Abstract
Background
Opioid analgesics are commonly used along with propofol during general anesthesia. Due to the dearth of data on the quality of anesthesia achieved with this combination, the present meta-analysis was carried out.
Methods
Electronic databases were searched for appropriate studies using a suitable search strategy. Randomized clinical trials comparing the combination of remifentanil/sufentanil/alfentanil with propofol with fentanyl and propofol, were included. The outcome measures were as follows: total propofol dose to achieve the desired general anesthesia; time of onset and duration of general anesthesia; depth of general anesthesia; and recovery time (time for eye-opening and time taken for extubation). Risk of bias was assessed and Forest plots were generated for eligible outcomes. The weighted mean difference [95% confidence intervals] was used as the effect estimate.
Results
Fourteen studies were included in the systematic review and 13 were included in the meta-analysis. Statistically significant differences were observed for remifentanil in comparison to fentanyl when combined with propofol: Propofol dose (in mg) -76.18 [-94.72, -57.64]; time of onset of anesthesia (min) -0.44 [-0.74, -0.15]; time taken for eye-opening (min) -3.95 [-4.8, -3.1]; and time for extubation (min) -3.53 [-4.37, -2.7]. No significant differences were observed for either sufentanil or alfentanil about the dose of propofol required and due to scanty data, pooling of the data could not be attempted for other outcome measures for either sufentanil or alfentanil.
Conclusion
To conclude, we found that remifentanil has a statistically significant anesthetic profile than fentanyl when combined with propofol. Scanty evidence for both alfentanil and sufentanil precludes any such confirmation.
Keywords: Opioids, anesthesia, propofol related pain, fentanyl induced cough, alfentanil, sufentanil
1. INTRODUCTION
Propofol is a general anesthetic drug widely used for day-care surgeries with the advantages of faster onset and shorter duration of anesthesia [1]. The main drawback associated with the administration of propofol alone is the injection-related pain reported in nearly 60% and even slightly more (85%) in children [2, 3]. Other adverse events related to propofol include systemic hypotension, allergy, hypertriglyceridemia and pancreatitis [4].
Fentanyl is a potent synthetic opioid analgesic used in combination with other drugs for producing balanced general anesthesia [5]. The main attributes of fentanyl are pain relief and sedation that are equally applicable to other drugs in the series such as remifentanil, alfentanil and sufentanil. Studies have shown that the combination of opioid analgesics with propofol decreases the incidence of propofol related pain as well as the severity [6]. Interestingly, the addition of propofol also decreases the incidence of fentanyl-induced cough [7]. Remifentanil is a highly potent opioid drug with the fastest onset of action (of about one minute) and a shorter elimination half-life of 10 minutes [8]. Similar activity has been observed with alfentanil and sufentanil [9]. Amidst the studies comparing pharmacodynamic effects of the above opioids, there is a dearth of data regarding the onset, duration and the extent of general anesthesia attained with the combination of opioid analgesics and propofol. Hence, we undertook the systematic review and meta-analysis to compare the profile of general anesthesia of remifentanil, sufentanil and alfentanil with fentanyl when combined with propofol.
2. METHODS
2.1. Information Sources and Search Strategy
The protocol for this review was registered with the International prospective register of systematic reviews (PROSPERO) with the registration number CRD 42016045622. The review protocol can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016045622. A thorough literature search was conducted and was completed on 14 August 2016. The primary database used was Medline (via PubMed), Cochrane central register of clinical trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE) and Google Scholar. The keywords used were Propofol [tiab] AND fentanyl [tiab]. This search was further supplemented by manual searching of relevant references from review articles and other eligible studies. We did not pose any limitation to any language or date in the present study.
2.2. Eligibility Criteria
Studies with randomized controlled design meeting the following requirements were included in the present study:
Type of participants- Any patient undergoing surgery or endoscopy under general anesthesia.
Type of intervention- A combination of either remifentanil or sufentanil or alfentanil with propofol.
Comparison- Combination of fentanyl and propofol.
Outcome- Total dose of propofol required to achieve the desired general anesthesia, time of onset and duration of general anesthesia, depth of general anesthesia and recovery time (time for eye-opening and time taken for extubation).
2.3. Study Procedure
Two authors independently screened the databases and reviewed the identified abstracts for suitability. Full-text articles were obtained following abstract screening for those found to be eligible to be included in the review. A pre-tested data extraction form was created and both the authors independently extracted the following data from each of the eligible studies as follows: trial site, year, trial methods, participants, interventions, and outcomes. Disagreement between the authors was resolved through discussion. The extracted data were analyzed using non-Cochrane mode in RevMan 5.3 software. The methodological quality of the trials was assessed using The Cochrane collaboration’s tool for assessing the risk of bias across the following six domains: sequence generation, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias. The judgment was categorized into the low, high or unclear risk of bias [8]. For continuous outcome measures, mean differences (MD) were considered for the final assessment from individual studies with 95% confidence interval (95% CI) as a measure to represent the deviation from the point estimate. Heterogeneity between the studies was assessed using Forest plot visually, I2 statistics wherein more than 30% was considered to have moderate to severe heterogeneity and Chi-square test with a statistical P-value of less than 0.10 to indicate statistical significance. Random-effect models were chosen in cases of moderate to severe heterogeneity otherwise, fixed-effect models were used. The present meta-analysis was conducted and presented in compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9].
3. RESULTS
3.1. Search Results
A total of 2879 articles were obtained, of which 14 studies [10-23] were found eligible to be included in the systematic review Fig. (1). Except one [10], all were also included in the meta-analysis. The key characteristics of the included studies are mentioned in Table 1. A summary of the risk of bias of the included studies is depicted in Fig. (2).
Fig. (1).
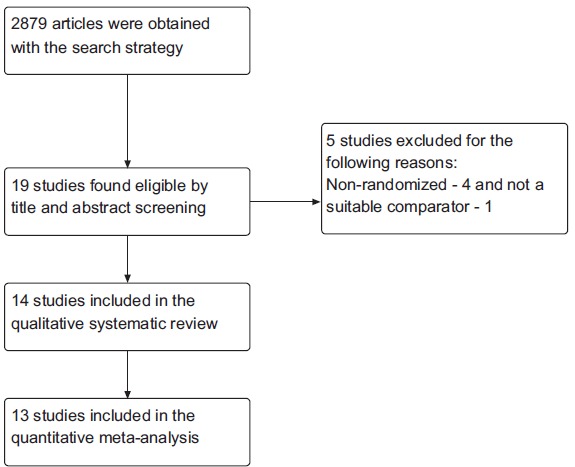
Study flow diagram. A total of 2879 studies obtained with the outlined search strategy and finally 14 were included in the systematic review and 13 in the meta-analysis.
Table 1. Key characteristics of the studies included in this systematic review.
| Study Id; Year and Country of Conduct of the Study | Participants | Intervention | Control | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Hui et al. [10]; 2002, Hong Kong | ASA I/II patients who have been scheduled for minor surgery. Those with anticipated difficult airway as determined by Mallampati score of > 3 were excluded. | Alfentanil (10 µg/kg) with propofol (2.5 mg/kg) 90 seconds prior to laryngeal mask airway insertion to 73 patients. | Fentanyl (1 µg/kg) with propofol (2.5 mg/kg) 90 seconds prior to laryngeal mask airway insertion to 67 patients. | Six variable scores for each of mouth opening, ease of insertion, swallowing, coughing, movement and laryngospasm was used to assess insertion conditions. | ||||
| Mannine et al. [11]; 2006, Canada | Patients undergoing awake craniotomy. |
Propofol infusion at the dose of 75 to 100 µg/kg/min with remifentanil infusion at a dose of 0.03-0.05 µg/kg/min to 25 participants. | Propofol infusion at the dose of 75 to 100 µg/kg/min with fentanyl infusion at a dose of 0.5-1 µg/kg bolus to 25 participants. | Total propofol dose, sedation and pain scores, mean arterial pressure, heart rate, SpO2, respiratory rate and intra-operative complications. | ||||
| Haytural et al. [12]; 2015, Turkey | ASA I-III patients who are undergoing elective endoscopic retrograde cholangiopancreatography aged between 18 and 70 years. Pregnant women, epileptics, those who have allergy to opioids or sedatives or underwent any surgery within the past 72 hours were excluded. | Remifentanil (0.05 µg/kg) with propofol (1.5 mg/kg) to 30 patients. | Fentanyl (1 µg/kg) with propofol (1.5 mg/kg) to 30 patients. Another group of patients were administered only propofol (1.5 mg/kg) but the data from this population was not considered for this review. | Total propofol dose, systolic, diastolic and mean arterial pressures, Ramsey scores and pain levels. | ||||
| Yong-hua et al [13]; 2005, China | Patients who have been scheduled for colonoscopy. | Remifentanil (0.05 µg/kg/min) with propofol 0.4 mg/kg loading dose and 0.2 mg/kg boluses intermittently to 15 patients. | Fentanyl (1 µg/kg) bolus with propofol 0.4 mg/kg loading dose and 0.2 mg/kg boluses intermittently to 15 patients. | Induction time of anesthesia, intubation time for colonoscopy, recovery time, stay in post anesthetic care unit, mean arterial pressure, heart rate, pulse oxygen saturation and respiratory rate. | ||||
| Srivastava et al. [14]; 2008, India | Patients of either sex, aged between 40 and 75 years requiring direct laryngoscopy under general anesthesia were recruited. Those with lipid allergy, difficult or long procedures were excluded. | Propofol (2.5 mg/kg) with sufentanil (0.25-0.5 µg/kg) to 22 patients. | Propofol (2.5 mg/kg) with fentanyl (1-1.5 µg/kg) to 23 patients. |
Conditions of insertion technique, recovery time, propofol dose, adverse events, mean arterial pressure and heart rate. | ||||
| Ryu et al. [15]; 2008, South Korea | ASA I/II women aged between 18 and 60 years undergoing routine hysteroscopic procedures were included. Those with chronic use of opioids or analgesics or history of sedative abuse or allergy were excluded. | Remifentanil at a bolus of 0.5 µg/kg followed by a continuous infusion of 0.05 µg/kg/min to 15 patients. Propofol was administered to achieve BIS of 60 to 80. | Fentanyl at 1 µg/kg bolus with an additional 0.5 µg/kg bolus dose in case of insufficient analgesia to 15 patients. Propofol was administered to achieve BIS of 60 to 80. | Total dose of propofol, systolic, diastolic and mean arterial pressures, adverse events, satisfaction score. | ||||
| Lysakowski et al. [16]; 2001, Switzerland | ASA I/II patients scheduled for elective surgery were included. Those on psychotropic drugs or obese were excluded. | Alfentanil, remifentanil and sufentanil were administered individually to three groups of patients (15 patients each) to produce the effect-site concentrations of 100 ng/ml for alfentanil, 6 ng/ml for remifentanil and 0.2 ng/ml for sufentanil. Target controlled infusion of propofol was started to increase predicted plasma concentration stepwise to 1, 2 and 4 µg/min using a pump with the kinetic set of Marsh for propofol. | Fentanyl was administered at a dose to obtain the effect-site concentration of 1.5 ng/ml to 15 patients. Target controlled infusion of propofol was started to increase predicted plasma concentration stepwise to 1, 2 and 4 µg/min using a pump with the kinetic set of Marsh for propofol. | BIS, effect-site concentrations and alertness and sedation scores. | ||||
| Study id; Year and Country of Conduct of the Study | Participants | Intervention | Control | Outcomes | ||||
| Kwak et al. [17]; 2006, South Korea | ASA I/II patients scheduled for third molar extraction under local anesthesia were included. Those with significant cardiovascular, respiratory or hepatic diseases, hypersensitivity to opioids, alcohol or drug abuse were excluded. | Alfentanil infusion was administered to 16 study participants. Propofol was administered in addition and the dose titrated based on the level of alertness and sedation scale. | Bolus fentanyl at a dose of 100 µg to 24 patients. Propofol was administered in addition and the dose titrated based on the level of alertness and sedation scale. | Duration of anesthesia, duration of surgery, total dose of propofol, hemodynamic changes, sedation and co-operation scores. | ||||
| Ho et al. [18]; 2012, Taiwan | Consecutive patients undergoing diagnostic esophagogastroduodenoscopy and colonoscopy were included. Those who were < 20 years of age, pregnant, ASA IV, history of allergies to propofol, soy beans or eggs, chronic lung disease, history of drug allergy or alcohol abuse, seizure disorder, sleep apnea or history of complications with previous sedation and inability to provide informed consent were excluded. | Alfentanil 0.5 mg with initial bolus of propofol (0.5 mg/kg) and dose titrated based on the level of sedation to 129 patients. | Fentanyl 0.05 mg with initial bolus of propofol (0.5 mg/kg) and dose titrated based on the level of sedation to 131 patients. | Propofol dose, awake time, recovery time, total anesthetic costs and hemodynamic parameters. | ||||
| Takayama et al. [19]; 2012, Japan | ASA I/II with age between 34 and 60 years within 15% ideal body weight, scheduled to undergo elective oral surgery or extraction of impacted teeth or cystectomy or open reduction of fractures or sequesterectomy or resection of leukoplakia under total intravenous anesthesia. Those with history of cardiac, pulmonary, hepatic or renal disease or disabling neuropsychiatric disorders were excluded. | Remifentanil infusion at a dose of 0.3 µg/kg/min to 20 patients. Propofol was initiated and the dose was adjusted based on the values of BIS. | Fentanyl 3 µg/kg bolus and 1 µg/kg every 30 minutes during surgery to 20 patients. Propofol was initiated and the dose was adjusted based on the values of BIS. | BIS, number of dots missed, maximum distance of the dots missed and average distance of the dots missed, duration of surgery and duration of anesthesia. | ||||
| Wilhelm et al. [20]; 2002, Germany |
Patients with ASA I-III scheduled for vertebral surgery were included. Those with history of cardiovascular or disabling central nervous system disease, hypersensitivity to opioids or substance abuse, pre-existing treatment with opioids, any psychiatric medication, history of difficult intubation or clinical signs of difficult airway management were excluded. | Propofol 2 mg/kg with remifentanil infustion at 0.5 µg/kg/min to 12 patients. | Propofol 2 mg/kg with fentanyl 1.5 µg/kg bolus to 12 patients. | Time to drop syringe, time for loss of eyelash reflex, induction dose of propofol, quality of induction of anesthesia and hemodynamic parameters. | ||||
| Ahonen et al. [21]; 2000, Finland | Patients undergoing coronary artery bypass graft surgery were included. Those with left ventricular ejection fraction of less than 40%, significant valvular dysfunction, renal or liver insufficiency, uncontrolled hypertension, treatment with either cytochrome P450 inducers or inhibitors, morbid obesity, anesthesia duration of more than 6 hours and re-operation were excluded. | Alfentanil 75 µg/kg and sufentanil 0.75 µg/kg were administered to independent groups (20 each) along with propofol 1-1.5 mg/kg. | Fentanyl 7.5 µg/kg with propofol 1-1.5 mg/kg to 20 patients. | Total propofol dose, total opioid dose, duration of anesthesia, time taken for shifting the patient from intensive care unit to ward, quantity of crystalloids administered and hemodynamic parameters. | ||||
| Coskun et al. [22]; 2010, Turkey | Patients with ASA I or II undergoing elective septorhinoplasty were included. Those less than 18 years or more than 65 years of age, receipt of analgesics or sedatives in the past 24 hours, diastolic blood pressure > 100 mmHg or systolic blood pressure < 100 mm Hg or signs of bradyarrhythmia were excluded. | Remifentanil bolus at 1 µg/kg followed by continuous infusion at 0.15 µg/kg/min to 20 patients. Propofol infusion was then commenced with a 3 µg/ml effect site concentration. | Fentanyl 3 µg/kg bolus with continuous infusion at 0.03 µg/kg/min to 20 patients. Propofol infusion was then commenced with a 3 µg/ml effect site concentration. | Onset and duration of anesthesia, total doses of propofol, fentanyl and remifentanil, duration of surgery and hemodynamic parameters. | ||||
| Del Gaudio et al. [23]; 2006, Italy | Patients scheduled for elective supratentorial craniotomy with Glasgow coma scale score of 15 with ASA I/II were included. Those who had undergone prior craniotomy and those with clinically serious pre-operative systemic diseases were excluded. |
Remifentanil 0.25 µg/kg/h to 20 patients. Propofol infusion was initiated to achieve a plasma level of 3 µg/ml in 15 minutes. | Fentanyl 2-3 µg/kg/h to 20 patients. Propofol infusion was initiated to achieve a plasma level of 3 µg/ml in 15 minutes. | Hemodynamic parameters, total doses of propofol, fentanyl and remifentanil, duration of anesthesia, time for extubation and time for responding after completion of anesthesia. | ||||
ASA – American Society of Anesthesiologist; BIS – bispectral index.
Fig. (2).
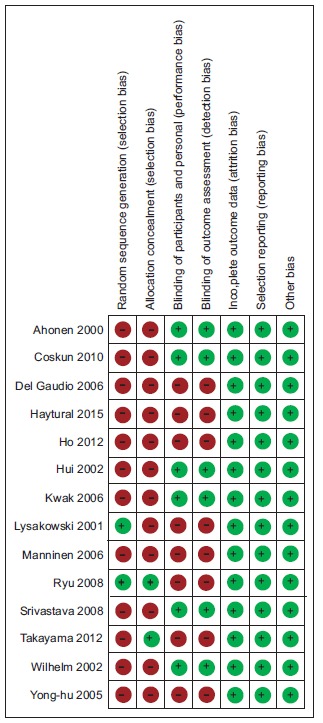
Risk of bias of the included studies. Red circle with minus symbol indicates the absence of reporting that specific element while green circle with plus symbol indicates the reporting of the same. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2. Pooled Results
3.2.1. Total Propofol Dose
Seven studies (284 participants) assessed the dose (in mg) of propofol required to achieve general anesthesia. The pooled estimate {mean difference of -76.18 [-94.72, -57.64]} favored the combination of remifentanil than fentanyl when combined with propofol Fig. (3). Similarly, three studies (340 participants) compared the total propofol dose required with alfentanil and fentanyl; and two studies (85 participants) compared the same between sufentanil and fentanyl groups. No significant differences were observed with the pooled estimates for either alfentanil {-6.32 [-13.23, 0.60]} or sufentanil {-4.01 [-9.85, 1.84]} when combined with propofol.
Fig. (3).
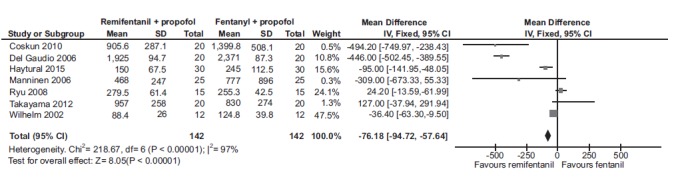
Forest plot of total propofol dose (mg) required to achieve general anesthesia when combined with either remifentanil and fentanyl. A statistically significant decrease in the required total dose of propofol was observed with remifentanil than fentanyl to achieve general anesthesia.
3.2.2. Time for Induction of Anesthesia
Three studies (94 patients) compared the induction time for anesthesia when remifentanil was combined with propofol to fentanyl. The pooled estimate was found to favor remifentanil {-0.44 [-0.74, -0.15]} Fig. (4). There were no studies comparing the induction time for alfentanil or sufentanil when combined with propofol.
Fig. (4).
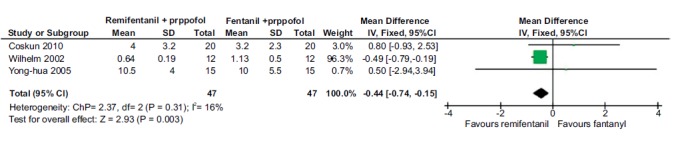
Forest plot of time taken for induction of anesthesia for remifentanil and fentanyl, in combination with propofol. A statistically significant difference was observed in the time taken for inducing anesthesia for remifentanil combination with propofol than fentanyl combination.
3.2.3. Duration of Anesthesia
Three studies (110 patients) compared the duration of anesthesia (in minutes) for remifentanil compared to fentanyl when combined with propofol. The pooled estimate was not found to be significantly different between the groups {2.37 [-2.52, 7.25]}. Only one study compared the duration of anesthesia with alfentanil and sufentanil and hence pooling of the results was not attempted.
3.2.4. Time Taken for Eye-opening
Three studies (120 participants) compared the time taken for eye-opening (in minutes) when remifentanil was combined with propofol compared to fentanyl and propofol. The pooled estimate was observed to favor remifentanil with the mean difference [95% confidence interval] of -3.95 [-4.8, -3.1] Fig. (5). Two studies (300 patients) compared the time taken for awakening when alfentanil was combined with propofol compared to fentanyl and propofol combination but the pooled estimate was not found to be statistically significant {-0.27 [-0.89, 0.34]}. Unfortunately, only one study compared this outcome parameter for sufentanil and so data pooling was not attempted.
Fig. (5).
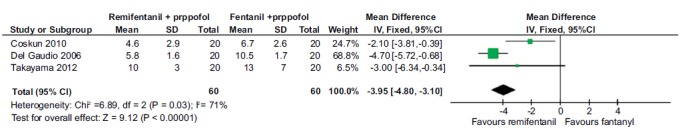
Forest plot of eye opening time (in minutes) for remifentanil compared to fentanyl with propofol. Time taken for opening eyes was found to be significantly lower with remifentanil when combined with propofol in comparison to fentanyl.
3.2.5. Time for Extubation
Three studies (120 patients) compared the time taken for extubation for remifentanil and fentanyl. The pooled mean difference [95% confidence interval] was -3.53 [-4.37, -2.7] favoring remifentanil Fig. (6). Only one study compared the time for extubation for alfentanil and sufentanil and so pooling of the results was not attempted.
Fig. (6).
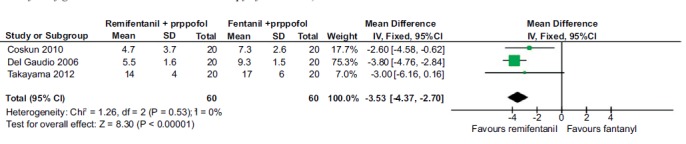
Forest plot of extubation time for remifentanil in comparison to fentanyl combination with propofol. A statistically significant reduction in the time taken for extubation was observed with remifentanil in comparison with fentanyl in combination with propofol.
3.2.6. Bispectral Index
Two studies (67 patients) compared the bispectral index when remifentanil was combined with propofol to fentanyl combination and the pooled estimate was not statistically significant {1.69 [-0.28, 3.65]}. Only one study compared the bispectral index with alfentanil and sufentanil and hence no pooling of the results was not attempted.
4. DISCUSSION
We conducted the present study to assess the profile of general anesthesia with remifentanil, sufentanil and alfentanil when combined with propofol and compared to fentanyl with propofol combination. A total of 14 studies were included in this review, and we observed that when remifentanil was combined with propofol, a significantly low dose of propofol was required to achieve general anesthesia. Moerover, the duration, onset and depth of general anesthesia were significantly more compared to fentanyl combination.
Combination of opioid analgesic with propofol has been shown to be an effective and safe method of analgosedation for patients undergoing mechanical ventilation [24]. Propofol has also been shown to be a better general anesthetic agent for successful insertion of laryngeal mask airway as it sufficiently suppresses the laryngeal reflexes leading to minimal coughing, gagging, and laryngospasm [25]. However, the incidence of pain following propofol administration is a major disadvantage, reducing the quality of anesthesia. The combination of opioid analgesics with propofol has been shown to prevent pain with a number needed to treat (NNT) of 3 to 4 in comparison to lidocaine where NNT was found to be 2.4 [26]. Combination of opioids with propofol has also been shown to improve the success of laryngeal mask airway insertion [27]. Additionally, the combination of propofol with fentanyl has been shown to decrease the incidence of fentanyl-induced cough more than lidocaine and NMDA-receptor antagonists in a meta-analysis [28]. Weisenberg et al. have observed a 31% increase in the mean number of hypotensive or bradycardia episodes requiring interventions with an increase of 0.3 mg/kg dose of propofol [25]. Combining an opioid analgesic lowers the total dose of propofol that is required, thereby reducing the risk of propofol-induced cardiac adverse events. We also found that the dose of propofol required to produce general anesthesia is significantly lower with remifentanil than fentanyl. In addition to duration, remifentanil also has a faster onset and recovery of
anesthesia when compared to fentanyl. Other favorable pharmacological aspects of remifentanil include minimal alteration of the pharmacokinetics, in patients with extremes of age or renal or hepatic dysfunction and ease of drug administration and titration [29]. Similarly, Kawano et al. [30]
have also shown that co-administration of remifentanil reduces the intra-operative blood loss significantly than fentanyl. Despite having a better anesthetic profile, Beers et al. have shown that the perioperative drug cost for remifentanil was $ 17.72 more than fentanyl [31]. However, cost-effectiveness data of the combination of opioid analgesics with propofol is lacking.
The strength of this review is that this is the first systematic compilation and pooled analysis of the existing literature regarding the use of opioids as adjuvants with propofol for obtaining general anesthesia. However, the review was also limited by the following: our search databases did not include EMBASE due to access constraints; dose variations in the propofol and individual opioids were not accounted for; due to paucity in the total number of studies with alfentanil and sufentanil, valid estimates could not be obtained; and most of the included studies had a high risk of bias in at least one of the domains.
CONCLUSION
To conclude, we found that remifentanil has a statistically significant anesthetic profile than fentanyl when combined with propofol. Scanty evidence for both alfentanil and sufentanil precludes any such confirmation.
ACKNOWLEDGEMENTS
We thank PROSPERO for registering the protocol of this review and Cochrane for using RevMan software for generating the pooled estimates and Forest plot.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
The present study was designed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.McKeage K., Perry C.M. Propofol: a review of its use in intensive care sedation of adults. CNS Drugs. 2003;17(4):235–272. doi: 10.2165/00023210-200317040-00003. [http://dx.doi.org/10.2165/00023210-200317040-00003]. [PMID: 12665397]. [DOI] [PubMed] [Google Scholar]
- 2.Jalota L., Kalira V., George E., et al. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342:d1110. doi: 10.1136/bmj.d1110. [DOI] [PubMed] [Google Scholar]
- 3.Morton N.S., Johnston G., White M., Marsch B.J. Propofol in paediatric anaesthesia. Paediatr. Anaesth. 1992;2:89–97. [http://dx.doi.org/10.1111/j.1460-9592.1992.tb00182.x]. [Google Scholar]
- 4.Marik P.E. Propofol: therapeutic indications and side-effects. Curr. Pharm. Des. 2004;10(29):3639–3649. doi: 10.2174/1381612043382846. [http://dx.doi.org/10.2174/1381612043382846]. [PMID: 15579060]. [DOI] [PubMed] [Google Scholar]
- 5.Peng P.W.H., Sandler A.N. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology. 1999;90(2):576–599. doi: 10.1097/00000542-199902000-00034. [http://dx.doi.org/10.1097/00000542-199902000-00034]. [PMID: 9952166]. [DOI] [PubMed] [Google Scholar]
- 6.Kizilcik N., Menda F., Bilgen S., Keskin O., Koner O. Effects of a fentanyl-propofol mixture on propofol injection pain: a randomized clinical trial. Korean J. Anesthesiol. 2015;68(6):556–560. doi: 10.4097/kjae.2015.68.6.556. [http://dx.doi.org/10.4097/kjae.2015.68.6.556]. [PMID: 26634078]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedighinejad A., Naderi Nabi B., Haghighi M., et al. Propofol is effec-tive to depress fentanyl-induced cough during of anesthesia. Anesth. Pain Med. 2013;2(4):170–173. doi: 10.5812/aapm.8383. [http://dx.doi.org/10.5812/aapm.8383]. [PMID: 24223355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 edition. Available from. www.cochrane-handbook.org
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [http://dx.doi.org/10.1016/j.jclinepi.2009.06.005]. [PMID: 19631508]. [DOI] [PubMed] [Google Scholar]
- 10.Hui J.K.L., Critchley L.A.H., Karmakar M.K., Lam P.K.K. Co-administration of alfentanil-propofol improves laryngeal mask airway insertion compared to fentanyl-propofol. Can. J. Anaesth. 2002;49(5):508–512. doi: 10.1007/BF03017932. [http://dx.doi.org/10.1007/BF03017932]. [PMID: 11983670]. [DOI] [PubMed] [Google Scholar]
- 11.Manninen P.H., Balki M., Lukitto K., Bernstein M. Patient satisfaction with awake craniotomy for tumor surgery: a comparison of remifentanil and fentanyl in conjunction with propofol. Anesth. Analg. 2006;102(1):237–242. doi: 10.1213/01.ANE.0000181287.86811.5C. [http://dx.doi.org/10.1213/01.ANE.0000181287.86811.5C]. [PMID: 16368836]. [DOI] [PubMed] [Google Scholar]
- 12.Haytural C., Aydınlı B., Demir B., et al. Comparison of propofol, propofol-remifentanil and propofol-fentanyl admin-istrations with each other used for the sedation of patients to undergo ERCP. BioMed Res. Int. 2015:2015465465. doi: 10.1155/2015/465465. [http://dx.doi.org/10.1155/2015/465465]. [PMID: 26576424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong-hua Y.A.O., Zi-lin W.U. Clinical investigation of total intravenous anesthesia with remifentanil and propofol for co-lonoscopy in the elderly. J. First Mil. Med. Univ. 2005;25:715–717. [PubMed] [Google Scholar]
- 14.Srivastava U., Mishra A.R., Sharma S., et al. Anaesthesia for direct laryngoscopy with propofol and fentanyl or sufentanil. Indian J. Otolaryngol. Head Neck Surg. 2008;60(4):314–316. doi: 10.1007/s12070-008-0106-x. [http://dx.doi.org/10.1007/s12070-008-0106-x]. [PMID: 23120572]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu J-H., Kim J-H., Park K-S., Do S-H. Remifentanil-propofol versus fentanyl-propofol for monitored anesthesia care during hysteroscopy. J. Clin. Anesth. 2008;20(5):328–332. doi: 10.1016/j.jclinane.2007.12.015. [http://dx.doi.org/10.1016/j.jclinane.2007.12.015]. [PMID: 18761238]. [DOI] [PubMed] [Google Scholar]
- 16.Lysakowski C., Dumont L., Pellegrini M., Clergue F., Tassonyi E. Effects of fentanyl, alfentanil, remifentanil and sufentanil on loss of consciousness and bispectral index during propofol induction of anaesthesia. Br. J. Anaesth. 2001;86(4):523–527. doi: 10.1093/bja/86.4.523. [http://dx.doi.org/10.1093/bja/86.4.523]. [PMID: 11573626]. [DOI] [PubMed] [Google Scholar]
- 17.Kwak H.J., Kim J.Y., Kwak Y.L., Park W.S., Lee K.C. Comparison of a bolus of fentanyl with an infusion of alfentanil during target-controlled propofol infusion in third molar extraction under conscious sedation. J. Oral Maxillofac. Surg. 2006;64(11):1577–1582. doi: 10.1016/j.joms.2005.11.112. [http://dx.doi.org/10.1016/j.joms.2005.11.112]. [PMID: 17052581]. [DOI] [PubMed] [Google Scholar]
- 18.Ho W.M., Yen C-M., Lan C-H., et al. Comparison between the recovery time of alfentanil and fentanyl in balanced propofol sedation for gastrointestinal and colonoscopy: a prospective, randomized study. BMC Gastroenterol. 2012;12:164. doi: 10.1186/1471-230X-12-164. [http://dx.doi.org/10.1186/1471-230X-12-164]. [PMID: 23170921]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama A., Yamaguchi S., Ishikawa K., et al. Recovery of psychomotor function after total intravenous anesthesia with remifentanil-propofol or fentanyl-propofol. J. Anesth. 2012;26(1):34–38. doi: 10.1007/s00540-011-1266-5. [http://dx.doi.org/10.1007/s00540-011-1266-5]. [PMID: 22048284]. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm W., Biedler A., Huppert A., et al. Comparison of the effects of remifentanil or fentanyl on anaesthetic induction characteristics of propofol, thiopental or etomidate. Eur. J. Anaesthesiol. 2002;19(5):350–356. doi: 10.1017/s026502150200056x. [http://dx.doi.org/10.1097/00003643-200205000-00006]. [PMID: 12095015]. [DOI] [PubMed] [Google Scholar]
- 21.Ahonen J., Olkkola K.T., Hynynen M., et al. Comparison of alfentanil, fentanyl and sufentanil for total intravenous anaesthesia with propofol in patients undergoing coronary artery bypass surgery. Br. J. Anaesth. 2000;85(4):533–540. doi: 10.1093/bja/85.4.533. [http://dx.doi.org/10.1093/bja/85.4.533]. [PMID: 11064610]. [DOI] [PubMed] [Google Scholar]
- 22.Coskun D., Celebi H., Karaca G., Karabiyik L. Remifentanil versus fentanyl compared in a target-controlled infusion of propofol anesthesia: quality of anesthesia and recovery profile. J. Anesth. 2010;24(3):373–379. doi: 10.1007/s00540-010-0898-1. [http://dx.doi.org/10.1007/s00540-010-0898-1]. [PMID: 20229001]. [DOI] [PubMed] [Google Scholar]
- 23.Del Gaudio A., Ciritella P., Perrotta F., et al. Remifentanil vs fentanyl with a target controlled propofol infusion in patients undergoing craniotomy for supratentorial lesions. Minerva Anestesiol. 2006;72(5):309–319. [PMID: 16675939]. [PubMed] [Google Scholar]
- 24.Tedders K.M., McNorton K.N., Edwin S.B. Efficacy and safety of analgosedation with fentanyl compared with traditional sedation with propofol. Pharmacotherapy. 2014;34(6):643–647. doi: 10.1002/phar.1429. [http://dx.doi.org/10.1002/phar.1429]. [PMID: 24753262]. [DOI] [PubMed] [Google Scholar]
- 25.Weisenberg M., Sessler D.I., Tavdi M., et al. Dose-dependent hemodynamic effects of propofol induction following brotizolam premedication in hypertensive patients taking angiotensin-converting enzyme inhibitors. J. Clin. Anesth. 2010;22(3):190–195. doi: 10.1016/j.jclinane.2009.07.008. [http://dx.doi.org/10.1016/j.jclinane.2009.07.008]. [PMID: 20400005]. [DOI] [PubMed] [Google Scholar]
- 26.Picard P., Tramèr M.R. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth. Analg. 2000;90(4):963–969. doi: 10.1097/00000539-200004000-00035. [http://dx.doi.org/10.1213/00000539-200004000-00035]. [PMID: 10735808]. [DOI] [PubMed] [Google Scholar]
- 27.Goyagi T., Tanaka M., Nishikawa T. Fentanyl decreases propofol requirement for laryngeal mask airway insertion. Acta Anaesthesiol. Scand. 2003;47(6):771–774. doi: 10.1034/j.1399-6576.2003.00123.x. [http://dx.doi.org/10.1034/j.1399-6576.2003.00123.x]. [PMID: 12803598]. [DOI] [PubMed] [Google Scholar]
- 28.Kim J.E., Min S.K., Chae Y.J., Lee Y.J., Moon B.K., Kim J.Y. Pharmacological and nonpharmacological prevention of fentanyl-induced cough: a meta-analysis. J. Anesth. 2014;28(2):257–266. doi: 10.1007/s00540-013-1695-4. [http://dx.doi.org/10.1007/s00540-013-1695-4]. [PMID: 23958914]. [DOI] [PubMed] [Google Scholar]
- 29.Beers R., Camporesi E. Remifentanil update: clinical science and utility. CNS Drugs. 2004;18(15):1085–1104. doi: 10.2165/00023210-200418150-00004. [http://dx.doi.org/10.2165/00023210-200418150-00004]. [PMID: 15581380]. [DOI] [PubMed] [Google Scholar]
- 30.Kawano H., Manabe S., Matsumoto T., et al. Comparison of intraoperative blood loss during spinal surgery using either remifentanil or fentanyl as an adjuvant to general anesthesia. BMC Anesthesiol. 2013;13(1):46. doi: 10.1186/1471-2253-13-46. [http://dx.doi.org/10.1186/1471-2253-13-46]. [PMID: 24304964]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beers R.A., Calimlim J.R., Uddoh E., Esposito B.F., Camporesi E.M. A comparison of the cost-effectiveness of remifentanil versus fentanyl as an adjuvant to general anesthesia for outpatient gynecologic surgery. Anesth. Analg. 2000;91(6):1420–1425. doi: 10.1097/00000539-200012000-00022. [http://dx.doi.org/10.1097/00000539-200012000-00022]. [PMID: 11093992]. [DOI] [PubMed] [Google Scholar]


