Abstract
Patients with cancer can go though many stages in their disease, including diagnosis, recurrence, metastasis, and treatment failure. Cancer stem cells (CSCs) are a subgroup of cells within tumors that may explain the mechanism by which tumors recur and progress. CSCs can both self‐renew and produce progenitor cells of more differentiated cancer cells as well as heterogeneously demonstrate resistance and the abilities to migrate and metastasize. These “stemness” characteristics are often the result of dysregulation of one or more pathways, which can be detected by various biomarkers. Although there has been considerable laboratory research conducted on CSCs, its relevance to the practicing oncologist may seem questionable. We sought to determine the clinical impact of CSCs on patients. A systematic literature search was conducted to identify analyses containing survival information based on the expression of known CSC biomarkers in any cancer. Overall, 234 survival analyses were identified, of which 82% reported that high expression of CSC biomarker(s) resulted in poor overall survival and/or disease‐free survival compared with low or no expression of the biomarker. Elevated stemness biomarker levels were also associated with decreased tumor differentiation, altered TNM stage, and increased metastasis. This analysis would suggest that CSCs have a clinical impact on patients and that practicing oncologists need to start considering incorporating CSC‐targeting therapies into their patients’ treatment regimens.
Implications for Practice
Cancer stem cells (CSCs) may occur at any stage of cancer and are implicated in the occurrence of resistance, recurrence, and metastasis. A systematic literature analysis has shown that the presence of CSCs, identified via the upregulation of stemness pathway biomarkers, results in reduced survival across all cancers studied. Several CSC‐targeting agents are currently approved, and several others are in clinical trials. Future treatment regimens will likely include CSC‐targeting agents to enable the elimination of these holdouts to current therapies.
Keywords: Cancer stem cells, Stemness, Stem cell state, Clinical impact, Survival, Recurrence, Biomarker
Short abstract
Cancer stem cells are a subgroup of cells within tumors that may explain the mechanism by which tumors recur and progress. This article reports on the clinical evidence to support cancer stem cells as a critical phenomenon that needs to be considered in clinical practice.
CSCs and Their Relevance to Practicing Oncologists
Cancer is a journey in which patients may experience multiple stages of diagnoses, from their initial diagnosis through possible recurrence, metastasis, and/or treatment failure (Fig. 1). Clinicians guide their patients throughout this journey regarding treatment options, expectations, and monitoring/testing.
Figure 1.
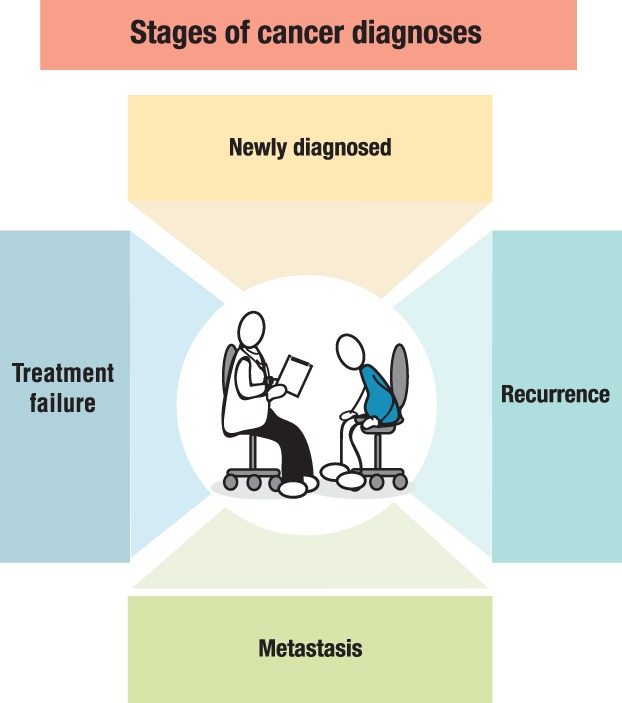
Stages of cancer diagnoses. Patient and oncologist encounter multiple types of diagnoses throughout the patient journey. Cancer stem cells may be present at any of these stages.
The rate of cancer deaths in the U.S. has actually declined 26% since the early 1990s 1, with 5‐year survival rates improving for most cancers 2. In 1975, the 5‐year survival rate for a patient diagnosed with acute myeloid leukemia (AML) was approximately 6%; by 2016, 27% of patients with AML survived 5 years 3. These improvements are due to better screening, earlier detection, and the availability of many new treatments. One critical improvement in the treatment of cancer is our increased understanding of it, in its many forms, allowing some treatments to be tailored specifically to a patient based on their genetics and epigenetic features. Despite these improvements, patients still succumb to cancer. However, this occurs often after the patient has endured one or multiple recurrences and many more rounds and types of treatment, developed treatment resistance, and ultimately experienced treatment failure.
Cancer stem cells (CSCs) may explain these phenomena. CSCs are a small group of cells within a tumor that appear to have tumor‐initiating abilities 4, 5. In addition, CSCs may be the cause of recurrence, they may promote resistance to treatment, and they can contain the mechanisms necessary for metastasis. CSCs appear to be an integral part of cancer and are potentially present in all stages of a patient's journey.
CSCs were first discovered in leukemic and breast cancer cells 6, 7. Since then, thousands of articles have been published discussing the discovery and characteristics of CSCs, their ability to cause cancer in xenografts, their resistance mechanisms, and their ability to support metastasis. Here, we report on the clinical evidence to support CSCs as a critical phenomenon that needs to be considered in clinical practice.
Cancer Stem Cells and Stemness Characteristics
When we think about stem cells, most people think about embryonic stem cells—pluripotent cells capable of indefinite self‐renewal coupled with the potential to differentiate into any cell type. However, there are also adult stem cells, a small number of undifferentiated cells existing in organs that help replenish cells within that organ as a result of normal cell turnover or injury. One example of this would be the stem cells in the crypts of the gastrointestinal tract, that constantly regulate the renewal of the crypt lining as cells migrate toward the mucosa. Self‐renewal is achieved through symmetric cell division creating two identical daughter cells, whereas asymmetric division will result in one daughter cell that preserves the stem cell identity while the other becomes the proliferating progenitor of differentiated cell types. The key factor that phenotypically differentiates CSCs from embryonic or adult stem cells is their unregulated, and primarily symmetric, cell division 8, 9.
Cancer cells within a tumor are heterogeneous. They differ in their morphology, genetics, cell surface markers, proliferation kinetics, tumorigenicity, and response to therapy 4. The original clonal evolution model or stochastic model of tumor growth assumed that every cancer cell contained the same potential to form a new tumor (Fig. 2). The cancer stem cell model of tumor growth asserts that a small subset of tumor cells have enriched tumor‐initiating capabilities 4, 5. CSCs are tumor cells that have acquired the ability to self‐renew and differentiate. This occurs primarily when pathways involved in early development are reactivated in the wrong place at the wrong time. Cancer cells that have acquired these stemness characteristics and have these pathways activated may also confer the ability to migrate, demonstrate resistance to treatment, and be able to initiate primary and secondary tumor growths.
Figure 2.
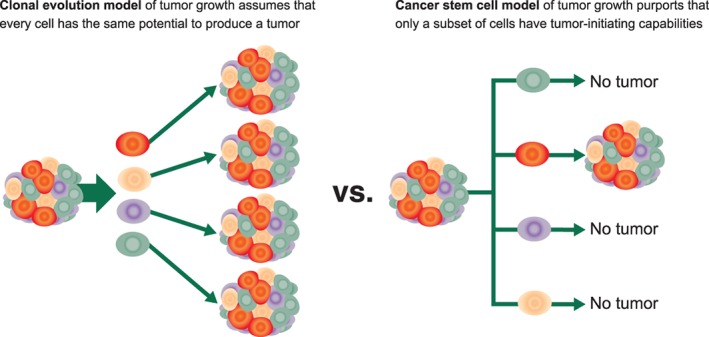
Clonal evolution model and cancer stem cell model of tumor growth (adapted from [4, 5]).
There are six major pathways that have been identified as bestowing stemness characteristics upon cells (Fig. 3) 10, 11, 12, 13, 14, 15, 16. These include the Hedgehog, JAK/STAT, Nanog, Notch, PI3K/Akt, and Wnt/β‐catenin pathways. All of these pathways are involved in conferring the ability of cells to self‐renew into identical daughter cells, thereby demonstrating immortality, as well as to differentiate into various types of cancer cells. Moreover, some of these pathways are also involved in migration, resistance, and tumor initiation and in the epithelial–mesenchymal transitions (EMT) and mesenchymal–epithelial transitions (MET) seen in metastasis 17, 18. The six pathways identified here are by no means an exhaustive list, and other pathways known or yet to be discovered may also contribute to these deleterious outcomes.
Figure 3.
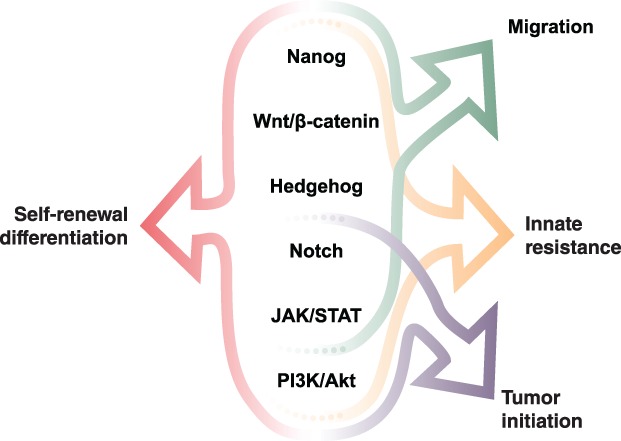
Key pathways identified as resulting in stemness characteristics when dysregulated 10, 11, 12, 13, 14, 15, 16.Abbreviations: JAK, Janus kinase; STAT, signal transducer and activator of transcription.
It should be noted that cancer stemness is highly heterogeneous. Different pathways may be expressed at different times in different tumor types, resulting in distinct stemness characteristics 19, 20, 21. Activation of stemness pathways can be identified in cells expressing distinct CSC markers. Biomarkers for CSCs are commonly identified by flow cytometry in single cell suspensions. However, in solid tumors biopsies or resection samples, CSC biomarkers can be measured using immunohistochemistry or reverse transcription polymerase chain reaction (PCR), while leukemia biomarkers have been primarily identified using reverse‐transcriptase PCR. Many cell surface biomarkers, such as CD44, CD133, and EpCAM, as well as the enzyme ALDH, have been identified in multiple cancer types and are well established as CSC biomarkers 22. Others are less well known, and the list of biomarkers indicating the activation of stemness pathways is continuously growing.
Clinical Impact of CSCs: Theoretical
Dysregulation of stemness pathways can explain many of the complications seen in patients during their cancer journey, including recurrence, resistance, and metastasis (Fig. 4). With the ability to self‐renew and differentiate, each CSC, by definition, is tumor initiating and able to establish a new tumor if the microenvironment niche is suitable.
Figure 4.
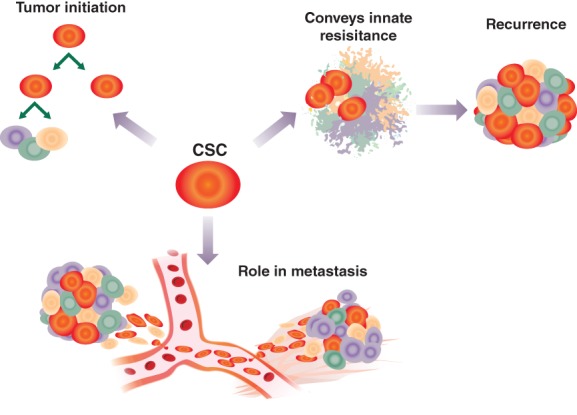
The presence of CSCs can have multiple outcomes for patients. CSCs can maintain themselves through self‐renewal, as well as differentiate. Activated stemness pathways can result in resistance to different therapies, allowing CSCs to re‐establish the tumor. Acquisition of migratory and invasive properties seen in epithelial–mesenchymal transitions coupled with tumor‐initiating capabilities enable tumors to metastasize.Abbreviation: CSC, cancer stem cell.
In the case of resistance, several stemness pathways confer resistance to various chemotherapeutics 8. Mechanisms for the innate resistance seen in CSCs include increased expression of ABC transporters, maintenance of low reactive oxygen species (ROS) levels, overexpression of antiapoptosis regulators to reduce apoptosis, activation of DNA repair systems to combat DNA‐targeting agents, and activation of multiple stemness pathways. CSCs tend to be slow growing when not activated, often in a quiescent stage of the cell cycle; chemotherapeutics typically target rapidly dividing cells and therefore may be ineffective against inactive CSCs. Finally, microenvironmental stimuli can also provide niches that appear favorable for CSC survival and subsequent activation (proliferation) 12, 23. Although conventional therapies can potentially kill off the majority of the bulk tumor cells, innate resistance will result in CSCs surviving and subsequently proliferating, thereby re‐establishing the tumor. This is one possible mechanism as to how tumors recur. Notably, this re‐established tumor will now contain a much higher fraction of resistant CSCs that will be much harder to treat. Radiotherapy can actually enhance stemness characteristics in nonstem cancer cells by inducing stemness pathways to be upregulated 24. This can result in CSCs with higher innate resistance to radiotherapy.
The process of metastasis is complicated with multiple steps. The dynamic stages of EMT and MET overlap with several stemness characteristics. For example, the migratory pathways of Wnt/β‐catenin and Hedgehog have been shown to be upregulated in cells undergoing EMT. Activation of these pathways would reduce cell–cell adhesion, allowing cells to migrate into the vasculature. Once cells lodge in another location, EMT inducers are suppressed. Increased cell–cell adhesion and changes in the microenvironment can enable colonization, while enhanced expression of tumor‐initiating pathways can result in circulating tumor cell cluster formation as well as accelerated growth of a metastatic colony 17, 18, 25.
Clinical Impact of CSCs: Literature Evidence
While many CSC analyses have been conducted in laboratory settings, establishing biomarkers and pathways, they are only relevant to the practicing oncologist if they have a deleterious effect on patients and could become an area of intervention. In order to determine the actual clinical impact of CSCs, a PubMed literature search was conducted using the terms “cancer stem cell” and “clinical.” A total of 1,715 publications were identified up to June 30, 2018. These were reviewed by the authors and included for further analysis if they contained survival information based on the expression of known CSC biomarkers. No unpublished material was included. Overall, 164 publications were identified as including data on overall survival (OS), disease‐free survival (DFS), and/or progression‐free survival (PFS). These publications included 234 survival analyses based on the expression of various biomarkers in different cancers. Of these, 24 were meta‐analyses, and 17 were based on database sources, 14 of which used the The Cancer Genome Atlas (TCGA) database. Overall, 87.6% (205) of the analyses included OS data.
Figure 5 summarizes the published evidence for each cancer type for each CSC biomarker for 225 of these analyses. The number of studies for each biomarker and the total number of subjects included in these studies is included as a guide as to the depth of the evidence reported to date. Where multiple meta‐analyses have been conducted, manuscripts were compared to report only the total number of subjects in the analyses without duplication. The most common cancer types studied were colorectal cancer and breast cancer. The most common biomarkers studied were ALDH1/ALDH1A1, CD44, and CD133.
Figure 5.
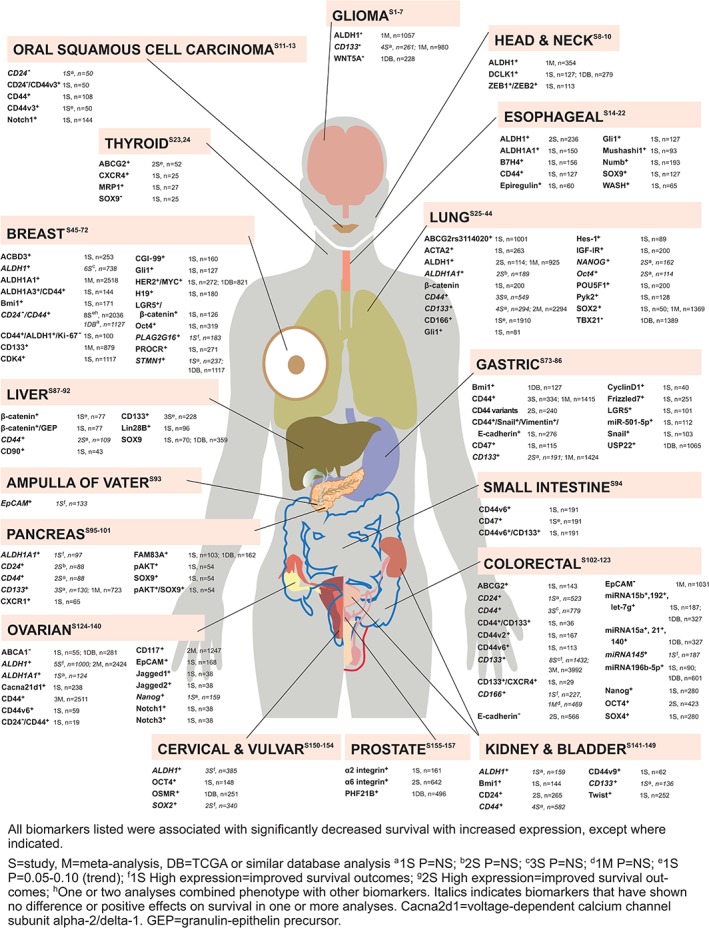
Clinical evidence reporting that stemness biomarkers are associated with poor survival in cancer. Published evidence of increased biomarker expression being associated with decreased overall survival is summarized under each cancer type [S1–S157]. See supplemetary material for analyses details and citations.
Of the 234 analyses, the vast majority (i.e., 192 analyses, 82.1%) reported that high expression of the biomarker(s) resulted in poor OS and/or DFS compared with low or no expression of the biomarker. This included eight studies that showed a trend for poor survival outcomes with high biomarker expression (.05 < p < .10). Elevated CSC markers have been associated with significantly reduced OS in the following cancers: breast, gastric, ovarian, colorectal, liver, glioblastoma, pancreatic, small cell and non‐small cell lung cancer, bladder, prostate, head and neck, oral squamous cell carcinoma, esophageal, thyroid, small intestine, kidney, cervical, and vulvar. In addition, overexpression of single biomarkers have shown poor OS in pediatric rhabdomyosarcoma 26, Ewing sarcoma 26, renal pelvis 27, osteosarcoma 28, 29, and melanoma 30; in acute myeloid leukemia, patients aged <70 years showed poorer OS with increased OCT4 expression in their bone marrow, whereas an analysis of all patients showed no difference in OS (these results not shown in figure) 31.
Based on the studies reported, none of the biomarkers appear to be pan‐cancer. The most commonly investigated biomarker, CD44, was studied in 47 analyses, including 4 meta‐analyses, in 11 different cancers. These included 8 studies on CD44 variants and 15 studies on CD44 combined with another biomarker, all of which showed poor survival outcomes. The specificity of identifying variants and biomarker combination that predict poor survival suggests that research is becoming increasingly targeted. In contrast, 10 studies showed no difference in survival based on CD44 expression; interestingly, these included all 3 lung cancer studies and all 3 colorectal cancer studies indicating that CD44 is not a pan‐CSC biomarker but does appear to be consistently associated with poor survival in breast and gastric cancers. For CD133, 27 analyses and 9 meta‐analyses were performed in nine different tumor types. Whereas eight of these analyses showed no difference in survival based on expression level and one study showed improved survival with high expression, it should be noted that all nine meta‐analyses plus three analyses of CD133 combined expression with another biomarker reported poor survival with elevated CD133 expression. These CD133 meta‐analyses would suggest poor outcomes for patients with breast, lung, gastric, pancreatic, and colorectal cancer. ALDH1/ALDH1A1 was investigated in 26 studies and 6 meta‐analyses in nine cancers, showing robust data to support poor outcomes in glioma, lung (for ALDH1, no difference for ADLH1A1), ovarian, and breast (for ALDH1A1). Whereas 10 studies showed no difference in survival or improved outcomes with high expression, all 6 meta‐analyses reported poor survival with high expression. This demonstrates the importance of meta‐analyses in confirming the prognostic findings from multiple individual studies.
Of note, 30 analyses and 1 meta‐analysis showed no difference in survival outcomes based on high or low biomarker expression, whereas 10 analyses showed an inverse relationship with high biomarker expression conferring positive effects on survival. These are indicated in italics in Figure 5. In several of these cases, different studies reported contradictory findings, where some studies showed poor OS with increased expression whereas others did not. There can be a variety of reasons for this observation. First, the expression level can differ depending on the stage of the cancer. For example, a single study showed high ALDH1 expression in ovarian cancer led to greater survival, whereas four studies and two meta‐analyses found that high ALDH1 resulted in poor survival. In this one outlier, high ALDH1 expression was significantly associated with early international federation of gynecology and obstetrics (FIGO) stage and the mucinous carcinoma histological subtype 32. Meta‐analyses of all ALDH1 in ovarian cancer studies, including Huang et al. 32, indicated that increased ALDH1 expression was associated with advanced FIGO stage and poor OS 33, 34. Second, an increase in a single biomarker does not always designate a cancer cell as a CSC. Ideally, cells identified as CSCs would have at least two or three stemness biomarkers with altered expression. Although most analyses only focused on a single biomarker, 23 reported the effects of coexpression of two to three biomarkers, with many including analyses of multiple biomarkers in the same population. All but one of these showed poor OS.
In many cases, analyses looked at specific aspects of the disease state and/or treatment stage. For example, elevated ALDH1 expression in breast cancer was found to be significantly associated with poor OS and/or DFS in estrogen receptor (ER)‐negative (ER−) tumors 35, after primary treatment 36, and when identified in lymph nodes 37. However, ALDH1 expression levels had no effect on OS in human epidermal growth receptor 2 (HER2)‐positive primary breast cancers 38 or on DFS in ipsilateral breast tumor recurrence 39. In contrast to Woodward 35, a small analysis of 23 primary breast cancer samples found no survival difference for ER− patients based on ALDH1 expression 40. This study also noted that ALDH1 and CD44 did not coexpress 40.
A more consistent predictor for poor outcomes in breast cancer appears to be the combination of CD44+/CD24− biomarkers. This phenotype has been associated with poor OS, DFS, and/or PFS in breast cancer in multiple analyses 36, 38, 41, 42, 43, 44. One large‐scale analysis of 1,127 patient samples showed no association between CD44+/CD24− and survival 45, whereas one report only showed poor outcomes in ER− patients exhibiting this phenotype, with a trend to showing better survival in ER‐positive patients 40. Further combination analyses reveal that CD44+/CD24− plus ALDH1A1, ALDH1A3, integrin α‐6, CD133, EpCAM, and Ki‐67− produces mixed results 44, 45, 46, 47, with different phenotypes being more or less prevalent in different types of tumors or stages.
These findings emphasize the importance of performing robust analyses in the form of meta‐analyses to confirm the prognostic importance of various biomarker phenotypes. However, it also highlights how the individual characteristics and status of patients can alter the outcomes, and these factors would be important diagnostically.
In addition, elevated stemness biomarker expression were typically associated with several other clinicopathological characteristics including decreased tumor differentiation, increased TNM stage, vascular invasion, depth of tumor invasion, lymph node metastasis, and distant metastasis.
It should be noted that the literature search would not have identified studies that did not include the search terms used and consequently were not included in the summary here. Notably, only one AML study appeared, and several meta‐analyses included studies that were not identified individually. This may be partly due to the term “cancer stem cell” not being used in many of the earlier publications. However, the studies we report on here help provide a snapshot of the impact of cancer stem cells (as identified by biomarkers) on survival.
Relevance of CSCs to Clinical Practice
Reported survival data overwhelmingly demonstrated that increased expression of CSC or stemness biomarkers is associated with poor clinical outcomes. In clinical practice, oncologists should be aware of the possible presence of CSCs whenever they see resistance or recurrence, and that although a dramatic reduction in tumor load in the early stages of treatment is encouraging, it does not indicate full eradication of a tumor. More importantly, resistant and recurrent tumor cells are more likely to demonstrate tumor‐initiating capabilities and remain far more challenging to treat.
Currently, there are no standardized protocols or tests applicable in clinical practice for assessing or quantifying the presence of CSC biomarkers in tumors. The analyses presented in this review were all performed as part of research and used nonstandardized research methodology to identify both the biomarker and its level of expression. There is still controversy surrounding the ideal methodology for reliably assessing the presence and expression levels of certain biomarkers, for example, ALDH, and thus far methods have not been standardized, particularly as CSCs are rare cell populations, at the limit of detectability. New clinical trials are starting to incorporate exploratory assays measuring “stemness” characteristics in tumor specimens; however, there is a need for developing Clinical Laboratory Improvement Amendments–standardized tests to accurately detect and robustly quantify CSC biomarker levels within tumor samples, such that reproducibility between laboratories and tumor sets can be ensured. With the advent of single cell technologies rapidly entering the clinical arena, characterization of single cell populations will become increasingly possible.
The discovery of CSC biomarkers has led to investigations into potential new avenues of targeted therapy. Although in the relatively early stages, several CSC‐targeted therapies are currently in development and a few are already approved (Table 1). It is important to note that the exact mechanism of action for each of these agents may not have been fully elucidated when first investigated in oncology trials. Some agents may have appeared to target specific stemness pathways in preclinical screening, but the multiplicity of downstream functions may reveal additional or alternative mechanisms not originally foreseen during clinical development. Moreover, some agents originally developed and tested in other diseases have been identified as having potential CSC‐targeting activity. It is therefore possible that some agents may target both CSCs and nonstem cancer cells. For these reasons, therapies may not be specifically described as CSC‐targeted agents.
Table 1.
CSC‐targeting agents: Approved and in phase II/III development
| CSC‐targeting agent | Mechanism of action//Target pathway | Phase | Company |
|---|---|---|---|
| Amcasertib (BBI‐503) | Serine‐threonine kinase inhibitor//Nanog and other CSC pathways 48 | II | Boston Biomedical Inc., Cambridge, MA |
| ChemoID assay | Diagnostic chemosensitivity assay of CSCs 49, 50 | III | Cordgenics LLC, Huntington, WV |
| Defactinib | FAK inhibitor//Wnt pathway 51, 52 | II | Verastem, Needham, MA |
| Duvelisib (IPI‐145, Copiktra) | Dual PI3K‐δ and PI3K‐γ inhibitor//PI3K/Akt pathway 53, 54 | Approved | Verastem, Needham, MA |
| Glasdegib (PF‐04449913) | SMO‐antagonist//Hedgehog pathway 55 | II/III | Pfizer, NY, NY |
| Napabucasin (BBI‐608) | NQO1‐bioactivator/STAT3//Nanog pathway 56, 57 | III | Boston Biomedical Inc., Cambridge, MA |
| Palbociclib (PD‐0332991, Ibrance) | CDK4/6 inhibitor//CSC self‐renewal 58 | Approved | Pfizer Labs, NY, NY |
| Plerixafor (Mozobil) | CXCR4 antagonist//CSC microenvironment 59 | Approved | Sanofi Genzyme, Cambridge, MA |
| Reparixin (repertaxin) | CXCR1 inhibitor//FAK/AKT/FOXO3A pathway 60 | II | Dompé Farmaceutici S.p.A, Milan, Italy |
| Sonedegib (LDE225, Odomzo) | SMO‐antagonist//Hedgehog pathway 55 | Approved | Sun Pharma Global FZE, Cranbury, NJ |
| Vismodegib (GDC‐0449, Erivedge) | SMO‐antagonist//Hedgehog pathway 55 | Approved | Genentech Inc., San Francisco, CA |
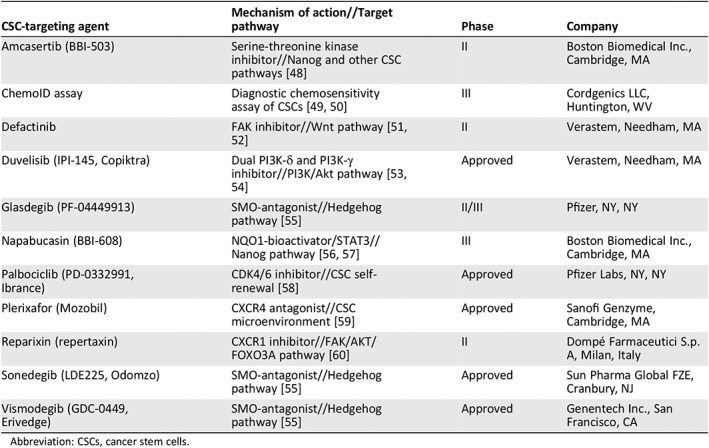
Abbreviation: CSCs, cancer stem cells.
The CSC‐targeted agents listed in Table 1 target several of the main pathways identified as conferring stemness characteristics. Investigative approaches include immunotherapy, targeting the CSC microenvironment, CSC surface biomarker antibodies, targeting CSC aberrant metabolism, and CSC vaccines. The first two approved agents known to target CSC‐related pathways were vismodegib and sonedegib, both of which inhibit Smoothened, thereby inhibiting the Hedgehog pathway, which is upregulated in basal cell carcinoma 55. However, disappointing results of Hedgehog inhibitors in pancreatic ductal adenocarcinoma (PDAC) have led to the halting of further development in metastatic PDAC 48. Duvelisib, also approved, inhibits PI3K‐δ, which is involved in the survival and proliferation of leukemia and lymphoma cells, as well as PI3K‐γ, which contributes to the differentiation and migration of cells 53, 54. Duvelisib is indicated for adult patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma 61. None of these approved agents are promoted as CSC‐targeting agents, but their involvement with CSC pathways would suggest potential efficacy in cells that have the relevant stemness pathway activated.
Palbociclib is the first CDK4/6 inhibitor to be approved for the treatment of cancer, specifically, hormone receptor–positive and HER2‐negative breast cancer 62, and is thought to disrupt the self‐renewal of breast CSCs 58. Plerixafor and reparixin both target CSC biomarkers; reparixin has demonstrated the ability to kill both bulk and CSCs in in vitro and xenograft studies, although its primary development pathway is for the treatment of pancreatic transplant patients 60. Plerixafor is approved for the mobilization of hematopoietic stem cells to the blood stream for collection and subsequent autologous transplantation in patients with non‐Hodgkin lymphoma and multiple myeloma 59.
Napabucasin appears to enhance the production of ROS in cancer cells with downstream effects on STAT3 signaling 48, 56, 57, known to be involved in multiple oncogenic pathways. Amcasertib is a serine‐threonine kinase inhibitor that inhibits Nanog and other stemness pathways 48. Defactinib is described as a FAK inhibitor, whose effect on the tumor microenvironment may enhance antitumor immunity 52, 53.
The ChemoID assay is in phase III trials in recurrent and resistant ovarian cancer and glioblastoma. This assay uses patient biopsies to test the chemosensitivity of individual patient bulk tumor cells and CSCs to currently available therapies to predict tumor response. This assay could potentially determine which therapies are most likely to kill both bulk tumor cells and CSCs in an individual patient 49, 50.
Outcomes from a CSC‐targeted therapy may look different than traditional treatments; for example, bulk tumor cells may not be reduced immediately. To achieve better clinical parameters of measurements, CSC‐targeting strategies will need to be combined with other bulk tumor–removing approaches such as surgery, chemotherapy, and radiotherapy. Ideally, CSC‐directed therapy should be used early in the patient journey, preferably soon after diagnosis when CSC populations are smaller and have had less exposure to treatments that could induce resistant pathways. Use of anti‐CSC therapy could also be used as maintenance therapy to prevent recurrence and prolong survival. Moreover, because CSCs are very much regulated by immune microenvironmental niches, combining the CSC‐ and cancer‐removal therapies with immune activation may provide new hope to eradicate cancer.
These therapies have the potential to prolong life with cancer even further than has already been achieved in the last few decades, reducing the incidence of recurrence and metastasis. Although there will be no “one shot” deal, such therapies will provide important options for patients. With several CSC‐targeting agents already approved, and many more in development, incorporating these agents into the various stages of the patient treatment journey may become the reality of daily treatment in the near future.
Conclusion
The cancer journey can take many routes. CSCs and the dysregulation of stemness pathways appear to be important contributors to cancer resistance, recurrence, and metastasis. The evidence presented here demonstrates that the activation of these stemness pathways results in poor OS outcomes for patients. Clinicians need to be aware of the presence and relevance of CSCs in tumors and stay abreast of new CSC‐targeted agents currently in development.
Author Contributions
Conception/design: Justin Lathia, Huiping Liu
Collection and/or assembly of data: Justin Lathia, Huiping Liu, Daniela Matei
Data analysis and interpretation: Justin Lathia, Huiping Liu, Daniela Matei
Manuscript writing: Justin Lathia, Huiping Liu, Daniela Matei
Final approval of manuscript: Justin Lathia, Huiping Liu, Daniela Matei
Disclosures
Justin Lathia: B*CURED, Silphium (C/A), Cleveland Clinic (IP); Daniela Matei: Genentech/Roche, AstraZeneca, Radius (C/A). Huiping Liu indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table
Supplemental Appendices
Acknowledgments
We thank Dr. Jeremy Rich for his valuable input into the initial concept of this manuscript and assistance in the literature analysis. We also thank Jodie Macoun, Ph.D., for editorial assistance and Mary Jo Roberts‐Braisted for preparation of the illustrations, funded by an unrestricted, educational grant from Boston Biomedical, Inc.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. National Cancer Institute . Cancer Statistics. Available at http://www.cancer.gov/about-cancer/understanding/statistics. Accessed March 26, 2019.
- 2. National Cancer Institute . Cancer Stat Facts: Cancer of any site. Available at https://seer.cancer.gov/statfacts/html/all.html. Accessed March 26, 2019.
- 3. National Cancer Institute . Cancer Statistics. Cancer Stat Facts: Leukemia‐AML. Available at https://seer.cancer.gov/statfacts/html/amyl.html. Accessed November 11, 2018.
- 4. Dick JE. Stem cell concepts renew cancer research. Blood 2008;112:4793–4807. [DOI] [PubMed] [Google Scholar]
- 5. Fanali C, Lucchetti D, Farina M et al. Cancer stem cells in colorectal cancer from pathogenesis to therapy: Controversies and perspectives. World J Gastroenterol 2014;20:923–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lapidot T, Sirard C, Vormoor J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645–648. [DOI] [PubMed] [Google Scholar]
- 7. Al‐Hajj M, Wicha MS, Benito‐Hernandez A et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reya T, Morrison SJ, Clarke MF et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–111. [DOI] [PubMed] [Google Scholar]
- 9. Verga Falzacappa MV, Ronchini C, Reavie LB et al. Regulation of self‐renewal in normal and cancer stem cells. FEBS J 2012;279:3559–3572. [DOI] [PubMed] [Google Scholar]
- 10. Jeter CR, Yang T, Wang J et al. Concise review: NANOG in cancer stem cells and tumor development: An update and outstanding questions. Stem Cells 2015;33:2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan Y, Liu F, Han L et al. HIF‐2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J Exp Clin Cancer Res 2018;37:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim JK, Jeon HY, Kim H. The molecular mechanisms underlying the therapeutic resistance of cancer stem cells. Arch Pharm Res 2015;38:389–401. [DOI] [PubMed] [Google Scholar]
- 13. Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochem Biophys Acta 2013;1830:2481–2495. [DOI] [PubMed] [Google Scholar]
- 14. Kroon P, Berry PA, Stower MJ et al. JAK‐STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem‐like cells. Cancer Res 2013;73:5288–5298. [DOI] [PubMed] [Google Scholar]
- 15. Hernandez‐Vargas H, Ouzounova M, Le Calvez‐Kelm F et al. Methylome analysis reveals Jak‐STAT pathway deregulation in putative breast cancer stem cells. Epigenetics 2011;6:428–439. [DOI] [PubMed] [Google Scholar]
- 16. Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am J Cancer Res 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 17. Yao D, Dai C, Peng S. Mechanism of the mesenchymal–epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res 2011;9:1608–1620. [DOI] [PubMed] [Google Scholar]
- 18. Fabregat I, Malfettone A, Soukupova J. New insights into the crossroads between EMT and stemness in the context of cancer. J Clin Med 2016;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 2013;34:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visvander JE, Lindeman GJ. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell 2012;10:717–728. [DOI] [PubMed] [Google Scholar]
- 21. Keysar SB, Jimeno A. More than markers: Biological significance of cancer stem cell‐defining molecules. Mol Cancer Ther 2010;9:2450–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ajani JA, Song S, Hochster HS et al. Cancer stem cells: The promise and the potential. Semin Oncol 2015;42(suppl 1):S1–S17. [DOI] [PubMed] [Google Scholar]
- 23. Kurtova AV, Xiao J, Mo Q et al. Blocking PGE2‐induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015;517:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghisolfi L, Keates AC, Hu X et al. Ionizing radiation induces stemness in cancer cells. PLoS One 2012;7:e43628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X, Taftaf R, Kawaguchi M et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient‐derived breast cancer models. Cancer Discov 2019;9:96‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zambo I, Hermanova M, Zapletalova D et al. Expression of nestin, CD133 and ABCG2 in relation to the clinical outcome in pediatric sarcomas. Cancer Biomark 2016;17:107–116. [DOI] [PubMed] [Google Scholar]
- 27. Liu J, Jin J, Yang Y et al. Prognostic impact of ALDH1 expression in transitional cell carcinoma of the renal pelvis. Anticancer Res 2015;35:4829–4836. [PubMed] [Google Scholar]
- 28. He A, Qi W, Huang Y et al. CD133 expression predicts lung metastasis and poor prognosis in osteosarcoma patients: A clinical and experimental study. Exp Ther Med 2012;4:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ren Z, Liang S, Yang J et al. Coexpression of CXCR4 and MMP9 predicts lung metastasis and poor prognosis in resected osteosarcoma. Tumour Biol 2016;37:5089–5096. [DOI] [PubMed] [Google Scholar]
- 30. Lukenda A, Dotlic S, Vukojevic N et al. Expression and prognostic value of putative cancer stem cell markers CD117 and CD15 in choroidal and ciliary body melanoma. J Clin Pathol 2016;69:234–239. [DOI] [PubMed] [Google Scholar]
- 31. Yin JY, Tang Q, Zhai LL et al. High expression of OCT4 is frequent and may cause undesirable treatment outcomes in patients with acute myeloid leukemia. Tumour Biol 2015;36:9711–9716. [DOI] [PubMed] [Google Scholar]
- 32. Huang R, Li X, Holm R et al. The expression of aldehyde dehydrogenase 1 (ALDH1) in ovarian carcinomas and its clinicopathological associations: A retrospective study. BMC Cancer 2015;15:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao W, Zang C, Zhang T et al. Clinicopathological characteristics and prognostic value of the cancer stem cell marker ALDH1 in ovarian cancer: A meta‐analysis. Onco Targets Ther 2018;11:1821–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tao Y, Li H, Huang R et al. Clinicopathological and prognostic significance of cancer stem cell markers in ovarian cancer patients: Evidence from 52 studies. Cell Physiol Biochem 2018;46:1716–1726. [DOI] [PubMed] [Google Scholar]
- 35. Woodward WA, Krishnamurthy S, Lodhi A et al. Aldehyde dehydrogenase1 immunohistochemical staining in primary breast cancer cells independently predicted overall survival but did not correlate with the presence of circulating or disseminated tumors cells. J Cancer 2014;5:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee HE, Kim JH, Kim YJ et al. An increase in cancer stem cell population after primary systemic therapy is a poor prognostic factor in breast cancer. Br J Cancer 2011;104:1730–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nogami T, Shien T, Tanaka T et al. Expression of ALDH1 in axillary lymph node metastases is a prognostic factor of poor clinical outcome in breast cancer patients with 1–3 lymph node metastases. Breast Cancer 2014;21:58–65. [DOI] [PubMed] [Google Scholar]
- 38. Seo AN, Lee HJ, Kim EJ et al. Expression of breast cancer stem cell markers as predictors of prognosis and response to trastuzumab in HER2‐positive breast cancer. Br J Cancer 2016;114:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shien T, Tanaka T, Tanabe M et al. Evaluation of ALDH1 expression in ipsilateral breast cancer recurrence. Oncol Lett 2017;13:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horimoto Y, Arakawa A, Sasahara N et al. Combination of cancer stem cell markers CD44 and CD24 is superior to ALDH1 as a prognostic indicator in breast cancer patients with distant metastases. PLoS One 2016;11:e0165253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oliveira RV, Souza VB, Souza PC et al. Detection of putative stem‐cell markers in invasive ductal carcinoma of the breast by immunohistochemistry: Does it improve prognostic/predictive assessments? Appl Immunohistochem Mol Morphol 2018;26:760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H, Wang L, Song Y et al. CD44+/CD24‐ phenotype predicts a poor prognosis in triple‐negative breast cancer. Oncol Lett 2017;14:5890–5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu C, Luo Y, Liu X et al. Clinical implications of CD44+/CD24‐ tumor cell ratio in breast cancer. Cancer Biother Radiopharm 2012;27:324–328. [DOI] [PubMed] [Google Scholar]
- 44. Yang F, Cao L, Sun Z et al. Evaluation of breast cancer stem cells and intratumor stemness heterogeneity in triple‐negative breast cancer as prognostic factors. Int J Biol Sci 2016;12:1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ali HR, Dawson SJ, Blows FM et al. Cancer stem cell markers in breast cancer: Pathological, clinical and prognostic significance. Breast Cancer Res 2011;13:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei H, Fu P, Yao M et al. Breast cancer stem cells phenotype and plasma cell‐predominant breast cancer independently indicate poor survival. Pathol Res Pract 2016;212:294–301. [DOI] [PubMed] [Google Scholar]
- 47. Da Cruz Paula A, Marques O, Sampaio R et al. Characterization of CD44+ALDH1+Ki‐67‐ cells in non‐malignant and neoplastic lesions of the breast. Anticancer Res 2016;36:4629–4638. [DOI] [PubMed] [Google Scholar]
- 48. Sonbol MB, Ahn DH, Bekaii‐Saab T. Therapeutic targeting strategies of cancer stem cells in gastrointestinal malignancies. Biomedicines 2019;7:pii:E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. ChemoID . NIH U.S. National Library of Medicine. Available at https://clinicaltrials.gov/ct2/results?cond=&term=ChemoID. Accessed June 7, 2019.
- 50. Howard CM, Valluri J, Alberico A et al. Analysis of chemopredictive assay for targeting cancer stem cells in glioblastoma patients. Transl Oncol 2017;10:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Serrels A, Lund T, Serrels B et al. FAK controls chemokine transcription, Tregs, and evasion of anti‐tumor immunity. Cell 2015;163:160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jiang H, Hegde S, Knolhoff BL et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016;22:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Flinn IW, O'Brien S, Kahl B et al. Duvelisib, a novel oral dual inhibitor of PI3K‐δ,γ, is clinically active in advanced hematologic malignancies. Blood 2018;131:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lampson BL, Brown JR. PI3Kδ‐selective and PI3Kα/δ‐combinatorial inhibitors in clinical development for B‐cell non‐Hodgkin lymphoma. Expert Opin Investig Drugs 2017;26:1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cortes JE, Gutzmer R, Kieran MW et al. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat Rev 2019;76:41–50. [DOI] [PubMed] [Google Scholar]
- 56. Froeling FEM, Swamynathan MM, Deschênes A et al. Bioactivation of napabucasin triggers reactive oxygen species–mediated cancer cell death; Clin Cancer Res: 2019;25:7162–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chang AY, Hsu E, Patel J et al. Evaluation of tumor cell‐tumor microenvironment component interactions as potential predictors of patient response to napabucasin. Mol Cancer Res 2019;17:1439–1434. [DOI] [PubMed] [Google Scholar]
- 58. Brooks MD, Burness ML, Wicha MS. Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell 2015;17:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mozobil (plerixafor injection) . Prescribing information. Cambridge, MA: Genzyme Corporation, 2017. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022311s001lbl.pdf. Accessed June 14, 2019. [Google Scholar]
- 60. Ginestier C, Liu S, Diebel ME et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 2010;120:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. COPIKTRA (duvelisib). Product information. Needham, MA: Verastem Inc., 2018. Available at http://www.verastem.com/wp-content/uploads/2018/08/prescribing-information.pdf. Accessed June 14, 2019.
- 62. Ibrance (palbociclib) capsules . Product information. New York: Pfizer, 2017. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207103s007lbl.pdf. Accessed June 14, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table
Supplemental Appendices


