Short abstract
This letter to the editor is in response to the letter from Brugu's and colleagues regarding the Warner et al. study of sicca syndrome induced by immune checkpoint inhibitor therapy.
We thank Ortiz Brugues et al. for their thoughtful synopsis, supporting data, and careful review of the literature on immune checkpoint inhibitor‐induced sicca (ICIS) 1. In general, we agree with the data that was presented but would like to address several of the critiques.
Although we did not find a correlation between grade and severity, we noted a spectrum of clinical and histologic severity in this heterogenous cohort. This relates in part to the use of a schema originally developed for grading the severity of minor salivary gland inflammation in patients with suspected or established Sjögren's syndrome. Even in Sjögren's syndrome, these have an imperfect correlation with degrees of salivary hypofunction, and this observation has suggested a role for alternative mechanisms of salivary dysfunction. In patients with ICIS, the use of the focus score is particularly problematic, because the glandular inflammation is often diffuse or seemingly minor despite the presence of clear‐cut glandular injury not tallied in routine histologic evaluation.
To illustrate the possible connection between presence of inflammation and response to therapy, we highlight three patients with thymic neoplasms within our cohort 2. Each presented with severe sialadenitis that occurred abruptly 2–3 months after the initiation of immune checkpoint inhibitor (ICI), as judged by the rapid onset of severe xerostomia. Patients 14 and 15 stopped immune checkpoint inhibitor (ICI) therapy and received prednisone and pilocarpine; both had subjective and objective improvement. In contrast, patient 16 chose not to stop ICI therapy and did not receive prednisone; neither subjective nor objective change was observed. Thus, we disagree with Ortiz Brugués et al. that the use of corticosteroids is premature, as there are no other options besides supportive care in ICIS, which we have found to be insufficient in these patients. Our immunohistochemistry studies suggest that the pathogenic inflammation may be targetable using anti‐inflammatory medications, such as prednisone, and in our experience, some patients do experience mild improvement. However, our study was not designed to test the efficacy of prednisone to reverse ICIS and more reflects how our own scalable approach to managing these patients changed.
Ortiz Brugués et al. noted that they “reported one patient with grade 3 xerostomia and no history of pre‐existing autoimmune disease who developed Sjögren's syndrome under ICI therapy according to American College of Rheumatology/European League Against Rheumatism criteria.” This comment raises two points:
The first is regarding ICIS in patients with serum autoantibodies to common markers of Sjögren's syndrome (SS). In our patient cohort, we also had four patients (7, 14, 15, 16) who had anti‐Sjögren's‐syndrome–related antigen A (SSA) antibodies during their evaluation for ICIS; in cases 7 and 16, these antibodies were known to be present before the initiation of ICI therapy 3. ICI therapy may have exacerbated the underlying autoimmune condition in those with known pre‐existing anti‐SSA antibodies. In the other cases, 14 and 15, we cannot say whether ICI therapy induced de novo anti‐SSA antibody formation or merely aggravated a pre‐existing autoimmune condition associated with previously undetected anti‐SSA antibodies.
The second point is regarding severity grading. Grading for “dry mouth” can be performed subjectively or objectively with CTCAE v5.0 criteria. We collected both types of data on every patient. In contrast to Ortiz Brugués et al., none of our patients had subjective grade 3 dry mouth (“xerostomia”). In our experience, dry mouth severity was always more severe when assessed objectively (salivary hypofunction), being found in all 20 of our patients, with 19 having grade 3 severity. It is not clear whether Ortiz Brugués et al. collected data regarding salivary hypofunction. This difference may certainly lead to underreporting and possibly undermanagement. Because practitioners have the choice to determine adverse event severity using either subjective or objective CTCAE criteria, we think that there is a clear opportunity to guide care and follow‐up for these patients by refining these criteria.
The authors also noted “some concern that systemic corticosteroids and/or discontinuation of immunotherapy may represent the first‐line therapy for grade 2–3 SS as suggested by Warner et al.” 1.
With regard to corticosteroids, we share the concern that corticosteroid therapy for immune‐related adverse events (irAE) could influence patient outcomes and support revision of management guidelines as new data emerge. Published data suggest that corticosteroid therapy, prior to initiation of ICI therapy and in doses equivalent to prednisone 10 mg/day or greater, may reduce progression‐free survival and overall survival (OS) 4. However, Ricciuti et al. controlled for those patients receiving steroids for cancer‐related (palliative) or cancer‐unrelated reasons 5. Patients with poorer prognoses at baseline more commonly received corticosteroids for cancer‐related indications (fatigue, dyspnea, etc.) and in fact exhibited worse outcomes in contrast to those patients who received corticosteroids for cancer unrelated reasons. The latter group of patients do approximately as well as those not receiving corticosteroids. However, Fucà et al. reported that early use of corticosteroids (days 1–30 post‐ICI) in metastatic non‐small cell lung cancer reduced OS when controlling for clinical covariates 6.
We disagree with Ortiz Brugués et al. that a graded approach to corticosteroid use in grade 2 or grade 3 ICIS is premature. Most patients in our cohort had grade 3 salivary hypofunction; pathogenic inflammation was found in at least one‐third of patients, and histopathologic changes suggestive of glandular injury were found in almost all patients. Clearly, well‐controlled prospective studies are necessary to assess the impact of steroid therapy both on recovery of glandular function and overall cancer survival. We anticipate that steroid sparring anti‐inflammatory medications (e.g., infliximab, tofacitinib) may also be attempted in patients with ICIS in the future.
During follow‐up, we observed subjective and objective improvements using our management algorithm in most patients. However, patients may accommodate to salivary hypofunction through lifestyle changes; this has been our experience with patients with Sjögren's syndrome. Thus, the slight‐to‐moderate improvements in some of our patients with ICIS may reflect accommodation rather than actual increases in salivary output. Indeed, the objective improvement we witnessed was not universal in those patients who reported improvement and who had follow‐up visits in our clinic (Fig. 1). Despite many patients reporting improvement, all remained objectively CTCAE v5.0 grade 3. Anecdotally, several of our patients who experienced a clinical response are >2 years out from ICI therapy cessation but remain severely dry, and their quality of life remains very low (see patient 4 2).
Figure 1.
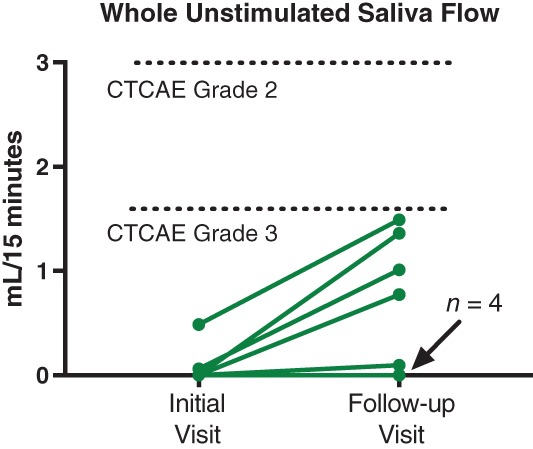
Some patients with immune checkpoint‐induced sicca experienced objective improvements in saliva flow (4/9) using a graded approach as described in Warner et al. 2. However, even with modest objective improvement in saliva flow, all patients remained CTCAE grade 3 using objective criteria for “dry mouth.”
Our management algorithm is modeled after those recommended for other irAEs, as detailed by the American Society of Clinical Oncology 7. We aimed to provide clinical context to aid practitioners who manage these patients. Our algorithm specifically suggests multidisciplinary evaluation and referral, including oral medicine, oral pathology, and oral oncology specialists and rheumatology. It is our opinion that this is in the best interest of the patients.
In summary, we believe that this is an evolving field that requires investigations into the mechanism of ICIS and prospective studies to evaluate the persistence of salivary and lacrimal exocrinopathy in these patients.
References
- 1. Ortiz Brugués A, Sibaud V, Herbault‐Barrés B et al. Sicca syndrome induced by immune checkpoint inhibitor therapy: Optimal management still pending. The Oncologist 2020;25:e391–e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warner BM, Baer AN, Lipson EJ et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. The Oncologist 2019;24:1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burbelo PD, Ferré EMN, Chaturvedi A et al. Profiling autoantibodies against salivary proteins in sicca conditions. J Dent Res 2019. Jul;98:772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arbour KC, Mezquita L, Long N et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small‐cell lung cancer. J Clin Oncol 2018;36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 5. Ricciuti B, Dahlberg SE, Adeni A et al. Immune checkpoint inhibitor outcomes for patients with non‐small‐cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 2019;37:1927–1934. [DOI] [PubMed] [Google Scholar]
- 6. Fucà G, Galli G, Poggi M et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non‐small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019;4:e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer JR, Lacchetti C, Thompson JA. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical practice guideline summary. J Oncol Pract 2018;14:247–249. [DOI] [PubMed] [Google Scholar]


