Abstract
Urine drug test (UDT) is an effective tool used in chronic opioid therapy to ensure patient adherence to treatment and detect nonmedical opioid use. The two main types of UDT used in routine clinical practice are the screening tests or immunoassays and the confirmatory tests or laboratory‐based specific drug identification tests such as gas chromatography–mass spectrometry, liquid chromatography–mass spectrometry, or tandem mass spectrometry. UDT produces objective data on some nonmedical opioid use that may otherwise go undetected, such as the use of undisclosed medications, the nonuse of prescribed medications, and the use of illegal drugs. It allows clinicians to initiate an open and effective conversation about nonmedical opioid use with their patients. However, the test has certain limitations that sometimes compromise its use. Its interpretation can be challenging to clinicians because of the complexity of the opioid metabolic pathways. Clear guidelines or recommendations regarding the use of UDT in cancer pain is limited. As a result, UDT appears to be underused among patients with cancer pain receiving opioid therapy. More studies are needed to help standardize the integration and use of UDT in routine cancer pain management.
Implications for Practice
Despite its potential benefits, urine drug testing (UDT) appears to be underused among patients with cancer pain receiving opioid therapy. This is partly because its interpretation can be challenging owing to the complexity of the opioid metabolic pathways. Information regarding the use of UDT in opioid therapy among patients with cancer is limited. This review article will improve clinician proficiency in UDT interpretation and assist oncologists in developing appropriate treatment plans during chronic opioid therapy.
Keywords: Urine drug test, Opioids, Cancer pain, Nonmedical opioid use, Opioid crisis
Short abstract
It is important for oncologists to use effective strategies in managing patients with cancer pain to reduce nonmedical opioid use while protecting those who merit unrestricted access to opioids for their pain. Strategies include initial screening to determine risk and effective monitoring strategies. This article discusses a key element of the universal precautions approach, urine drug testing.
Introduction
The opioid overdose crisis has generated much concern in the medical community and the general public, resulting in increased efforts to mitigate inappropriate opioid use while ensuring optimal pain relief in patients with chronic pain. The U.S. Surgeon General issued an unprecedented letter to all physicians in 2016 calling for ways to end the opioid crisis 1. The U.S. government has declared the opioid crisis as a nationwide public health emergency 2. The Joint Commission released new public standards for pain management in 2017 that specifically highlight the importance of safe opioid prescribing practices 3. The Center for Disease Control and Prevention 4 and the American Society of Clinical Oncology 5 also released guidelines for pain management with specific emphasis on safe opioid practices.
Although a significant proportion may be attributable to nonprescription opioids such as illicitly manufactured fentanyl, prescription opioids still continue to contribute to opioid overdose rates. Prescription opioids alone are responsible for 46 overdose deaths every day and over 40% of all opioid‐related overdose deaths 6. Regrettably, there is a perception that physicians are mainly responsible for the current climate despite their probable well‐intentioned efforts. According to data from a recent national poll, most people blamed the opioid crisis on physicians (38%), followed by those who illegally sell opioids (28%) and the pharmaceutical industry (13%) 7. Moreover, the majority of respondents believed that physicians bear the most responsibility for fighting the opioid epidemic (47%), followed by the pharmaceutical industry (29%) and the law enforcement industry (12%) 7. The opioid crisis and its associated public health and legal implications may potentially impact oncologists either directly or indirectly. It has therefore become more important to employ effective strategies in managing patients with cancer pain in order to reduce nonmedical opioid use (NMOU) while protecting those that merit unrestricted access to opioids for their pain.
To achieve this, oncologists are encouraged to adopt the universal precautions approach, which generally entails initial screening of all patients to determine their level of risk for NMOU, and implementation of effective ongoing monitoring strategies 8. Urine drug testing (UDT) is a key element of this approach and has been endorsed in numerous opioid prescribing guidelines 4, 5, 9. In this article, we will discuss the main types of UDT and their interpretation. We also review the literature on its use among patients with cancer pain.
Types of UDT
A variety of biological specimens are used in performing drug testing, including urine, blood, sweat, saliva, hair, and nails. Urine is currently the most widely used and extensively validated specimen in clinical settings. It is considered the gold standard mainly because of its adequate specificity, sensitivity, ease of administration, and cost 10. Concentrations of drugs and their metabolites also tend to be high in the urine, resulting in relatively longer detection times compared with other specimens such as serum. Drugs are usually detectable within 1–3 days after ingestion. Oral fluid (saliva) is also widely available as point‐of‐care testing with comparable costs and equivalent results to UDT, but lower detection rates have been reported for benzodiazepines and some opioids such as hydromorphone and oxymorphone 11. Moreover, it has a relatively shorter window of detection than UDT (12–48 hours) 12. An advantage with oral fluid is that patients are easily monitored when they are providing a sample, and this significantly decreases the possibility for test subversion. Hair and nail testing may not detect drugs until 5–7 days after ingestion, with a typical detection window as long as 90 days. It is more expensive than urine or oral fluid testing and therefore infrequently used in clinical practice.
UDT is an effective tool used in chronic opioid therapy to ensure patient adherence to treatment, and also to detect NMOU or illicit drug use 13. There are typically two main types of UDT used in routine clinical practice. The screening tests or immunoassays use antibodies to detect the presence of a particular drug or metabolite in a urine sample. They are designed to classify substances as either present or absent according to a predetermined cutoff threshold. They are economical, able to test for drugs rapidly, and have adequate sensitivity (Table 1). However, they have a low specificity. Most of them can only recognize classes of drugs (class assays) and are unable to distinguish between drugs in the same class. Moreover, most of them miss compounds such as oxycodone and synthetic opioids such as fentanyl and methadone 14. However, there are some immunnoassays that are specifically directed toward the detection of compounds such as oxycodone, methadone, fentanyl, and buprenorphine (analyte‐specific assays) 14.
Table 1.
Main advantages and disadvantages of the types of urine drug test
| Advantages | Disadvantages |
|---|---|
| Immunoassays | |
| Less expensive | Cannot distinguish between drugs in the same classa |
| Rapid turnaround time | Cannot detect compounds such as oxycodone and synthetic opioidsa |
| Readily available in the office setting as point‐of‐care testing | High variations in cut‐off concentrations of testing devices from different manufacturers |
| Adequate sensitivity | High false positivity because of cross‐reactivities of the opioids with other substances |
| Specific drug identification testsb | |
| Able to identify the specific drug of interest | More expensive |
| Highly sensitive and specific | Slower turnaround time |
| No cross reactivities of opioids with other compounds | High level of expertise required |
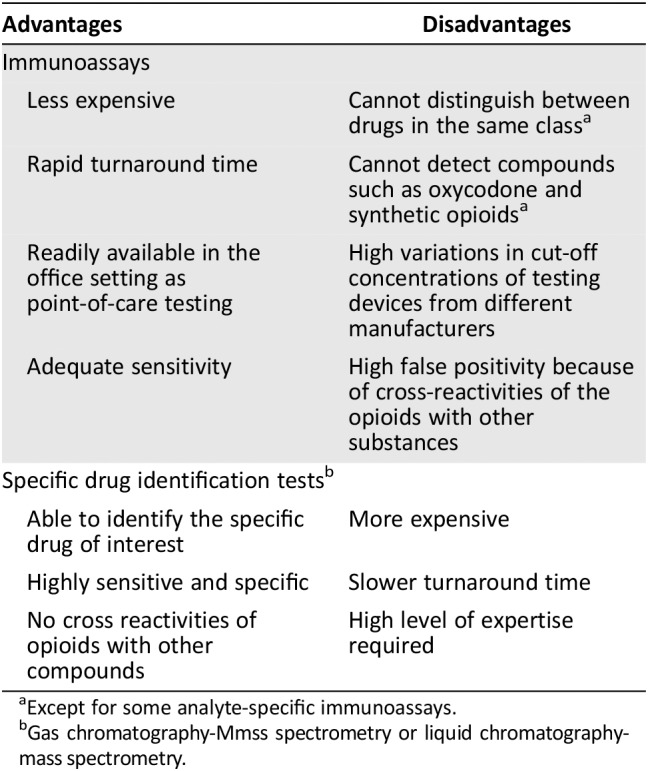
Except for some analyte‐specific immunoassays.
Gas chromatography‐Mmss spectrometry or liquid chromatography‐mass spectrometry.
There are high variations in cutoff concentrations of different testing devices from different manufacturers. Therefore, false negative results can occur if a sample has a low drug concentration or the test has a relatively high cutoff caliberation. Immunoassays are also subject to false positive results because of cross‐reactivities of the opioids with other substances such as quinolones, diphenhydramine, poppy seeds, chlorpromazine, rifampin, dextromethorphan, and quinine 13, 15.
The confirmatory tests or laboratory‐based specific drug identification tests such as gas chromatography–mass spectrometry (GC‐MS), liquid chromatography–mass spectrometry (LC‐MS), or tandem mass‐spectrometry (LC‐MS/MS) use techniques that separate the drug or drug metabolite from other analytes or adulterants (chromatography) and then identify it based on its molecular structure and properties (mass spectrometry). They are more sensitive and specific. They do not produce false positive results caused by cross‐reactivity or false negatives because they precisely identify the individual parent drug as well as its metabolites. However, they are relatively more expensive, require a high level of expertise in processing the samples, and have a slower turnaround time than screening tests 14. GC‐MS is considered the gold standard for confirmatory test 16, but LC‐MS/MS is very widely used because it is associated with less drug interference and can be performed with smaller urine volumes as compared with GC‐MS 16.
A hybrid immunoassay/LC‐MS test is available, which measures each drug with either the immunoassay or LC‐MS approach, depending on which one is more accurate and faster. This has been found to prevent the need for the stepwise initial immunoassay and subsequent confirmatory testing 17. Another technique bypasses immunoassay testing and directly uses the LC‐MS/MS for screening and confirmatory retesting if needed. An advantage with this technique is that the LC‐MS/MS screens for more drugs than any immunoassay can identify. It is also usually less susceptible to adulteration and dilution as compared to immunoassay 18.
Most clinicians consider it rational to use the laboratory‐based specific drug identification tests for both preliminary and follow‐up testing because of their relatively more favorable sensitivity and specificity values, which potentially makes them cost‐effective in the end 19. Ultimately, the best confirmation of a presumptive abnormal immunoassay test is patient validation of the results, in which case a further confirmatoy test is avoided and cost is reduced.
UDT Interpretation
Interpretation of UDT results can be challenging because of the complexity of the opioid metabolic pathways. Evidence suggests that clinicians have insufficient knowledge regarding UDT interpretation 20, 21. In a study to determine the knowledge of UDT interpretation among 114 physicians attending an opioid education conference, none of the physicians who used the test in their practice answered all seven questions administered in the survey correctly. Only 30% of them answered more than half the questions correctly. Physicians who use UDT did not perform any better in the test than those who do not use UDT 22. In a similar study among 359 adolescent medicine physicians, most of whom use UDT in their clinical practice, only 10% answered all survey questions correctly, and 75% responded to at least one items incorrectly. Only 12% knew that oxycodone cannot be detected by most opioid screening immunoassays 23. Another study was conducted among 99 internal medicine physicians to examine the relationship between participant knowledge regarding UDT interpretation and confidence in their ability to interpret the results 20. Overall, participants demonstrated poor knowledge regarding UDT interpretation, with a mean score of 3 out of 7 (SD, 1.2). Interestingly, 73% of those who felt confident in their UDT interpretive ability had a knowledge score of 3 or lower 20.
A good knowledge and understanding of the opioid metabolic pathways is needed for correct interpretation of UDT. The potential error is to misinterpret the presence of metabolites that are also pharmaceutical agents. Figure 1 suggests the most likely opioids that can be expected in the urine after giving a prescribed opioid. The following key caveats may be helpful when interpreting UDT results:
Morphine in urine may also be indicative of heroin use (especially if it also tests positive for 6‐monoacetylmorphine), poppy seeds, or codeine.
Morphine can also produce small amounts of hydromorphone.
Rarely, a small amount of codeine may be present in the urine with morphine ingestion because of manufacturing impurities. In such cases, a disproportionately high amount of morphine should be present.
Codeine metabolizes to morphine, but the reverse does not occur.
Codeine can produce a small amount of hydrocodone. Therefore the presence of small amounts of hydrocodone in urine containing a high concentration of codeine should not be interpreted as evidence of hydrocodone misuse.
Because hydromorphone is also a metabolite of hydrocodone, it may also be detectable with codeine ingestion.
Rarely, a small amount of hydrocodone may be present in the urine with oxycodone ingestion because of manufacturing impurities. In such cases, a disproportionately high amount of oxycodone should be present.
Oxycodone ingestion may only have oxymorphone detected in urine because oxymorphone has a longer half‐life than oxycodone.
The dose of opioid ingested cannot be extrapolated from drug screen results even with a quantitative test such as GC/MS because of interindividual and intraindividual variabilities in volumes of distribution and drug metabolism 24.
Quantitative values of opioids in urine may sometimes be helpful. For instance, high concentrations of a parent drug without its metabolites is highly suggestive of tampering.
Figure 1.
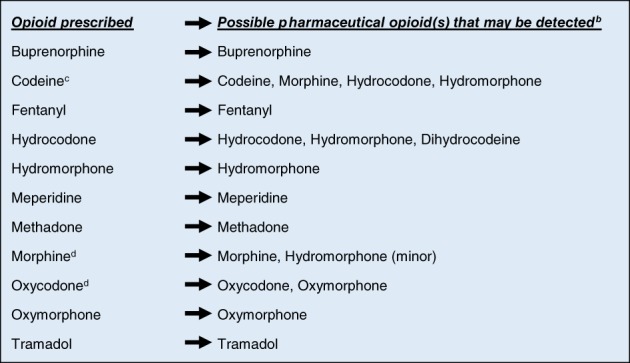
Possible urine drug test findings based on opioid prescribed.a
a Using confirmatory tests such as Gas Chromatography/Mass Spectrometry, Liquid Chromatography/Mass Spectrometry.
b The pharmaceutical opioid and/or its metabolites.
c As contained in medications such as Tylenol #3 and Tylenol #4.
d Rarely, morphine may have codeine impurities and oxycodone may have hydrocodone impurities.
Note: the presence of 6‐monoacetylmorphine in addition to morphine metabolites in the urine is indicative of recent heroin ingestion.
Occasionally, patients may tamper with the urine in order to avoid detection. This will usually result in a false negative test. Urine tampering can be achieved via a variety of in vivo and in vitro techniques. In vivo methods include ingestion of sodium bicarbonate, diuretics, salicylates, and commercial body “cleansers” to change the chemical composition or concentration (specific gravity) of urine. In vitro methods include specimen adulteration or substitution with drug‐free human, animal, or synthetic urine 25. Tampering can usually be detected by measuring the urine creatinine, pH, specific gravity, or conducting a urine adulteration panel.
UDT Application in Routine Clinical Care
UDT results should not be interpreted in isolation but should be used as part of a comprehensive evaluation of patients to arrive at the right diagnosis, develop appropriate treatment plans, and support the therapeutic decision‐making process. There are wide variations among various groups regarding who should undergo UDT and how frequent the test should be ordered. As a result, no universal standardized protocol currently exists. Some guidelines recommend UDT testing at least once a year for all patients receiving opioid therapy regardless of the level of risk 4, every 6 months to 2 years for low‐risk patients, one to three times a year for moderate risk patients, and at least two to four times a year for high‐risk patients 26, 27, 28.
Others recommend UDT annually for low‐risk patients, at least twice a year for moderate risk, and at least three times a year for high‐risk patients 19. Additional monitoring may be performed based on clinical judgement and regardless of risk level. Other authors have also proposed UDT at baseline 13, then random testing once within 3 months, then 6–12 months after initiation 29. Most agree that baseline UDT should be done either before initiation of opioid therapy or within the first 3 months of the initial visit.
UDT has some potential advantages when used effectively during clinical practice. It may help detect certain problematic situations that may otherwise go undetected such as the use of undisclosed medications, the nonuse of prescribed medications, and the use of illicit drugs. It may produce the most objective data on drug taking behavior and therefore supplements patient self‐reporting. It provides the opportunity for physicians to initiate an open and effective conversation about NMOU. Many studies in the chronic noncancer pain population have observed benefits from the use of UDT including increased patient adherence and reductions in NMOU indicators 30, 31, 32, 33. One systematic review confirmed that there is some evidence supporting the effectiveness of urine drug testing as well as opioid treatment agreements in reducing NMOU among patients with chronic pain 34. However, a few other studies did not show any significant benefit 35.
Just like with many other test devices, UDT has its own limitations that may sometime compromise its ultimate value. A normal UDT result may present the clinician with a false sense of assurance that substance misuse is nonexistent. A normal result does not guarantee normal drug taking behavior, because patients who chemically cope with opioids may have a normal UDT but might be using opioids in an excessive or maladaptive manner 8. Similarly, an abnormal result does not necessarily “diagnose” substance use disorder until other potential causes have been explored. Misinterpretation of UDT results may have negative consequences for the patient such as unfair loss of opioid privileges, deterioration of physician‐patient relationship, potentially distressing opioid withdrawal syndromes, and compromised ability to receive appropriate therapy from future physicians because providers who take over the patient's care may request medical records from previous providers before continuing with opioid prescriptions 22. Sometimes, these issues may result in the involvement of law enforcement. Some patients may view physician requests for UDT as a punitive measure or as a disproportionate act of physician self‐protection at the expense of optimal patient care 22. Studies are needed to better determine the cost‐effectiveness of these tests 36, 37 because the financial 38, 39 and logistic burden imposed on patients by conducting expensive tests may potentially cause psychological or emotional harm. It is difficult to determine the actual out‐of‐pocket cost of these tests, as they vary significantly depending on patient's geographic location, insurance policy, the laboratory used, and the facility where the test was ordered 40. According to the 2019 Medicare Clinical Laboratory Fee Schedule, the reimbursement rate for a 9‐panel immunoassay drug test is $65 whereas a 1‐ to 7‐panel definitive drug testing is $114 and an 8‐ to 14‐panel definitive testing is $157. Comparatively, reimbursement for complete blood count with differential test is $9, and comprehensive metabolic panel is $12 41.
In communicating the rationale for UDT to patients, clinicians should emphasize that the key reason for the test is to ensure patient safety and improve effectiveness of therapy. It should be explained using the concept of universal precautions and as part of routine clinic procedure or standard of care. The results of an abnormal UDT should be used to initiate a dialog with the patient. Clinicians should have an open and nonjudgmental conversation with the patient. Other measures that may be taken when NMOU is detected with the assistance of the UDT include additional confirmation testing, increased patient monitoring, setting appropriate boundaries, decreasing the time interval between follow‐ups, limiting the overall quantity and doses, switching to a nonopioid or adjuvant analgesic, using nonpharmacological interventions, evaluation and treatment of any underlying psychological comorbidity, and referral to specialist clinicians for management of NMOU 13.
UDT Use in Patients with Cancer
UDT is recommended by most clinical guidelines in chronic noncancer pain management 9, 13, 42, 43, 44. Among patients with cancer, standard guidelines or recommendations regarding its use in opioid therapy were rare until recently. The American Society of Clinical Oncology now endorses the use of UDT in opioid risk assessment, stratification,and adherence monitoring among cancer survivors 5. The most recent National Comprehensive Cancer Network guidelines for Adult Cancer Pain recommend UDT at baseline and during treatment to help document opioid analgesic adherence, detect illicit drug use, and identify opioid diversion 45. This is important because there is growing clinical evidence suggesting that patients with cancer may be at higher risk of NMOU than was previously thought 46, 47, 48. At least one in five patients with cancer might be at risk for opioid use disorder 48.
UDT appears to be underused among patients with cancer pain receiving opioid therapy (Table 2). In a study conducted by our team among 1,058 consecutive outpatients with cancer pain seen at our supportive care clinic, only 6% underwent UDT, of which 54% had abnormal results 46. In another study, only 2.4% of 8,727 patients seen at a chronic cancer pain clinic underwent UDT, of which 58% were abnormal 49. A study among 323 patients with cancer attending an outpatient pain and palliative care clinic found that only 5% of the patient visits included a UDT ordering. Of those tests, 56% had abnormal results 50. A limitation of these studies was that the tests were conducted among a select cohort of patients who already have a high propensity for NMOU. In spite of this limitation, the rates of abnormality are considerably high and are comparable to findings among patients with noncancer pain 13, 51, 52, 53, 54, 55, 56, 58.
Table 2.
Rate of urine drug test ordering and abnormality in patients with cancer
| Study | Type of study | Patient selection for test | Rate of UDT ordering | Rate of UDT abnormality |
|---|---|---|---|---|
| Barclay et al. 2014 58 | Retrospective | Based on level of riska | 40% | 46% |
| Childers et al. 2014 50 | Retrospective | Based on level of riskb | 4% | 56% |
| Rauenzahn et al. 2017 47 | Retrospective | Based on level of riskb | 40% | 73% |
| Arthur et al. 2016 46 | Retrospective | Based on level of riskb | 6% | 54% |
| Koyyalagunta et al. 2017 49 | Retrospective | Based on level of riskb | 2.4% | 58% |
| Arthur et al. 2018 57 | Retrospective | Random, regardless of risk levela | – | 28% |
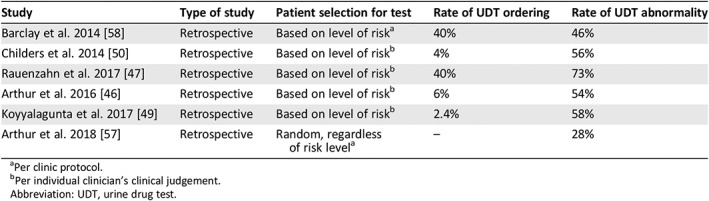
Per clinic protocol.
Per individual clinician's clinical judgement.
Abbreviation: UDT, urine drug test.
Efforts to standardize the integration and use of UDT in routine cancer pain management are much needed. The high rates of UDT abnormalities in patients with cancer raise concerns about the possibility of under detection, missed opportunities, or delays in detecting NMOU during clinic visits, especially among individuals who appear to be at minimal risk and therefore do not undergo closer monitoring. Our team implemented a policy to perform UDT in a randomly selected small number of outpatients regardless of their risk profile. We conducted a study to assess the feasibility of this process and also compare the results with those of patients who underwent UDT based on their high‐risk profiles (targeted group) 57. We found that 98% of patients who were randomly approached for UDT cooperated, indicating that this process of random patient selection was highly feasible. Approximately 28% of the randomly selected patients and 43% of the targeted group had abnormal UDT results (p = .01). When marijuana was excluded from the list of abnormal results, 17% of the random group and 39% of the targeted group had abnormal UDT result. These findings suggest a comparatively high rate of abnormality even among patients who were randomly selected for screening regardless of their risk profile. This process of such random screening needs to be investigated to better determine its utility in routing clinical care. This study is a key step in our efforts to better understand and define the timing and frequency of UDT implementation among patients with cancer especially because they differ from the patients without cancer with regard to symptom burden, needs, and expectations.
Conclusion
UDT is an effective test used in the monitoring of patient adherence to opioid therapy and detection of NMOU. Two main types of the test exist. The laboratory‐based specific drug identification test appears to be more preferable to the immunoassay because of its superior advantages, although it is relatively more expensive and has a slower turnaround time. Interpretation of UDT results can be challenging and requires a good knowledge and understanding of the opioid metabolic pathways. The test has some limitations that may sometimes compromise its usefulness, so clinicians should be aware of them in order to use the test more effectively. Despite its potential benefits, UDT appears to be underused among patients with cancer receiving chronic opioid therapy. Further studies are needed to better determine its utility in routing cancer pain management.
Disclosures
The author indicated no financial relationships
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Murthy VH. Ending the opioid epidemic—A call to action. N Engl J Med 2016;375:2413–2415. [DOI] [PubMed] [Google Scholar]
- 2. Report of the President's Commission on Combating Drug Addiction and the Opioid Crisis. Washington, DC: The White House; 2017. [Google Scholar]
- 3. Pain management standards for accredited organizations . The Joint Commission. 2018. [Google Scholar]
- 4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315:1623–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paice JA, Portenoy R, Lacchetti C et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical practice guideline. J Clin Oncol 2016;34:3325–3345. [DOI] [PubMed] [Google Scholar]
- 6. Seth P, Scholl L, Rudd RA et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015‐2016. MMWR Morb Mortal Wkly Rep 2018;67:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blendon RJ, Benson JM. The public and the opioid‐abuse epidemic. N Engl J Med 2018;378:407–411. [DOI] [PubMed] [Google Scholar]
- 8. Arthur J, Bruera E. Balancing opioid analgesia with the risk of nonmedical opioid use in patients with cancer. Nat Rev Clin Oncol 2019;16:213–226. [DOI] [PubMed] [Google Scholar]
- 9. Chou R. 2009 Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: What are the key messages for clinical practice? Pol Arch Med Wewn 2009;119:469–477. [PubMed] [Google Scholar]
- 10. Manchikanti KN, Manchikanti L, Damron KS et al. Increasing deaths from opioid analgesics in the United States: An evaluation in an interventional pain management practice. J Opioid Manag 2008;4:271–283. [DOI] [PubMed] [Google Scholar]
- 11. Heltsley R, Depriest A, Black DL et al. Oral fluid drug testing of chronic pain patients. II. Comparison of paired oral fluid and urine specimens. J Anal Toxicol 2012;36:75–80. [DOI] [PubMed] [Google Scholar]
- 12. Pre‐employment drug test positives increase more than 5%, according to new data from Quest Diagnostics Drug Testing Index. Quest Diagnostics. March 7, 2013. [Google Scholar]
- 13. Owen GT, Burton AW, Schade CM et al. Urine drug testing: Current recommendations and best practices. Pain Physician 2012;15(suppl):ES119–ES133. [PubMed] [Google Scholar]
- 14. Magnani B, Kwong T. Urine drug testing for pain management. Clin Lab Med 2012;32:379–390. [DOI] [PubMed] [Google Scholar]
- 15. Zacher JL, Givone DM. False‐positive urine opiate screening associated with fluoroquinolone use. Ann Pharmacother 2004;38:1525–1528. [DOI] [PubMed] [Google Scholar]
- 16. Mikel C, Pesce A, West C. A tale of two drug testing technologies: GC‐MS and LC‐MS/MS. Pain Physician 2010;13:91–92. [PubMed] [Google Scholar]
- 17. McMillin GA, Marin SJ, Johnson‐Davis KL et al. A hybrid approach to urine drug testing using high‐resolution mass spectrometry and select immunoassays. Am J Clin Pathol 2015;143:234–240. [DOI] [PubMed] [Google Scholar]
- 18. Drug testing : A white paper of the American Society of Addiction Medicine. Chevy Chase, MD: American Society of Addiction Medicine, 2013. [Google Scholar]
- 19. Argoff CE, Alford DP, Fudin J et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: Consensus recommendations. Pain Med 2018;19:97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Starrels JL, Fox AD, Kunins HV et al. They don't know what they don't know: Internal medicine residents’ knowledge and confidence in urine drug test interpretation for patients with chronic pain. J Gen Intern Med 2012;27:1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reisfield GM, Webb FJ, Bertholf RL et al. Family physicians’ proficiency in urine drug test interpretation. J Opioid Manag 2007;3:333–337. [DOI] [PubMed] [Google Scholar]
- 22. Reisfield GM, Bertholf R, Barkin RL et al. Urine drug test interpretation: What do physicians know? J Opioid Manag 2007;3:80–86. [DOI] [PubMed] [Google Scholar]
- 23. Levy S, Harris SK, Sherritt L et al. Drug testing of adolescents in ambulatory medicine: Physician practices and knowledge. Arch Pediatr Adolesc Med 2006;160:146–150. [DOI] [PubMed] [Google Scholar]
- 24. Nafziger AN, Bertino JS, Jr . Utility and application of urine drug testing in chronic pain management with opioids. Clin J Pain 2009;25:73–79. [DOI] [PubMed] [Google Scholar]
- 25. Reisfield GM, Salazar E, Bertholf RL. Rational use and interpretation of urine drug testing in chronic opioid therapy. Ann Clin Lab Sci 2007;37:301–314. [PubMed] [Google Scholar]
- 26. Manchikanti L, Kaye AM, Knezevic NN et al. Responsible, safe, and effective prescription of opioids for chronic non‐cancer pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017;20:S3–S92. [PubMed] [Google Scholar]
- 27. Peppin JF, Passik SD, Couto JE et al. Recommendations for urine drug monitoring as a component of opioid therapy in the treatment of chronic pain. Pain Med 2012;13:886–896. [DOI] [PubMed] [Google Scholar]
- 28. Washington State Agency Medical Directors’ Group . Interagency Guideline on Prescribing Opioids for Pain. Olympia, WA: Department of Health, 2015. [Google Scholar]
- 29. Christo PJ, Manchikanti L, Ruan X et al. Urine drug testing in chronic pain. Pain Physician 2011;14:123–143. [PubMed] [Google Scholar]
- 30. Yee DA, Hughes MM, Guo AY et al. Observation of improved adherence with frequent urine drug testing in patients with pain. J Opioid Manag 2014;10:111–118. [DOI] [PubMed] [Google Scholar]
- 31. Manchikanti L, Manchukonda R, Damron KS et al. Does adherence monitoring reduce controlled substance abuse in chronic pain patients? Pain Physician 2006;9:57–60. [PubMed] [Google Scholar]
- 32. Manchikanti L, Manchukonda R, Pampati V et al. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician 2006;9:123–129. [PubMed] [Google Scholar]
- 33. Pesce A, West C, Rosenthal M et al. Illicit drug use in the pain patient population decreases with continued drug testing. Pain Physician 2011;14:189–193. [PubMed] [Google Scholar]
- 34. Starrels JL, Becker WC, Alford DP et al. Systematic review: Treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med 2010;152:712–720. [DOI] [PubMed] [Google Scholar]
- 35. Turner JA, Saunders K, Shortreed SM et al. Chronic opioid therapy risk reduction initiative: impact on urine drug testing rates and results. J Gen Intern Med 2014;29:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melanson SEF, Petrides AK. Economics of Pain Management Testing. J Appl Lab Med 2018;2:587–597. [DOI] [PubMed] [Google Scholar]
- 37. Hammett‐Stabler CA, Pesce AJ, Cannon DJ. Urine drug screening in the medical setting. Clin Chim Acta 2002;315:125–135. [DOI] [PubMed] [Google Scholar]
- 38. Gilbert JW, Wheeler GR, Mick GE et al. Urine drug testing in the treatment of chronic noncancer pain in a Kentucky private neuroscience practice: The potential effect of Medicare benefit changes in Kentucky. Pain Physician 2010;13:187–194. [PubMed] [Google Scholar]
- 39. Gilbert JW, Wheeler GR, Mick GE et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent medicare policy changes in kentucky. Pain Physician 2010;13:167–186. [PubMed] [Google Scholar]
- 40. Kale N. Urine drug tests: Ordering and interpreting results. Am Fam Physician 2010. 9;99:33–39. [PubMed] [Google Scholar]
- 41. Clinical laboratory fee schedule . Centers for Medicare and Medicaid Services. Available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/index.html. 2019. Accessed August 30, 2019.
- 42. Michna E, Ross EL, Hynes WL et al. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage 2004;28:250–258. [DOI] [PubMed] [Google Scholar]
- 43. Chou R, Fanciullo GJ, Fine PG et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug‐related behaviors: A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009;10:131–146. [DOI] [PubMed] [Google Scholar]
- 44. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain 2002;18:S76–S82. [DOI] [PubMed] [Google Scholar]
- 45. NCCN Clinical Practice Guidelines in Oncology : Adult Cancer Pain (version 1.2018). Plymouth Meeting, PA: National Comprehensive Cancer Network, 2018.
- 46. Arthur JA, Edwards T, Lu Z et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer 2016;122:3732–3739. [DOI] [PubMed] [Google Scholar]
- 47. Rauenzahn S, Sima A, Cassel B et al. Urine drug screen findings among ambulatory oncology patients in a supportive care clinic. Support Care Cancer 2017;25:1859–1864. [DOI] [PubMed] [Google Scholar]
- 48. Carmichael AN, Morgan L, Del Fabbro E. Identifying and assessing the risk of opioid abuse in patients with cancer: An integrative review. Subst Abuse Rehabil 2016;7:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koyyalagunta D, Bruera E, Engle MP et al. Compliance with opioid therapy: Distinguishing clinical characteristics and demographics among patients with cancer pain. Pain Medicine 2018;19:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Childers JW, King LA, Arnold RM. Chronic pain and risk factors for opioid misuse in a palliative care clinic. Am J Hosp Palliat Care 2015;32:654–659. [DOI] [PubMed] [Google Scholar]
- 51. Cook RF, Bernstein AD, Arrington TL et al. Methods for assessing drug use prevalence in the workplace: A comparison of self‐report, urinalysis, and hair analysis. Int J Addict 1995;30:403–426. [DOI] [PubMed] [Google Scholar]
- 52. Fishbain DA, Cutler RB, Rosomoff HL. et al. Validity of self‐reported drug use in chronic pain patients. Clin J Pain 1999;15:184–191. [DOI] [PubMed] [Google Scholar]
- 53. Hariharan J, Lamb GC, Neuner JM. Long‐term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007;22:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. West R, Pesce A, West C et al. Observations of medication compliance by measurement of urinary drug concentrations in a pain management population. J Opioid Manag 2010;6:253–257. [DOI] [PubMed] [Google Scholar]
- 55. Manchikanti L, Cash KA, Damron KS et al. Controlled substance abuse and illicit drug use in chronic pain patients: An evaluation of multiple variables. Pain Physician 2006;9:215–225. [PubMed] [Google Scholar]
- 56. Michna E, Jamison RN, Pham LD et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain 2007;23:173–179. [DOI] [PubMed] [Google Scholar]
- 57. Arthur JA, Lu Z, Hui D et al. Random versus targeted urine drug testing among cancer patients receiving opioid therapy at a supportive care clinic. J Clin Oncol 2018;36:221a. [Google Scholar]
- 58. Barclay JS, Owens JE, Blackhall LJ. Screening for substance abuse risk in cancer patients using the Opioid Risk Tool and urine drug screen. Support Care Cancer 2014;22:1883–1888. [DOI] [PubMed] [Google Scholar]


