Abstract
The detection of lymph node metastasis affects the management of patients with primary breast cancer significantly in terms of staging, treatment, and prognosis. The main goal for the radiologist is to determine and detect the presence of metastatic disease in nonpalpable axillary lymph nodes with a positive predictive value that is high enough to initially select patients for upfront axillary lymph node dissection. Features that are suggestive of axillary adenopathy may be seen with different imaging modalities, but ultrasound is the method of choice for evaluating axillary lymph nodes and for performing image‐guided lymph node interventions. This review aims to provide a comprehensive overview of the available imaging modalities for lymph node assessment in patients diagnosed with primary breast cancer.
Implications for Practice
The detection of lymph node metastasis affects the management of patients with primary breast cancer. The main goal for the radiologist is to detect lymph node metastasis in patients to allow for the selection of patients who should undergo upfront axillary lymph node dissection. Features that are suggestive of axillary adenopathy may be seen with mammography, computed tomography, and magnetic resonance imaging, but ultrasonography is the imaging modality of choice for evaluating axillary lymph nodes. A normal axillary lymph node is characterized by a reniform shape, a maximal cortical thickness of 3 mm without focal bulging, smooth margins, and, depending on size, a discernable central fatty hilum.
Keywords: Axilla, Lymph nodes, Magnetic resonance imaging, Ultrasonography, Positron emission tomography
Short abstract
This review provides a comprehensive overview of the available imaging modalities for lymph node assessment in patients diagnosed with primary breast cancer.
Introduction
The detection of lymph node metastasis affects the management of patients with primary breast cancer significantly in terms of staging, treatment, and prognosis 1. Formerly, axillary lymph node dissection (ALND) in clinically positive axilla was the state‐of the‐art procedure to determine staging and achieve regional control in patients with breast cancer. However, its associated comorbidities (lymphedema, restriction of arm and shoulder movement, numbness of upper arm skin, etc.) have spurred efforts in the last decades to provide a highly selective approach for the assessment of lymph node involvement 2, 3, 4. Sentinel lymph node biopsy is currently the most accurate method for axillary staging 5. Since 2005, it has been recommended by the American Society of Clinical Oncology as an initial alternative to upfront ALND in patients with early‐stage breast cancer, ensuring that only women with positive findings in the sentinel nodes would undergo complete dissection 6. Recently, data from the American College of Surgeons Oncology Group Z0011 trial suggested that even patients with a stage T1 or T2 tumor and one or two positive sentinel lymph nodes with no extracapsular extension can be spared ALND 1, 7.
The radiologist has an important role in the preoperative imaging of the axilla and sampling of abnormal lymph nodes. The goal is to determine and detect the presence of metastatic disease in nonpalpable axillary lymph nodes (low or high tumor burden) with a positive predictive value that is high enough to initially select patients for upfront ALND. Imaging characteristics that are indicative of axillary lymph node metastatic involvement can be seen with mammography (MG), computed tomography (CT), and magnetic resonance imaging (MRI). Nevertheless, ultrasonography (US) is the method of choice for axillary lymph nodes assessment and for performing image‐guided lymph node interventions.
In this review, we aim to provide a comprehensive overview of the available imaging modalities for lymph node assessment in patients diagnosed with primary breast cancer. First, we introduce the basic anatomy of the axilla, as this serves as an important knowledge base to understand the metastatic pathways of spread, and then we briefly summarize the N‐staging guidelines from the latest edition of the TNM staging guidelines for Breast Cancer from the American Joint Commission on Cancer (AJCC) 8. We then focus on providing details on the interpretation of suspicious nodal features from the available imaging modalities.
In Table 1 we report the main advantages and disadvantages of the imaging modalities to assess lymph node involvement in primary breast cancer. Considering the growing importance of radiomics and radiogenomics, we provide insight into current research involving these applications in the detection of metastatic lymph nodes from breast cancer. Finally, we present our conclusions regarding for future directions that can be expected for lymphatic mapping in primary breast cancer.
Table 1.
Advantages and disadvantages of all modalities for lymph node imaging in primary breast cancer, with the reported range of sensibility and specificities
| Modality | Advantages | Disadvantages | SE | SP | Pooled estimates |
|---|---|---|---|---|---|
| PE | Accessible, primary care | Low sensitivity for nodal assement | 30% | 93% | NA |
| MG | Inital imaging modality, ubiquitously available | Moderate sensitivity for nodal assement | 66.9% | 80.8% | NA |
| US | Low cost, ubiquitously available, method of choice for nodal assesment biopsy guidance | Operator dependent | 87% | 53%–97% |
SE 48.8%–87.1% SP 55.6%–97.3% |
| CT | Not recommended for primary nodal staging | Moderate sensitivity, low specificity | 72% | 40% | NA |
| MRI | Potential for LN‐specific MRI contrast agents | Moderate sensitivity and specificity, limited ability to obtain a complete visualization of the axilla with breast MRI exam, requires dedicated axillary protocol to achieve good sensitivity and high specificty | 77% | 90% |
SE 75%–80% SP 89%–91% |
| PET/CT | Useful in identifying advanced axillary disease and metastatic nodal spread outside the axilla such as IMN | Low spatial resolution and lack in anatomic details | 64% | 93% |
SE 59%–69% SP 90%–95% |
| PET/MRI | Improves the diagnostic performance of axillary nodal staging in patients with clinically node‐positive breast cancer | Limited availablity, high costs | 77% | 100% | NA |
| SPECT/CT | Precise anatomic localization of sentinel lymph nodes | More expensive and not as broadly installed than planar lymphoscintigraphy | 75% | 90% | NA |
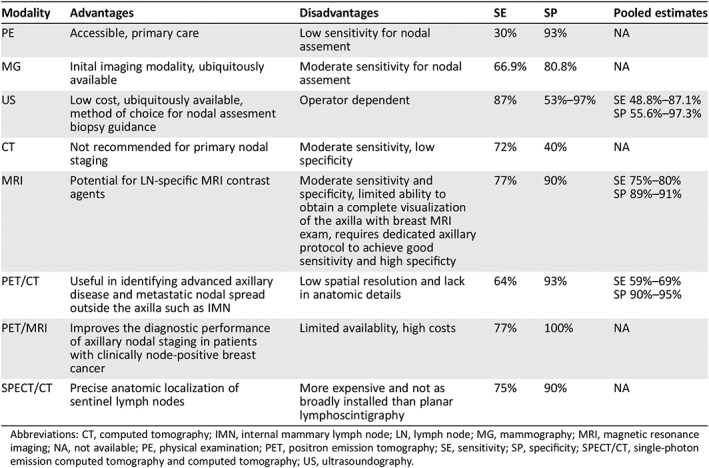
Abbreviations: CT, computed tomography; IMN, internal mammary lymph node; LN, lymph node; MG, mammography; MRI, magnetic resonance imaging; NA, not available; PE, physical examination; PET, positron emission tomography; SE, sensitivity; SP, specificity; SPECT/CT, single‐photon emission computed tomography and computed tomography; US, ultrasoundography.
Basic Anatomy of the Axilla
A basic knowledge of the anatomy of the axilla is important to accurately identify the location of abnormal lymph nodes using any cross‐sectional imaging techniques. As early as 1994, Giuliano et al. demonstrated that the status of the sentinel lymph node accurately reflects the status of the entire axillary basin draining a primary breast tumor 9. In fact, malignant cells first enter the nodes (regional spread) through an afferent lymphatic deposit in the subcapsular sinus, growing at this location and eventually replacing the local normal nodal architecture before spreading to a distant body region 10.
The axilla is a pyramidal space situated between the upper aspect of the thoracic wall and the medial aspect of the arm. The axilla is divided into three regions or levels by the pectoralis minor muscle (supplemental online Fig. A) 11:
Level I: Lymph nodes infero‐lateral to the pectoralis minor (Berg's level 1);
Level II: Lymph nodes behind the pectoralis minor (Berg's level 2);
Level III: Lymph nodes supero‐medial to the pectoralis minor (Berg's level 3).
The breast lymphatics are separate from those of the underlying torso, with a subareolar plexus of lymphatics and a small number of large lymphatic vessels draining into axillary lymph nodes. Breast lymphatic drainage comprises superficial, deep, and perforating systems. The superficial system drains to the axilla, usually to level II. The deep system drains to the axilla and also anastomoses with the perforating system that drains to the internal mammary nodes. The perforating system does not connect with the superficial system. From a practical standpoint, drainage generally proceeds in order from level I to level II, to level III, and finally into the thorax 10.
An alternate to the axilla is the drainage to the internal mammary lymph node (IMN) chain. This runs from the anterior phrenic nodes at the diaphragm to its termination in the thoracic venous system on the right and the thoracic duct on the left, and it follows the course of the internal mammary artery and vein between the pleura/endothoracic fascia and the chest wall near the sternal margin. The IMNs are located in the first through sixth intercostal spaces, and they are largest in the first three spaces. Usually, normal internal mammary lymph nodes measure less than 6 mm (supplemental online Fig. A).
Metastases to the IMNs generally occur after a tumor has metastasized to the axilla; nodal staging is considered N3b and therefore indicates stage IIIC disease.
Past studies of extended radical mastectomy in operable breast cancer showed a prevalence of positive IMNs in 8% to 20% of patients, but the axilla also was involved in most of these patients 12.
Isolated metastases to the IMNs occur in 1%–5% of breast cancers and usually come from deep or medial tumors. In the absence of axillary metastases, involvement of the IMNs is considered N2 disease. There is no survival benefit to surgical treatment of internal mammary node metastases, and because of the morbidity, dissection of the nodes is not usually performed. However, the presence of IMN metastases, either in isolation or with concomitant axillary disease, does have prognostic significance and also carries a small but definite risk of local recurrence. Long‐term survival is reduced in patients with isolated internal mammary node metastases and is reduced even further in patients with both internal mammary and axillary metastases. In addition, standard tangential beam radiation therapy of the breast does not necessarily include the internal mammary nodes. Radiation treatment planning may therefore be altered if metastases of the internal mammary nodes are identified 13.
Histopathologic Staging of the Axilla
Lymph node staging for breast cancer has changed and evolved over the years with the advent of new techniques. From the mere identification of only gross deposits of cancer cells in the lymph nodes, we are now finding microscopic areas of cancer spread with histopathology.
Thus, according to the 8th edition of the AJCC Cancer Staging Manual 8, an isolated area of cancer spread that is smaller than 0.2 mm (or that has fewer than 200 cells) does not change the stage but is recorded with the abbreviation “i+” that reflects the presence of isolated tumor cells. If the area of cancer spread is at least 0.2 mm (or 200 cells) but not larger than 2 mm, the area is labeled as a micrometastasis and cells are counted only if there are no larger areas of cancer spread. Areas of cancer spread larger than 2 mm change the N stage. The abbreviation “mol+” is used if a molecular test, Reverse transcription polymerase chain reaction, was used to find the cancer that is not otherwise detected 14. It may be stressed that the use of pathologic (microscopic) confirmatory methods of nodal involvement before the removal of the primary tumor results in a clinical N category. Qualifiers for either fine‐needle aspiration cytology or core‐needle biopsy (f) and sentinel node biopsy (sn) are to be added after the N category, to reflect this degree of confidence in nodal staging and to differentiate it from staging based on palpation or imaging (e.g., cN1(f) or cN1(sn) vs. cN1). The pN category mandates the definitive removal of the primary tumor and lymph node(s) 8. It has to be noted that the 8th edition of the AJCC Cancer Staging Manual also allows for pN0(mol+) and pN0(mol−) categories to reflect isolated tumor cells either detected or tested but undetected by nonmorphological means 15. In the supplemental online table, the N‐staging from the 8th edition of TNM staging for breast cancer is summarized.
Metastatic Spread and the Need for Axillary Treatment
Metastasis is a challenging clinical problem, considered the leading cause of death in breast cancer. Lymph nodes are the first regional site of metastasis and nodal disease is critical for staging and prognosis and for predicting increased mortality in many cancer types including the breast. Once a migratory cell has detached from the tumor, it may intravasate into blood vessels or lymphatics. Either route of dissemination can lead to venous circulation, as lymphatics drain into blood, most commonly through the left lymphatic duct (thoracic duct) or the right lymphatic duct, and then subsequently into the subclavian veins 16. However, although the potential of one detached cell to metastasize is limitless, only very few metastases eventually develop. Tumor cells forming metastases either have the autonomous characteristics while still in the primary tumor to metastasize or will acquire the needed changes induced by the environmental conditions. The potential to metastasize is not solely dependent on tumor cell traits but also modulated by the host cells, in particular platelets and bone marrow–derived cells. Tumors almost invariably invade lymph nodes in sequence, starting with the nearest (sentinel or draining) node, followed by increasingly distal ones 5.
Axillary Lymph Node Assessment
Physical Examination
Physical examination (PE) has a low accuracy in predicting nodal involvement from breast cancer 17. The likelihood of axillary lymph node metastases as determined by PE only is difficult to predict and subject to a large proportion of false‐positive and false‐negative results reported sensitivity of 30%, specificity of 93%, positive predictive value (PPV) of 76%, and negative predictive value (NPV) of 67% 18. Furthermore, Lanng et al., in a study of 301 consecutive patients with breast cancer undergoing either axillary dissection or sentinel node procedure, demonstrated that PE of axillary lymph nodes as a criterion for offering the sentinel node procedure is of little value 19.
Mammography and Tomosynthesis
MG is the standard imaging modality for screening for breast disease. On the mammogram, the normal axilla is seen as an area with almost fatty density, sometimes containing small normal or reactive lymph nodes and accessory breast tissue. The sensitivity, specificity, PPV, NPV, and accuracy for mammography were 66.9%, 80.8%, 41.3%, 92.3%, and 78.4% 20. It is not considered reliable for the evaluation of lymph node involvement in the setting of a recent breast cancer diagnosis because of limited spatial resolution and because parts of the axillary area may not be visualized (Fig. 1) 21. Valente et al. conducted a retrospective study of 244 consecutive patients diagnosed with invasive breast carcinoma and found that MG had the highest false‐negative rate in detecting lymph node involvement in patients with breast cancer 5. However, MG can raise the suspicion of malignancy by identifying enlarged nodes (lymphadenopathy) in specific cases such as lymphoma or carcinoma of unknown primary.
Figure 1.
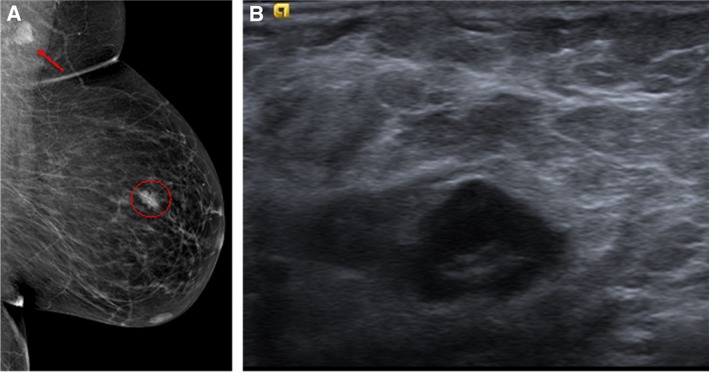
Female patient aged 40 years with an invasive ductal carcinoma in the left breast. (A): Left digital mammography, mediolateral‐oblique projection, demonstrates a high‐density lymph node in axilla and an irregular mass, spiculated, in the upper quadrants. (B): Ultrasonography confirmed the suspicious nature of the mass (Breast Imaging – Reporting and Data System [BI‐RADS] score 5) and a single enlarged lymph node in the axilla suspicious for metastatic involvement.
Digital breast tomosynthesis (DBT) is also of limited value for assessing axillary nodes. The overall haziness of the axillary region on some mediolateral oblique or lateral images is a limitation from synthesizing multiple homogeneous images. The pectoral muscle has homogeneous soft‐tissue contrast without much variation; therefore, the soft‐tissue attenuation will dominate the axillary region. In addition, high‐attenuation materials in the axillary region (i.e., the shoulder) may cause more prominent artifacts compared with those at full‐field digital mammography, which are likely caused by the DBT image acquisition arc, which amplifies high‐attenuation artifacts 22.
Ultrasound
US is the method of choice worldwide to assess lymph node involvement in patients with known or suspected breast cancer 23. In the identification of nodal metastasis, morphologic criteria are more important than size criteria, which have an overall lower accuracy 7. A normal axillary lymph node is characterized by a reniform shape, a maximal cortical thickness of 3 mm without focal bulging, smooth margins, and, depending on size, a discernable central fatty hilum (supplemental online Fig. B) 23. Morphologic characteristics predictive of malignancy are cortical thickness greater than 2.5–3.0 mm, focal cortical lobulation, loss of the fatty hilum, a round shape, and abnormal cortical blood flow (nonhilar flow; Fig. 2) 24, 25. According to the literature, US can be highly specific if morphologic characteristics are used, with a sensitivity ranging from 26% to 76% and a specificity of 88%–98% for depicting nonpalpable metastatic lymph nodes 21. If the diagnosis is based on size criteria only, sensitivity and specificity are 49%–87% and 55%–97%, respectively 21, 26. In another study, Sidibé et al. 27 confirmed that when lymph node size more than 5 mm was taken as a presumptive criterion of invasion, sensibility and specificity of ultrasonography varied from 66.1% to 87.1% and from 44.1% to 97.9%, respectively, whereas when the lymph node morphology was the mean criterion of axilla invasion, these parameters varied respectively from 40.5% to 92.3% and from 55.6% to 95.2%. The main problem we see here is that there is no guide, consensus, or Breast Imaging – Reporting and Data System (BI‐RADS) score for lymph node assessment on US, and this is the imaging method upon which the majority of diagnoses in daily practice relies. We consider changes in morphology, cortical thickness including nonhilar vascularity, and hilar displacement.
Figure 2.
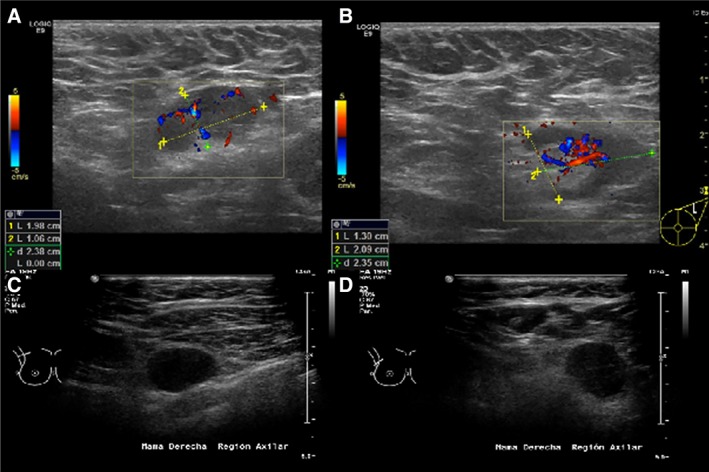
Sonographic morphologic characteristics that are predictors of malignancy. These include cortical thickness greater than 2.5–3.0 mm (A, C, D), focal cortical lobulation (B), loss of the fatty hilum (C, D), a round shape (C, D), and abnormal blood flow (A, B).
US‐guided lymph node sampling, such as fine‐needle aspiration (US‐FNA) and core‐needle biopsy (US‐CNB), is indispensable for confirming the presence of a metastasis in a node suspicious on imaging (supplemental online Fig. C). US‐FNA is quick, well tolerated, and associated with minimal morbidity. US‐FNA has a moderate sensitivity, that is, 25%–87.2% (the sensitivity depends on the experience of both the operator and the pathologist). It has a specificity of 100% 1, 28. Because US‐CNB has been shown to be equally safe as US‐FNA but has a higher sensitivity, reaching 94% in some cases, several institutions have abandoned US‐FNA in favor of US‐CNB 1, 28
Elastography may improve the sensitivity and specificity of axilla sampling in breast cancer 29, 30, 31. Evans et al. 32 demonstrated that using shear wave elastography (SWE), nodal involvement rates ranged from 7% for tumors with a mean stiffness less than 50 kPa to 41% for tumors with a mean stiffness greater than 150 kPa, indicating that the mean stiffness on SWE is an independent predictor of axilla metastasis.
To date, there is no consensus in marking the biopsied node with a clip 33. However, marking nodes with biopsy‐confirmed metastatic disease in patients undergoing neoadjuvant treatment improves the pathologic evaluation for residual nodal disease after chemotherapy 34.
Magnetic Resonance Imaging
MRI has a minor role in the evaluation of axillary lymph nodes. The main reason is that it offers limited ability to obtain a complete visualization of the axilla when dedicated breast coils are used. Furthermore, axillary lymph nodes occasionally can be obscured by a pulsation artifact from the heart, which is worse at levels II and III.
It has been demonstrated that morphologic features on magnetic resonance (MR) images have limited accuracy for the diagnosis of axillary metastases, and as with other modalities, nodal size is not a useful parameter. MRI features suspicious for malignancy include cortical thickening, loss of fatty hilum, round shape, or a long axis to short axis ratio of less than 2 (Fig. 3) 12, 35, 36. The presence of perifocal edema, defined as the area with marked T2 prolongation in the fat surrounding a node, has been shown to have the highest positive predictive value for malignancy (100%) among predefined quantitative and qualitative descriptors 37.
The presence of perifocal edema, defined as the area with marked T2 prolongation in the fat surrounding a node, has been shown to have the highest positive predictive value for malignancy (100%) among predefined quantitative and qualitative descriptors.
Figure 3.
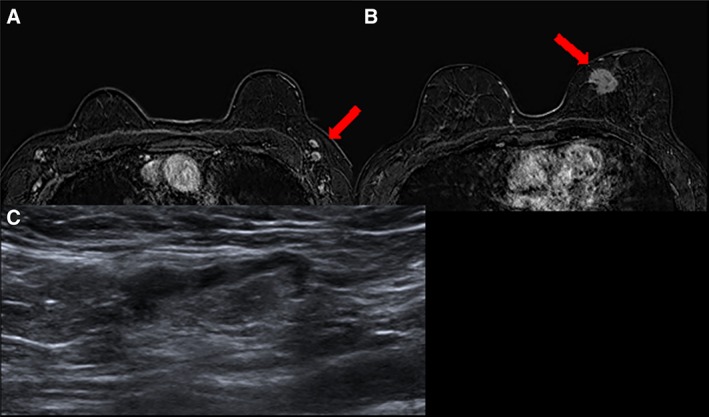
MRI and sonographic characteristics of benign nodes. T1 fat‐saturated magnetic resonance imaging 2 minutes after administration of contrast media (A) in a woman aged 68 years with a suspicious mass in the median‐inner quadrant of the left breast (B, red arrow) showing bilateral enlarged nodes with central fatty hilum. (C): The targeted ultrasound demonstrated normal appearance of one of the nodes. A fine‐needle aspiration was performed with a cytologic diagnosis: polymorphous lymphoid population, negative for malignant cells.
After the injection of contrast medium, nodes enhance rapidly and homogeneously with a characteristic signal intensity that is higher at the periphery of a node than at its center (rim enhancement) 37, 38, 39, 40. However, radiologists cannot simply rely on the evaluation of kinetic curves (signal intensity over time), as a type III washout is not always predictive of metastasis. Dedicated protocols for axillary MRI have shown diagnostic success 41, 42. Nevertheless, although the use of dedicated axillary protocols can increase the sensitivity (84%), specificity (95%), PPV (100%), and NPV (95%) compared with standard MR examinations (sensitivity 82% and specificity 82.6%), they require additional scanning time not feasible in clinical practice 43. Unenhanced T1‐weighted MRI and diffusion‐weighted imaging (DWI) techniques have shown high accuracy (85% and 80%, respectively) for the detection of nodal metastases 44. The Dixon‐based fat‐suppressed sequence, achieving a homogeneous and excellent fat suppression, has demonstrated an overall improved image quality, especially in the axilla region, compared with spectral fat suppression techniques 45. Ultra‐small superparamagnetic iron oxide (USPIO)–enhanced T2‐weighted sequences have shown the most promising results, with a sensitivity of 84.7% and a mean specificity of 95% (Fig. 4) 46. These particles are composed of small size particles of an iron oxide crystalline core, coated with a low‐molecular‐weight dextran, that cross the capillary wall into the interstitial space and then drain via lymphatic vessels to lymph nodes, where they are actively taken up by the macrophages in benign nodes. When USPIO accumulates in a benign node, the particles’ superparamagnetic property causes a loss in signal intensity owing to significant shortening of the T2* relaxation time 47. However, although USPIO particles can serve as a contrast agent for lymph node imaging, they are not currently approved in the U.S. After intravenous USPIO administration, normal lymph nodes show an avid uptake of particles, causing a signal void because of the magnetic susceptibility effects of ferromagnetic iron oxide. Thus, metastatic lymph nodes appear brighter than normal nodes on USPIO‐enhanced images. Because of the limited spatial resolution, this technique is invaluable in detection of occult micrometastasis (defined as smaller than 1 mm), but overall its clinical use remains uncertain 46.
Figure 4.
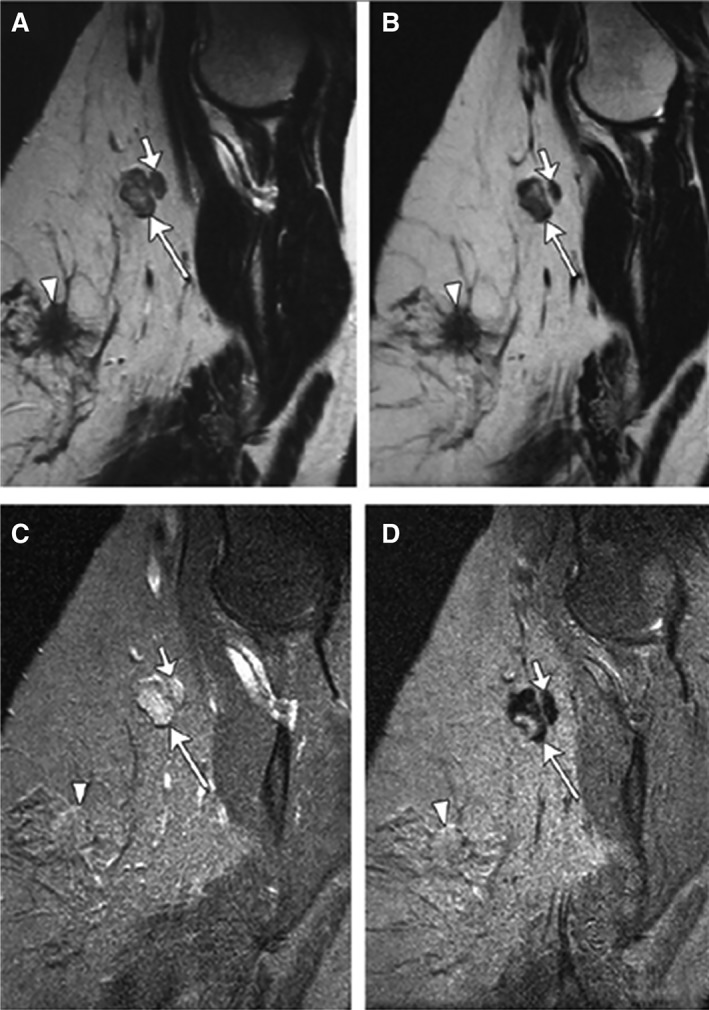
Partial SI decrease in metastatic lymph node in a woman aged 45 years with primary stage pT2N1 tumor. Sagittal nonenhanced (A) and ultra‐small superparamagnetic iron oxide (USPIO)–enhanced (B) T2‐weighted fast spin echo magnetic resonance (MR) images (7,600/120) as well as nonenhanced (C) and USPIO‐enhanced (D) T2*‐weighted fast field echo MR images (683/14) of left axilla show a 1.4 × 0.9 cm metastatic lymph node (large arrow) with partial signal intensity (SI) decrease after USPIO administration. Adjacent nonmetastatic node (small arrow) shows homogeneous SI decrease after USPIO administration. Primary tumor is seen. Reproduced, with permission, from Memarsadeghi et al., Axillary lymph node metastases in patients with breast carcinomas: Assessment with nonenhanced versus USPIO‐enhanced MR imaging. Radiology 2006;241:367–377 46. © 2006 RSNA.
In our experience, during preoperative breast MRI, adding a turbo‐spin echo T2‐weighted sequence in coronal view to the classic protocol 48, 49 aids in assessment of the axilla.
Computed Tomography
Although multidetector CT scans have a limited role in breast cancer staging, Cheung et al. 50 reported sensitivity, specificity, PPV, NPV, and accuracy of 72%, 40%, 85.7%, 22.2%, and 66.7%, respectively, in diagnosing axillary lymph node (ALN) metastases after neoadjuvant chemotherapy. They also suggested that multidetector CT can potentially serve the role of alerting radiologists or clinicians to the possibility of false‐negative nodal micrometastases on postchemotherapy multidetector CT, especially in patients with node‐positive disease on the initial multidetector CT examination 50. In a recent study, Chen et. al 51 evaluated the predictive value of preoperative multidetector‐row computed tomography (MDCT) for ALN metastasis in patients with breast cancer. In 148 cases with preoperative MDCT and ALN surgery, 61 (41.2%) cases had ALN metastasis. The cortical thickness in metastatic ALN was significantly thicker than that in nonmetastatic ALN (7.5 ± 5.0 mm vs. 2.6 ± 2.8 mm; p < .001). Multilogistic regression analysis indicated that cortical thickness of >3 mm (odds ratio [OR], 12.32; 95% confidence interval [CI], 4.50–33.75; p < .001) and nonfatty hilum (OR, 5.38; 95% CI, 1.51–19.19; p = .009) were independent predictors for ALN metastasis. The sensitivity, specificity, and area under the curve (AUC) of MDCT for ALN metastasis prediction based on combined‐variated analysis were 85.3%, 87.4%, and 0.893 (95% CI, 0.832–0.938; p < .001), respectively. Chen et al. concluded that cortical thickness (>3 mm) and nonfatty hilum of MDCT were independent predictors for ALN metastasis. MDCT can be a potent imaging tool for predicting ALN metastasis in breast cancer 51.
In cases when traditional techniques fail in identifying lymph nodes within the axilla, CT guidance for localization in the axilla is an option if the clip is not visible on US or mammogram 52. In the case that the axillary clip cannot be identified on US and the location is very high up in the axilla, a noncontrast CT limited to the axillary region can help to recognize the previously biopsied lymph node. This is especially true in patients undergoing neoadjuvant chemotherapy, in whom the localization of the clip within the node can be difficult even in MRI.
Kim et al. 53 studied breast cancer to assess the reliability of sentinel lymph node biopsy (SLNB) for axillary restaging after NAC to determine the possibility of image‐guided marker‐clip placement in axillary lymph nodes. In 20 patients they placed a marker‐clip at a clinically positive axillary lymph node under US guidance before initiation of NAC. Preoperative localization of marker‐clipped lymph nodes was performed, and the localized lymph nodes were removed by SLNB. Then they compared the postoperative results of the marker‐clipped lymph nodes, sentinel nodes, and axillary lymph nodes. A total of 24 marker clips were inserted, and 23 marker‐clipped lymph nodes were successfully retrieved during surgery (identification rate, 23/24, 95.8%). In the 11 patients with pathologically confirmed metastatic marker‐clipped lymph nodes, four became negative after NAC, and seven maintained metastatic residues on the marker‐clipped lymph nodes. Three of the seven patients had metastatic residues on the axillary lymph nodes, and two of the three patients also had negative sentinel nodes. Marker‐clipped nodes accurately predicted the axillary nodal status in these two patients compared with sentinel nodes alone. Kim et al. concluded that image‐guided marker‐clip placement on positive axillary lymph nodes before NAC and removal with SLNB are technically feasible. This technique can improve the accuracy of the residual disease evaluation on the axilla, especially in patients with negative SLNB results, and can identify candidates for limited axillary surgery after NAC 53.
Positron Emission Tomography/Computerized Tomography
Positron emission tomography (PET) using 18F‐fluorodeoxyglucose (FDG) enables the identification of the increased glucose metabolism that is typical of malignant tumors 54. Low spatial resolution and lack of anatomic details are the main limitations of PET, rendering it an insufficient tool for detecting metastases, with sensitivity from 25% to 84%. On the other hand, PET devices combined with CT, that is, PET/CT scanners, are useful in identifying advanced axillary disease and metastatic nodal spread outside the axilla, especially in the internal mammary chain, with high sensitivity (80%–94%) and specificity (86%–90%) 55. In patients with breast cancer who will undergo neoadjuvant chemotherapy, the use of PET/CT as a staging procedure prevents unnecessary sentinel lymph node biopsies, enables axillary response monitoring during or after neoadjuvant chemotherapy, and guides treatment planning by detecting occult nodal and distant metastases (Fig. 5) 56. Nevertheless, PET/CT is not yet sufficiently sensitive for the detection of primary breast cancer or for the evaluation of axillary lymph nodes in early‐stage breast cancer (stage I and II). PET/CT has a low accuracy for micrometastases, with a drop in sensitivity to 33% 57. Thus, it cannot be used as a standard tool for axillary staging of operable breast cancer.
Figure 5.
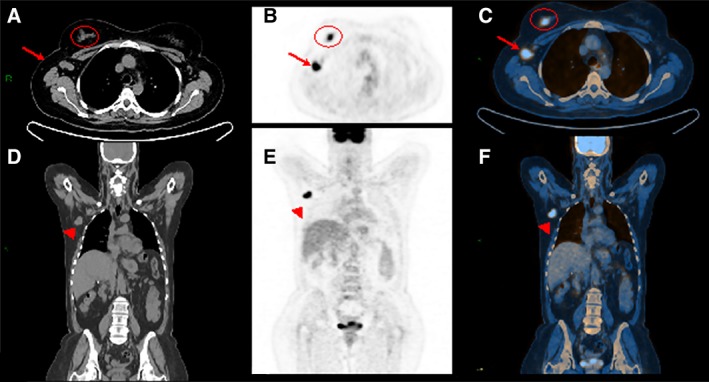
A woman aged 45 years with invasive ductal cancer of the right breast. Computed tomography (CT) of the whole body. Axial (A) and coronal (D) views show enlarged node in the right axilla (A, arrow; C) associated with an irregular mass within the right breast (A, circle). Whole body positron emission tomography (PET) examination. Axial (B) and coronal (E) views demonstrate high fluorodeoxyglucose uptake for both the mass and the enlarged node in the axilla that is also confirmed by hybrid imaging PET/CT (C, F).
Other high‐resolution breast‐specific devices have been introduced as an alternative to MRI, especially when a contraindication is found, such as positron emission mammography (PEM). By enabling the coregistration of mammography and emission FDG images of the breast, PEM is a valid method for local staging of early breast cancer. However, although the data are limited concerning its application, preliminary results have shown low accuracy of this modality for axillary staging 58.
Positron Emission Tomography/Magnetic Resonance Imaging
PET/MRI has been designed to combine the specificity obtained from functional imaging, that is, PET, with the superior sensitivity obtained from MRI to provide relevant information and achieve a higher diagnostic accuracy in a single session 59. Van Nijnatten et al. 60 investigated the value of dedicated axillary 18F‐FDG PET/MRI in comparison with standard imaging modalities for axillary nodal staging in patients with clinical suspicion of lymph node metastasis. Twelve patients underwent axillary US and dedicated axillary hybrid 18F‐FDG PET/MRI. Nine of the 12 patients also underwent whole body PET/CT. The authors measured the maximum standardized uptake values (SUVmax) for the primary breast tumor and the most FDG‐avid axillary lymph node. They found no significant difference in mean SUVmax for the primary tumor and the most FDG‐avid axillary lymph node when comparing dedicated axillary PET/MRI and PET/CT. Dedicated axillary hybrid PET/MRI changed nodal staging from conventional imaging as follows: 40% compared with US, 75% compared with T2‐weighted MRI, 40% compared with contrast‐enhanced MRI, and 22% compared with PET/CT.
For N‐staging, PET/MRI showed similar results of diagnostic accuracy with PET/CT (86% vs. 88%) and tended to be higher than MRI (80%). Another study showed that PET/MRI provided lower sensitivity for primary tumor than MRI (77% vs. 100%) but higher specificity (100% vs. 67%). For N‐staging, the difference between PET/MRI and MRI was not statistically significant 61, 62, 63.
Initial data indicate dedicated axillary 18F‐FDG hybrid PET/MRI of the diagnostic workup has the potential to improve the diagnostic performance of axillary nodal staging in patients with clinically node‐positive breast cancer, but further studies will be needed to corroborate and expand the current evidence.
Single‐Photon Emission Computed Tomography/Computed Tomography
The recent introduction of integrated single‐photon emission computed tomography and computed tomography (SPECT/CT) scanners was mainly due to the need for an imaging method that can offer more precise anatomic localization of sentinel lymph nodes. These systems have yielded broad consensus, especially in the evaluation of sentinel nodes in patients with complex lymphatic drainage, extra‐axillary metastatic spread, or an increased body mass index 64, and provide information on the drainage pattern when conventional imaging is inconclusive (nonvisualization or unclear location of the nodes). By enhancing the topographic orientation, SPECT/CT is thus more valuable than planar lymphoscintigraphy for sentinel lymph node detection; it has shown higher accuracy in the case of enlarged solid lymph nodes or normal‐sized nodes with intense tracer uptake or uptake as high as that in muscles (sensitivity 75%, specificity 90%) (Fig. 6) 65.
By enhancing the topographic orientation, SPECT/CT is thus more valuable than planar lymphoscintigraphy for sentinel lymph node detection; it has shown higher accuracy in the case of enlarged solid lymph nodes or normal‐sized nodes with intense tracer uptake or uptake as high as that in muscles (sensitivity 75%, specificity 90%).
Figure 6.
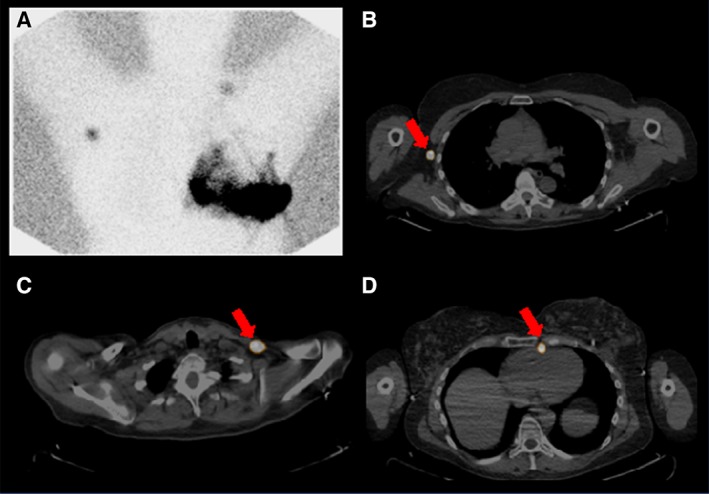
Single‐photon emission computed tomography (SPECT) of the breast. A patient aged 50 years after left lumpectomy with left axillary nodal dissection with recurrent left breast cancer undergoing left breast lymphoscintigraphy. Lymphoscintigraphy shows extensive heterogeneous tracer accumulation throughout the skin of the left breast, including multiple serpiginous trails of activity as well as irregular accumulations (A). Additionally, isolated foci are seen in the right axillary region and lower left neck. SPECT and computed tomography localize these two foci to the right axilla (B) and left supraclavicular region (C). Additionally, a left internal mammary node was visualized (D).
A Perspective of the Future: Radiomics and Radiogenomics Era
Radiomics, a new and rapidly evolving field of research, converts medical images into quantifiable data such as phenotypic characteristics of the entire tumor 66, 67. To date, radiomics research in breast imaging has mainly focused on dynamic contrast‐enhanced magnetic resonance imaging 20, 68, 69, 70, 71 and the assessment of the primary tumor for the differentiation of molecular breast cancer subtypes, correlation with recurrences scores, or correlation with individual gene signatures 72, 73, 74. Recently, radiomics and radiogenomics analyses have also focused on nodal assessment with encouraging results. In a recently published study in 2018, Dong et al. reported the potential of radiomics analysis extrapolated from T2‐weighted fat suppression (T2w‐FS) and DWI for the preoperative prediction of sentinel lymph nodes 75. The authors found AUCs from 0.770 to 0.863 for both the T2w‐FS model and DWI models and concluded that full utilization of breast cancer–specific textural features extracted from anatomical and functional MR images improves the performance of radiomics in predicting sentinel lymph node metastasis, providing a noninvasive approach in clinical practice.
Using a different approach, Han et al. 76 developed a radiomic nomogram for preoperative prediction of axillary lymph node metastasis in patients with breast cancer. Based on a radiomic signature and clinical features, a nomogram was developed that showed excellent predictive ability for lymph node metastasis (AUC 0.84 and 0.87 in training and validation sets, respectively). Another radiomic signature was constructed to distinguish the number of metastatic lymph nodes (fewer than two positive nodes vs. more than two positive nodes), which also showed moderate performance (AUC 0.79). The authors concluded that both nomogram and radiomic signatures can be used as tools to assist clinicians in assessing lymph node metastasis in patients with breast cancer (Fig. 7).
Figure 7.
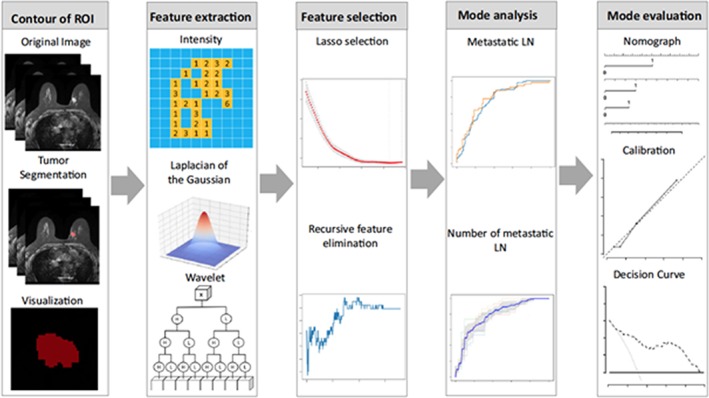
Radiomics workflow. Reprinted by permission from Springer Nature, from Han et al., Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer. European Radiology 2019;7:3820–3829 76. © 2019 European Society of Radiology.
Abbreviations: LN, lymph node; ROI, region of interest.
Multiparametric MRI is not the only method in which the potential of radiomics in predicting node involvement has been tested. Yang et al. 77 developed a mammography‐based radiomics nomogram for the preoperative prediction of axillary lymph node metastasis in 147 patients with breast cancer. The authors extracted radiomics features from each patient's mammography images and incorporated the radiomics signature with the clinicopathologic risk factors into a nomogram. Suo et al. 78 explored the diagnostic value of quantitative radiomics features from elastography and B‐mode for axillary lymph node metastasis in 158 patients with breast cancer. The authors extracted a total of 428 features, consisting of morphologic features from B‐mode and intensity features and gray‐level co‐occurrence matrix features from the dual modalities; they found a sensitivity, specificity, and accuracy of 86.96%, 85.51%, and 86.34%, respectively.
The presented results demonstrate that the fields of radiomics and radiogenomics need further development but that they have already shown to open new frontiers for the assessment of lymph nodes in patients with breast cancer.
Conclusion
US of the axilla is the method of choice for the assessment of metastatic involvement of lymph nodes in all patients with highly suspicious lesions or with a known breast cancer. It must be noted that the accuracy of US is moderate but also that its accuracy can be improved with image‐guided percutaneous sampling. In the case of inconclusive conventional images (nonvisualization or unclear location of the nodes), SPECT/CT may add valuable information and is routinely used for surgical planning. To improve pretreatment assessment of lymph nodes in patients with breast cancer, new avenues such as functional imaging with PET/CT, PET/MRI, the use of specific contrast agents, and radiomics and radiogenomics have been explored with encouraging results. However, further studies will be necessary confirm their clinical value.
Author Contributions
Conception/design: Maria Adele Marino, Daly Avendano, Pedro Zapata, Christopher C. Riedl, Katja Pinker
Provision of study material or patients: Maria Adele Marino, Daly Avendano, Pedro Zapata, Christopher C. Riedl, Katja Pinker
Collection and/or assembly of data: Maria Adele Marino, Daly Avendano, Pedro Zapata, Christopher C. Riedl, Katja Pinker
Data analysis and interpretation: Maria Adele Marino, Daly Avendano, Pedro Zapata, Christopher C. Riedl, Katja Pinker
Manuscript writing: Maria Adele Marino, Daly Avendano, Pedro Zapata, Christopher C. Riedl, Katja Pinker
Final approval of manuscript: Maria Adele Marino, Daly Avendano, Pedro Zapata, Christopher C. Riedl, Katja Pinker
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table
Supplemental Figures
Acknowledgments
The authors acknowledge the support in manuscript writing and editing from Joanne Chin, who performed this work as part of her responsibilities as a full‐time Editor/Grant Writer employed by Memorial Sloan Kettering Cancer Center (no conflicts of interest). This work was supported in part through the U.S. National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Humphrey KL, Saksena MA, Freer PE et al. To do or not to do: Axillary nodal evaluation after ACOSOG Z0011 trial. Radiographics 2014;34:1807–1816. [DOI] [PubMed] [Google Scholar]
- 2. Krag DN, Anderson SJ, Julian TB et al. Technical outcomes of sentinel‐lymph‐node resection and conventional axillary‐lymph‐node dissection in patients with clinically node‐negative breast cancer: Results from the NSABP B‐32 randomised phase III trial. Lancet Oncol 2007;8:881–888. [DOI] [PubMed] [Google Scholar]
- 3. Carlson GW, Wood WC. Management of axillary lymph node metastasis in breast cancer: Making progress. JAMA 2011;305:606–607. [DOI] [PubMed] [Google Scholar]
- 4. Fisher B, Jeong JH, Anderson S et al. Twenty‐five‐year follow‐up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567–575. [DOI] [PubMed] [Google Scholar]
- 5. Valente SA, Levine GM, Silverstein MJ et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol 2012;19:1825–1830. [DOI] [PubMed] [Google Scholar]
- 6. Lyman GH, Giuliano AE, Somerfield MR et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early‐stage breast cancer. J Clin Oncol 2005;23:7703–7720. [DOI] [PubMed] [Google Scholar]
- 7. Farrell TPJ, Adams NC, Stenson M et al. The Z0011 trial: Is this the end of axillary ultrasound in the pre‐operative assessment of breast cancer patients? Eur Radiol 2015;25:2682–2687. [DOI] [PubMed] [Google Scholar]
- 8. Cserni G, Chmielik E, Cserni B et al. The new TNM‐based staging of breast cancer. Virchows Arch 2018;472:697–703. [DOI] [PubMed] [Google Scholar]
- 9. Giuliano AE, Kirgan DM, Guenther JM et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994;220:391–398; discussion 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manca G, Volterrani D, Mazzarri S et al. Sentinel lymph node mapping in breast cancer: A critical reappraisal of the internal mammary chain issue. Q J Nucl Med Mol Imaging 2014;58:114–126. [PubMed] [Google Scholar]
- 11. Berg JW. The significance of axillary node levels in the study of breast carcinoma. Cancer 1955;8:776–778. [DOI] [PubMed] [Google Scholar]
- 12. Ecanow JS, Abe H, Newstead GM et al. Axillary staging of breast cancer: What the radiologist should know. Radiographics 2013;33:1589–1612. [DOI] [PubMed] [Google Scholar]
- 13. Byrd DR, Dunnwald LK, Mankoff DA et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol 2001;8:234–240. [DOI] [PubMed] [Google Scholar]
- 14. Giuliano AE, Connolly JL, Edge SB et al. Breast cancer ‐ major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:290–303. [DOI] [PubMed] [Google Scholar]
- 15. Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th ed. Hoboken, NJ: Wiley‐Blackwell, 2017. [Google Scholar]
- 16. Labelle M, Hynes RO. The initial hours of metastasis: The importance of cooperative host‐tumor cell interactions during hematogenous dissemination. Cancer Discov 2012;2:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrada J, Iyer RB, Atkinson EN et al. Relative value of physical examination, mammography, and breast sonography in evaluating the size of the primary tumor and regional lymph node metastases in women receiving neoadjuvant chemotherapy for locally advanced breast carcinoma. Clin Cancer Res 1997;3:1565–1569. [PubMed] [Google Scholar]
- 18. Majid S, Tengrup I, Manjer J. Clinical assessment of axillary lymph nodes and tumor size in breast cancer compared with histopathological examination: A population‐based analysis of 2,537 women. World J Surg 2013;37:67–71. [DOI] [PubMed] [Google Scholar]
- 19. Lanng C, Hoffmann J, Galatius H et al. Assessment of clinical palpation of the axilla as a criterion for performing the sentinel node procedure in breast cancer. Eur J Surg Oncol 2007;33:281–284. [DOI] [PubMed] [Google Scholar]
- 20. Dong Y, Feng Q, Yang W et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2‐weighted fat‐suppression and diffusion‐weighted MRI. Eur Radiol 2018;28:582–591. [DOI] [PubMed] [Google Scholar]
- 21. Choi HY, Park M, Seo M et al. Preoperative axillary lymph node evaluation in breast cancer: Current issues and literature review. Ultrasound Q 2017;33:6–14. [DOI] [PubMed] [Google Scholar]
- 22. Ratanaprasatporn L, Chikarmane SA, Giess CS. Strengths and weaknesses of synthetic mammography in screening. Radiographics 2017;37:1913–1927. [DOI] [PubMed] [Google Scholar]
- 23. Bedi DG, Krishnamurthy R, Krishnamurthy S et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: In vitro sonographic study. AJR Am J Roentgenol 2008;191:646–652. [DOI] [PubMed] [Google Scholar]
- 24. Cho N, Moon WK, Han W et al. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: Node‐to‐node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol 2009;193:1731–1737. [DOI] [PubMed] [Google Scholar]
- 25. Yang WT, Chang J, Metreweli C. Patients with breast cancer: Differences in color Doppler flow and gray‐scale US features of benign and malignant axillary lymph nodes. Radiology 2000;215:568–573. [DOI] [PubMed] [Google Scholar]
- 26. Alvarez S, Añorbe E, Alcorta P et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review. AJR Am J Roentgenol 2006;186:1342–1348. [DOI] [PubMed] [Google Scholar]
- 27. Sidibé S, Coulibaly A, Traoré S et al. Role of ultrasonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review [in French]. Mali Med 2007;22:9–13. [PubMed] [Google Scholar]
- 28. Rao R, Lilley L, Andrews V et al. Axillary staging by percutaneous biopsy: Sensitivity of fine‐needle aspiration versus core needle biopsy. Ann Surg Oncol 2009;16:1170–1175. [DOI] [PubMed] [Google Scholar]
- 29. Tsai WC, Lin CKJ, Wei HK et al. Sonographic elastography improves the sensitivity and specificity of axilla sampling in breast cancer: A prospective study. Ultrasound Med Biol 2013;39:941–949. [DOI] [PubMed] [Google Scholar]
- 30. Chang W, Jia W, Shi J et al. Role of elastography in axillary examination of patients with breast cancer. J Ultrasound Med 2018;37:699–707. [DOI] [PubMed] [Google Scholar]
- 31. Zhao Q, Sun JW, Zhou H et al. Pre‐operative conventional ultrasound and sonoelastography evaluation for predicting axillary lymph node metastasis in patients with malignant breast lesions. Ultrasound Med Biol 2018;44:2587–2595. [DOI] [PubMed] [Google Scholar]
- 32. Evans A, Rauchhaus P, Whelehan P et al. Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer? Breast Cancer Res Treat 2014;143:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuehn T, Bauerfeind I, Fehm T et al. Sentinel‐lymph‐node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol 2013;14:609–618. [DOI] [PubMed] [Google Scholar]
- 34. Caudle AS, Yang WT, Krishnamurthy S et al. Improved axillary evaluation following neoadjuvant therapy for patients with node‐positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J Clin Oncol 2016;34:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee SC, Jain PA, Jethwa SC et al. Radiologist's role in breast cancer staging: Providing key information for clinicians. Radiographics 2014;34:330–342. [DOI] [PubMed] [Google Scholar]
- 36. Mortellaro VE, Marshall J, Singer L et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging 2009;30:309–312. [DOI] [PubMed] [Google Scholar]
- 37. Baltzer PAT, Dietzel M, Burmeister HP et al. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? Evaluation of an extended protocol in an initial prospective study. AJR Am J Roentgenol 2011;196:W641–W647. [DOI] [PubMed] [Google Scholar]
- 38. Baltzer PAT, Kapetas P, Marino MA et al. New diagnostic tools for breast cancer. Memo 2017;10:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leithner D, Moy L, Morris EA et al. Abbreviated MRI of the breast: Does it provide value? J Magn Reson Imaging 2019;49e85–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marino MA, Helbich T, Baltzer P et al. Multiparametric MRI of the breast: A review. J Magn Reson Imaging 2018;47:301–315. [DOI] [PubMed] [Google Scholar]
- 41. Murray AD, Staff RT , Redpath TW et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: Comparison with pathology of excised nodes. Br J Radiol 2002;75:220–228. [DOI] [PubMed] [Google Scholar]
- 42. Dietzel M, Baltzer PA, Vag T et al. Application of breast MRI for prediction of lymph node metastases ‐ systematic approach using 17 individual descriptors and a dedicated decision tree. Acta Radiol 2010;51:885–894. [DOI] [PubMed] [Google Scholar]
- 43. Kuijs VJL, Moossdorff M, Schipper RJ et al. The role of MRI in axillary lymph node imaging in breast cancer patients: A systematic review. Insights Imaging 2015;6:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scaranelo AM, Eiada R, Jacks LM et al. Accuracy of unenhanced MR imaging in the detection of axillary lymph node metastasis: Study of reproducibility and reliability. Radiology 2012;262:425–434. [DOI] [PubMed] [Google Scholar]
- 45. Clauser P, Pinker K, Helbich TH et al. Fat saturation in dynamic breast MRI at 3 Tesla: Is the Dixon technique superior to spectral fat saturation? A visual grading characteristics study. Eur Radiol 2014;24:2213–2219. [DOI] [PubMed] [Google Scholar]
- 46. Memarsadeghi M, Riedl CC, Kaneider A et al. Axillary lymph node metastases in patients with breast carcinomas: Assessment with nonenhanced versus USPIO‐enhanced MR imaging. Radiology 2006;241:367–377. [DOI] [PubMed] [Google Scholar]
- 47. Narayanan P, Iyngkaran T, Sohaib SA et al. Pearls and pitfalls of MR lymphography in gynecologic malignancy. Radiographics 2009;29:1057–1069; discussion 1069–1071. [DOI] [PubMed] [Google Scholar]
- 48. Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007;244:356–378. [DOI] [PubMed] [Google Scholar]
- 49. Pinker K, Marino MA, Meyer‐Baese A et al. Multiparametric and molecular imaging of breast tumors with MRI and PET/MRI [in German]. Radiologe 2016;56:612–621. [DOI] [PubMed] [Google Scholar]
- 50. Cheung YC, Chen SC, Hsieh IC et al. Multidetector computed tomography assessment on tumor size and nodal status in patients with locally advanced breast cancer before and after neoadjuvant chemotherapy. Eur J Surg Oncol 2006;32:1186–1190. [DOI] [PubMed] [Google Scholar]
- 51. Chen CF, Zhang YL, Cai ZL et al. Predictive value of preoperative multidetector‐row computed tomography for axillary lymph nodes metastasis in patients with breast cancer. Front Oncol 2018;8:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Samreen N, Lee CU, Bhatt AA. Nonconventional options for tumor localization in breast and axillary lymph nodes: A pictorial how‐to. J Clin Imaging Sci 2018;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim EY, Byon WS, Lee KH et al. Feasibility of preoperative axillary lymph node marking with a clip in breast cancer patients before neoadjuvant chemotherapy: A preliminary study. World J Surg 2018;42:582–589. [DOI] [PubMed] [Google Scholar]
- 54. Marino MA, Helbich TH, Blandino A et al. The role of positron emission tomography in breast cancer: A short review. Memo 2015;2:130–135. [Google Scholar]
- 55. Fuster D, Duch J, Paredes P et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol 2008;26:4746–4751. [DOI] [PubMed] [Google Scholar]
- 56. Koolen BB, Valdés Olmos RA, Elkhuizen PHM et al. Locoregional lymph node involvement on 18F‐FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res Treat 2012;135:231–240. [DOI] [PubMed] [Google Scholar]
- 57. Avril N, Rosé CA, Schelling M et al. Breast imaging with positron emission tomography and fluorine‐18 fluorodeoxyglucose: Use and limitations. J Clin Oncol Off J Am Soc Clin Oncol 2000;18:3495–3502. [DOI] [PubMed] [Google Scholar]
- 58. Narayanan D, Madsen KS, Kalinyak JE et al. Interpretation of positron emission mammography: Feature analysis and rates of malignancy. AJR Am J Roentgenol 2011;196:956–970. [DOI] [PubMed] [Google Scholar]
- 59. Taneja S, Jena A, Goel R et al. Simultaneous whole‐body 18F‐FDG PET‐MRI in primary staging of breast cancer: A pilot study. Eur J Radiol 2014;83:2231–2239. [DOI] [PubMed] [Google Scholar]
- 60. van Nijnatten TJA, Goorts B, Vöö S et al. Added value of dedicated axillary hybrid 18F‐FDG PET/MRI for improved axillary nodal staging in clinically node‐positive breast cancer patients: A feasibility study. Eur J Nucl Med Mol Imaging 2018;45:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kwon HW, Becker AK, Goo JM et al. FDG whole‐body PET/MRI in oncology: A systematic review. Nucl Med Mol Imaging 2017;51:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Botsikas D, Kalovidouri A, Becker M et al. Clinical utility of 18F‐FDG‐PET/MR for preoperative breast cancer staging. Eur Radiol 2016;26:2297–2307. [DOI] [PubMed] [Google Scholar]
- 63. Grueneisen J, Nagarajah J, Buchbender C et al. Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast cancer: A comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Invest Radiol 2015;50:505–513. [DOI] [PubMed] [Google Scholar]
- 64. Lerman H, Lievshitz G, Zak O et al. Improved sentinel node identification by SPECT/CT in overweight patients with breast cancer. J Nucl Med 2007;48:201–206. [PubMed] [Google Scholar]
- 65. Novikov SN, Krzhivitskii PI, Kanaev SV et al. Axillary lymph node staging in breast cancer: Clinical value of single photon emission computed tomography‐computed tomography (SPECT‐CT) with 99mTc‐methoxyisobutylisonitrile. Ann Nucl Med 2015;29:177–183. [DOI] [PubMed] [Google Scholar]
- 66. Valdora F, Houssami N, Rossi F et al. Rapid review: Radiomics and breast cancer. Breast Cancer Res Treat 2018;169:217–229. [DOI] [PubMed] [Google Scholar]
- 67. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images are more than pictures, they are data. Radiology 2016;278:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pinker K, Shitano F, Sala E et al. Background, current role, and potential applications of radiogenomics. J Magn Reson Imaging 2018;47:604–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Grimm LJ. Breast MRI radiogenomics: Current status and research implications. J Magn Reson Imaging 2016;43:1269–1278. [DOI] [PubMed] [Google Scholar]
- 70. Saha A, Harowicz MR, Mazurowski MA. Breast cancer MRI radiomics: An overview of algorithmic features and impact of inter‐reader variability in annotating tumors. Med Phys 2018;45:3076–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saha A, Harowicz MR, Wang W et al. A study of association of Oncotype DX recurrence score with DCE‐MRI characteristics using multivariate machine learning models. J Cancer Res Clin Oncol 2018;144:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sutton EJ, Oh JH, Dashevsky BZ et al. Breast cancer subtype intertumor heterogeneity: MRI‐based features predict results of a genomic assay. J Magn Reson Imaging 2015;42:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li H, Zhu Y, Burnside ES et al. Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2016;2:16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li H, Zhu Y, Burnside ES et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology 2016;281:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dong Y, Feng Q, Yang W et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2‐weighted fat‐suppression and diffusion‐weighted MRI. Eur Radiol 2018;28:582–591. [DOI] [PubMed] [Google Scholar]
- 76. Han L, Zhu Y, Liu Z et al. Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer. Eur Radiol 2019;29:3820–3829. [DOI] [PubMed] [Google Scholar]
- 77. Yang J, Wang T, Yang L et al. Preoperative prediction of axillary lymph node metastasis in breast cancer using mammography‐based radiomics method. Sci Rep 2019;9:4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suo J, Zhang Q, Chang W et al. Evaluation of axillary lymph node metastasis by using radiomics of dual‐modal ultrasound composed of elastography and B‐mode [in Chinese]. Zhongguo Yi Liao Qi Xie Za Zhi 2017;41:313–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table
Supplemental Figures


