Abstract
Objective
Headache associated with ischemic stroke is poorly understood. To gain further insight, we systematically reviewed studies examining the prevalence and characteristics of new-onset poststroke headache.
Methods
Medline and PubMed databases were queried. A total of 1,812 articles were identified. Of these, 50 were included in this systematic review. Twenty were included in a meta-analysis and meta-regression.
Results
Headache occurred in 6%–44% of the ischemic stroke population. Most headaches had tension-type features, were moderate to severe, and became chronic in nature. Meta-analysis using an inverse-variance heterogeneity model revealed a pooled prevalence of 0.14 (95% confidence interval [CI] 0.07–0.23) with heterogeneity among studies. Metaregression revealed a significant association between prevalence and study location, the source population's national human development index (HDI), and study quality. We found higher prevalence in European (0.22, 95% CI 0.14–0.30) and North American (0.15, 95% CI 0.05–0.26) studies compared with Middle Eastern and Asian studies (0.08, 95% CI 0.01–0.18). However, within each region, populations from countries with higher HDI (p = 0.03) and studies with higher quality (p = 0.001) had lower prevalence. Calculated crude odds ratios (ORs) showed that posterior circulation stroke (pooled OR 1.92, 95% CI 1.4–2.64; n = 7 studies) and female sex (pooled OR 1.25, 95% CI 1.07–1.46; n = 11 studies) had greater odds of headache associated with ischemic stroke.
Conclusions
Taken together, these data suggest that headache is common at the onset of or shortly following ischemic stroke and may contribute to poststroke morbidity. Better understanding of headache associated with ischemic stroke is needed to establish treatment guidelines and inform patient management.
Ischemic stroke affects approximately 800,000 individuals annually in the United States alone and accounts for 1 in every 20 deaths.1 Stroke is among the top 20 conditions contributing to years lived with disability. While the focus after stroke is often on recovery of neurologic function and reducing the risk of recurrence, the emergence of comorbid conditions like poststroke headache is often overlooked and undertreated.
Evidence suggests a link between stroke and headache disorders. For example, migraine with aura, a primary headache disorder, is associated with a twofold higher risk of ischemic stroke.2,3 While the associations between a preexisting history of migraine and stroke are well-known, the epidemiology, predictive potential, and treatment underlying new onset poststroke headache or headache as a symptom heralding stroke remain unclear. While headache as a presenting feature of cerebrovascular conditions, such as venous sinus thrombosis, cervical artery dissection, reversible cerebral vasoconstriction syndrome, and vasculitis, has been well-described, few studies focus on new-onset and persistent headache after ischemic stroke in the absence of these conditions.
Prior studies report a wide range of prevalence of headache associated with ischemic stroke.4–6 New-onset headache presenting at the time of acute ischemic stroke is a predictor of persistent headache 6 months after the stroke.7 Indeed, headache after stroke has been characterized as a common form of chronic poststroke pain.8,9 Given the high prevalence of stroke in the United States, poststroke headache can be a significant cause of disability. However, the dearth of epidemiologic and outcome-based studies limits our understanding and treatment of persistent poststroke headache. Therefore, we undertook a systematic literature review and meta-analysis of stroke onset and poststroke headache, its presentation, epidemiology, and clinical outcomes.
Methods
Systematic review strategy
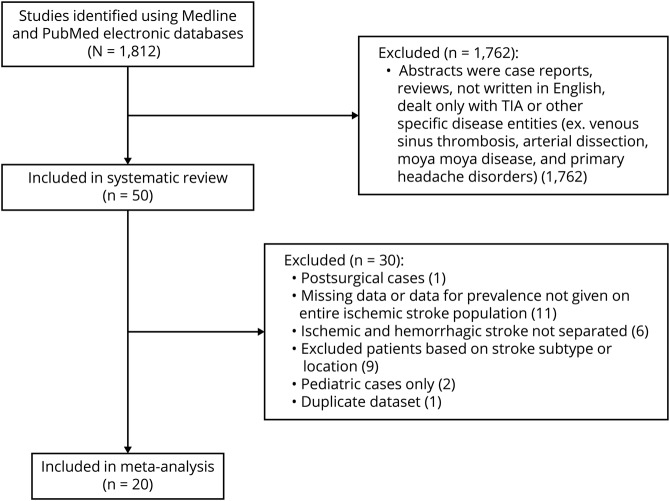
Medline and PubMed electronic databases were queried using the search terms “headache after stroke” or “poststroke headache” or “onset headache and stroke” and “headache and stroke.” Abstracts of observational and clinical studies written in English and containing the search terms in the title or abstract body were reviewed. If the articles found using the search terms referenced epidemiologic or outcomes data from other primary research articles, those articles were also included. A total of 1,812 articles on stroke and headache were thus identified. To determine the epidemiology of onset and poststroke headache and its characteristics, retrospective and prospective studies were included after abstracts were reviewed by A.M.H. and F.K. Case reports and reviews, articles without an abstract, articles on specific disease entities such as venous sinus thrombosis, arterial dissection, moyamoya disease, and primary headache disorders (i.e., migraine headache), and articles that included only intracranial hemorrhage or TIA were excluded. Of 1,812 articles, 50 primary research articles published between 1993 and 2018 were included in the systematic review (figure 1). Of the 50 primary research articles published between 1993 and 2018 included in this study, 38 were prospective cohort studies and 11 were retrospective, representing diverse populations throughout the world.
Figure 1. Flow diagram of study inclusion/exclusion.
Search strategies generated 1,812 articles using the search terms detailed in the Methods. Of these, 20 studies were included in the meta-analysis of prevalence.
Ischemic stroke was defined based on either clinical or radiologic criteria. Headache associated with ischemic stroke (HAIS) was variably defined among the studies. Some defined it as a presenting symptom during an ischemic stroke, onset simultaneous with or in close temporal relationship to stroke onset. Some described it as a concomitant symptom or accompaniment, present at admission to the hospital, and others used specific time windows with onset ranging from 24 to 72 hours before or after stroke onset.
Meta-analysis
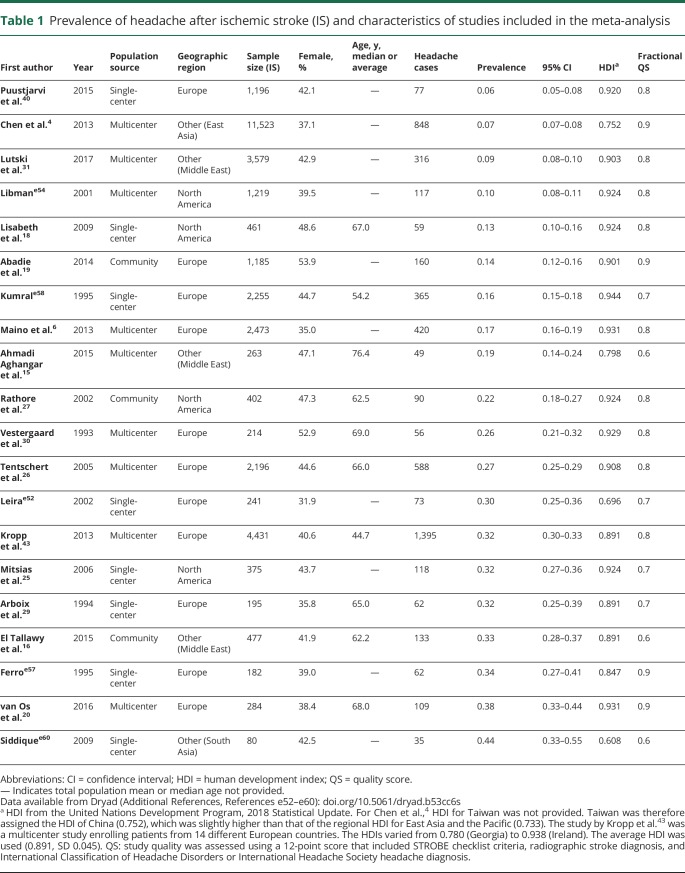
Of the 50 primary research articles in the systematic review, studies were included in the meta-analysis if they met the following criteria: enrolled adult participants (age >18 years) with ischemic stroke and presented prevalence data for the entire ischemic stroke population enrolled in the study. Articles were excluded if they only enrolled patients with a specific stroke subtype or location, if they only enrolled patients with stroke and headache, if there was no distinction between hemorrhagic and ischemic stroke in headache prevalence estimates, if the study only enrolled pediatric patients (age < 18 years), if prevalence could not be calculated from the data given, or if it was a duplicate population. In the case of duplicate population data, the article with the highest enrollment was included in the meta-analysis. Twenty studies were included in the meta-analysis (table 1 and figure 1).
Table 1.
Prevalence of headache after ischemic stroke (IS) and characteristics of studies included in the meta-analysis
We extracted data on study location, dates of enrollment, year of publication, study design, population (hospital or community-based), age, sex, headache prevalence, use of headache classification criteria, subgroup analysis including prevalence values by stroke location and sex, and data for quality assessment from these studies. We generated pooled prevalence estimates using an inverse variance heterogeneity model where the weights assigned to each study vary inversely with the square of the standard error.10 This model has an advantage over the random effects model that may yield faulty pooled estimates when there is high heterogeneity among studies. As heterogeneity or differences between studies increases, the random effects model tends towards an unweighted pooled estimate. The result of high heterogeneity on the random effects model is decreased coverage of the confidence interval (CI) and an overconfident estimate because of underestimation of the error. To overcome this shortcoming and allow for a pooled estimate in studies suffering from heterogeneity, we used an inverse variance heterogeneity model to determine the pooled estimate.10,11 Nevertheless, the results of the random effects model are also provided.
Meta-regression of heterogeneity
Heterogeneity among the studies was assessed using the I2 statistic and Cochran Q. To help understand the sources of heterogeneity, meta-regression analysis was performed using study characteristics as moderator variables, including year of publication, population source (community-based, single-center, or multicenter), and geographic region (North American, European, and Other, including the Middle East and Asia). Because the reporting of headache after stroke may vary depending on health literacy and awareness of the source population, we included the United Nations national human development index (HDI), which incorporates life expectancy, education, and gross national income per capita, for each study's source population as a variable in the meta-regression. For studies that included patients from multiple countries, the average HDI of these countries was used. For one study from Taiwan,4 HDI for mainland China was used as there was no HDI published for Taiwan. Finally, we also included study quality as an independent variable in the form of a score derived in part from the STROBE checklist for observational study reporting12 (study design, location, date and period of recruitment, eligibility criteria, diagnostic criteria, assessment of bias, description of statistical methods and confounding variables, reporting of demographic data and subgroup analysis, discussion of study limitations, generalizability of study results to the population, and assessment of missing data for subgroup analyses). We added 2 other criteria relevant to stroke and headache epidemiology (use of International Classification of Headache Disorders [ICHD] or International Headache Society criteria for diagnosis of headache, use of CT or MRI for diagnosis of stroke). Each criterion was assigned 0 if not present and 1 if present. The quality score (QS) for each study was expressed as a fraction by dividing the total points by the highest possible score (12).
Patient characteristics as risk factors for headache after stroke
To determine the effect of stroke location (posterior vs anterior circulation) and sex on ischemic stroke headache, data from studies that reported headache prevalence in these subgroups were used to calculate a crude odds ratio (OR) using 2 × 2 tables for each study and combined using a fixed effects heterogeneity model to generate a pooled estimate.
Publication bias
Publication bias was graphically represented with funnel and doi plots.13
Statistical analysis
Meta-regression data were generated using Meta-XL software (Epigear; epigear.com/index_files/metaxl.html) and analyzed using Stata Statistical Software.
p ≤ 0.05 was considered significant. The pooled estimates and 95% CIs are given. The effect of posterior circulation stroke or female sex was considered significant if the CI of the pooled estimate did not contain the null value of 1.
Data availability
Data will be provided to other investigators upon request made to the corresponding author, A.M.H., in accordance with International Committee of Medical Journal Editors requirements.
Results
Meta-analysis
Prevalence of HAIS
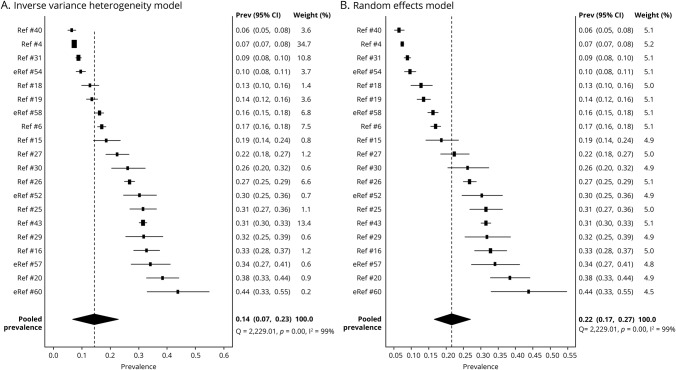
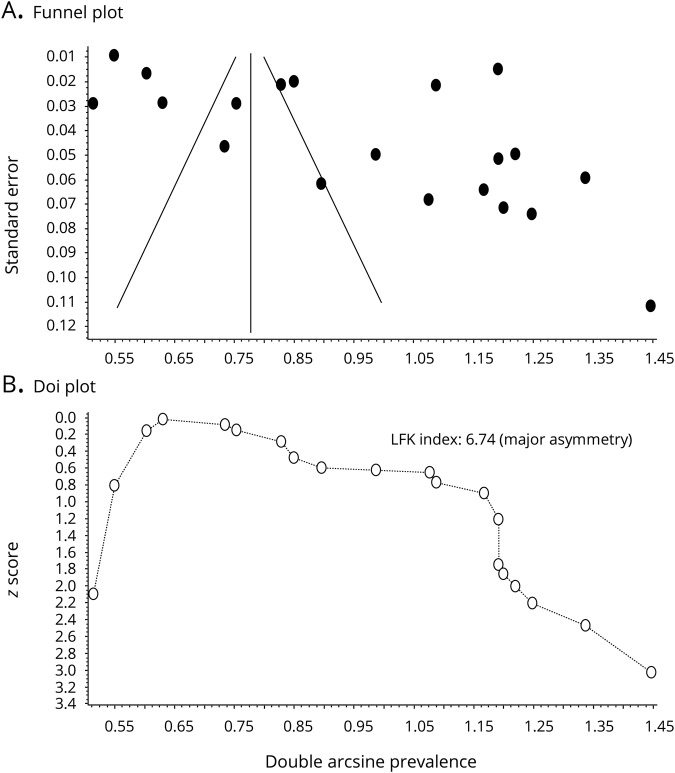
We performed a meta-analysis of 20 prospective studies (8 single-center, 9 multicenter, and 3 community-based) to estimate the prevalence of HAIS (table 1). Studies included 11 European, 4 North American, and 5 Asian or Middle Eastern populations. Enrollment ranged between 80 and 11,523 participants. The ratio of women ranged between 32% and 54%, whereas the average or median age ranged between 44.7 and 76.4 years, when reported. The prevalence of HAIS was between 0.06 and 0.44. An inverse variance heterogeneity model showed a pooled prevalence rate of 0.14 (95% CI 0.07–0.23; figure 2); this model was chosen over a random effects model to better account for the high degree of heterogeneity in the prevalence estimates among the studies (I2 99%, Cochran Q statistic 2,229). The random effects model yielded a higher pooled prevalence of 0.22 (95% CI 0.17–0.27; figure 2). Publication bias may have influenced the pooled prevalence estimate given the wide distribution of studies on the funnel plot and the asymmetric doi plot (figure 3).
Figure 2. Pooled prevalence of ischemic stroke headache.
(A) Inverse variance heterogeneity model: forest plot shows prevalence by individual study and the % weight, which is inversely proportional to the squared value of the standard error. The pooled prevalence of ischemic stroke–related headache is 14%. (B) Random effects model: pooled prevalence of ischemic stroke headache using the random effects model yields a higher pooled estimate (22%). Because of the high degree of heterogeneity, the random effects model tends to unweight the studies, as demonstrated by the % weight values falling between a narrow range of 4.5–5.2. CI = confidence interval.
Figure 3. Assessment of publication bias.
Given broad scatter of the funnel plot (A) and asymmetry of the doi plot (B), the prevalence estimate is likely affected by publication bias.
Sources of heterogeneity
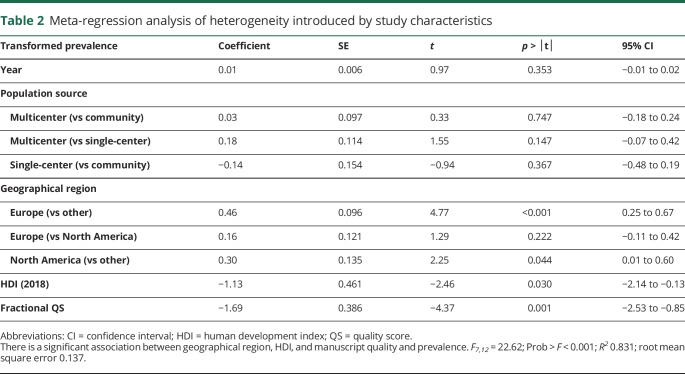
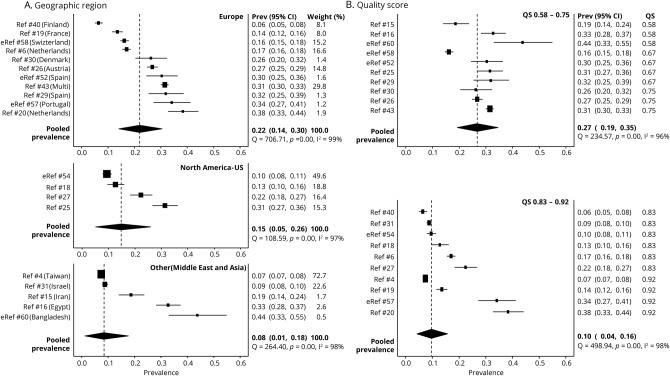
To better understand the contribution of study characteristics to the heterogeneity, we performed a meta-regression using the variables publication year, population source, geographic region, HDI, and QS. The initial model showed a significant association with the double arcsine-transformed prevalence (R2 = 0.831, p < 0.001), suggesting that one or more of the variables included in the model could predict the prevalence (table 2). To determine which study characteristics likely contribute to the heterogeneity, we examined the relationship between the individual moderator variables and the prevalence. There was no significant effect of publication year or population source on the prevalence. However, geographic region was significantly associated with prevalence. Studies performed in Europe (p < 0.001) or North America (p = 0.044) had a higher HAIS prevalence as compared to those performed in other regions (i.e., Asia and the Middle East) when Other was taken as the referent level. When North America was used as the referent level, there was no significant difference in prevalence between European and North American studies (table 2). The pooled prevalence rate of HAIS for studies performed in Europe was 0.22 (95% CI 0.14–0.30), North America was 0.15 (95% CI 0.05–0.26), and Other (Middle East and Asia) was 0.08 (95% CI 0.01–0.18; figure 4) The pooled prevalence of the Other category is heavily influenced by a single Taiwanese study4 because of the large sample size and narrow CI.
Table 2.
Meta-regression analysis of heterogeneity introduced by study characteristics
Figure 4. Study characteristics: geographic region and quality score (QS).
(A) Headache prevalence varies depending on regional location. European studies had higher prevalence values as compared to studies from other regions (Middle East and Asia). Likewise, studies whose source population was from the United States had higher prevalence values than those from Middle East and Asia. (B) Studies with a lower QS report a higher headache prevalence as compared to studies with higher QS (QS − fractional QS). CI = confidence interval.
After controlling for the geographic region, increasing HDI was associated with lower HAIS prevalence (p = 0.030; table 2). The United Nations HDI was not available for Taiwan. Therefore, the HDI for mainland China (0.752) was used for the Taiwanese study in the meta-regression.4 Since this study is heavily weighted, we examined if changing the HDI for this study influenced the association between prevalence and HDI in our meta-regression. If the HDI for Taiwan is changed from 0.75 to 0.7, 0.8, or 0.85, the association between the HDI and prevalence remains significant (p = 0.029, p = 0.027, and p = 0.020, respectively).
After controlling for the other variables, higher manuscript quality score was associated with lower HAIS prevalence (p = 0.001). Studies with the lowest quality score of 0.58 had a pooled prevalence of 0.29, whereas those with the highest quality score of 0.92 had a prevalence of 0.09 (figure 4).
Risk factors
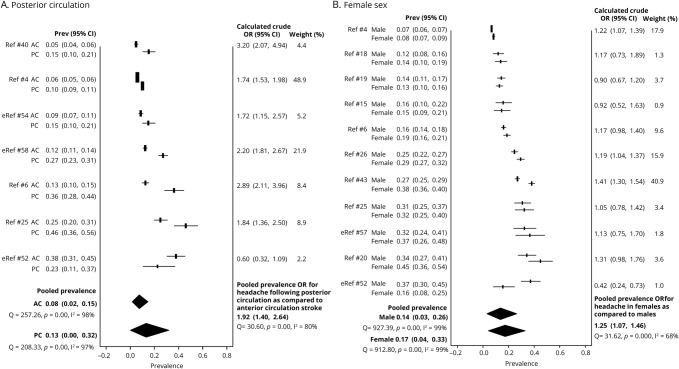
A higher rate of HAIS has been reported with posterior circulation strokes and in women. Therefore, we compared the pooled prevalence and crude ORs of HAIS in anterior vs posterior circulation strokes. In the meta-analysis, a majority of studies that specified stroke location reported an almost 2-fold higher prevalence of HAIS in posterior circulation strokes (pooled OR 1.92, 95% CI 1.4–2.64; figure 5). A majority of studies that specified sex showed a modestly higher odds of HAIS in women (pooled OR 1.25, 95% CI 1.07–1.46; figure 5). Age was not reported as a dichotomous or stratified variable in most studies, and therefore, was not included in the meta-analysis.
Figure 5. Patient characteristics: posterior circulation (PC) stroke and female sex are associated with greater odds of ischemic stroke headache.
(A) Headache prevalence for PC and anterior circulation (AC) stroke is reported for each study. The pooled headache prevalence for PC stroke is higher than that for AC stroke. The odds of having headache is 2-fold higher with PC stroke as compared with AC stroke. (B) Headache prevalence for female and male participants is reported for each study. There is a modestly higher prevalence of stroke-related headache in women as compared to men with a pooled odds ratio (OR) of 1.25. CI = confidence interval.
Systematic review
We further conducted a systematic review of the literature on HAIS of 50 articles, including the 20 used for the meta-analysis, to gain insight on timing, duration, characteristics, and outcome of HAIS, and the effect of age and stroke subtype.4,6,7,14–50,e51–e60
Prevalence, time of onset, and duration of headache in relation to stroke symptom onset
The prevalence of headache in the larger systematic review was within the range found in the meta-analysis.4,6,14–30 ICHD-3 defines HAIS as either acute or persistent and if “headache has developed in very close temporal relation to other symptoms and/or clinical signs of ischemic stroke, or has led to the diagnosis of ischemic stroke.”e61 Of the 16 studies that described the timing of headache assessment, 12 studies evaluated headache within 3–4 days of stroke symptoms,6,15,20,22–25,30,35,49,e55,e57 and the remaining 4 within 8–14 days.14,34,37,48 The headache onset occurred prior to, simultaneous with, or after stroke symptom onset. In one study, 86% of patients reported headache onset on the day of stroke symptoms; the remainder had headache onset 2–5 days after.48 In another study exclusively of lacunar infarction, 93% reported headache occurring simultaneously with stroke symptoms and only 7% occurring prior.24 In a third study, 31% had headache prior to, 11% simultaneous with, and 45% after the onset of stroke symptoms (1% within seconds, 10% within minutes, and 34% within hours). The timing was unknown in the remaining 13%.20 Altogether, these data suggest that most patients experience headache symptoms on the day of stroke presentation.
The initial headache duration varied from 1 to 4 days in the 3 studies in which it was reported.20,24,48 Similarly, few studies examined headache persistence during follow-up (on average >3 months) as defined by ICHD-3, with a wide prevalence of persistent headache ranging from 1% to 23%.14,17,23,45 In one cohort, only 1%–2% had persistent headache at 48–175 days after stroke.14 In another cohort, 12% had persistent headache at 3 years follow-up, 81.2% of whom had 2–14 headache days per month, and 18.8% reported daily headache.17 In a study of 299 participants, headache persisted in 23% of the cohort at both 3 and 6 months.23 In a small study of 21 patients with severe strokes that required hemicraniectomy, 42.9% continued to have headache an average of 3 years after stroke, although it is unclear if this was related to the severity of the stroke or persistent postoperative pain.32 Altogether, these data suggest that HAIS can persist for months, and in some, even years.
Headache characteristics
In most studies, HAIS had tension-type features (50%–80% of headaches) though the quality of the pain appeared to be severe and intractable.7,24,26,50,e51 In one cohort, 66% described pressure, aching, or soreness, while only 17% had throbbing pain.20 Migraine-like symptoms were less common: photophobia occurred in 13%, phonophobia in 8%, nausea in 17%, and vomiting in 16% of patients with headache. Similarly, in a different cohort, 80% of patients had bilateral and pressure-like headache, whereas only 16% had throbbing and 10% had stabbing pain.37 In this study, 30% had photophobia, 24% phonophobia, and 28% nausea or vomiting. In a third cohort, 50% of patients with HAIS had tension-type features while 31% had migrainous features.17 Despite the paucity of migraine-like symptoms, more than 50% reported moderate to severe pain intensity. Few studies attempted to employ ICHD criteria to better categorize the poststroke headache quality. In one study examining 124 patients using ICHD criteria, the headache was described as tension-type, with predominantly bilateral (59%–75%) and anterior or frontal location (42%–69%).48 Over the first week after stroke onset, 53%–67% of patients reported pressure, 11%–24% reported throbbing, and 0%–9% reported stabbing pain. Taken together, these data suggest that while HAIS is more often associated with tension-type features (i.e., less often migraine-like), the headache intensity tends to be more severe.
Influence of age on HAIS
Most studies found an association between younger age and headache at stroke onset.4,22–24,26,39,43,47 In a prospective study of first ever ischemic stroke, 12.2% of those younger than 50 developed HAIS, compared with 8.5% of those older than 50 (p < 0.002).31 In another population, 45.8% of younger patients (average age 42) developed HAIS vs 20.2% of older patients (average age 75, p < 0.05).21 Contradicting these reports, 2 studies found no association.18,20 Taken together, these data suggest that younger adult stroke patients (<50 years) are more prone to develop HAIS. There are very few studies dedicated to examining onset headache in the pediatric stroke population. In pediatric stroke studies, 24%–55% of the population had onset headache.35,42
Influence of stroke location and etiology on HAIS
Consistent with the findings of the meta-analysis, other studies also reported an association between posterior circulation stroke and HAIS.22,28,36,43,e59,e62 In a small cohort study of 57 posterior circulation strokes, a large majority (79%) had accompanying headache.36 In a larger multicenter study, headache was associated with strokes involving the vertebrobasilar circulation (OR 2.07, 95% CI 1.48–2.9; p < 0.001) but not posterior cerebral artery territory (OR 1.17, 95% CI 0.85–1.63), suggesting higher risk with posterior fossa strokes.43
Ischemic lesions involving putative brain regions responsible for pain processing may also contribute to HAIS. In a study enrolling 100 patients with ischemic stroke, headache was associated with infarcts involving insular and somatosensory cortical brain regions.37 In another small prospective study, headache intensity was associated with strokes involving the posterior insula and operculum, while cranial autonomic symptoms accompanying headache were found in strokes affecting the parietal lobe, somatosensory cortex, and middle temporal cortex.34
HAIS may be associated with cortical as opposed to subcortical or deep strokes. In one study, headache accompanying minor ischemic strokes was positively associated with cortical infarcts (OR 1.78, 95% CI 1.31–2.41; p = 0.0001) and negatively associated with small, deep, or subcortical infarcts (OR 0.58, 95% CI 0.44–0.76; p = 0.00006). The prevalence of headache was 12% in those with small deep infarcts vs 29% in those with cortical infarcts.e62 These data are consistent with other studies examining cortical vs deep or subcortical infarct location.6,e57
The stroke subtype may also be associated with HAIS. Most studies report a greater association between HAIS and cardioembolic4,23,e53 and large vessel stroke4,23 as compared to small vessel/lacunar stroke.22,24,e57 In one study, small vessel occlusion was associated with a lower prevalence of headache (prevalence ratio [PR] 0.72, 95% CI 0.64–0.81; p < 0.001) as compared to those caused by large vessel atherosclerosis (PR 1.28, 95% CI 1.14–1.44; p < 0.001) or cardioembolism (PR 1.27, 95% CI 1.0–1.61; p < 0.05).4 Similar to this and other studies,e57 one study found that headache was more common in those with nonlacunar stroke (9.9%) as compared to those with lacunar stroke (6.3%) (p = 0.03).22 In another study that separated patients into cardioembolic and noncardioembolic subgroups, cardioembolic stroke was associated with a higher prevalence of HAIS (36.8% vs 28.8%; p < 0.05).e53 Conflicting data have also been reported.26 The rates of HAIS for cardioembolic, small vessel, large vessel, and undetermined stroke causes vary considerably among the 4 studies that reported these prevalence values (cardioembolic 4.4%–38.9%, large vessel 4.9%–40.8%, small vessel 5.5%–24.9%, and undetermined etiology 0%–35%). Given the wide and overlapping rates, the presence of headache at stroke onset does not provide further insight into stroke mechanism.4,22,26,29
Altogether, these studies suggest that HAIS is associated with posterior circulation and cortical strokes and that HAIS characteristics (quality, intensity, and accompanying symptoms) may be associated with stroke involving brain regions implicated in neural networks responsible for pain processing. HAIS prevalence may be higher in strokes of cardioembolic or large vessel origin.
Stroke outcomes
HAIS was associated with stroke misdiagnosis in several studies.38,41,44 In one retrospective study, HAIS was 3 times more common in cases with a missed diagnosis (OR 2.99, 95% CI 1.74–5.14),38 whereas another showed that HAIS was present in 55% of the patients with a probable missed diagnosis of stroke.41 One study linked HAIS to early signs of stroke on CT and higher levels of CSF glutamate, interleukin-6, and nitric oxide metabolites, raising the possibility that headache could signify more severe neuroinflammation or early stroke progression.e52 Despite this, HAIS did not appear to be associated with worse stroke outcomes. In one cohort, initial stroke severity and 3-month functional outcomes did not differ between patients with and without HAIS.20 In a larger study, patients with HAIS showed a greater improvement in deficits at discharge and a better functional outcome at 1 month.4 HAIS also did not appear to affect the risk of any recurrent stroke in a 14-year follow-up study (OR 0.97, 95% CI 0.76–1.24).6
Discussion
To our knowledge, this is the first meta-analysis of HAIS prevalence and risk factors. Our meta-analysis and systematic review show that new onset headache is common in the ischemic stroke population. The overall prevalence suggests that approximately 14% of adult patients with ischemic stroke have headache at the time of or shortly following their stroke diagnosis. That the headache often persists for months to years, can be continuous or daily, and can be moderate to severe in intensity implicate significant disability associated with HAIS and punctuate the need for evidence-based treatment approaches. The meta-analysis and systematic review also highlight the predominant patient-related phenotypes associated with HAIS including younger age, female sex, nonlacunar cortical stroke syndromes, and involvement of the posterior fossa.
The data presented in this study add to the previously published systematic reviews by integrating prior studies and using statistical methods to provide a more precise estimate of headache prevalence in the stroke population. The reproducible association between headache and ischemic stroke, and the close temporal relationship between the headache and stroke symptom onset, strongly argue against the headache being a random occurrence. Indeed, the pooled point prevalence of HAIS assessed at the time of stroke in studies included in the meta-analysis was at least as high as the point prevalence of all headache disorders, which ranges from 5.7%e63 to 16.4%.e64
There are several putative explanations for the association between posterior circulation stroke and HAIS. One possible explanation is a difference in trigeminal and autonomic innervation of the posterior cerebral vessels.e65 Indeed, differences in the innervation pattern of the meninges overlying the posterior cerebral cortices and cerebellume66 raise the possibility that strokes involving these areas may have a greater ability to stimulate pain-sensing trigeminal and cervical dorsal root ganglion afferent fibers innervating the coverings overlying these regions. The posterior circulation may have differential cerebral autoregulatione67 and become more susceptible to fluctuations in vasomotor tone and permeability. There may be increases in posterior fossa compartmental pressures following ischemic stroke as compared to anterior strokes. Finally, this association may also be related to migraine as a confounding variable. In migraineurs with aura, there is an increased risk of ischemic stroke, particularly involving the posterior circulation.
Migraine was not systematically assessed in many of the studies included in these meta-analyses.
Therefore, it is also possible that the increased prevalence of headache is related to a higher proportion of migraineurs in the subpopulation of patients with posterior circulation strokes as compared to anterior circulation strokes. Similarly, the modest association between female sex and HAIS could be related to a sex or hormonal influence on the development of trigeminal pain, and reflect a confounding effect of a preexisting migraine in this population. Nevertheless, the predominantly tension-type features could distinguish HAIS from a migraine attack. In fact, the difference in headache quality indicates that even in migraine patients with ischemic stroke, HAIS is probably a new secondary headache diagnosis.
The mechanisms underlying HAIS remain unclear. While an overlap with migraine pathogenesis (i.e., activation of trigeminovascular afferents or meningeal inflammation) has been postulated, that HAIS is qualitatively different from migraine suggests otherwise. It is unknown if and to what extent traction and mechanical forces, electrical events like spreading depolarization, platelet-derived factors, serotonergic mechanisms, or peptide-containing nociceptors contribute to HAIS.
There are several study-related limitations. For example, most studies did not state whether ICHD criteria were used, potentially leading to differences in when and how HAIS was defined. Most studies did not assess for preexisting diagnoses of primary headache disorders, potentially leading to misclassification bias. Recall bias was less likely because we included only prospective studies in the meta-analysis. While studies from multiple institutions and regions included in the meta-analysis increase the likelihood of generalizability of the pooled estimate, a single study with a lower headache prevalence of 8% contributed the largest weight to the pooled prevalence, raising the possibility for underestimation. Most studies excluded patients with severe stroke syndromes and aphasia, creating selection bias possibly contributing to underestimation. Finally, while the inverse variance heterogeneity model provides better coverage of the CI as compared to the random effects model in situations of high heterogeneity,10 well-defined cohort studies with clear inclusion/exclusion criteria and implementation of standard diagnostic criteria for HAIS would more appropriately address this problem.
HAIS is a common disorder that contributes to the global health burden of stroke. However, because of study limitations and methodologic heterogeneity between studies, the results presented herein underscore the need for future prospective studies to better estimate the true HAIS incidence and prevalence. In particular, we recommend that future cohort studies (1) report clear inclusion and exclusion criteria; (2) use ICHD criteria to define HAIS; (3) use ICHD criteria to define a preexisting history of migraine or other primary headache disorder; (4) report descriptive characteristics of HAIS, including demographic information and headache quality, severity, timing, and accompanying symptoms; and (5) report HAIS rates by ischemic stroke etiology and location. The overall disability and reduced quality of life associated with this diagnosis remain poorly understood. Therefore, cohort studies assessing the effect of HAIS on poststroke disability and quality of life are also needed. Finally, no clinical trials on poststroke headache treatments were found in this review of the literature. While tricyclic antidepressants and anticonvulsants have been suggested for central poststroke pain syndrome,e68 there is a regrettable lack of evidence supporting the use of daily medications aimed at reducing poststroke headache frequency. Future research should therefore be directed towards clinical trials of the efficacy of established headache preventive medications for the treatment of HAIS and secondary prevention approaches.
Acknowledgment
The authors thank Dr. Daniel Lackland for discussions.
Glossary
- CI
confidence interval
- HAIS
headache associated with ischemic stroke
- HDI
human development index
- ICHD
International Classification of Headache Disorders
- OR
odds ratio
- QS
quality score
Appendix. Authors
Study funding
Support is provided to A.M.H. by the Phyllis and Jerome Lyle Rappaport Foundation, Building Interdisciplinary Careers in Women's Health Award (NIH, 5K12HD051959-13), and the Training in Research for Academic Neurologists to Sustain Careers and Enhance the Numbers of Diverse Scholars (TRANSCENDS), National Institute of Neurological Disorders and Stroke/NIH Award through the American Academy of Neurology (R25 NS098999-02); and to C.A. by the Foundation Leducq, Heitman Foundation, and the Ellison Foundation Awards.
Disclosure
A.M. Harriott: support is provided by the Phyllis and Jerome Lyle Rappaport Foundation, Building Interdisciplinary Careers in Women's Health Award (NIH, 5K12HD051959-13), and the Training in Research for Academic Neurologists to Sustain Careers and Enhance the Numbers of Diverse Scholars (TRANSCENDS), National Institute of Neurological Disorders and Stroke/NIH Award through the American Academy of Neurology (R25 NS098999-02). F. Karakaya reports no disclosures relevant to the manuscript. C. Ayata: support is provided by the Foundation Leducq, Heitman Foundation, and the Ellison Foundation Awards. Go to Neurology.org/N for full disclosures.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics: 2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurth T, Diener HC. Migraine and stroke: perspectives for stroke physicians. Stroke 2012;43:3421–3426. [DOI] [PubMed] [Google Scholar]
- 3.Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia 2015;35:165–181. [DOI] [PubMed] [Google Scholar]
- 4.Chen PK, Chiu PY, Tsai IJ, et al. Onset headache predicts good outcome in patients with first-ever ischemic stroke. Stroke 2013;44:1852–1858. [DOI] [PubMed] [Google Scholar]
- 5.Edmeads J. The headache of ischemic cerebrovascular disease. Headache 1979;19:345–349. [DOI] [PubMed] [Google Scholar]
- 6.Maino A, Algra A, Koudstaal PJ, et al. Concomitant headache influences long-term prognosis after acute cerebral ischemia of noncardioembolic origin. Stroke 2013;44:2446–2450. [DOI] [PubMed] [Google Scholar]
- 7.Widar M, Samuelsson L, Karlsson-Tivenius S, Ahlstrom G. Long-term pain conditions after a stroke. J Rehabil Med 2002;34:165–170. [DOI] [PubMed] [Google Scholar]
- 8.Zorowitz RD. Recovery patterns of shoulder subluxation after stroke: a six-month followup study. Top Stroke Rehabil 2001;8:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Widar M, Ek AC, Ahlstrom G. Caring and uncaring experiences as narrated by persons with long-term pain after a stroke. Scand J Caring Sci 2007;21:41–47. [DOI] [PubMed] [Google Scholar]
- 10.Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials 2015;45:130–138. [DOI] [PubMed] [Google Scholar]
- 11.Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials II: the quality effects model. Contemp Clin Trials 2015;45:123–129. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ 2012;344:d7762. [DOI] [PubMed] [Google Scholar]
- 14.Paolucci S, Iosa M, Toni D, et al. Prevalence and time course of post-stroke pain: a multicenter prospective hospital-based study. Pain Med 2016;17:924–930. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadi Aghangar A, Bazoyar B, Mortazavi R, Jalali M. Prevalence of headache at the initial stage of stroke and its relation with site of vascular involvement: a clinical study. Caspian J Intern Med 2015;6:156–160. [PMC free article] [PubMed] [Google Scholar]
- 16.El Tallawy HN, Farghaly WM, Badry R, et al. Epidemiology and clinical presentation of stroke in Upper Egypt (desert area). Neuropsychiatr Dis Treat 2015;11:2177–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen AP, Marcussen NS, Klit H, Kasch H, Jensen TS, Finnerup NB. Development of persistent headache following stroke: a 3-year follow-up. Cephalalgia 2015;35:399–409. [DOI] [PubMed] [Google Scholar]
- 18.Lisabeth LD, Brown DL, Hughes R, Majersik JJ, Morgenstern LB. Acute stroke symptoms: comparing women and men. Stroke 2009;40:2031–2036. [DOI] [PubMed] [Google Scholar]
- 19.Abadie V, Jacquin A, Daubail B, et al. Prevalence and prognostic value of headache on early mortality in acute stroke: the Dijon Stroke Registry. Cephalalgia 2014;34:887–894. [DOI] [PubMed] [Google Scholar]
- 20.van Os HJ, Mulder IA, van der Schaaf IC, et al. Role of atherosclerosis, clot extent, and penumbra volume in headache during ischemic stroke. Neurology 2016;87:1124–1130. [DOI] [PubMed] [Google Scholar]
- 21.Kes VB, Jurasic MJ, Zavoreo I, Lisak M, Jelec V, Matovina LZ. Age and gender differences in acute stroke hospital patients. Acta Clin Croat 2016;55:69–78. [DOI] [PubMed] [Google Scholar]
- 22.Pollak L, Shlomo N, Korn Lubetzki I; National Acute Stroke Israeli Survey Group. Headache in stroke according to the National Acute Stroke Israeli Survey. Acta Neurol Scand 2017;135:469–475. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AP, Marcussen NS, Klit H, Andersen G, Finnerup NB, Jensen TS. Pain following stroke: a prospective study. Eur J Pain 2012;16:1128–1136. [DOI] [PubMed] [Google Scholar]
- 24.Arboix A, Garcia-Trallero O, Garcia-Eroles L, Massons J, Comes E, Targa C. Strokerelated headache: a clinical study in lacunar infarction. Headache 2005;45:1345–1352. [DOI] [PubMed] [Google Scholar]
- 25.Mitsias PD, Ramadan NM, Levine SR, Schultz L, Welch KM. Factors determining headache at onset of acute ischemic stroke. Cephalalgia 2006;26:150–157. [DOI] [PubMed] [Google Scholar]
- 26.Tentschert S, Wimmer R, Greisenegger S, Lang W, Lalouschek W. Headache at stroke onset in 2196 patients with ischemic stroke or transient ischemic attack. Stroke 2005;36:e1–e3. [DOI] [PubMed] [Google Scholar]
- 27.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: findings from the Atherosclerosis Risk in Communities Study. Stroke 2002;33:2718–2721. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen HS, Jespersen HF, Nakayama H, Raaschou HO, Olsen TS. Headache in stroke: the Copenhagen Stroke Study. Neurology 1994;44:1793–1797. [DOI] [PubMed] [Google Scholar]
- 29.Arboix A, Massons J, Oliveres M, Arribas MP, Titus F. Headache in acute cerebrovascular disease: a prospective clinical study in 240 patients. Cephalalgia 1994;14:37–40. [DOI] [PubMed] [Google Scholar]
- 30.Vestergaard K, Andersen G, Nielsen MI, Jensen TS. Headache in stroke. Stroke 1993;24:1621–1624. [DOI] [PubMed] [Google Scholar]
- 31.Lutski M, Zucker I, Shohat T, Tanne D. Characteristics and outcomes of young patients with first-ever ischemic stroke compared to older patients: the National Acute Stroke Israeli Registry. Front Neurol 2017;8:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budhdeo S, Kolias AG, Clark DJ, Chari A, Hutchinson PJ, Warburton EA. A retrospective cohort study to assess patient and physician reported outcome measures after decompressive hemicraniectomy for malignant middle cerebral artery stroke. Cureus 2017;9:e1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojaghihaghighi S, Vahdati SS, Mikaeilpour A, Ramouz A. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J Emerg Med 2017;8:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifert CL, Schonbach EM, Zimmer C, et al. Association of clinical headache features with stroke location: an MRI voxel-based symptom lesion mapping study. Cephalalgia 2018;38:283–291. [DOI] [PubMed] [Google Scholar]
- 35.DeLaroche AM, Sivaswamy L, Farooqi A, Kannikeswaran N. Pediatric stroke clinical pathway improves the time to diagnosis in an emergency department. Pediatr Neurol 2016;65:39–44. [DOI] [PubMed] [Google Scholar]
- 36.Owolabi LF, Ibrahim A, Musa I. Infratentorial posterior circulation stroke in a Nigerian population: clinical characteristics, risk factors, and predictors of outcome. J Neurosci Rural Pract 2016;7:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifert CL, Schonbach EM, Magon S, et al. Headache in acute ischaemic stroke: a lesion mapping study. Brain 2016;139:217–226. [DOI] [PubMed] [Google Scholar]
- 38.Brandler ES, Sharma M, McCullough F, et al. Prehospital stroke identification: factors associated with diagnostic accuracy. J Stroke Cerebrovasc Dis 2015;24:2161–2166. [DOI] [PubMed] [Google Scholar]
- 39.Arboix A, Estevez S, Rouco R, Oliveres M, Garcia-Eroles L, Massons J. Clinical characteristics of acute lacunar stroke in young adults. Expert Rev Neurother 2015;15:825–831. [DOI] [PubMed] [Google Scholar]
- 40.Puustjarvi V, Strbian D, Tiainen M, Curtze S, Tatlisumak T, Sairanen T. Recognition of posterior circulation stroke. Acta Neurol Scand 2015;131:389–393. [DOI] [PubMed] [Google Scholar]
- 41.Newman-Toker DE, Moy E, Valente E, Coffey R, Hines AL. Missed diagnosis of stroke in the emergency department: a cross-sectional analysis of a large population-based sample. Diagnosis 2014;1:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallick AA, Ganesan V, Kirkham FJ, et al. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol 2014;13:35–43. [DOI] [PubMed] [Google Scholar]
- 43.Kropp P, Holzhausen M, Kolodny E, et al. Headache as a symptom at stroke onset in 4,431 young ischaemic stroke patients: results from the “Stroke in Young Fabry Patients (SIFAP1) study.” J Neural Transm (Vienna) 2013;120:1433–1440. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Chao Y, Zhao X, et al. Factors associated with delayed presentation in patients with TIA and minor stroke in China: analysis of data from the China National Stroke Registry (CNSR). Neurol Res 2013;35:517–521. [DOI] [PubMed] [Google Scholar]
- 45.Klit H, Finnerup NB, Overvad K, Andersen G, Jensen TS. Pain following stroke: a population-based follow-up study. PLoS One 2011;6:e27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Searls DE, Pazdera L, Korbel E, Vysata O, Caplan LR. Symptoms and signs of posterior circulation ischemia in the New England Medical Center Posterior Circulation Registry. Arch Neurol 2012;69:346–351. [DOI] [PubMed] [Google Scholar]
- 47.Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. Factors associated with the steep increase in late-midlife stroke occurrence among US men. J Stroke Cerebrovasc Dis 2008;17:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdelho A, Ferro JM, Melo T, Canhao P, Falcao F. Headache in acute stroke: a prospective study in the first 8 days. Cephalalgia 2008;28:346–354. [DOI] [PubMed] [Google Scholar]
- 49.Arboix A, Grau-Olivares M, Garcia-Eroles L, Massons J, Comes E, Targa C. Clinical implications of headache in lacunar stroke: relevance of site of infarct. Headache 2006;46:1172–1180. [DOI] [PubMed] [Google Scholar]
- 50.Widar M, Ek AC, Ahlstrom G. Coping with long-term pain after a stroke. J Pain Symptom Manage 2004;27:215–225. [DOI] [PubMed] [Google Scholar]
- Additional references e51–e68 available at 10.5061/dryad.b53cc6s [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided to other investigators upon request made to the corresponding author, A.M.H., in accordance with International Committee of Medical Journal Editors requirements.