Short abstract
Commenting on a recently published article on sicca syndrome linked to immune checkpoint inhibitor therapy, this letter to the editor shares another viewpoint on the management of this immune‐related adverse event.
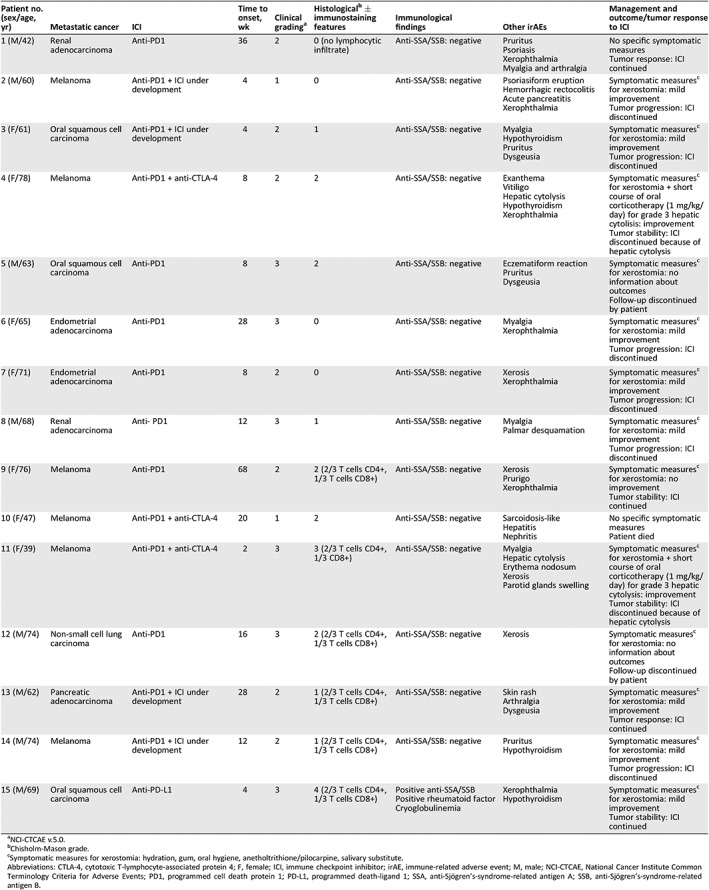
We read with great interest the recent work by Warner et al. 1, who reported 20 patients developing sicca syndrome (SS) associated with immune checkpoint inhibitor (ICI) therapy. They characterized clinicopathologic features of SS in patients treated with ICIs. We also evaluated 15 patients with metastatic cancer who developed SS under ICIs (anti‐programmed cell death protein 1/programmed death‐ligand 1, anti‐cytotoxic T‐lymphocyte‐associated protein 4 or ICI under development, either in monotherapy or in combination; Table 1). Accessory salivary gland biopsy was performed in all cases (Fig. 1), suggesting an absence of correlation between SS clinical grade induced by ICIs and histologic classification. Like Warner et al., our ancillary immunostaining analysis suggested that ICI‐related SS is mediated by an immunologic mechanism through the triggering of cytotoxic CD4+/CD8+ T‐cell activation (Fig. 2A–2C).
Table 1.
Clinical, histological, and immunostaining features of sicca syndrome in patients treated with ICIs
| Patient no. (sex/age, yr) | Metastatic cancer | ICI | Time to onset, wk | Clinical gradinga | Histologicalb ± immunostaining features | Immunological findings | Other irAEs | Management and outcome/tumor response to ICI |
|---|---|---|---|---|---|---|---|---|
| 1 (M/42) | Renal adenocarcinoma | Anti‐PD1 | 36 | 2 | 0 (no lymphocytic infiltrate) | Anti‐SSA/SSB: negative |
|
|
| 2 (M/60) | Melanoma | Anti‐PD1 + ICI under development | 4 | 1 | 0 | Anti‐SSA/SSB: negative |
|
|
| 3 (F/61) | Oral squamous cell carcinoma | Anti‐PD1 + ICI under development | 4 | 2 | 1 | Anti‐SSA/SSB: negative |
|
|
| 4 (F/78) | Melanoma | Anti‐PD1 + anti‐CTLA‐4 | 8 | 2 | 2 | Anti‐SSA/SSB: negative |
|
|
| 5 (M/63) | Oral squamous cell carcinoma | Anti‐PD1 | 8 | 3 | 2 | Anti‐SSA/SSB: negative |
|
|
| 6 (F/65) | Endometrial adenocarcinoma | Anti‐PD1 | 28 | 3 | 0 | Anti‐SSA/SSB: negative |
|
|
| 7 (F/71) | Endometrial adenocarcinoma | Anti‐PD1 | 8 | 2 | 0 | Anti‐SSA/SSB: negative |
|
|
| 8 (M/68) | Renal adenocarcinoma | Anti‐ PD1 | 12 | 3 | 1 | Anti‐SSA/SSB: negative |
|
|
| 9 (F/76) | Melanoma | Anti‐PD1 | 68 | 2 | 2 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 10 (F/47) | Melanoma | Anti‐PD1 + anti‐CTLA‐4 | 20 | 1 | 2 | Anti‐SSA/SSB: negative |
|
|
| 11 (F/39) | Melanoma | Anti‐PD1 + anti‐CTLA‐4 | 2 | 3 | 3 (2/3 T cells CD4+, 1/3 CD8+) | Anti‐SSA/SSB: negative |
|
|
| 12 (M/74) | Non‐small cell lung carcinoma | Anti‐PD1 | 16 | 3 | 2 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 13 (M/62) | Pancreatic adenocarcinoma | Anti‐PD1 + ICI under development | 28 | 2 | 1 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 14 (M/74) | Melanoma | Anti‐PD1 + ICI under development | 12 | 2 | 1 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 15 (M/69) | Oral squamous cell carcinoma | Anti‐PD‐L1 | 4 | 3 | 4 (2/3 T cells CD4+, 1/3 T cells CD8+) |
Positive anti‐SSA/SSB Positive rheumatoid factor Cryoglobulinemia |
|
|
NCI‐CTCAE v.5.0.
Chisholm‐Mason grade.
Symptomatic measures for xerostomia: hydration, gum, oral hygiene, anetholtrithione/pilocarpine, salivary substitute.
Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte‐associated protein 4; F, female; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; M, male; NCI‐CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; PD1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; SSA, anti‐Sjögren's‐syndrome‐related antigen A; SSB, anti‐Sjögren's‐syndrome‐related antigen B.
Figure 1.
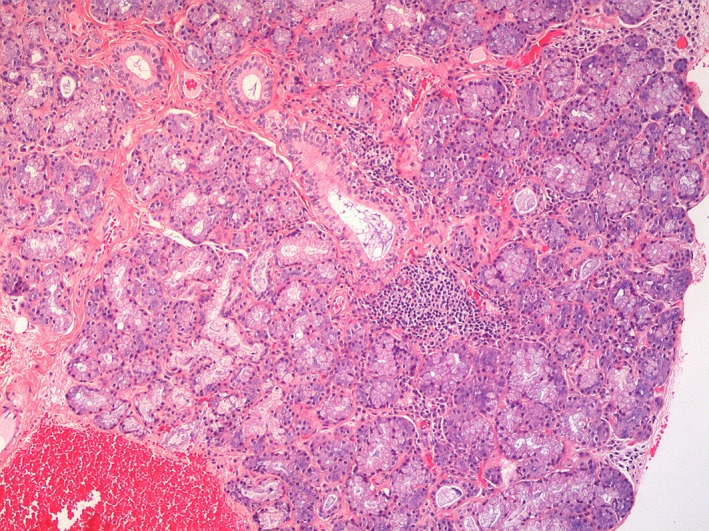
Lymphohistiocytic infiltrate surrounding salivary glands (hematoxylin and eosin staining, original magnification ×10).
Figure 2.
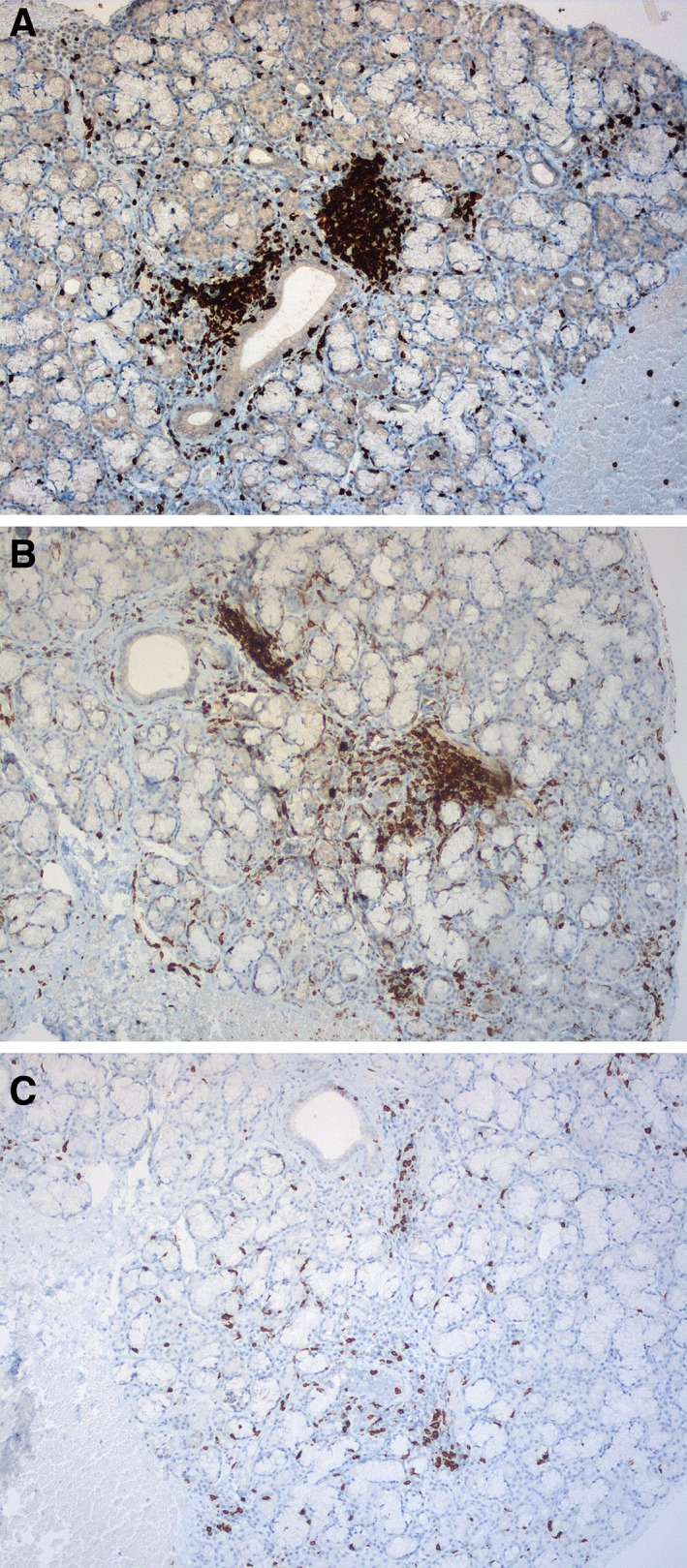
Immunostaining showing CD3‐positive T‐cell infiltrate (original magnification ×10) (A), CD4‐positive T‐cell infiltrate (original magnification ×10) (B) and CD8‐positive T‐cell infiltrate (original magnification ×10) (C).
However, we reported one patient with grade 3 xerostomia (Fig. 3) and no history of pre‐existing autoimmune disease who developed Sjögren's syndrome under ICI therapy according to American College of Rheumatology/European League Against Rheumatism criteria 2.
Figure 3.
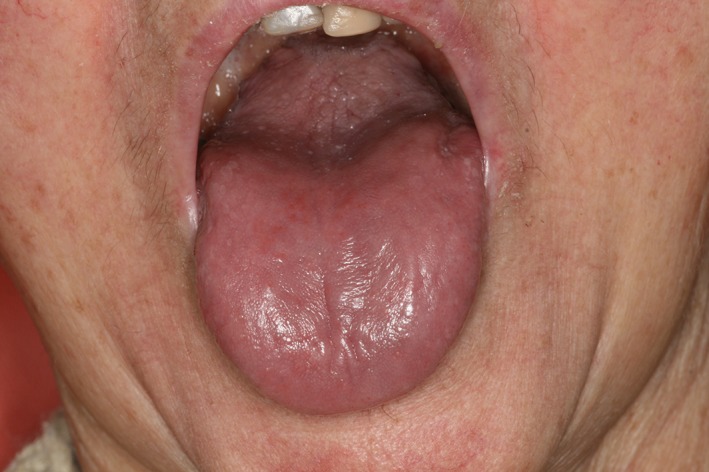
Grade 3 xerostomia induced by anti‐programmed death‐ligand 1.
We have some concern that systemic corticosteroids and/or discontinuation of immunotherapy may represent the first‐line therapy for grade 2–3 SS, as suggested by Warner et al. Although most of their patients treated with oral corticosteroids seemed to ameliorate (8/10), objective improvement in whole unstimulated saliva flow remained of mild benefit. Moreover, ICIs were held simultaneously in eight of the improved patients, so the impact of oral corticosteroids alone is difficult to assess. Furthermore, the only patient treated with oral corticosteroids without holding ICI (patient 4) did not ameliorate. Also, SS improved without oral corticosteroids in two patients (patients 17 and 18) in whom ICIs were stopped as a result of completed protocol. We observed a slight to moderate progressive improvement after discontinuation of ICIs in eight of our patients (because of either tumor progression or other severe immune‐related adverse events [irAEs]). Two of our patients were significantly relieved by a short course of prednisolone (1 mg/kg/day) prescribed for grade 3 transaminitis.
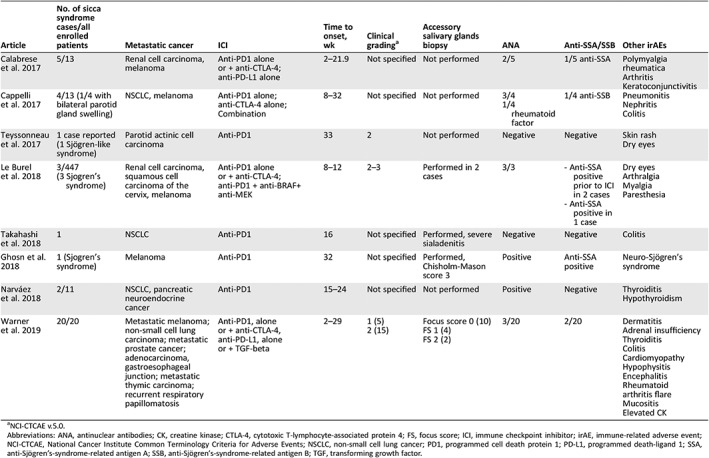
As SS is immune related, we can speculate that oral corticosteroids may benefit patients with ICI‐induced SS. However, proposing oral corticosteroids for grade 2–3 SS seems premature (Table 2) 6, 7, 8, 9, 10. In our series, basic oral care 5 without oral corticosteroids led to a mild improvement in almost half of our patients.
Table 2.
| Article | No. of sicca syndrome cases/all enrolled patients | Metastatic cancer | ICI | Time to onset, wk | Clinical gradinga | Accessory salivary glands biopsy | ANA | Anti‐SSA/SSB | Other irAEs |
|---|---|---|---|---|---|---|---|---|---|
| Calabrese et al. 2017 |
|
Renal cell carcinoma, melanoma | Anti‐PD1 alone or + anti‐CTLA‐4; anti‐PD‐L1 alone | 2–21.9 | Not specified | Not performed | 2/5 | 1/5 anti‐SSA |
|
| Cappelli et al. 2017 |
|
NSCLC, melanoma | Anti‐PD1 alone; anti‐CTLA‐4 alone; Combination | 8–32 | Not specified | Not performed |
3/4 1/4 rheumatoid factor |
1/4 anti‐SSB |
|
| Teyssonneau et al. 2017 |
|
Parotid actinic cell carcinoma | Anti‐PD1 | 33 | 2 | Not performed | Negative | Negative |
|
| Le Burel et al. 2018 |
(3 Sjogren's syndrome) |
Renal cell carcinoma, squamous cell carcinoma of the cervix, melanoma | Anti‐PD1 alone or + anti‐CTLA‐4; anti‐PD1 + anti‐BRAF+ anti‐MEK | 8–12 | 2–3 | Performed in 2 cases | 3/3 |
‐ Anti‐SSA positive prior to ICI in 2 cases ‐ Anti‐SSA positive in 1 case |
|
| Takahashi et al. 2018 |
|
NSCLC | Anti‐PD1 | 16 | Not specified | Performed, severe sialadenitis | Negative | Negative |
|
| Ghosn et al. 2018 |
|
Melanoma | Anti‐PD1 | 32 | Not specified | Performed, Chisholm‐Mason score 3 | Positive | Anti‐SSA positive |
|
| Narváez et al. 2018 |
|
NSCLC, pancreatic neuroendocrine cancer | Anti‐PD1 | 15–24 | Not specified | Not performed | Positive | Negative |
|
| Warner et al. 2019 |
|
Metastatic melanoma; non‐small cell lung carcinoma; metastatic prostate cancer; adenocarcinoma, gastroesophageal junction; metastatic thymic carcinoma; recurrent respiratory papillomatosis | Anti‐PD1, alone or + anti‐CTLA‐4, anti‐PD‐L1, alone or + TGF‐beta | 2–29 |
1 (5) 2 (15) |
Focus score 0 (10) FS 1 (4) FS 2 (2) |
3/20 | 2/20 |
|
aNCI‐CTCAE v.5.0.
Abbreviations: ANA, antinuclear antibodies; CK, creatine kinase; CTLA‐4, cytotoxic T‐lymphocyte‐associated protein 4; FS, focus score; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; NCI‐CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; NSCLC, non‐small cell lung cancer; PD1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; SSA, anti‐Sjögren's‐syndrome‐related antigen A; SSB, anti‐Sjögren's‐syndrome‐related antigen B; TGF, transforming growth factor.
Neither interruption nor discontinuation of ICIs due to oral symptoms was required in any of our cases. Holding ICIs in patients who develop SS, a non‐life‐threatening adverse event, should be a case‐by‐case multidisciplinary issue, because ICIs may potentially impact tumor response and/or overall survival. Prospective randomized studies with objective measures are required to assess the impact of high‐dose oral corticosteroids (0.5–1 mg/kg/day) on the management of this irAE, in order to define the optimal dose and duration of the treatment.
In the meantime, we would propose a new algorithm adapted from the one by Warner et al. and according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0:
GRADE 1: Basic oral care. Maintain ICI therapy and reassess within 2 weeks.
GRADE 2: Reinforce grade 1 management. Discuss introduction of pilocarpine/anetholtrithione. Maintain ICI therapy and reassess within 2 weeks.
INTOLERABLE GRADE 2: Consider grade 3 management.
GRADE 3: Refer to Grade 2 management. Multidisciplinary decision to introduce oral corticosteroids (0.5–1 mg/kg/day) for 4 weeks. Maintain ICI therapy and reassess within 2 to 4 weeks.
PERSISTENT GRADE 3: Multidisciplinary decision to interrupt ICI and reassess within 2 to 4 weeks.
Disclosures
Ariadna Ortiz Brugués: Pierre Fabre (C/A); Vincent Sibaud: Bristol‐Myers Squibb, Pierre Fabre, Novartis, Incyte, Roche (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Warner BM, Baer AN, Lipson EJ et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. The Oncologist 2019;24:1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shiboski CH, Shiboski SC, Seror R et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data‐driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 3. Le Burel S, Champiat S, Routier E et al. Onset of connective tissue disease following anti‐PD1/PD‐L1 cancer immunotherapy. Ann Rheum Dis 2018;77:468–470. [DOI] [PubMed] [Google Scholar]
- 4. Ghosn J, Vicino A, Michielin O et al. A severe case of neuro‐Sjögren's syndrome induced by pembrolizumab. J Immunother Cancer 2018;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vigarios E, Epstein JB, Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer 2017;25:1713–1739. [DOI] [PubMed] [Google Scholar]
- 6. Calabrese C, Kirchner E, Kontzias K et al. Rheumatic immune‐related adverse events of checkpoint therapy for cancer: Case series of a new nosological entity. RMD Open 2017;3:e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cappelli, LC , Gutierrez AK, Baer AN et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teyssonneau D, Cousin S, Italiano A. Gougerot‐Sjogren‐like syndrome under PD‐1 inhibitor treatment. Ann Oncol 2017;28:3108. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi S, Chieko X, Sakai T et al. Nivolumab‐induced sialadenitis. Respirol Case Rep 2018;6:e00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Narváez J, Juarez‐López P, LLuch J et al. Rheumatic immune‐related adverse events in patients on anti‐PD‐1 inhibitors: Fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev 2018;17:1040–1045. [DOI] [PubMed] [Google Scholar]


