. Bone‐marrow relapse in paediatric acute lymphoblastic leukaemia. Lancet Oncol 2008;9:873–883. [DOI] [PubMed] [Google Scholar]
- 6. Seeger K, Adams HP, Buchwald D et al. TEL‐AML1 fusion transcript in relapsed childhood acute lymphoblastic leukemia: The Berlin‐Frankfurt‐Münster Study Group. Blood 1998;91:1716–1722. [PubMed] [Google Scholar]
- 7. Fielding AK, Richards SM, Chopra R et al.; Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group . Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007. 1;109:944–950. [DOI] [PubMed] [Google Scholar]
- 8. Tilly H, Gomes da Silva M, Vitolo U et al.; ESMO Guidelines Committee . Diffuse large B‐cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2015;26(suppl 5):v116–v125. [DOI] [PubMed] [Google Scholar]
- 9. Pfreundschuh M, Kuhnt E, Trümper L et al.; MabThera International Trial (MInT) Group . CHOP‐like chemotherapy with or without rituximab in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: 6‐year results of an open‐label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013–1022. [DOI] [PubMed] [Google Scholar]
- 10. Coiffier B, Sarkozy C. Diffuse large B‐cell lymphoma: R‐CHOP failure ‐ what to do? Hematology Am Soc Hematol Educ Program 2016;2016:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gisselbrecht C, Glass B, Mounier N et al. Salvage regimens with autologous transplantation for relapsed large B‐cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crump M, Kuruvilla J, Couban S et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem‐cell transplantation for relapsed and refractory aggressive lymphomas: NCIC‐CTG LY.12. J Clin Oncol 2014;32:3490–3496. [DOI] [PubMed] [Google Scholar]
- 13. Schuster SJ, Svoboda J, Chong EA et al. Chimeric antigen receptor T cells in refractory B‐cell lymphomas. N Engl J Med 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuster SJ, Bishop MR, Tam C et al. Sustained disease control for adult patients with relapsed or refractory diffuse large B‐cell lymphoma: An updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel. Blood 2018;132(suppl 1):1684A. [Google Scholar]
- 15. Maude SL, Laetsch TW, Buechner J et al. Tisagenlecleucel in children and young adults with B‐cell lymphoblastic leukemia. N Engl J Med 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeha S, Gaynon PS, Razzouk BI et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol 2006;24:1917–1923. [DOI] [PubMed] [Google Scholar]
- 17. Rabin R, de Charro F. EQ‐5D: A measure of health status from the EuroQol Group. Ann Med 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 18. Neelapu SS, Tummala S, Kebriaei P et al. Chimeric antigen receptor T‐cell therapy ‐ assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Den Neste E, Schmitz N, Mounier N et al. Outcomes of diffuse large B‐cell lymphoma patients relapsing after autologous stem cell transplantation: An analysis of patients included in the CORAL study. Bone Marrow Transplant 2017;52:216–221. [DOI] [PubMed] [Google Scholar]
- 20. Crump M, Neelapu SS, Umar Farooq U, et al. Outcomes in refractory diffuse large B‐cell lymphoma: Results from the international SCHOLAR‐1 study. Blood 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]


