Abstract
Background
This study aimed to assess the characteristics of breakthrough cancer pain (BTcP) in patients receiving low doses of opioids for background pain in comparison with patients receiving at least 60 mg of oral morphine equivalents (OME).
Materials and Methods
Patients with advanced cancer receiving less than 60 mg/day of OME with episodes of BTcP were included in the analysis (group L). Data were compared with patients receiving doses of opioids ≥60 mg of OME (group H). Pain intensity, current analgesic therapy, number of BTcP episodes, intensity of BTcP, its predictability and triggers, onset duration, interference with daily activities, BTcP medications, and time to meaningful pain relief were collected. Adverse effects imputable to a BTcP medication were recorded.
Results
A total of 1,418 and 2,474 patients were included in groups L and H, respectively. A lower number of BTcP episodes (p = .005), a lower BTcP intensity (p = .0001), a faster BTcP onset (p = .024), and a longer time to meaningful pain relief after taking a BTcP medication (p = .009) were found in group L as compared with group H. In group L, BTcP interference on daily activity was less than in group H (p = .009). Patients in group L were less likely to be prescribed an opioid as BTcP medication in comparison with patients in group H (p = .0001). Opioid doses used for BTcP were significantly higher in group H. Patients in group L were more likely to be less satisfied (p = .003) than patients in group H. No adverse effects of severe intensity were reported in both groups.
Conclusion
Patients receiving lower doses of opioids exhibit some differences in BTcP presentation: fewer episodes with lower intensity and a faster onset, a longer time to meaningful pain relief, and less satisfaction with BTcP medication. A relevant percentage of patients was receiving fentanyl preparations normally reserved for patients receiving higher doses of opioids.
Implications for Practice
Breakthrough pain is present in patients receiving low doses of opioids. It has its own peculiarities: less frequent, lower intensity, faster onset, longer time to meaningful pain relief, and less satisfaction with medication. Many patients were prescribed fentanyl preparations, which are normally reserved for patients receiving higher doses of opioids.
Keywords: Breakthrough cancer pain, Opioids, Doses
Short abstract
Breakthrough cancer pain has been defined as a peak of pain intensity of short duration in patients with stable analgesia provided by round‐the‐clock analgesics. This study assessed the characteristics of breakthrough cancer pain in patients receiving less than 60 mg/day of oral morphine equivalents for their background pain, compared to those observed in patients receiving higher doses.
Introduction
Breakthrough cancer pain (BTcP) has traditionally been defined as a peak of pain intensity of short duration in patients with stable and acceptable analgesia provided by analgesics given around the clock 1. Other than oral opioids, the treatment of BTcP has recently been based on the use of rapid‐onset opioids, such as fentanyl, delivered by transmucosal routes, which provide different drug availabilities 2, 3. Many studies have shown superiority over oral opioids in term of effectiveness and rapidity 4. The lowest‐strength dose of each system is recommended to start dose titration in patients receiving at least 60 mg of oral morphine equivalents (OME) for background pain 5. Thus, many studies assessing fentanyl products as BTcP medication have focused on patients receiving doses of opioids equivalent to the third step of World Health Organization analgesic ladder, that is, 60 mg/day of OME.
Many epidemiological studies of BTcP, however, have included patients receiving nonopioids or opioids for moderate pain 6, 7, 8, 9 for which these drugs are potentially contraindicated, as they should be only prescribed to patients tolerating 60 mg/day of OME 5. BTcP has never been properly assessed in this population and no information exists on how BTcP is managed. The aim of this study was to assess the characteristics of BTcP in patients receiving less than 60 mg/day of OME for their background pain, compared with those observed in patients receiving higher doses of OME. The secondary outcome was to assess which drugs and doses are commonly used in this group of patients.
Materials and Methods
This was a secondary analysis of a prospective, multicenter, national study 10, performed in a large number of patients during a period of 24 months in 32 centers, including different settings such as palliative care, oncology, radiotherapy, and pain therapy. Patients were seen in outpatient clinics, as inpatients, and in day hospital. The original study was approved by each local ethical committee, and written informed consent was obtained from each patient.
Inclusion criteria were as follows: age ≥18 years, stable and well‐controlled background pain (pain intensity ≤4 on a 0–10 numerical scale), a cancer diagnosis, and the presence of BTcP episodes well distinguished from background pain with moderate to severe intensity. The diagnosis of BTcP was based on an algorithm previously reported 2, 11, 12. Exclusion criteria were unstable or uncontrolled background pain (>4/10), peaks of minor pain intensity (<5/10), and poor collaboration.
Age, gender, setting, primary cancer, ongoing anticancer treatment, and Karnofsky status were recorded. From the entire sample, patients receiving opioids in doses of <60 mg/day of OME (group L) and ≥60 mg of OME (group H) for background pain were selected.
The following data were collected: average pain intensity in the last 24 hours (on a numerical scale 0–10); opioids used for background pain and their doses, expressed as OME; the number of BTcP episodes per day, its intensity, onset, duration, and predictability; medication and doses used for BTcP; mean time to meaningful pain relief after taking a BTcP medication; patient's satisfaction with BTcP medications measured on a verbal scale (1 = unsatisfied, 4 = very satisfied); and adverse effects and their intensity (0 = none, 1 = mild, 2 = moderate, 3 = severe) attributed to opioids given for BTcP.
Statistical Analysis
Sample descriptive statistics have been provided for both outcomes and explanatory variables. Association patterns have been assessed using chi‐square tests for categorical variables. Correlations have been calculated and tested using Spearman correlations for ordinal variables and, if requested, point‐biserial correlations between binary and continuous variables. The statistical significance level was set at 5%. Continuous variables groups' comparisons have been carried out using t tests with Satterwhite's adjustment for deviations from homoscedasticity. The analysis was carried out using the statistical software STATA, version 14 (StataCorp, College Station, TX).
Results
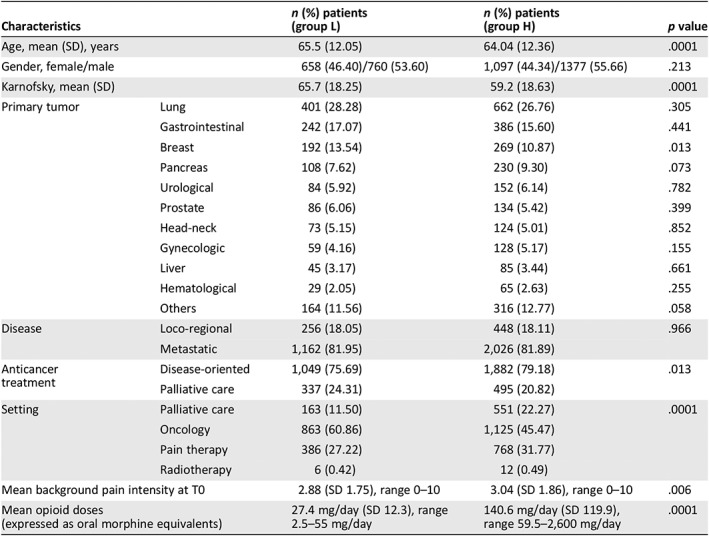
From 4,016 patients surveyed in the study period, 124 patients were not receiving opioid analgesics for background pain; 1,418 patients were included in group L (36.43%), and 2,474 patients (63.57%) were included in group H. Thus, the analysis was performed in 3,892 patients. The general characteristics of these patients are described in Table 1. Statistical differences in age, Karnofsky, anticancer treatment, primary tumor, and background pain intensity were found between the two groups (Table 1).
Table 1.
Characteristics of patients who were receiving low and high doses of opioids (L and H groups)
| Characteristics | n (%) patients (group L) | n (%) patients (group H) | p value | |
|---|---|---|---|---|
| Age, mean (SD), years | 65.5 (12.05) | 64.04 (12.36) | .0001 | |
| Gender, female/male | 658 (46.40)/760 (53.60) | 1,097 (44.34)/1377 (55.66) | .213 | |
| Karnofsky, mean (SD) | 65.7 (18.25) | 59.2 (18.63) | .0001 | |
| Primary tumor | Lung | 401 (28.28) | 662 (26.76) | .305 |
| Gastrointestinal | 242 (17.07) | 386 (15.60) | .441 | |
| Breast | 192 (13.54) | 269 (10.87) | .013 | |
| Pancreas | 108 (7.62) | 230 (9.30) | .073 | |
| Urological | 84 (5.92) | 152 (6.14) | .782 | |
| Prostate | 86 (6.06) | 134 (5.42) | .399 | |
| Head‐neck | 73 (5.15) | 124 (5.01) | .852 | |
| Gynecologic | 59 (4.16) | 128 (5.17) | .155 | |
| Liver | 45 (3.17) | 85 (3.44) | .661 | |
| Hematological | 29 (2.05) | 65 (2.63) | .255 | |
| Others | 164 (11.56) | 316 (12.77) | .058 | |
| Disease | Loco‐regional | 256 (18.05) | 448 (18.11) | .966 |
| Metastatic | 1,162 (81.95) | 2,026 (81.89) | ||
| Anticancer treatment | Disease‐oriented | 1,049 (75.69) | 1,882 (79.18) | .013 |
| Palliative care | 337 (24.31) | 495 (20.82) | ||
| Setting | Palliative care | 163 (11.50) | 551 (22.27) | .0001 |
| Oncology | 863 (60.86) | 1,125 (45.47) | ||
| Pain therapy | 386 (27.22) | 768 (31.77) | ||
| Radiotherapy | 6 (0.42) | 12 (0.49) | ||
| Mean background pain intensity at T0 | 2.88 (SD 1.75), range 0–10 | 3.04 (SD 1.86), range 0–10 | .006 | |
| Mean opioid doses (expressed as oral morphine equivalents) | 27.4 mg/day (SD 12.3), range 2.5–55 mg/day | 140.6 mg/day (SD 119.9), range 59.5–2,600 mg/day | .0001 | |
Pain mechanism was mixed, nociceptive, and neuropathic in 881 (62.13%), 433 (30.54%), and 104 (7.33%) in group L, respectively. No statistical differences with group H were found (p = .193).
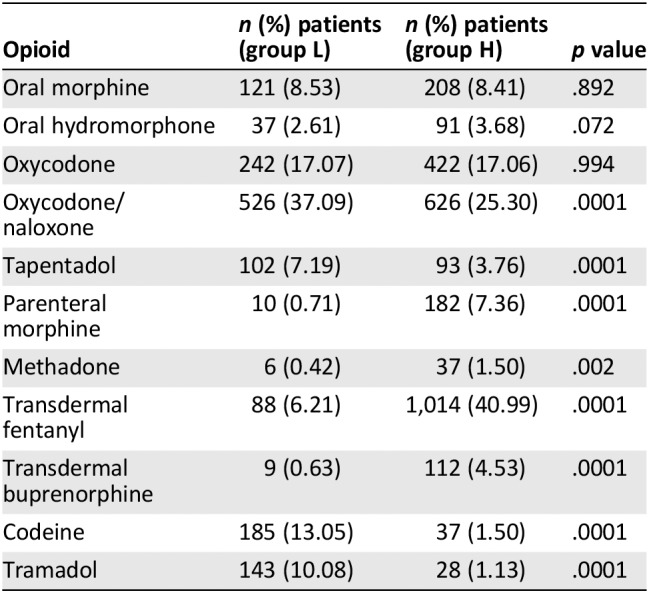
Drugs administered for background pain in group L included anti‐inflammatory drugs (n = 105, 7.40%), paracetamol (n = 549, 45.11%), weak opioids (codeine and tramadol, n = 328, 23.13%), oral morphine (n = 121, 8.53%), oral hydromorphone (n = 37, 2.61%), oral oxycodone (n = 242, 17.07%), oral oxycodone/naloxone (n = 526, 37.09%), oral tapentadol (n = 102, 7.19%), parenteral morphine (n = 10, 0.71%), oral methadone (n = 6, 0.42%), transdermal fentanyl (n = 88, 6.21%), and transdermal buprenorphine (n = 9, 0.63%). No significant differences were found in the use of nonsteroidal anti‐inflammatory drugs between L and H groups (7.4% vs. 8.85%, p = .116). Indeed, significant differences were found in the use of paracetamol (38.72% vs. 27.00, p = .0001), codeine (13.05% vs. 1.50%, p = .0001), and tramadol (10.08% vs. 1.13%, p = .0001). Oral oxycodone/naloxone and oral tapentadol were more frequently used in group L, whereas parenteral morphine, oral methadone, transdermal fentanyl, and transdermal buprenorphine were more frequently used in patients in group H. Opioids used for background pain in groups L and H are reported in Table 2.
Table 2.
Opioids used for background pain in patients who were receiving low and high doses of opioids (L and H groups)
| Opioid | n (%) patients (group L) | n (%) patients (group H) | p value |
|---|---|---|---|
| Oral morphine | 121 (8.53) | 208 (8.41) | .892 |
| Oral hydromorphone | 37 (2.61) | 91 (3.68) | .072 |
| Oxycodone | 242 (17.07) | 422 (17.06) | .994 |
| Oxycodone/naloxone | 526 (37.09) | 626 (25.30) | .0001 |
| Tapentadol | 102 (7.19) | 93 (3.76) | .0001 |
| Parenteral morphine | 10 (0.71) | 182 (7.36) | .0001 |
| Methadone | 6 (0.42) | 37 (1.50) | .002 |
| Transdermal fentanyl | 88 (6.21) | 1,014 (40.99) | .0001 |
| Transdermal buprenorphine | 9 (0.63) | 112 (4.53) | .0001 |
| Codeine | 185 (13.05) | 37 (1.50) | .0001 |
| Tramadol | 143 (10.08) | 28 (1.13) | .0001 |
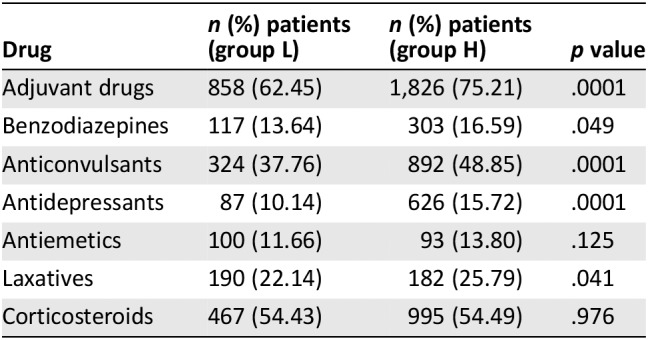
Eight hundred fifty‐eight patients (62.45%) of group L were receiving adjuvant drugs, including benzodiazepines (n = 117, 13.64%), anticonvulsants (n = 324, 37.76%), antidepressants (n = 87, 10.14%), antiemetics (n = 100, 11.66%), laxatives (n = 190, 22.14%), and corticosteroids (n = 467, 54.43%). Adjuvant drugs were more frequently used in patients receiving higher doses of opioids than in group L (75.21 vs. 62.45%, p = .0001; Table 3).
Table 3.
Adjuvant drugs used for background pain in patients who were receiving low and high doses of opioids (L and H groups)
| Drug | n (%) patients (group L) | n (%) patients (group H) | p value |
|---|---|---|---|
| Adjuvant drugs | 858 (62.45) | 1,826 (75.21) | .0001 |
| Benzodiazepines | 117 (13.64) | 303 (16.59) | .049 |
| Anticonvulsants | 324 (37.76) | 892 (48.85) | .0001 |
| Antidepressants | 87 (10.14) | 626 (15.72) | .0001 |
| Antiemetics | 100 (11.66) | 93 (13.80) | .125 |
| Laxatives | 190 (22.14) | 182 (25.79) | .041 |
| Corticosteroids | 467 (54.43) | 995 (54.49) | .976 |
BTcP
The mean number of BTcP episodes in group L was 2.27 per day (SD 1.41, range 1–10); 954 patients (67.33%) had 1–2 episodes per day, 383 patients (27.03%) had 3–4 episodes per day, and 81 patients (5.65%) had ≥5 episodes per day. In group H, 63.42%, 30.44%, and 6.15% had 1–2 episodes per day, 3–4 episodes per day, and ≥5 episodes per day, respectively. A lower number of BTcP episodes was found in group L as compared with group H (p = .005).
The mean intensity of BTcP in group L was 7.34 (SD 1.27, range 5–10), which was significantly lower in comparison with group H (7.62 [SD 1.26], p = .0001). The mean duration of untreated episodes in group L was 41.85 minutes (SD 35.21). No statistical differences between the two groups were found (p = .451).
BTcP was predictable in 446 patients (31.45%) in group L. No differences between the two groups were found (31.45% vs. 29.39%, p = .176). The triggers of predictable BTcP were movement (62.11%), ingestion of food (16.37%), procedures (9.64%), and cough (11.43%), which were similar to those found in group H.
In group L, BTcP onset was short (≤10 minutes) in 1,006 patients (70.94%), whereas 412 patients (29.06%) exhibited a slower onset of BTcP (>10 minutes). Patients in group L were more likely to have a faster onset of BTcP in comparison with group H patients (67.46%, p = .024).
In group L, BTcP interference with daily activity was mild, much, and very much in 248 (17.80%), 838 (60.16%), and 303 (21.75%) patients, respectively. In group L, BTcP interference on daily activity was less than in group H: very much 21.75% versus 32.68%. The difference was significant (p = .009).
In group L, 1,161 patients (81.93%) were prescribed a BTcP medication; these patients were less likely to be prescribed a BTcP medication in comparison with patients in group H (90.54%, p = .0001). Of these, 779 (67.10%) and 1,958 patients (87.45%) were prescribed an opioid for BTcP (p = .0001; Table 4). The mean doses of opioids prescribed for BTcP in groups L and H are reported in Table 5. The differences were highly significant.
Table 4.
Number of patients using opioid as breakthrough cancer pain medication in patients who were receiving low doses and high doses of opioids (L and H groups)
| Opioids used for breakthrough pain | n (%) patients receiving low doses | n (%) patients receiving high doses | p value |
|---|---|---|---|
| OTFC | 28 (3.59) | 100 (5.11) | .003 |
| FBT | 91 (11.68) | 338 (17.26) | .0001 |
| FBST | 106 (13.60) | 460 (23.49) | .0001 |
| FPNS | 242 (31.06) | 555 (28.34) | .010 |
| INFS | 10 (1.28) | 30 (1.53) | .220 |
| Oral morphine | 230 (29.52) | 275 (14.04) | .0001 |
| Parenteral morphine | 72 (9.24) | 200 (10.21) | .001 |
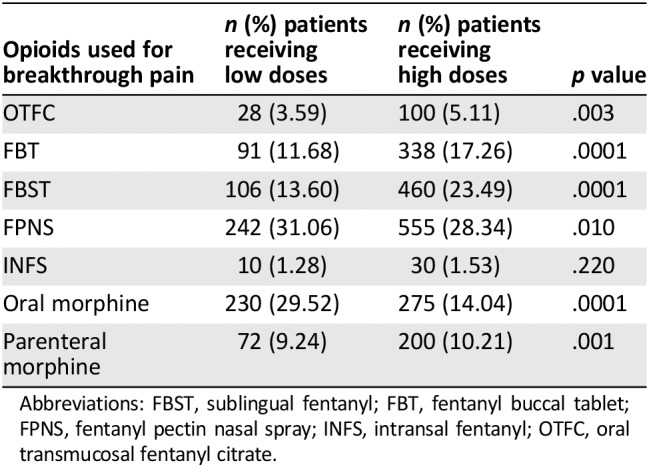
Abbreviations: FBST, sublingual fentanyl; FBT, fentanyl buccal tablet; FPNS, fentanyl pectin nasal spray; INFS, intransal fentanyl; OTFC, oral transmucosal fentanyl citrate.
Table 5.
Opioid doses used for breakthrough cancer pain in patients who were receiving low doses and high doses of opioids (L and H groups)
| Opioids used for breakthrough pain | Mean dose (group L) | Mean dose (group H) | p value |
|---|---|---|---|
| OTFC | 248 μg (137.09) | 433 μg (290.26) | .0001 |
| FBT | 148 μg (72.05) | 260 μg (194.53) | .0001 |
| FBST | 160 μg (96.29) | 247 μg (177.41) | .0001 |
| FPNS | 118 μg (59.04) | 187 μg (139.21) | .0001 |
| INFS | 75 μg (26.35) | 106 μg (53.71) | .019 |
| Oral morphine | 8.75 mg (3.23) | 14.62 mg (10.11) | .0001 |
| Parenteral morphine | 7.18 mg (3.29) | 10.26 mg (6.28) | .0001 |
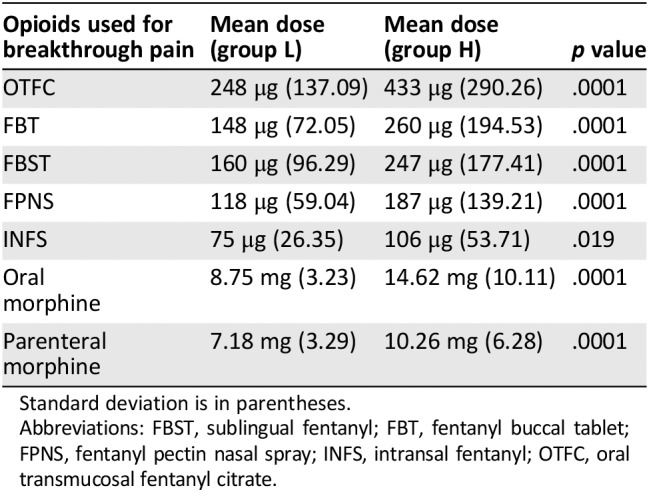
Standard deviation is in parentheses.
Abbreviations: FBST, sublingual fentanyl; FBT, fentanyl buccal tablet; FPNS, fentanyl pectin nasal spray; INFS, intransal fentanyl; OTFC, oral transmucosal fentanyl citrate.
The mean meaningful time for pain relief after a BTcP medication was 17.52 minutes (SD 14.07) in group L. Significant differences with group H were found (15.96 [SD 13.99], p = .009).
Patients’ Satisfaction
In patients receiving BTcP medications, the grade of satisfaction in group L was not satisfied, neutral, satisfied, and much satisfied in 89 (7.79%), 160 (14.00%), 793 (69.38%), and 101 (8.84%) cases, respectively. In patients receiving BTcP medications, the grade of satisfaction in group H was not satisfied, neutral, satisfied, and much satisfied in 248 (11.27%), 282 (12.82%), 1,437 (65.32%), and 233 (10.59%) cases, respectively.
Patients of group L were more likely to be less satisfied (8.84% vs. 10.59%) and more neutral than group H patients (14.00% vs. 12.82%, p = .003).
Adverse Effects
Ten patients (1.44%) and 43 patients (3.50%) reported adverse effects related to opioid medication used for BTcP in groups L and H, respectively (p = .008).
Side effects were headache, nausea, vomiting, and confusion. Only two patients had severe side effects, particularly nausea and confusion, and only three patients had moderate nausea. In group L, 1 patient reported headache, 3 patients confusion, 2 patients nausea, and 1 patient vomiting; in group H, 1 patient reported headache, 17 patients confusion, 6 patients nausea, and 3 patients vomiting.
Discussion
The findings of this study provided interesting information regarding BTcP in patients receiving low doses of opioids. In comparison with patients in group H, patients in group L exhibited a lower number of episodes per day, a lower BTcP intensity, a shorter BTcP onset, less interference with daily activity, a longer time to meaningful pain relief, and less satisfaction with BTcP medication.
There are different explanations for these findings. It is likely that these patients were on an early stage of disease and the pain syndrome was less aggressive, therefore requiring lower doses of opioids for an adequate background analgesia. The lower number of episodes with a lower intensity explains the less interference with daily activity. On the other hand, this subgroup of patients was less frequently prescribed a BTcP medication, with oral morphine being one of the most frequent options, as a result of existing guidelines and prescription requirements for patients with relatively low opioid exposure. Therefore, it is also expected that oral morphine would be used more frequently in group L, which could explain the longer time to meaningful pain relief, as well as the less satisfaction with BTcP medication compared with group H, in which fentanyl products, known to have a shorter onset of effect, were more often prescribed.
There are other relevant observations. Unexpectedly, fentanyl transmucosal products were also given in group L, although in a lower percentage of cases, suggesting an off‐label use in more than half of patients. Prescription regulations require that the minimal strength of fentanyl products should be given in patients tolerant to at least 60 mg of OME, for the risk of adverse effects 4, 5. Nevertheless, the percentage of patients reporting adverse effects was lower compared with that found in group H. This finding suggests that transmucosal fentanyl products, even at the lowest strength, are tolerated by patients receiving less than the traditional 60 mg/day of OME, that is, the minimal opioid dose reported by pioneer regulatory studies for the use of fentanyl products 4, 5.
There are few comparative data, as this topic has not been specifically addressed in literature. Although patients on low doses of opioids have been included in many epidemiological studies of BTcP 6, 7, 8, 9, no specific characteristics of BTcP have ever been reported. One controlled trial comparing 5 mg of subcutaneous morphine with 100 μg of sublingual fentanyl also included patients using less than 60 mg of OME daily dose and found minimal incidence of opioid adverse effects and no relationship between previous opioid exposure and adverse effects incidence 13, 14. One study assessed the effects of sublingual fentanyl in doses lower than the minimal strength commonly used for patients receiving at least 60 mg/day of OME 15. Doses of 67 μg of sublingual fentanyl for BTcP in patients who were receiving lower doses of opioids for background analgesia were safe and effective. Differently from the doses of fentanyl products found in this study, which were slightly higher, these low doses of fentanyl were in some way proportional to the lower doses of opioids used for background pain.
There are some limitations in this study. This was a secondary analysis of a large group of patients included for assessing the characteristics and factors influencing the presentation of BTcP. The large number of patients recruited in different settings, however, reproduced exactly what is happening in the real world, particularly with the use of fentanyl products that, based on actual recommendations, should not be used in this subgroup of patients, even at the existent minimal strengths. Quality of data entry was guaranteed by sharing the definitions regarding BTcP characteristics in an investigator meeting and continuous data monitoring by a web platform for which each center received a specific investigator manual 10. Finally, the study was performed only in patients with BTcP, so that the prevalence of this phenomenon in this population remains unknown.
Conclusion
Patients receiving low doses of opioids have BTcP characterized by a lower number of episodes per day, a lower BTcP intensity, a shorter BTcP onset, a longer time to meaningful pain relief, less satisfaction with BTcP medication, and less interference with daily activity, compared with patients receiving higher doses. Other than oral opioids, patients were prescribed off‐label fentanyl products. Although this prescription was not associated with relevant adverse effects, caution is needed about such potentially harmful practices, especially in the presence of well‐established guidelines, and lack of information about amount of submucosal fentanyl products dosing for BTcP relief in relation to total OME dose. In patients receiving a median OME of 60 mg/day, that is about half of patients who were receiving low opioid doses for background pain, proportional doses of oral morphine were effective for controlling of BTcP 16. Further studies should redefine the best treatment of BTcP and dosing for this subgroup of patients.
Author Contributions
Conception/design: Sebastiano Mercadante, Augusto Caraceni
Provision of study material or patients: IOPS group
Collection and/or assembly of data: Francesco Masedu, Teresa Scipioni, Federica Aielli
Data analysis and interpretation: Sebastiano Mercadante, Augusto Caraceni
Manuscript writing: Sebastiano Mercadante, Augusto Caraceni
Final approval of manuscript: Sebastiano Mercadante, Augusto Caraceni, Francesco Masedu, Teresa Scipioni, Federica Aielli
Disclosures
The authors indicated no financial relationships.
Acknowledgments
The ORIGINAL study was sponsored by Molteni, Italy. Data were independently analyzed by the IOPS MS Scientific Committee. Data for secondary analysis were independently managed by authors. The data sets generated and/or analyzed during the current study are available from the corresponding author on request.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Mercadante S, Portenoy RK. Breakthrough cancer pain: Twenty‐five years of study. Pain 2016;157:2657–2663. [DOI] [PubMed] [Google Scholar]
- 2. Davies A, Zeppetella G, Andersen S et al. Multi‐centre European study of breakthrough cancer pain: Characteristics and patient perceptions of current and potential management strategies. Eur J Pain 2011;15:756–763. [DOI] [PubMed] [Google Scholar]
- 3. Mercadante S, Marchetti P, Cuomo A et al. Breakthrough pain and its treatment: Critical review and recommendations of IOPS (Italian Oncologic Pain Survey) expert group. Support Care Cancer 2016;24:961–968. [DOI] [PubMed] [Google Scholar]
- 4. Mercadante S. Pharmacotherapy for breakthrough cancer pain. Drugs 2012;72:181–190. [DOI] [PubMed] [Google Scholar]
- 5. Davies AN, Dickman A, Reid C et al. The management of cancer‐related breakthrough pain: Recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 2009;13:331–338. [DOI] [PubMed] [Google Scholar]
- 6. Zeppetella G, O'Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J Pain Symptom Manage 2000;20:87–92. [DOI] [PubMed] [Google Scholar]
- 7. Gómez‐Batiste X, Madrid F, Moreno F et al. Breakthrough cancer pain: Prevalence and characteristics in patients in Catalonia, Spain. J Pain Symptom Manage 2002;24:45–52. [DOI] [PubMed] [Google Scholar]
- 8. Petzke F, Radbruch L, Zech D et al. Temporal presentation of chronic cancer pain: Transitory pains on admission to a multidisciplinary pain clinic. J Pain Symptom Manage 1999;17:391–401. [DOI] [PubMed] [Google Scholar]
- 9. Swanwick M, Haworth M, Lennard RF. The prevalence of episodic pain in cancer: A survey of hospice patients on admission. Palliat Med 2001;15:9–18. [DOI] [PubMed] [Google Scholar]
- 10. Mercadante S, Marchetti P, Cuomo A et al. Factors influencing the clinical presentation of breakthrough pain in cancer patients. Cancers (Basel) 2018;10. pii: E175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Løhre ET, Klepstad P, Bennett MI et al. From “breakthrough” to “episodic” cancer pain? A European Association for Palliative Care Research Network Expert Delphi survey toward a common terminology and classification of transient cancer pain exacerbations. J Pain Symptom Manage 2016;51:1013–1019. [DOI] [PubMed] [Google Scholar]
- 12. Mercadante S, Marchetti P, Cuomo A et al. Breakthrough cancer pain: Preliminary data of The Italian Oncologic Pain Multisetting Multicentric Survey (IOPS‐MS). Adv Ther 2017;34:120–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zecca E, Brunelli C, Centurioni F et al. Fentanyl sublingual tablets versus subcutaneous morphine for the management of severe cancer pain episodes in patients receiving opioid treatment: A double‐blind, randomized, noninferiority trial. J Clin Oncol 2017;35:759–765. [DOI] [PubMed] [Google Scholar]
- 14. Ricchini F, Caraceni A, Zecca E et al. Effect of opioid exposure on efficacy and tolerability of sublingual fentanyl and subcutaneous morphine for severe cancer pain episodes. Secondary analysis from a double‐blind double‐dummy, randomized trial. J Pain Symptom Manage 2019;58:587–595. [DOI] [PubMed] [Google Scholar]
- 15. Mercadante S, Adile C, Cuomo A et al. The use of low doses of a sublingual fentanyl formulation for breakthrough pain in patients receiving low doses of opioids. Support Care Cancer 2017;25:645–649. [DOI] [PubMed] [Google Scholar]
- 16. Azhar A, Kim YJ, Haider A et al. Response to oral immediate‐release opioids for breakthrough pain in patients with advanced cancer with adequately controlled background pain. The Oncologist 2019;24:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]


