Abstract
Nanotechnology-based delivery systems have been considered a promising approach for topical application, considering their characteristics of penetration into/across the skin. The present review aimed to evaluate the recent international scenario of patents concerning the use of nanotechnology-based delivery systems as skin penetration enhancers. A survey of recent patent documents was conducted by using the Espacenet patent database including the terms “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat*” and “nano*” with the truncation symbol (*) in the abstract of documents. A total of 110 patents were published from 2008 to 2018, with 94 technologies being considered. The results demonstrated an increase in innovations concerning nanotechnology-based delivery systems as skin penetration enhancers in recent years. Most patent applicants are from China (60.6%) and Korea (21.3%), and companies (68%) were the most prominent owners. The majority of patent applications (76%) were intended for cosmetic purposes; the types of products and nanostructures were also investigated. Overall results demonstrated the increased interest around the world in patenting products involving skin permeation promotion and nanotechnology for pharmaceutical and, mainly, for cosmetics purposes.
Keywords: Nanotechnology-based delivery systems, patents, penetration enhancers, skin delivery, cosmetic purposes, nanostructures.
1. INTRODUCTION
The skin is the largest organ in the human body. It is an important protective barrier against external agents (chemicals, microorganisms, ultraviolet radiation, and others) and contributes significantly to the human body’s homeostasis. It is composed of three main functional layers: the epidermis (divided into the stratum corneum, stratum granulosum, stratum spinosum, and stratum basale), dermis, and hypodermis. In particular, the structure of the stratum corneum in the epidermis is mainly formed from nonviable corneocytes rich in fatty acids, ceramides, cholesterol, and cholesterol esters, that altogether represent the main mechanism of the skin’s protective barrier function, including the prevention of water loss [1-5].
On the other hand, although the stratum corneum is an important protective barrier in the skin, it also represents the main limitation to compound penetration and drug absorption by this route [1-4]. In the last decade, the search for different strategies to overcome the stratum corneum barrier, in order to improve skin penetration or permeation and achieve dermal or transdermal delivery of drugs or actives for therapeutic/cosmetic purposes, has gained attention [6-13].
Skin penetration enhancement strategies include the use of physical (electroporation, iontophoresis, high-voltage, laser-light pulse sources, sonophoresis, and others) and chemical methods (cyclodextrins, lectin, fatty acids, urea, glycols, surfactants, terpenes, and others). Nevertheless, in many cases, these strategies are correlated with unexpected risks of skin irritation or sensitization [4, 9]. Recently, the use of nanotechnology-based delivery systems for pharmaceutical or cosmetic topical applications has been considered a promising approach, taking into account that the small size (nanometer scale) could facilitate passage through biological barriers such as skin [7, 14-16].
Considering their characteristics of penetration into/across the skin, nanostructures have been proposed as topical vehicles for a variety of therapeutic and cosmetic applications [17-19]. The main interest of using such vehicles is the potential for improvement of the percutaneous absorption of drugs and/or bioactive compounds [20]. The main types of nanostructures used in skin delivery are liposomes [21], nanoemulsions [14], lipid nanocapsules [22], solid lipid nanoparticles [23], and polymeric nanoparticles [4]. Different drugs/actives have been proposed, including anti-inflammatory drugs [24, 25], vitamins [26], antibacterials [27], antifungals [28], antioxidants [29], and sunscreens [30]. Besides the promotion of drug/active absorption, it has been claimed that nanostructures could also protect against drug/ activity instability and could achieve prolonged and/or controlled sustained release, improving the effect in the skin [31].
As can be observed in the literature, many scientific articles have described skin penetration enhancers, including the use of nanotechnology delivery systems, highlighting their importance in the skin delivery field. Thus, numerous researchers have written review articles in order to assess the state of the art of the scientific use of nanotechnology-based delivery systems as skin permeation promoters [6, 14, 20, 21]. However, it is well-known that to evaluate the state of the art of a specific topic, the technologies protected through patents must also be investigated. Patents represent a fundamental tool for determining knowledge production and dissemination between the university and private sectors [32-35]. Regarding the use of nanotechnology in skin penetration enhancers, to the best of our knowledge, no systematic review has been developed exploring this specific field.
In this context, the present study was conducted aiming to evaluate the recent international scenario of patent technologies concerning the use of nanotechnology-based delivery systems as skin penetration enhancers. The importance of this study is mainly justified as the state of the art regarding this field has not been completely explored, and technological innovations are particularly important in terms of the competitiveness of innovative delivery systems, supporting the Research and Development (R&D) of new products and avoiding the uncertainty of investment.
2. DATA SURVEY
As reported by Rother [36], seven steps are recommended for the elaboration of a systematic review, including question formulation, study location, data collection, critical evaluation of studies, data analysis and presentation, data interpretation, and review enhancement. Table 1 shows the methodology of the systematic review employed in the present study.
Table 1. Methodology of systematic review elaboration.
| Steps | Answers |
|---|---|
| Question Formulation (Hypothesis) | How is the scenario of patent technologies involving nanotechnology-based delivery systems as skin penetration enhancers? |
| Study Location | International patents |
| Data Collection | Patent database: Espacenet (EPO) (Accessed on July 25th, 2019) Scientific articles database: Web of Knowledge (Accessed on August 8th, 2019) Strategies of search: 1) Keyword in the title: skin Keywords in abstract: promot* or enhanc* and penetrat* or absorp* or permeat* No time restriction 2) Keyword in the title: skin Keywords in abstract: promot* or enhanc* and penetrat* or absorp* or permeat* and nano* No time restriction 3) Keyword in the title: skin Keywords in abstract: promot* or enhanc* and penetrat* or absorp* or permeat* and nano* Period: 2008 - 2018 |
| Critical Evaluation of Studies | Tabulation of patents of the third search strategy. Accounting by technology and not by patents (avoid repeated counting). Exclusion of patent technologies that do not fit the article purpose. Division of patent technologies by categories (year, country, type of applicants, types of products, types of nanostructure). |
| Data Analysis and Presentation | Graphs and tables elaboration. Discussion of results trying to understand the data obtained, and comparison with scenarios from other sources (scientific papers). |
| Review Enhancement | Search perspective every 5 years. |
3. IMPORTANCE OF PATENTS
In order to explore the existing state of the art of a specific field, both scientific and technology indicators are important aspects to be investigated [34]. Technological innovation is recognized as a significant strategy for development and growth for all categories of companies. Intellectual Property (IP) is an important protection to inventors and industries from their innovations and creations being taken or explored by third parties in an unauthorized manner. One system to guarantee IP rights is patenting product or process [32, 35, 37, 38].
So, while scientific indicators are represented by published articles, technological indicators are commonly represented by patent data analysis [33]. Patents are one of the most significant innovation and inventive parameters attempted to devise the intrinsic value of an original technological invention [32, 37, 38]. Patent documents mainly comprise the application date and bibliographical details, as well as the novelties of the invention and its areas of application [35]. Technological changes have been found to have a critical impact on industry competitiveness, especially causing an impact in the industrial R&D sector [32, 37, 38].
4. OVERVIEW OF PATENTS TECHNOLOGIES
The first survey of patent documents using the international Espacenet (EPO) database included the terms “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat*” with the truncation symbol (*) in the abstract. A total of 3386 patents were found. Further, in order to analyze the patents that correlated to the use of nanotechnology-based delivery systems as skin penetration enhancers, a second analysis was performed crossing these results with the keyword “nano*” (representing all nanotechnologies involved). In this search without time restriction, a total of 142 patents were found. Of these, 77.43% were published between 2008 and 2018 Fig. (1A), totalizing 110 patents. For comparison purposes, a search was also performed in the Web of Knowledge database using the same terms “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat” with the truncation symbol (*) in the abstract, crossing these results with the keyword “nano*”. For the period 2008 to 2018, 439 scientific articles were found, as shown in Fig. (1B).
Fig. (1).
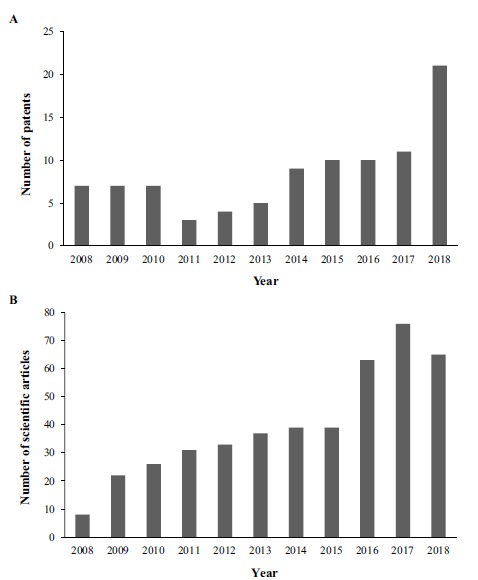
Comparison between (A) annual distribution of patents with keywords “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract, found in the Espacenet (EPO) database between 2008 and 2018 and (B) number of scientific articles with title's keywords “skin” and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract, found in the Web of Knowledge database between 2008 and 2018. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In Fig. (1A), an increase in the number of patents published in recent years can be observed, although, we observed a constancy in the number of patents published in the first three years (2008-2010) and a slight decline in the following years. This low number may also be correlated with the low number of scientific articles published in the same period, as shown by Fig. (1B). From 2013 to 2017, the number of patents shows a slight growth, highlighting a jump in the number of patents filed from 2017 to 2018, doubling from 11 to 21 patents. This growth in the number of patents can also be explained by the significant increase in the number of scientific articles published during the same period, demonstrating the increased interest in researching and patenting products involving skin permeation promotion and nanotechnology [2, 9, 14, 39].
The abstracts from the 110 patents were analyzed, and those that did not represent the subject of interest of this study were excluded. A total of 10 patents were excluded, examples including patents that involve cellulose nanofibers for food or for enhancing the characteristics of construction material; to improve the leather tanning process; a nanocarrier polymer to improve the extraction method of a plant or the use of a nanometer in radiation. Also, patents from the same applicants and with the same contents were grouped and counted as one technology (Table 2). So, from 110 patents found between 2008 and 2018, 94 technologies were considered for the other steps of this study.
Table 2. Patents with the same technologies found during the search in Espacenet with the keywords “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract. Data from patents between 2008 and 2018.
| Name of Technology | Patents Considered in Data Analysis |
Patents not Considered
Because are Repeated from the Same Technology |
Applicant / Country |
|---|---|---|---|
| Nanoliposome using esterified lecithin and method for preparing the same, and composition for preventing or treating skin diseases comprising the same | TWI325325 (B) | US2009263473 (A1); US8685440 (B2) | DAEWOONG CO LTD [KR] |
| Systems and methods for skin rejuvenation | US8568749 (B2) | US2013345307 (A1); US9241887 (B2) US2016101029 (A1); US9782334 (B2) |
SESVALIA USA LLC [US] |
| Multilamellar nanoliposome which contains skin lipid components, and preparation method therefor | KR20160106508 (A) | CN107405282 (A) | AMOREPACIFIC CORP [CN] |
| Approaches for improving skin hydration or moisturization |
WO2017048807 (A1) | US2018256482 (A1) | JRX BIOTECHNOLOGY INC [US] |
| Topical herbal formulation for treatment of acne and skin disorders | CA2746566 (A1) | SG172455 (A1) | SUNEV PHARMA SOLUTION LTD [IN] |
To visualize the most prominent countries involved in technological innovation regarding skin permeation promoters and nanotechnology, the 94 technologies were organized by the country in which patents were developed, as presented in Fig. (2). The results demonstrated that China and Korea were the most prominent countries, with 60.6% and 21.3% of technologies, respectively. Comparing these results with the recent data from World Intellectual Property Indicators (WIPO) concerning the worldwide ranking of patents in various areas, the positions of these countries corroborate the world scenario, with few distinctions, given that the top 10 patent offices with the most filings in 2017 were, in the following order: China, United States of America, Japan, Korea, Germany, France, United Kingdom, Switzerland, Netherlands, and Italy. The data also reveal a very significant increase in the number of filings in China, in first place for patent filings, trademarks, utility models, industrial design, and cultivars, accounting for 43.6% of all patents filed in the world [40].
Fig. (2).
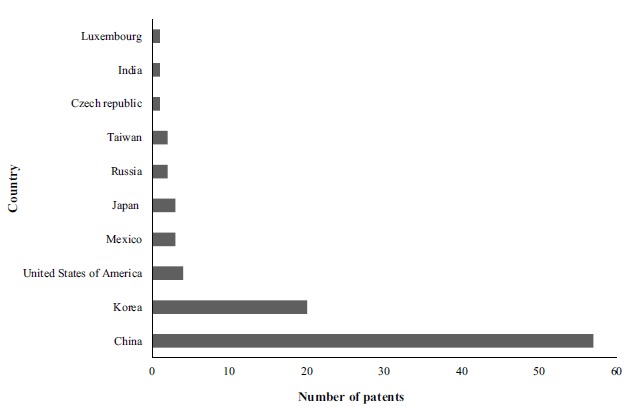
Relationship between the number of technologies by depositing countries with the keywords “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract, found in the Espacenet (EPO) database between 2008 and 2018. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In order to better evaluate the types of patent applicants, the 94 technologies were also grouped according to the type of holder, divided into three categories: company, independent applicant and university. According to the results shown in Fig. (3), the most prominent applicants were companies, representing 68% of patent holdings. Following that were independent applicants (18%) and universities (14%).
Fig. (3).
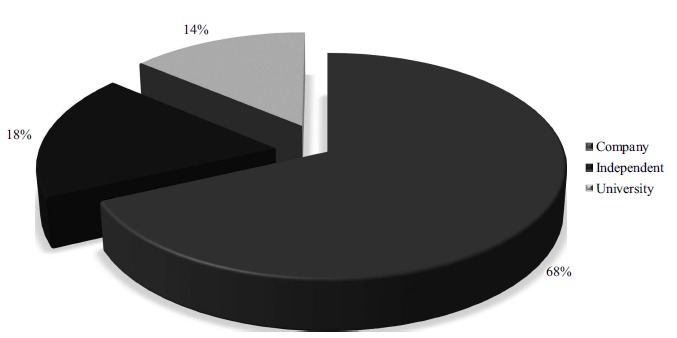
Relationship between the number of technologies and applicants type for patents with keywords “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract, found in the Espacenet (EPO) database between 2008 and 2018. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Considering the company applicants, some companies stand out because they have more than one technology protected by patents. The Chinese Nantong Snakebite Therapy Res Inst® is the holder of patents from eight different technologies, all of them filed in China. The Japanese FujiFilm Corp.® is the holder of patents from three different technologies filed in countries other than Japan, such as the United States of America and in Europe. The Korean Hwajin Biocosmetics Co. Ltd.® is the holder of patents from two technologies, both filed in Korea. The Chinese Beijing Laimiruike Technology Dev. Co. Ltd.® is the holder of patents from two technologies, both filed in China. Other companies that stand out are those that have one technology but filed in more than five patent offices, generating families of patents. Examples are Daewoong Co. Ltd.® from Korea, Euro Celtique SA® from Luxembourg, Sunev Pharma Solution Ltd.® from India, Sesvalia USA LLC® and JRX Biotechnology INC® from the United States of America.
Regarding university applicants, no institute presented more than one patent, and the technologies are not protected in the office of more than one country. Considering the localization of universities, eight are in China, one is in the United States of America, one from the Czech Republic, one in Korea, one in Russia, and one in Taiwan. Regarding independent applicants, only the inventor Wasserteil Raquel Lustbader presents more than one technology, representing three patents in total, all filed in Mexico.
The 94 technologies of the present survey were further organized into two major clusters according to their application: (1) cosmetics and (2) pharmaceutical purposes, presented in Table 3 and Fig. (4).
Table 3. Patents separated by clusters after examination of abstract documents obtained in Espacenet database, between the years of 2008 - 2018 with interested keywords.
| Cluster | Patent Number | ||
|---|---|---|---|
|
Cluster 1 Cosmetic |
TWI325325 (B) JP2008297241 (A) JP2008255020 (A) JP2008247814 (A) KR100912462 (B1) KR100858628 (B1) TW200914063 (A) KR101036961 (B1) CN101474153 (A) KR100958094 (B1) CN101411678 (B) CN101401775 (B) CN101874762 (B) US8568749 (B2) KR20100092922 (A) KR101023041 (B1) CN101664372 (B) CN202060058 (U) CN102091013 (B) CN102784084 (B) CN102579289 (A) CN102552073 (A) KR101193606 (B1) KR101337454 (B1) |
CN103142448 (A) CN103110534 (B) CN102988257 (B) CN102988200 (B) CN104116651 (A) CN103966685 (B) CN103565743 (B) CN103520007 (A) MX2014006945 (A) TWI593426 (B) CN105055186 (A) KR101536996 (B1) CN104644459 (A) CN104606078 (A) CN104398395 (A) KR101702482 (B1) CZ306274 (B6) KR20160106508 (A) CN105755817 (A) MX2014009967 (A) MX2014008660 (A) CN105496886 (B) CN105473200 (A) CN105231999 (B) |
CN107281045 (A) CN206401924 (U) CN106860188 (A) KR101719811 (B1) WO2017048807 (A1) CN106474373 (A) KR20180133237 (A) CN108938546 (A) KR101956553 (B1) CN108524411 (A) CN108379204 (A) KR20180079673 (A) CN108272670 (A) KR20180067901 (A) KR101865841 (B1) KR20180047152 (A) KR101850421 (B1) KR101860555 (B1) CN107898699 (A) CN207044803 (U) CN206999797 (U) CN107595715 (A) CN107582496 (A) |
|
Cluster 2 Pharmaceutical |
CN101200816 (B) CA2723307 (C) CA2746566 (A1) WO2010039310 (A1) CN102090904 (B) RU2554219 (C2) KR101437563 (B1) CN104055720 (A) |
CN103948960 (B) KR101419602 (B1) CN104987485 (A) RU2559938 (C2) CN204092654 (U) CN105853335 (A) CN107412882 (A) CN106691888 (A) |
CN206217260 (U) CN106492278 (A) CN106427100 (A) CN108998856 (A) CN108842222 (A) CN207535410 (U) CN107753747 (A) |
Fig. (4).
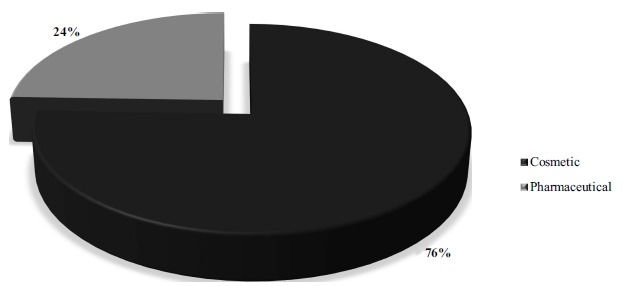
Relationship of the number of technologies with keywords “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract, found in the Espacenet (EPO) database between 2008 and 2018 according to the Clusters 1 (Cosmetic) and Cluster 2 (Pharmaceutical). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Analyzing the use of nanotechnology as skin penetration enhancers, the impact of the cosmetic segment is clear, showing that 76% of patents on technology filed in the last 10 years are for cosmetic use Fig. (4). Cosmetics are legally defined as “any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odors”, according the European Commission [41]. The cosmetic market has been increasingly promising for all segments of the economy as its growth in the last 20 years has been approximately 4.5% by year. This scenario proves its continued and consistent growth, even in unfavorable economic conditions [42-44].
So, to keep up with the evolution of this market, companies, independent applicants, and universities have also increasingly invested in research and innovation in this segment to stay ahead in this highly competitive universe, where more efficient options are expected by the consumer [45, 46]. According to the latest data published by Mintel (2018) in the cosmetics segment, the country leading the global market in product launches per year is the United States of
America (1st place) followed by China (2nd place), which corroborates the results found for the top-ranking countries for nanotechnology patents as skin penetration enhancers [47].
The main purpose of patents in the cosmetic field is to treat recurrent skin problems and employ nanotechnology in order to promote transdermal absorption of the bioactive components, and occasionally to increase their stability. In general, protected technologies have as their target purpose: (a). treatment of acne; (b). treatment of hyperpigmentation of the skin; (c). sunscreen; (d). retarding the process of aging (anti-aging), (e). acting by fighting the production of free radicals (antioxidant); f. enhancing skin hydration or moisturization. These results are in accordance with the scientific literature, in which similar targets in the cosmetic segment have also been published [48-54].
Regarding pharmaceutical applications, the number of published patents is smaller than for the cosmetic segment, representing 24% of the total patent technologies. However, the development of pharmaceutical products using nanotechnology-based delivery systems as skin penetration enhancers and drug absorption skin promoter has been continuous over the years. The study and development of these delivery systems have allowed the release of drugs while providing one or more functionalities, including protecting the drug from the biological environment and, masking toxicity to cells and tissues, as well as, being able to promote prolonged and controlled release in order to accumulate the drug at the target site [55-57].
Analyzing the patents presented in Table 2, all protected technologies for pharmaceutical use are mainly concerned with the manufacture of products employing at least one of the following nanostructures: nanoemulsions, nanoparticles, nano-elements, or liposomes. In general, protected technologies have as their purpose the treatment of immune system dysfunctions that can cause different types of skin lesions such as psoriasis, allergies, eczema, and inflammatory processes of different natures. Another important use of these technologies is related to the treatment of different microbiological infections, presenting antifungal, antibacterial, and antiviral activity. Altogether, the pharmaceutical application technologies are in accordance with the scientific literature that also reports the relevance of nanotechnology in a wide range of skin diseases [18, 24, 28, 31, 58-63].
Other categorizations of the 94 technologies were performed, such as by the kind of products and the nanostructures used in the products. Fig. (5) shows the percentage of technologies classified as a formulation, skin adhesive, equipment, fiber, or artificial skin. Fig. (6) shows the percentage of technologies that use nano-elements, nanoemulsions, liposomes, nanocapsules, protein nanoparticles, carbon nanotubes, and others. It is important to mention that, for some technologies, there is no information about the type of nanostructure used in the abstract, and because of that they were classified as “non-explicited”.
Fig. (5).
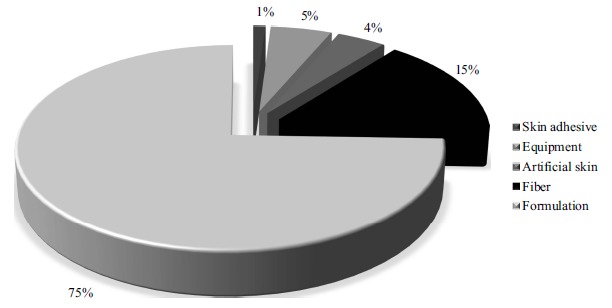
Relationship of the number of technologies with keywords “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract, found in the Espacenet (EPO) database between 2008 and 2018 according to the kind of products. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (6).
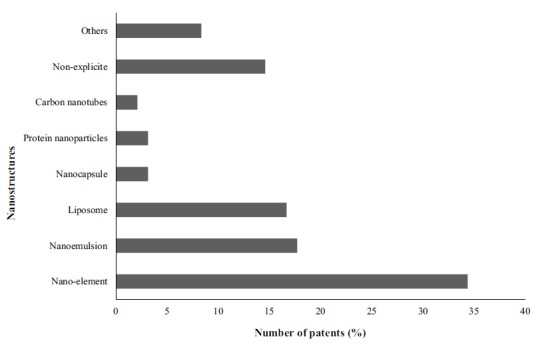
Relationship of the number of technologies with keywords “skin” in the title and “promot* or enhanc* and penetrat* or absorp* or permeat* and nano*” in the abstract, found in the Espacenet (EPO) database between 2008 and 2018 according to the type of nanostructures used in the products. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The only invention classified in the group of “skin adhesive” corresponds to 1% of data, and the purpose of the product is to increase skin adhesiveness through interaction of the product and the skin lipid layer effectively collecting the active ingredient (KR20180133237 (A)). The nanostructure present in the product is nanocapsules with an amphiphilic block copolymer and a bionic surface-active agent and further comprising an additional useful active ingredient.
The patents classified as “equipment” represent 5% of technologies. The inventions are related to a skin massager (KR101036961 (B1)), a device for activating the ionic components of electrolytes in skin (KR101437563 (B1)), a skin laser (RU2559938 (C2)), electrode sensors (CN105231999 (B)), and a vibrating device (CN206401924 (U)). Most of them are involved in the enhancement of drug absorption, in which the substance could be present in the equipment or could be administered after the use of the equipment. Also, one of these technologies reports the use of a device for skin makeup purposes. The main nanostructures employed in equipment are carbon nanotubes and nanoelements.
Technologies involving skin tissue engineering were observed in patents classified as “artificial skin”, corresponding to 4% of technologies, and “fiber”, corresponding to 15% of technologies. Most innovations employed systems to enhance drug absorption, for which antimicrobial agents were the most cited, and to promote better skin lesion regeneration. A great number of these technologies employed nanoelements in the products.
Nanoelements represent the most patented nanostructures from the present survey (34.4%). According to the Commission Recommendation, a “nanomaterial means a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1-100 nm” [64]. Herein, included in this category were the technologies that employed nano-silver, nano-crystalline cellulose, nanometer pearl particles, nano-pearl fibers, nanometer collagen particles, nano-titanium dioxide, nano-selenium, nano-chitin, nano-calamine, and nano-zirconium phosphate, among others. The nanoelement most used in technologies was silver nanoparticles, important in the area of nanomedicine due to the antibacterial capacity of silver [65, 66]. Nano-silver can be incorporated into devices (dressings, bandages, catheters, and implants) as well as soaps for treating acne and dermatitis [65]. Among the protected technologies, silver nanoparticles are mainly developed for pharmaceutical purposes, in the form of artificial skin and fibers for antibacterial activity, but they were also present in other technologies belonging to the group of “formulations”.
Finally, the greatest number of technologies was categorized as “formulation”, corresponding to 75% of inventions. It is important to highlight that the composition of products depends on the interest of use, physical characteristics, and type of nanostructure employed [67]. Analyzing these technologies according to the nanostructure used in the composition, it is possible to verify the wide use of nanoemulsions, liposomes, nanocapsules and protein nanoparticles.
Nanoemulsions are Oil-in-Water (O/W) or Water-in-Oil (W/O) nanodispersion of two immiscible liquids stabilized by an amphiphilic surfactant system. Their droplet size ranges from 10 to 1000 nm, they are thermodynamically stable, optically clear with a milky white color. Such systems have been considered as a potential lipid-nanotechnology-based delivery system for topical administration and an alternative to incorporating lipophilic drugs/actives [68-70]. Eighteen patents were found using nanoemulsions. Regarding pharmaceutical application, only two patents were found, one for eczema/ psoriasis (CA2746566 (A1)) and another for transdermal drug delivery (KR101419602 (B1)). The other 16 patents were correlated with cosmetic application, and indications include: anti-aging (CN101874762 (B); CN102784084 (B); CN107595715 (A); CN103110534 (B); KR101956553 (B1); CN106860188 (A); KR101850421 (B1); CA2746566 (A1); CN104644459 (A)), enhancement of skin permeation/absorption (TW200914063 (A); KR101719811 (B1); CN105473200 (A)), enhancement of skin hydration or moisturization (WO2017048807 (A1); CN107898699 (A); KR101956553 (B1)), whitening or lighting the skin (CN101401775 (B); CN104606078 (A); CN107898699 (A)), and antioxidant activity (CN101664372 (B); KR101850421 (B1)).
Liposomes are small vesicles comprising amphipathic lipids organized in one or more concentric bilayers, basically formed spontaneously when a lipid is brought into contact with an aqueous phase. Their size can range from 25 to 2500 nm; they are thermodynamically stable and lamellar structures mainly classified as multilamellar or unilamellar vesicles. Such systems are biocompatible, biodegradable, and present low toxicity. Additionally, they have been considered as suitable skin delivery systems to trap hydrophilic, hydrophobic, or amphipathic drugs/ actives [21, 71, 72]. Sixteen patents were found using liposome, one for pharmaceutical application (transdermal drug vehicle KR101419602 (B1)) and 15 for cosmetics. Indications for use in cosmetics include: enhancement skin of permeation/absorption (TWI325325 (B); CN103520007 (A); KR20160106508 (A); CN104116651 (A)), enhancement skin moisturization (KR101023041 (B1); TWI325325 (B); CN102988200 (B); MX2014009967 (A); CN105496886 (B); KR100912462 (B1); KR20160106508 (A)), minimizing skin irritation (KR100858628 (B1); CN102988257 (B)), anti-aging (MX2014006945 (A); KR100912462 (B1); KR20160106508 (A); MX2014008660 (A); KR100912462 (B1); KR100912462 (B1); KR20160106508 (A)), suppressing melanogenesis (KR100912462 (B1)), improving skin immunity and acne treatment (KR101702482 (B1)), and hair growing promotion (KR100912462 (B1)).
Nanocapsules are polymeric nanoparticles that present a typical core-shell structure wherein the drug is kept to a reservoir or within a cavity covered by a polymer. The loaded drug/active can be lipophilic or hydrophobic, and in liquid or solid form or as a molecular dispersion, with a high drug efficiency of encapsulation. Their particle size usually ranges from 100 to 500 nm, not exceeding 1,000 nm [73-75]. Concerning the types of nanostructures, only 2 patents use nanocapsules as a formulation, both being used for cosmetic purposes: one for skincare, to improve the permeability and selective absorption function of the skin (CN104398395 (A)), and the other for skin wrinkles (KR101865841 (B1)).
Another type of nanostructure also reported in the “formulation” technologies category is protein nanoparticles, which can derive from several nanomaterials. Protein nanoparticles have been actively used especially regarding their unique functionalities and properties, including biodegradability, metabolization, and capability to suffer surface modifications, that may facilitate attachment of the drug and targeting ligands (site-specific action) [76-78]. In total, only three patents were found using protein nanoparticles and all referred to cosmetic application, one indicated for acne treatment (JP2008297241 (A)), one for anti-aging (JP2008255020 (A)), and one for bleaching (JP2008247814 (A)), all from Japanese FujiFilm Corp.®.
The technologies of nanostructures cited only once were classified as “others” and include a nano-sponge sheet for a rapid allergic skin test detector (CN102090904 (B)), a nanolipid carrier for epidermal growth factor (CN101474153 (A)), a nanosuspension applied for skin moisturization and soothing (KR101337454 (B1)), lipid nanostructures applied for hair growth (KR101536996 (B1)), and solid lipid nanoparticles for skin lightening (KR101860555 (B1)).
CONCLUSION
The use of nanotechnology-based delivery systems as skin penetration/permeation enhancers can be considered an attractive strategy for pharmaceutical and, mainly, for cosmetics topical application.
CURRENT & FUTURE DEVELOPMENTS
Skin delivery still represents a great challenge due to the limited penetration and absorption by this route. In the last decade, various studies proposed different strategies to improve skin penetration/permeation and to achieve dermal or transdermal delivery of drugs or bioactive compounds for therapeutic/cosmetic purposes. Nanotechnology-based delivery systems have been suggested to overcome this feature. In this study, a systematic review from recent patent technologies published from 2008 to 2018, concerning the use of nanotechnology-based delivery systems as skin penetration enhancers was performed for the first time. The overall data indicate an increase in the number of patents published in recent years, demonstrating the perspective for growth interest around the world in patenting products involving skin permeation promotion and nanotechnology for pharmaceutical and mainly for cosmetics purposes (anti-aging, antioxidant, moisturization, whitening, and others).
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The authors gratefully acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES - Finance Code 001) for the scholarships and the financial support.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Cosco D., Celia C., Cilurzo F., Trapasso E., Paolino D. Colloidal carriers for the enhanced delivery through the skin. Expert Opin. Drug Deliv. 2008;5(7):737–755. doi: 10.1517/17425247.5.7.737. [http://dx.doi.org/10.1517/17425247.5.7.737]. [PMID: 18590459]. [DOI] [PubMed] [Google Scholar]
- 2.Mota A.H., Rijo P., Molpeceres J., Reis C.P. Broad overview of engineering of functional nanosystems for skin delivery. Int. J. Pharm. 2017;532(2):710–728. doi: 10.1016/j.ijpharm.2017.07.078. [http://dx.doi.org/10.1016/j.ijpharm.2017.07.078]. [PMID: 28764984]. [DOI] [PubMed] [Google Scholar]
- 3.Lane M.E. Skin penetration enhancers. Int. J. Pharm. 2013;447(1-2):12–21. doi: 10.1016/j.ijpharm.2013.02.040. [http://dx.doi.org/10.1016/j.ijpharm.2013.02.040]. [PMID: 23462366]. [DOI] [PubMed] [Google Scholar]
- 4.Yotsumoto K., Ishii K., Kokubo M., Yasuoka S. Improvement of the skin penetration of hydrophobic drugs by polymeric micelles. Int. J. Pharm. 2018;553(1-2):132–140. doi: 10.1016/j.ijpharm.2018.10.039. [http://dx.doi.org/10.1016/j.ijpharm.2018.10.039]. [PMID: 30339944]. [DOI] [PubMed] [Google Scholar]
- 5.Amjadi M., Mostaghaci B., Sitti M. Recent advances in skin penetration enhancers for transdermal gene and drug delivery. Curr. Gene Ther. 2017;17(2):139–146. doi: 10.2174/1566523217666170510151540. [http://dx.doi.org/10.2174/1566523217666170510151540]. [PMID: 28494734]. [DOI] [PubMed] [Google Scholar]
- 6.Souto E.B., Doktorovova S., Boonme P. Lipid-based colloidal systems (nanoparticles, microemulsions) for drug delivery to the skin: Materials and end-product formulations. J. Drug Deliv. Sci. Technol. 2011;21:43–54. [http://dx.doi.org/10.1016/S1773-2247(11)50005-X]. [Google Scholar]
- 7.Souto E.B., Müller R.H. Cosmetic features and applications of lipid nanoparticles (SLN, NLC). Int. J. Cosmet. Sci. 2008;30(3):157–165. doi: 10.1111/j.1468-2494.2008.00433.x. [http://dx.doi.org/10.1111/j.1468-2494.2008.00433.x]. [PMID: 18452432]. [DOI] [PubMed] [Google Scholar]
- 8.Lane M.E. Nanoparticles and the skin- Applications and limitations. J. Microencapsul. 2011;28(8):709–716. doi: 10.3109/02652048.2011.599440. [http://dx.doi.org/10.3109/02652048.2011.599440]. [PMID: 21767116]. [DOI] [PubMed] [Google Scholar]
- 9.Sala M., Diab R., Elaissari A., Fessi H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018;535(1-2):1–17. doi: 10.1016/j.ijpharm.2017.10.046. [http://dx.doi.org/10.1016/j.ijpharm.2017.10.046]. [PMID: 29111097]. [DOI] [PubMed] [Google Scholar]
- 10.Ganceviciene R., Liakou A.I., Theodoridis A., Makrantonaki E., Zouboulis C.C. Skin anti-aging strategies. Dermatoendocrinol. 2012;4(3):308–319. doi: 10.4161/derm.22804. [http://dx.doi.org/10.4161/derm.22804]. [PMID: 23467476]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torin H.J., Sivaloganathan S., Kohandel M., Foldvari M. Drug delivery through the skin: Molecular simulations of barrier lipids to design more effective noninvasive dermal and transdermal delivery systems for small molecules, biologics, and cosmetics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011;3(5):449–462. doi: 10.1002/wnan.147. [http://dx.doi.org/10.1002/wnan.147]. [PMID: 21595050]. [DOI] [PubMed] [Google Scholar]
- 12.Lademann J., Richter H., Schanzer S., et al. Penetration and storage of particles in human skin: Perspectives and safety aspects. Eur. J. Pharm. Biopharm. 2011;77(3):465–468. doi: 10.1016/j.ejpb.2010.10.015. [http://dx.doi.org/10.1016/j.ejpb.2010.10.015]. [PMID: 21056659]. [DOI] [PubMed] [Google Scholar]
- 13.Vitorino C., Sousa J., Pais A. Overcoming the skin permeation barrier: Challenges and opportunities. Curr. Pharm. Des. 2015;21(20):2698–2712. doi: 10.2174/1381612821666150428124053. [http://dx.doi.org/10.2174/1381612821666150428124053]. [PMID: 25925125]. [DOI] [PubMed] [Google Scholar]
- 14.Nastiti C.M.R.R., Ponto T., Abd E., Grice J.E., Benson H.A.E., Roberts M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics. 2017;9(4):1–25. doi: 10.3390/pharmaceutics9040037. [http://dx.doi.org/10.3390/pharmaceutics9040037]. [PMID: 28934172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karadzovska D., Brooks J.D., Monteiro-Riviere N.A., Riviere J.E. Predicting skin permeability from complex vehicles. Adv. Drug Deliv. Rev. 2013;65(2):265–277. doi: 10.1016/j.addr.2012.01.019. [http://dx.doi.org/10.1016/j.addr.2012.01.019]. [PMID: 22342772]. [DOI] [PubMed] [Google Scholar]
- 16.de Matos S.P., Teixeira H.F., de Lima Á.A.N., Veiga-Junior V.F., Koester L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: A review. Biomolecules. 2019;9(4):1–19. doi: 10.3390/biom9040138. [http://dx.doi.org/10.3390/biom9040138]. [PMID: 30959802]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez-Román R., Naik A., Kalia Y.N., Guy R.H., Fessi H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release. 2004;99(1):53–62. doi: 10.1016/j.jconrel.2004.06.015. [http://dx.doi.org/10.1016/j.jconrel.2004.06.015]. [PMID: 15342180]. [DOI] [PubMed] [Google Scholar]
- 18.Batheja P., Sheihet L., Kohn J., Singer A.J., Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release. 2011;149(2):159–167. doi: 10.1016/j.jconrel.2010.10.005. [http://dx.doi.org/10.1016/j.jconrel.2010.10.005]. [PMID: 20950659]. [DOI] [PubMed] [Google Scholar]
- 19.Ganesan P., Choi D.K. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int. J. Nanomedicine. 2016;11:1987–2007. doi: 10.2147/IJN.S104701. [http://dx.doi.org/10.2147/IJN.S104701]. [PMID: 27274231]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta M., Agrawal U., Vyas S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012;9(7):783–804. doi: 10.1517/17425247.2012.686490. [http://dx.doi.org/10.1517/17425247.2012.686490]. [PMID: 22559240]. [DOI] [PubMed] [Google Scholar]
- 21.Fang J.Y., Hwang T.L., Huang Y.L. Liposomes as vehicles for enhancing drug delivery via skin routes. Curr. Nanosci. 2006;2:55–70. [http://dx.doi.org/10.2174/157341306775473791]. [Google Scholar]
- 22.Ngwuluka N.C., Pillay V., Choonara Y.E., et al. Fabrication, modeling and characterization of multi-crosslinked methacrylate copolymeric nanoparticles for oral drug delivery. Int. J. Mol. Sci. 2011;12(9):6194–6225. doi: 10.3390/ijms12096194. [http://dx.doi.org/10.3390/ijms12096194]. [PMID: 22016653]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Küchler S., Herrmann W., Panek-Minkin G., et al. SLN for topical application in skin diseases- Characterization of drug-carrier and carrier-target interactions. Int. J. Pharm. 2010;390(2):225–233. doi: 10.1016/j.ijpharm.2010.02.004. [http://dx.doi.org/10.1016/j.ijpharm.2010.02.004]. [PMID: 20153414]. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y.K., Yang S.H., Chen C.C., Kao H.C., Fang J.Y. Using imiquimod-induced psoriasis-like skin as a model to measure the skin penetration of anti-psoriatic drugs. PLoS One. 2015;10(9):e0137890. doi: 10.1371/journal.pone.0137890. [http://dx.doi.org/10.1371/journal.pone.0137890]. [PMID: 26355594]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domínguez-Villegas V., Clares-Naveros B., García-López M.L., Calpena-Campmany A.C., Bustos-Zagal P., Garduño-Ramírez M.L. Development and characterization of two nano-structured systems for topical application of flavanones isolated from Eysenhardtia platycarpa. Colloids Surf. B Biointerfaces. 2014;116:183–192. doi: 10.1016/j.colsurfb.2013.12.009. [http://dx.doi.org/10.1016/j.colsurfb.2013.12.009]. [PMID: 24463153]. [DOI] [PubMed] [Google Scholar]
- 26.Jenning V., Gysler A., Schäfer-Korting M., Gohla S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000;49(3):211–218. doi: 10.1016/s0939-6411(99)00075-2. [http://dx.doi.org/10.1016/S0939-6411(99)00075-2]. [PMID: 10799811]. [DOI] [PubMed] [Google Scholar]
- 27.Liu J., Hu W., Chen H., Ni Q., Xu H., Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007;328(2):191–195. doi: 10.1016/j.ijpharm.2006.08.007. [http://dx.doi.org/10.1016/j.ijpharm.2006.08.007]. [PMID: 16978810]. [DOI] [PubMed] [Google Scholar]
- 28.Alomrani A.H., Shazly G.A., Amara A.A.A.F., Badran M.M. Itraconazole-hydroxypropyl-β-cyclodextrin loaded deformable liposomes: In vitro skin penetration studies and antifungal efficacy using Candida albicans as model. Colloids Surf. B Biointerfaces. 2014;121:74–81. doi: 10.1016/j.colsurfb.2014.05.030. [http://dx.doi.org/10.1016/j.colsurfb.2014.05.030]. [PMID: 24937135]. [DOI] [PubMed] [Google Scholar]
- 29.Marafon P., Fachel F.N.S., Dal Prá M., et al. Development, physico-chemical characterization and in-vitro studies of hydrogels containing rosmarinic acid-loaded nanoemulsion for topical application. J. Pharm. Pharmacol. 2019;71(8):1199–1208. doi: 10.1111/jphp.13102. [http://dx.doi.org/10.1111/jphp.13102]. [PMID: 31124591]. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro-Riviere N.A., Wiench K., Landsiedel R., Schulte S., Inman A.O., Riviere J.E. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: An in vitro and in vivo study. Toxicol. Sci. 2011;123(1):264–280. doi: 10.1093/toxsci/kfr148. [http://dx.doi.org/10.1093/toxsci/kfr148]. [PMID: 21642632]. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Mottaleb M.M.A., Moulari B., Beduneau A., Pellequer Y., Lamprecht A. Nanoparticles enhance therapeutic outcome in inflamed skin therapy. Eur. J. Pharm. Biopharm. 2012;82(1):151–157. doi: 10.1016/j.ejpb.2012.06.006. [http://dx.doi.org/10.1016/j.ejpb.2012.06.006]. [PMID: 22728016]. [DOI] [PubMed] [Google Scholar]
- 32.Nemitz M.C., Argenta D.F., Koester L.S., Bassani V.L., von Poser G.L., Teixeira H.F. The international scenario of patents concerning isoflavones. Trends Food Sci. Technol. 2016;49:85–95. [http://dx.doi.org/10.1016/j.tifs.2016.01.008]. [Google Scholar]
- 33.Ernst H. Patent information for strategic technology management. World Pat. Inf. 2003;25:233–242. [http://dx.doi.org/10.1016/S0172-2190(03)00077-2]. [Google Scholar]
- 34.Basberg B.L. Patents and the measurement of technological change: A survey of the literature. Res. Policy. 1987;16:131–141. [http://dx.doi.org/10.1016/0048-7333(87)90027-8]. [Google Scholar]
- 35.Noh H., Jo Y., Lee S. Keyword selection and processing strategy for applying text mining to patent analysis. Expert Syst. Appl. 2015;42:4348–4360. [http://dx.doi.org/10.1016/j.eswa.2015.01.050]. [Google Scholar]
- 36.Napoleão de RB. Systematic x Narrative Review. Acta Paul. Enferm. 2007;20(2):6–7. [Google Scholar]
- 37.Frietsch R., Schmoch U., van Looy B., et al. The value and indicator function of patents. Stud Zum Dtsch Innov. 2011;40:969–985. [Google Scholar]
- 38.Idris K. Intellectual property: A power tool for economic growth. World Pat. Inf. 2003;25:359–360. [http://dx.doi.org/10.1016/S0172-2190(03)00075-9]. [Google Scholar]
- 39.Roberts M.S., Mohammed Y., Pastore M.N., et al. Topical and cutaneous delivery using nanosystems. J. Control. Release. 2017;247:86–105. doi: 10.1016/j.jconrel.2016.12.022. [http://dx.doi.org/10.1016/j.jconrel.2016.12.022]. [PMID: 28024914]. [DOI] [PubMed] [Google Scholar]
- 40.World Intellectual Property Indicators. World Intellect Prop Organ. 2018 [Google Scholar]
- 41.Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off J. Eur. Union. 2009;2009:342–359. [Google Scholar]
- 42.Yapar E.A. Intellectual property and patent in cosmetics. Marmara Pharm. J. 2017;21:419–424. [http://dx.doi.org/10.12991/marupj.306786]. [Google Scholar]
- 43.Galenytska K. Euromonitor Consumer Trends. 2019;2019:21. [Google Scholar]
- 44.Łopaciuk A., Łoboda M. Global Beauty Industry Trends in the 21st Century. Active Citizenship by Knowledge Management & Innovation: Proceedings of the Management; Knowledge and Learning International Conference; 2013. [Google Scholar]
- 45.Bom S., Jorge J., Ribeiro H.M., Marto J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019;225:270–290. [http://dx.doi.org/10.1016/j.jclepro.2019.03.255]. [Google Scholar]
- 46.Thoma G. Trademarks and the patent premium value: Evidence from medical and cosmetic products. World Pat. Inf. 2015;41:23–30. [http://dx.doi.org/10.1016/j.wpi.2015.02.003]. [Google Scholar]
- 47.Global Beauty & Personal Care Trends. 2018 [Google Scholar]
- 48.Loo Ch., Basri M., Ismail R., et al. Effect of compositions in Nanostructured Lipid Carriers (NLC) on skin hydration and occlusion. Int. J. Nanomedicine. 2013;8:13–22. doi: 10.2147/IJN.S35648. [PMID: 23293516]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong M., Chen X.G., Kweon D.K., Park H.J. Investigations on skin permeation of hyaluronic acid based nanoemulsion as transdermal carrier. Carbohydr. Polym. 2011;86:837–843. [http://dx.doi.org/10.1016/j.carbpol.2011.05.027]. [Google Scholar]
- 50.Budhiraja A., Dhingra G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015;22(6):723–730. doi: 10.3109/10717544.2014.903010. [http://dx.doi.org/10.3109/10717544.2014.903010]. [PMID: 24786487]. [DOI] [PubMed] [Google Scholar]
- 51.Balestrin L.A., Bidone J., Bortolin R.C., Moresco K., Moreira J.C., Teixeira H.F. Protective effect of a hydrogel containing Achyrocline satureioides extract-loaded nanoemulsion against UV-induced skin damage. J. Photochem. Photobiol. B. 2016;163:269–276. doi: 10.1016/j.jphotobiol.2016.08.039. [http://dx.doi.org/10.1016/j.jphotobiol.2016.08.039]. [PMID: 27599114]. [DOI] [PubMed] [Google Scholar]
- 52.Back P.I., Furtado L.R., Nemitz M.C., et al. Skin permeation and oxidative protection effect of soybean isoflavones from topical nanoemulsions. A comparative study of extracts and pure compounds. AAPS PharmSciTech. 2018;19(7):3029–3039. doi: 10.1208/s12249-018-1133-x. [http://dx.doi.org/10.1208/s12249-018-1133-x]. [PMID: 30084071]. [DOI] [PubMed] [Google Scholar]
- 53.Frombach J., Unbehauen M., Kurniasih I.N., et al. Core-multishell nanocarriers enhance drug penetration and reach keratinocytes and antigen-presenting cells in intact human skin. J. Control. Release. 2019;299:138–148. doi: 10.1016/j.jconrel.2019.02.028. [http://dx.doi.org/10.1016/j.jconrel.2019.02.028]. [PMID: 30797867]. [DOI] [PubMed] [Google Scholar]
- 54.Mahmoud N.N., Alkilany A.M., Dietrich D., Karst U., Al-Bakri A.G., Khalil E.A. Preferential accumulation of gold nanorods into human skin hair follicles: Effect of nanoparticle surface chemistry. J. Colloid Interface Sci. 2017;503:95–102. doi: 10.1016/j.jcis.2017.05.011. [http://dx.doi.org/10.1016/j.jcis.2017.05.011]. [PMID: 28502717]. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H., Zhai Y., Yang X., Zhai G. Breaking the skin barrier: Achievements and future directions. Curr. Pharm. Des. 2015;21(20):2713–2724. doi: 10.2174/1381612821666150428124406. [http://dx.doi.org/10.2174/1381612821666150428124406]. [PMID: 25925124]. [DOI] [PubMed] [Google Scholar]
- 56.Steinbach O. An industry update: The latest developments in therapeutic delivery covering February 2019. Ther. Deliv. 2019;10(6):337–341. doi: 10.4155/tde-2019-0088. [http://dx.doi.org/10.4155/tde-2019-0023]. [PMID: 31161890]. [DOI] [PubMed] [Google Scholar]
- 57.Ren T., Yang X., Wu N., Cai Y., Liu Z., Yuan W. Sustained-release formulation of levodopa methyl ester/benserazide for prolonged suppressing dyskinesia expression in 6-OHDA-leisoned rats. Neurosci. Lett. 2011;502(2):117–122. doi: 10.1016/j.neulet.2011.07.042. [http://dx.doi.org/10.1016/j.neulet.2011.07.042]. [PMID: 21835223]. [DOI] [PubMed] [Google Scholar]
- 58.Nemitz M.C., Moraes R.C., Koester L.S., Bassani V.L., von Poser G.L., Teixeira H.F. Bioactive soy isoflavones: Extraction and purification procedures, potential dermal use and nanotechnology-based delivery systems. Phytochem. Rev. 2014;14:849–869. [http://dx.doi.org/10.1007/s11101-014-9382-0]. [Google Scholar]
- 59.Al-Subaie M.M., Hosny K.M., El-Say K.M., Ahmed T.A., Aljaeid B.M. Utilization of nanotechnology to enhance percutaneous absorption of acyclovir in the treatment of herpes simplex viral infections. Int. J. Nanomedicine. 2015;10:3973–3985. doi: 10.2147/IJN.S83962. [PMID: 26109856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Vargas B.A., Bidone J., Oliveira L.K., Koester L.S., Bassani V.L., Teixeira H.F. Development of topical hydrogels containing genistein-loaded nanoemulsions. J. Biomed. Nanotechnol. 2012;8(2):330–336. doi: 10.1166/jbn.2012.1386. [http://dx.doi.org/10.1166/jbn.2012.1386]. [PMID: 22515085]. [DOI] [PubMed] [Google Scholar]
- 61.Bidone J., Argenta D.F., Kratz J., et al. Antiherpes activity and skin / mucosa distribution of flavonoids from Achyrocline satureioides extract incorporated into topical nanoemulsions. BioMed Res. Int. 2015;2015:238010. doi: 10.1155/2015/238010. [http://dx.doi.org/10.1155/2015/238010]. [PMID: 26101767]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng L., Gong Z., Lu Y., et al. A UPLC-MS/MS method for simultaneous determination of danshensu, protocatechuic aldehyde, rosmarinic acid, and ligustrazine in rat plasma, and its application to pharmacokinetic studies of Shenxiong glucose injection in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;997:210–217. doi: 10.1016/j.jchromb.2015.06.008. [http://dx.doi.org/10.1016/j.jchromb.2015.06.008]. [PMID: 26118621]. [DOI] [PubMed] [Google Scholar]
- 63.Akhtar N., Verma A., Pathak K. Topical delivery of drugs for the effective treatment of fungal infections of skin. Curr. Pharm. Des. 2015;21(20):2892–2913. doi: 10.2174/1381612821666150428150456. [http://dx.doi.org/10.2174/1381612821666150428150456]. [PMID: 25925110]. [DOI] [PubMed] [Google Scholar]
- 64.European Commission Recommendation of 18 October 2011 on the definition of nanomaterial, 2011/696/EU. Off J. Eur. Union. 2011;2011:54. [Google Scholar]
- 65.Pulit-Prociak J., Grabowska A., Chwastowski J., Majka T.M., Banach M. Safety of the application of nanosilver and nanogold in topical cosmetic preparations. Colloids Surf. B Biointerfaces. 2019;183(1):110416. doi: 10.1016/j.colsurfb.2019.110416. [http://dx.doi.org/10.1016/j.colsurfb.2019.110416]. [PMID: 31398622]. [DOI] [PubMed] [Google Scholar]
- 66.Contado C. Nanomaterials in consumer products: A challenging analytical problem. Front Chem. 2015;3:48. doi: 10.3389/fchem.2015.00048. [http://dx.doi.org/10.3389/fchem.2015.00048]. [PMID: 26301216]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes G.A. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1:22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Singh Y., Meher J.G., Raval K., et al. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [http://dx.doi.org/10.1016/j.jconrel.2017.03.008]. [PMID: 28279798]. [DOI] [PubMed] [Google Scholar]
- 69.Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech. 2015;5:123–7. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thakur N., Garg G., Sharma P.K., Kumar N. Nanoemulsions: A review on various pharmaceutical application. Glob. J. Pharmacol. 2012;6:222–225. [Google Scholar]
- 71.Sheoran R., Khokra S.L., Chawla V., Dureja H. Recent patents, formulation techniques, classification and characterization of liposomes. Recent Pat. Nanotechnol. 2019;13(1):17–27. doi: 10.2174/1872210513666181127110413. [http://dx.doi.org/10.2174/1872210513666181127110413]. [PMID: 30479223]. [DOI] [PubMed] [Google Scholar]
- 72.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., et al. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [http://dx.doi.org/10.1186/1556-276X-8-102]. [PMID: 23432972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frank L.A., Contri R.V., Beck R.C.R., Pohlmann A.R., Guterres S.S. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7(5):623–639. doi: 10.1002/wnan.1334. [http://dx.doi.org/10.1002/wnan.1334]. [PMID: 25641603]. [DOI] [PubMed] [Google Scholar]
- 74.Mora-Huertas C.E., Fessi H., Elaissari A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010;385(1-2):113–142. doi: 10.1016/j.ijpharm.2009.10.018. [http://dx.doi.org/10.1016/j.ijpharm.2009.10.018]. [PMID: 19825408]. [DOI] [PubMed] [Google Scholar]
- 75.Crespy D., Lv L.P., Landfester K. Redefining the functions of nanocapsule materials. Nanoscale Horizons. 2016;1:268–271. doi: 10.1039/c5nh00112a. [http://dx.doi.org/10.1039/C5NH00112A]. [DOI] [PubMed] [Google Scholar]
- 76.Baimanov D., Cai R., Chen C. Understanding the chemical nature of nanoparticle-protein interactions. Bioconjug. Chem. 2019;30(7):1923–1937. doi: 10.1021/acs.bioconjchem.9b00348. [http://dx.doi.org/10.1021/acs.bioconjchem.9b00348]. [PMID: 31259537]. [DOI] [PubMed] [Google Scholar]
- 77.Choi W.I., Lee J.H., Kim J.Y., Kim J.C., Kim Y.H., Tae G. Efficient skin permeation of soluble proteins via flexible and functional nano-carrier. J. Control. Release. 2012;157(2):272–278. doi: 10.1016/j.jconrel.2011.08.013. [http://dx.doi.org/10.1016/j.jconrel.2011.08.013]. [PMID: 21867735]. [DOI] [PubMed] [Google Scholar]
- 78.Verma D., Gulati N., Kaul S., Mukherjee S., Nagaich U. Protein based nanostructures for drug delivery. J. Pharm. (Cairo) 2018;2018:9285854. doi: 10.1155/2018/9285854. [http://dx.doi.org/10.1155/2018/9285854]. [PMID: 29862118]. [DOI] [PMC free article] [PubMed] [Google Scholar]


