Abstract
Objective
To determine whether quantitative polysomnographic REM sleep without atonia (RSWA) distinguishes between cognitive impairment phenotypes.
Background
Neurodegenerative cognitive impairment in older adults predominantly correlates with tauopathy or synucleinopathy. Accurate antemortem phenotypic diagnosis has important prognostic and treatment implications; additional clinical tools might distinguish between dementia syndromes.
Methods
We quantitatively analyzed RSWA in 61 older adults who underwent polysomnography including 46 with cognitive impairment (20 probable synucleinopathy), 26 probable non-synucleinopathy (15 probable Alzheimer disease, 11 frontotemporal lobar dementia), and 15 age- and sex-matched controls. Submentalis and anterior tibialis RSWA metrics and automated REM atonia index were calculated. Group statistical comparisons and regression were performed, and receiver operating characteristic curves determined diagnostic RSWA thresholds that best distinguished synucleinopathy phenotype.
Results
Submentalis—but not anterior tibialis RSWA—was greater in synucleinopathy than nonsynucleinopathy; several RSWA diagnostic thresholds distinguished synucleinopathy with excellent specificity including submentalis tonic, 5.6% (area under the curve [AUC] 0.791); submentalis any, 15.0% (AUC 0.871); submentalis phasic, 10.8% (AUC 0.863); and anterior tibialis phasic, 31.4% (AUC 0.694). In the subset of patients without dream enactment behaviors, submentalis RSWA was also greater in patients with synucleinopathy than in those without synucleinopathy. RSWA was detected more frequently by quantitative than qualitative methods (p = 0.0001).
Conclusion
Elevated submentalis RSWA distinguishes probable synucleinopathy from probable nonsynucleinopathy in cognitively impaired older adults, even in the absence of clinical dream enactment symptoms.
Classification of evidence
This study provides Class III evidence that quantitative RSWA analysis is useful for distinguishing cognitive impairment phenotypes. Further studies with pathologic confirmation of dementia diagnoses are needed to confirm the diagnostic utility of RSWA in dementia.
Determination of dementia etiology can be clinically challenging due to considerable overlap in cognitive and behavioral changes seen in Alzheimer disease (AD) dementia, dementia with Lewy bodies (DLB), and frontotemporal dementia (FTD).1–4 AD is largely associated with abnormal accumulation of tau protein, whereas DLB occurs secondary to accumulation of α-synuclein.5 FTD may evolve due to tau protein accumulation but may also occur due to other genetic mutations, all of which are tau-negative.6 However, there is considerable pathologic overlap in AD and DLB, and patients often have pathology different from clinical diagnoses.5,7–9
REM sleep behavior disorder (RBD) is strongly associated with synucleinopathy-related neurodegeneration.10 RBD is present in 76% of DLB cases, compared with 4% of non-DLB cases in one large pathologically based series.11 By contrast, the frequency of RBD in patients with clinically diagnosed AD is low, with previous studies showing between 4.7% and 27% of patients with probable AD having REM sleep without atonia (RSWA), the neurophysiologic substrate of RBD.12,13 Pathologic studies have found that 34.3% of patients with RBD had both DLB and AD pathology, whereas only 3.5% had solely AD pathology.13 RBD has occurred in only one reported FTD case.14
RSWA on polysomnography is required for RBD diagnosis.7,15 No prior studies quantitatively measuring RSWA in a series of patients with DLB or FTD and only 2 studies of RSWA quantification in patients with AD have been published.12,13 We aimed to determine whether quantitative RSWA could distinguish the etiology of clinically diagnosed mild cognitive impairment and dementia.
Methods
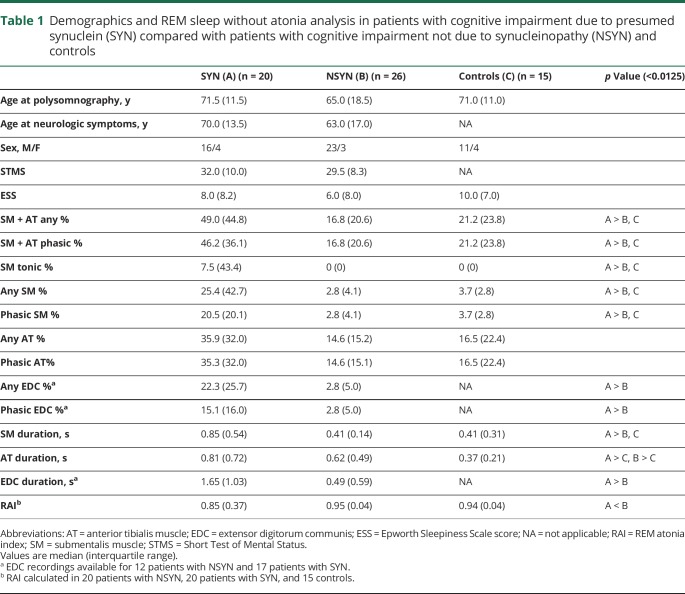
We retrospectively analyzed RSWA in patients who underwent polysomnography at the Mayo Clinic Center for Sleep Medicine between 2008 and 2015 if they (1) met published consensus diagnostic criteria for amnestic mild cognitive impairment (aMCI), nonamnestic mild cognitive impairment (naMCI),16 AD dementia,17 behavioral variant frontotemporal dementia (bvFTD), primary progressive aphasia (PPA), primary progressive apraxia of speech (PPAOS),18–20 or DLB21 and (2) had one or more additional objective tests supportive of clinical diagnosis of dementia including imaging with FDG-PET or 123I-FP-CIT SPECT scan (DaTscan), neuropsychological testing, or positive CSF amyloid/tau biomarkers. We divided 46 patients into 2 groups based on clinically presumed underlying dementia pathology: probable synucleinopathy (SYN, n = 20) and probable nonsynuclein etiologies (NSYN, n = 26). The SYN groups included 14 patients with DLB and 6 with naMCI, while the NSYN group included 15 with probable AD (12 AD, 3 aMCI) and 11 with probable FTD (9 bvFTD, 1 PPA, and 1 PPAOS). Two patients with bvFTD had mutations in progranulin (c.154delA in exon 3, Ala9Asp), and 2 other patients with bvFTD had pathology-proven disease (both of whom were negative for MAPT and GRN mutations). Of patients with DLB with available pathology, 6 had diffuse Lewy body disease, and 1 had limbic type Lewy body disease. All SYN and NSYN patients had symptomatic cognitive impairment at the time of polysomnography. Thirty-two patients had a clinical diagnosis prior to polysomnography while the remaining 14 patients had polysomnography prior to neurodegenerative syndrome diagnosis. We included 15 age- and sex-matched controls with primary snoring or mild obstructive sleep apnea (OSA) (apnea–hypopnea index [AHI] ≤5) for RSWA comparison including some that were previously published.22–24
Chart review confirmed clinical diagnosis and age, sex, and other clinical, neuroimaging, and demographic factors were extracted. Patients and controls were excluded with a REM AHI of ≥15 or total REM time <5 minutes. Several SYN and NSYN patients were receiving antidepressant medications, clonazepam, memantine, donepezil, or carbidopa/levodopa at the time of polysomnography. Diagnostic polysomnography studies were analyzed for patients without clinically meaningful sleep-disordered breathing, while in patients with mild OSA, REM periods were drawn from therapeutic continuous positive airway pressure polysomnography studies to minimize arousal artifact due to sleep-disordered breathing, as per previously published methods.23,25,26
Analysis of REM sleep muscle activity
Polysomnography recording and RSWA scoring methods were as previously reported.23,24 Scorers were blinded to each group and individual patient data. All scorers had high interrater reliability against a gold standard record, as previously described,18,21 with a kappa score of 0.92 for this study. Briefly, conventionally clinically sampled EMG channels, including submentalis (SM) and anterior tibialis (AT) muscles, were analyzed for all participants. Extensor digitorum communis (EDC) was recorded only in patients with clinical concern for dream-enactment behavior (DEB), and was analyzed when available (n = 29). Phasic (duration between 0.1 and 14.9 seconds, with an amplitude of greater than 4 times background EMG) and any percent muscle activity were calculated separately for SM, AT, and EDC muscles, and combined in the SM + AT.23–25
Tonic muscle activity (>15 seconds of muscle activity continuously greater than double the background EMG voltage, or for measured SM voltage ≥10 µV) was scored only in the SM.23–25 The duration of each phasic muscle-activity burst during REM sleep was directly measured and averaged to yield phasic muscle-burst duration by our previous methods.23,24 Any 3-second mini-epoch containing a breathing-related or spontaneous arousal was excluded from analysis.23 The automated REM atonia index (RAI) for the SM muscle was also calculated using HypnoLab sleep-scoring software.27 Prior to RAI analysis, 30-second epochs containing a breathing-related artifact or arousal were excluded, and the SM signal was notch filtered at 60 Hz and rectified.23,27 Five patients with AD and 1 with FTD were excluded from RAI analysis due to SM artifact that would have artificially reduced RAI values, whereas artifact remained clearly distinguishable from REM sleep muscle activity for inclusion of these 6 patients in the visual/manual analyses.
Statistical analysis
Clinical, demographic, and polysomnographic data are presented as medians, interquartile ranges, and frequencies. Quantitative variables were analyzed using nonparametric Kruskal-Wallis tests; χ2 tests were used to analyze categorical variables with JMP statistical software (JMP, version 12; SAS Institute Inc., Cary, NC). Relationships between clinical independent variables and dependent tonic, phasic, any muscle activity, RAI, and phasic muscle-burst duration were analyzed utilizing multivariable linear or logistic regression. A post hoc Bonferroni correction was applied for multiple tests, setting experiment-wise α level at p < 0.0125. Receiver operating characteristic (ROC) curves were calculated for combined phasic and any percent muscle activities, as well as phasic and any percent muscle activities for SM and AT muscles. In addition, ROC curves were calculated for SM tonic muscle-activity percentage and phasic muscle-burst durations in both muscles. Area under the curve (AUC) was calculated for each analysis, and cutoff diagnostic threshold values were chosen that yielded the highest specificity with reasonable sensitivity distinguishing SYN from NSYN etiology. We also analyzed the frequency of RSWA detected by quantitative RSWA muscle-activity percentages that fulfilled previously defined RBD diagnostic cutoff thresholds23 against that of qualitative visual RSWA determination alone as interpreted by a board-certified sleep medicine physician. For each of the 46 patients, we rated each quantitative and qualitative method for RSWA determination as a positive (present) or negative (absent) categorical variable, determined the degree of agreement between methods using Cohen kappa, and analyzed whether there was a significant difference in RSWA frequency determination between the 2 methods using Fisher exact test.
Standard protocol approvals, registrations, and patient consents
The Mayo Clinic Institutional Review Board approved this study, and participating patients (or their legally authorized representatives) provided written consent to use their medical information.
Data sharing
All relevant data have been shared and published in this article. Data regarding case ascertainment and methodology on case identification have been published previously.23,24
Results
Clinical and demographic data
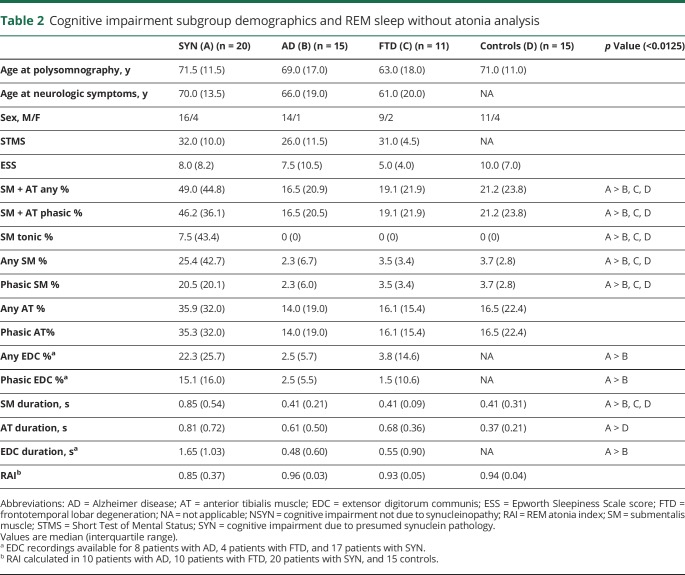
There were no differences in sex or age at polysomnography or at neurologic symptom onset between the SYN and NSYN patient subgroups (table 2). Patients with aMCI and naMCI were similar in age, as were patients in the probable AD, DLB, and FTD subgroups. SYN and NSYN groups were no different in measures of sleepiness (Epworth Sleepiness Scale [ESS]) or cognition (as determined by The Kokmen Short Test of Mental Status [STMS])28 within 3 months of polysomnography. The most common sleep complaints for patients with SYN were RBD (65%), excessive daytime sleepiness (20%), and sleep-disordered breathing (15%). Within the NSYN group, sleep-disordered breathing was the most common presenting sleep complaint for patients with AD (67%), followed by nocturnal motor behaviors (20%) and excessive daytime sleepiness (13%), while for patients with FTD, sleep complaints included sleep-disordered breathing (64%), excessive daytime sleepiness (27%), and insomnia (9%). Sixteen (80%) patients with SYN had a history of DEB beginning at an average age of 65.4 ± 9.2 years, with 10 (62%) having DEB evolve prior to or in conjunction with cognitive changes, and 6 (38%) having developed DEB after cognitive changes began. In the NSYN group, only 2 (13%) patients with AD had a history of DEB (1 before and 1 after cognitive symptoms evolved), and no patient with FTD had DEB history. There were no differences between the SYN and NSYN groups in frequency of OSA, restless legs syndrome, or periodic limb movement disorder (PLMD) diagnoses, or in history of depression or antidepressant use.
Table 2.
Cognitive impairment subgroup demographics and REM sleep without atonia analysis
Memory difficulties were the initial neurologic symptom in 12 (60%) of the patients with SYN, followed by executive dysfunction (4 patients, 20%), gait changes (3 patients, 15%), and hallucinations (1 patient, 5%). In the NSYN group, initial neurologic symptoms in patients with AD were memory impairment (10 patients, 66%), word-finding difficulties (3 patients, 20%), and cognitive/personality changes (2 patients, 14%). In patients with FTD, 5 (45%) patients presented with behavioral changes, 3 (27%) had memory impairment with word-finding difficulties, and 3 (27%) had executive dysfunction.
At the time of polysomnography, 3 (20%) patients with AD, 3 (27%) patients with FTD, and 4 (20%) patients with SYN were taking antidepressant medications. Five (33%) patients with AD, 1 (9%) patient with FTD, and 8 (40%) patients with SYN were taking memantine or donepezil. In addition, 3 (15%) patients with SYN were taking clonazepam, 3 (15%) were taking melatonin, and 4 (20%) were taking carbidopa/levodopa at polysomnography. No controls received centrally acting drugs at the time of polysomnography.
RSWA analysis
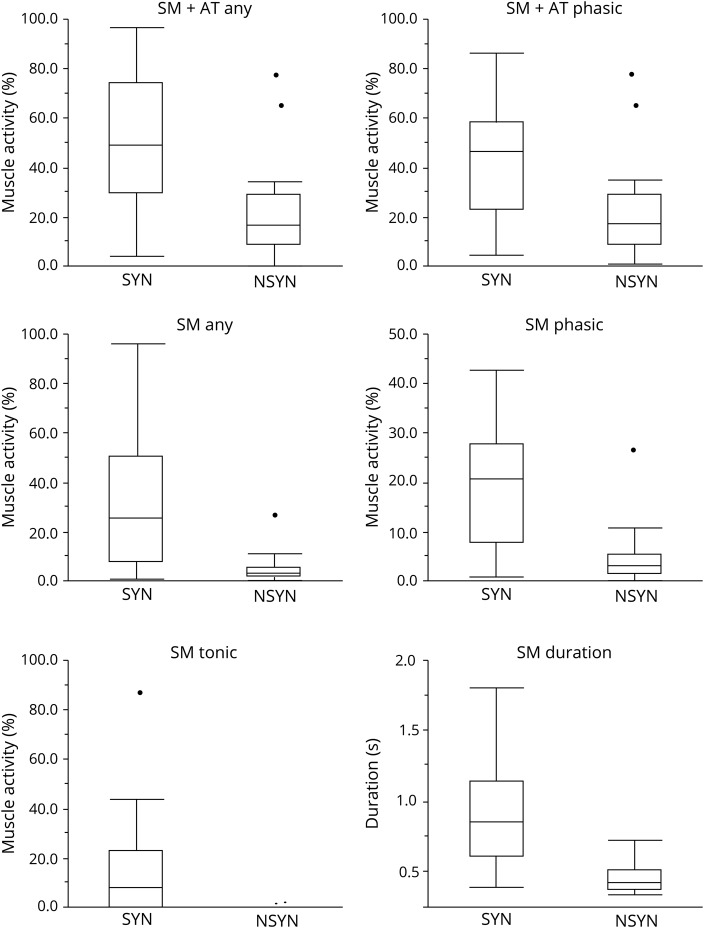
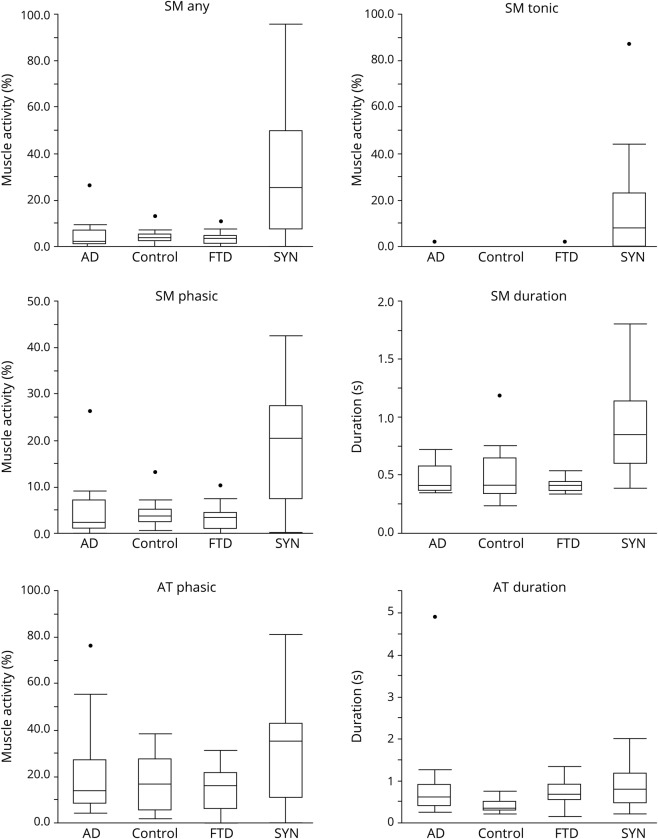
All RSWA measures were greater in SYN than NSYN patients and controls (all p <0.0125), including SM + AT phasic and any muscle activity, SM any, phasic, and tonic muscle activity, SM duration, and EDC phasic/any muscle activity and duration (table 1 and figure 1). In the SYN groups, RSWA was similar between naMCI and DLB, while in the NSYN group, RSWA was similar between aMCI and AD. On subgroup analysis, patients with SYN had greater SM + AT phasic and any muscle activity, SM any, phasic and tonic muscle activity, and SM duration than patients with NSYN (including both AD and FTD subgroups) or controls (all p values <0.0125) (table 2 and figure 2).
Table 1.
Demographics and REM sleep without atonia analysis in patients with cognitive impairment due to presumed synuclein (SYN) compared with patients with cognitive impairment not due to synucleinopathy (NSYN) and controls
Figure 1. Box plots of REM sleep without atonia comparisons between cognitive impairment due to presumed synuclein pathology (SYN) and cognitive impairment due to presumed nonsynuclein pathology (NSYN ).
AT = anterior tibialis muscle; SM = submentalis muscle.
Figure 2. Box plots of REM sleep without atonia comparisons among dementia subgroups.
AD = Alzheimer disease; FTD = frontotemporal degeneration; SYN = cognitive impairment due to presumed synuclein pathology; SM = submentalis muscle; AT = anterior tibialis muscle.
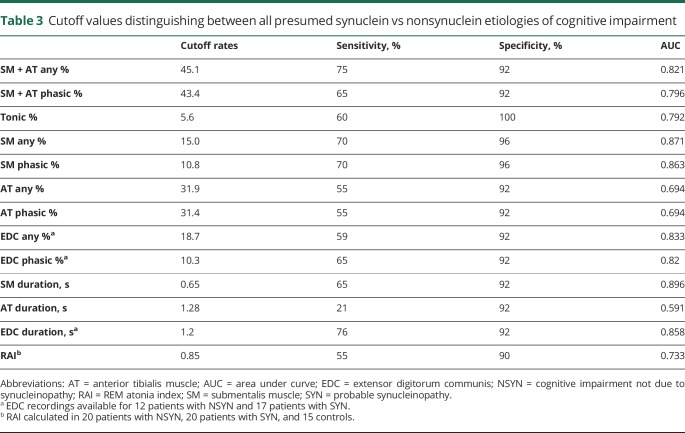
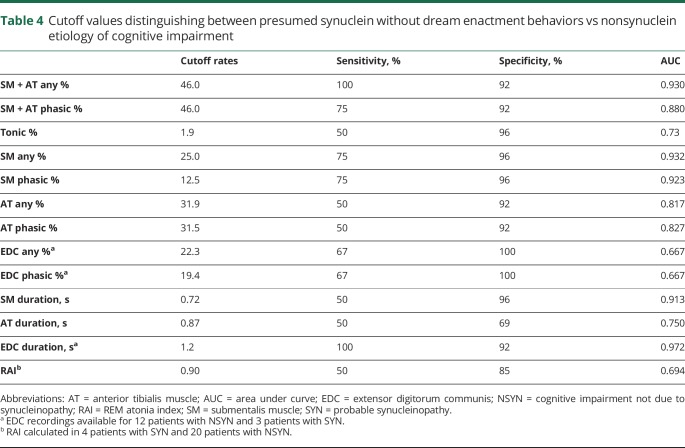
ROC analyses demonstrating RSWA diagnostic thresholds that distinguished all SYN from NSYN patients, with and without DEB, can be found in tables 3 and 4, respectively. The SYN group was associated with higher SM phasic/any, SM tonic, and SM muscle-activity duration, adjusted for age at polysomnography, sex, REM AHI, antidepressant use, and %of mini-epochs (%ME) excluded. With addition of Periodic Limb Movements Index (PLMI) to the model, both the SYN group and PLMI were associated with SM + AT phasic/any muscle activity, controlling for age, sex, REM AHI, and antidepressant use. AT phasic/any muscle activity % was associated with PLMI, adjusting for age, sex, group, REM AHI, antidepressant use, and %ME excluded.
Table 3.
Cutoff values distinguishing between all presumed synuclein vs nonsynuclein etiologies of cognitive impairment
Table 4.
Cutoff values distinguishing between presumed synuclein without dream enactment behaviors vs nonsynuclein etiology of cognitive impairment
Antidepressant use was not associated with SM or AT muscle activity or duration in univariate analysis. In addition, there were no group differences between those receiving or not receiving antidepressants. There were no statistically significant differences in RSWA between patients taking acetylcholinesterase inhibitors, clonazepam, or carbidopa/levodopa compared with those not taking these medications. Neither STMS nor ESS was associated with RSWA.
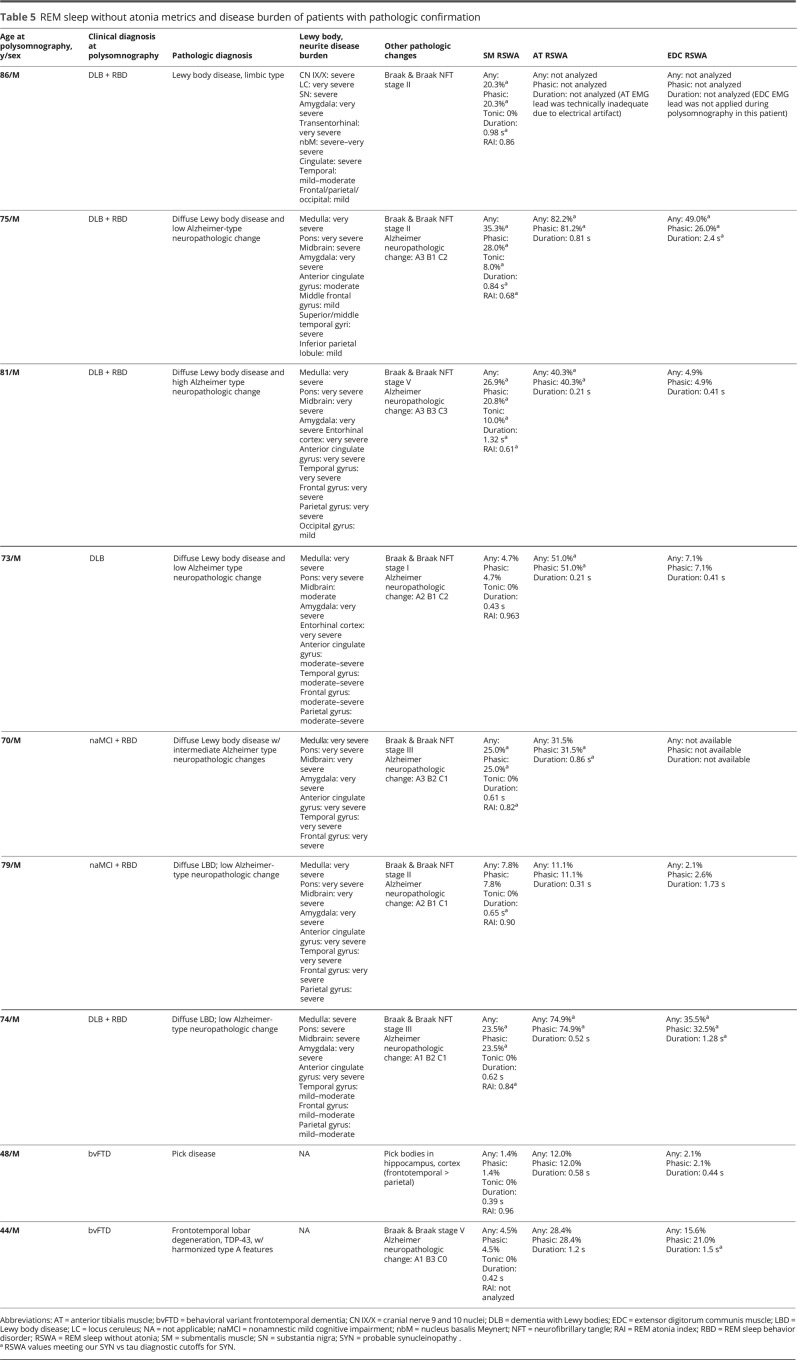
SM + AT any, SM phasic, and muscle activity percentage, as well as SM duration, were greater in 4 patients with SYN with no history of DEB when compared with all patients with NSYN (p < 0.0125). SM tonic muscle activity was also nonsignificantly increased in patients with SYN without DEB compared with patients with NSYN (p = 0.0132). On subgroup analysis, all measures of RSWA in SYN without DEB were greater when compared with FTD (p values all <0.0125), and there was nonsignificantly greater RSWA in patients with SYN without DEB than in patients with AD for all measures except for RAI, EDC phasic/any, and AT and EDC duration. Individual RSWA data of pathologically confirmed patients, including RSWA values that met SYN vs NSYN diagnostic cutoffs, are shown in table 5.
Table 5.
REM sleep without atonia metrics and disease burden of patients with pathologic confirmation
Patients with DLB had lower REM percentage compared with controls, while patients with FTD had lower total sleep time than controls. AHI was also higher than in both patients with AD and controls. There were no differences in PLMI between the SYN or NSYN patients or controls (data not shown).
For the 46 patients with cognitive impairment, quantitative and qualitative RSWA determination had only weak agreement (Cohen κ = 0.54). Both qualitative and quantitative methods identified RSWA presence in 16 (34.7%) patients, while both methods found absent RSWA in 19 (41.3%) patients. Quantitative RSWA identified RSWA presence in 10 (21.7%) patients that was missed and determined as absent by qualitative interpretation (as interpreted by a boarded sleep physician), while only 1 (2.2%) patient was identified as RSWA-positive by qualitative RSWA determination that did not meet quantitative RSWA diagnostic cutoffs (p = 0.0001).
Discussion
SM RSWA was strongly associated with presumed synucleinopathy in older adults with cognitive impairment, even in the absence of clinical dream enactment. Several SM RSWA thresholds distinguished SYN from NSYN in older adults with cognitive impairment with good sensitivity and excellent specificity (all AUC >0.8 in ROC analyses). No AT RSWA thresholds had an AUC >0.70.
Quantitative RSWA could prove to be a useful additional diagnostic tool in the growing armamentarium of diagnostic technology to aid in the accurate antemortem differential diagnosis of dementia etiologies. Given the potential for neuroleptic sensitivity in patients with DLB, accurate distinction of DLB from AD and FTD is clinically important to avoid this complication.21 Further, as future targeted therapies directed at disease-specific molecules are studied in clinical trials, accurate clinical diagnosis is paramount to ensure accurate patient selection. Recent neuroimaging studies have suggested that medial temporal lobe AV-1451 tau uptake on PET scans accurately distinguishes AD from probable DLB.29 Abnormalities on 123I-FP-CIT SPECT (DaTscan) are highly sensitive and specific for a diagnosis of DLB.30 A recent study showed that 123I-metaiodobenzylguanidine myocardial scintigraphy is 93% sensitive and 100% specific for distinguishing DLB from AD and bvFTD.31 Further, preservation of hippocampal volume on MRI studies in patients with MCI is associated with progression to probable DLB compared with AD.32 The addition of polysomnography and quantitative RSWA analysis to this sophisticated group of neuroimaging tools as an additional noninvasive tool for distinction between major neurocognitive disorder phenotypes may enable accurate antemortem diagnosis of synucleinopathy neurodegeneration. Advantages of polysomnography as an additional potential diagnostic application for cognitive impairment in adults include the evaluation and treatment of comorbid sleep-disordered breathing or PLMD, treatable factors that also contribute to overall poorer cognitive performance,33,34 as well as accurate diagnosis of parasomnia behaviors in REM and NREM sleep linked to injury potential for both the patient and his or her bed partner.7,35,36
Interestingly, the SM-duration cutoff of 0.65 seconds is similar to our previously determined threshold that accurately distinguished idiopathic RBD (0.66 seconds) and symptomatic RBD associated with Parkinson disease (0.65 seconds) from controls.23,24 The reproducibility of this highly similar RSWA phasic burst duration threshold across studies suggests this measure could have value as an antemortem SYN biomarker. Additional validation in future studies with pathologic confirmation is needed. The RSWA diagnostic thresholds are also consistent with the high prevalence of clinical RBD (80%) in our patients with SYN, and low frequency of RBD and very low amounts of RSWA in patients with AD and FTD. These findings suggest limited disruption of REM sleep-atonia control networks in nonsynuclein etiologies of cognitive impairment, and provide antemortem evidence for the selective vulnerability of brainstem nuclei involved in synucleinopathies.37
We found no difference in AT phasic/any muscle activity percentages or durations between any patient subgroups or controls with an AUC <0.70, suggesting AT RSWA is unable to distinguish between dementia subtypes. This is similar to previous studies that have found a lower sensitivity/specificity for the diagnosis of RBD vs controls in the AT muscle, suggesting that the SM appears to be relatively specific for underlying synucleinopathy, while the AT is by comparison relatively nonspecific.23–25 We also found an association between PLMI and AT muscle activity. This is unsurprising, since we include REM PLM-like muscle activity in our AT RSWA analysis, given the difficulties distinguishing REM PLM-like activity from true periodic limb movements of non-REM sleep, and lack of reliable or defined methodology to enable ready distinction of REM PLMs from REM phasic muscle activity associated with RBD.23,24 PLMI was similar between patient groups and controls, suggesting that neither PLMI nor AT RSWA is useful to distinguish between cognitive impairment subtypes or controls.
Our study found similar RSWA levels between patients with AD and patients with FTD and controls. Previous RSWA studies of patients with AD utilized different RSWA methods than ours and have shown conflicting results. One report found no difference in SM RSWA between 15 patients with probable AD and 15 controls, while the other found increased SM muscle activity in 33% of patients with AD who had screened positive for RBD by history, but lacked controls.12,13 In our current study, 2 patients with AD (13%) had a history of DEB, similar to the percentage reported in another study,13 but did not meet previously published RSWA cutoffs for any muscle, alone or combined.23,24 Similarly, only 1 patient with AD met previously published SM tonic cutoffs for a diagnosis of RBD; however, this patient did not have a history of DEB.23 No patients with FTD had a history of DEB or met RBD diagnostic cutoffs, providing quantitative evidence for normal REM muscle atonia in FTD, which further substantiates the paucity of RBD in FTD.14 The lack of RSWA seen in AD/FTD provides evidence for the selective vulnerability of brainstem nuclei (especially the locus subcoeruleus/sublateral dorsal nucleus) secondary to presumed synuclein aggregation as suggested by the Braak hypothesis.38
Our study was underpowered to determine RSWA differences between aMCI and naMCI subtypes. However, naMCI subtypes had non–statistically significant elevations in all RSWA types in all muscles studied, which may have reached significance with greater numbers. Given that the preservation of hippocampal volume has been proven predictive of MCI conversion to probable DLB, future studies combining RSWA analysis and hippocampal volumes in patients with MCI may be even more accurate in dementia prediction than either measure alone.32
In this study, quantitative RSWA determination was superior to visual RSWA interpretation alone, since quantitative RSWA meeting previously defined RBD diagnostic thresholds was present in 10 (21.7) unique patients who were missed by qualitative visual RSWA interpretation, while qualitative RSWA inspection identified only 1 (2.2%) patient who did not meet specified quantitative cutoffs (p = 0.0001).23 This discrepancy in RSWA detection suggests that visual RSWA determination of polysomnography alone may not be sufficient to detect clinically important RSWA amounts, at least in some clinical settings. We are aware of only one previously published study that systematically compared qualitative RSWA detection during routine clinical polysomnography interpretation to quantitative RSWA methods for the determination of isolated/incidental RSWA.39 This study found that only 12%–14% of patients with qualitatively determined RSWA on polysomnography met phasic and any quantitative RSWA diagnostic cutoffs for RBD, showing that these 2 methods for RSWA determination may yield substantially different conclusions.39 Further systematic studies comparatively analyzing quantitative and qualitative RSWA methods in various clinical settings are needed.
Strengths of our study include relatively large patient subgroups and novel analysis of RSWA in patients with DLB and FTD, which, to our knowledge, have not been reported previously. Further, our findings of normal REM sleep muscle tone in AD and FTD corroborate findings of previous RSWA studies in AD and provide evidence in support of the dearth of case reports of RBD in patients with FTD.12–14 Finally, our study provides methodology for an accurate dementia diagnosis with therapeutic potential (i.e., identification and treatment of comorbid sleep disorders that may worsen cognition in patients with dementia) that other diagnostic modalities do not offer.
Our study has several limitations. Our sample was drawn from a single tertiary care sleep center and is therefore subject to referral and sampling biases. Since patients were diagnosed based on clinical and neuroimaging characteristics with pathologic confirmation available in only 2 patients with FTD and 7 patients with DLB (2 of whom had coexisting intermediate–high Alzheimer pathologic changes), there may have been pathologic overlap between groups, particularly in AD and SYN.13 In addition, clinical polysomnography results were available for review by the clinical neurologists who made SYN vs NSYN diagnoses, which may have led to incorporation bias by influencing the clinical dementia diagnosis that we subsequently used to categorize this study cohort. However, 16/26 (61.5%) patients with NSYN and 16/20 (80%) patients with SYN received their clinical dementia syndrome diagnosis prior to polysomnography, making incorporation bias less likely to have significantly affected our primary RSWA analysis results. However, clinical history of these patients, including probable dementia syndrome diagnosis, was likely known by the clinical polysomnographers who interpreted and reported the presence or absence of qualitative RSWA, which may have affected our secondary analysis comparing qualitative vs quantitative RSWA determination. A further limitation was that several of our patients with cognitive impairment were receiving CNS-active medications including antidepressants (which have been shown to increase phasic AT muscle activity) as well as donepezil, memantine, carbidopa/levodopa, and clonazepam.40 However, in our regression models, there were no differences in RSWA between patients receiving medications and those who were not. Since our polysomnograms were recorded in clinical practice, where medication discontinuation is impractical and in many cases unsafe, our data represent a naturalistic representation of patients with cognitive impairment as typically encountered in neurology and sleep clinics. Another limitation was sampling of only the SM and AT muscles for analyses. Historically, most clinical sleep laboratories, including ours, have not routinely recorded EMG from EDC or other arm muscles, unless there is clinical suspicion for RBD. Accordingly, EDC was unavailable in our control patients, and we were unable to analyze group differences or determine diagnostic cutoffs between SYN and controls for the EDC, although we have included data regarding the EDC, including cutoffs between the SYN and NSYN groups, for completeness. Others have found that arm muscles are the most sensitive and specific sites to sample for RBD diagnosis,19 and future studies should more systematically aim to determine the diagnostic utility of arm muscles in patients with cognitive impairment. Finally, the RAI, typically a highly sensitive and specific method for RBD diagnosis, did not perform as well as manual RSWA analysis of the SM in this study,22–24 which may have been due to the exclusion of 6 patients (5 AD and 1 FTD) with excessive SM artifact. These cases were excluded because automated RAI cannot distinguish artifacts from phasic or tonic REM sleep-muscle activity, unlike visual analysis methods. Future larger studies using RAI will need to be done in older adults with cognitive impairment to determine the diagnostic utility of this technique in this patient population.
SM RSWA may be useful as another diagnostic tool to distinguish SYN from other etiologies of neurodegenerative cognitive impairment in older adults, even in the absence of clinical dream enactment. REM sleep muscle atonia appears normally preserved in most patients with suspected AD dementia or frontotemporal dementia, and RSWA in patients with clinical AD may represent comingling of synucleinopathy. Future studies of quantitative RSWA analysis in patients with pathologically proven dementia will be necessary to corroborate these findings and to validate RSWA as a potentially useful diagnostic tool in antemortem dementia diagnosis.
Acknowledgment
The authors thank the Mayo Clinic Alzheimer's Disease Research Center for providing care to many of the patients analyzed in this project, which is supported by the National Institutes on Aging and the Mangurian Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or PDF-APDA. The authors also thank Lea Dacy, Department of Neurology, Mayo Clinic, for secretarial support.
Glossary
- AD
Alzheimer disease
- AHI
apnea–hypopnea index
- aMCI
amnestic mild cognitive impairment
- AT
anterior tibialis
- AUC
area under the curve
- bvFTD
behavioral variant frontotemporal dementia
- DEB
dream-enactment behavior
- DLB
dementia with Lewy bodies
- EDC
extensor digitorum communis
- ESS
Epworth Sleepiness Scale
- FTD
frontotemporal dementia
- %ME
% of mini-epochs
- naMCI
nonamnestic mild cognitive impairment
- NSYN
cognitive impairment not due to synucleinopathy
- OSA
obstructive sleep apnea
- PLMD
periodic limb movement disorder
- PLMI
Periodic Limb Movements Index
- PPA
primary progressive aphasia
- PPAOS
primary progressive apraxia of speech
- RAI
REM atonia index
- RBD
REM sleep behavior disorder
- ROC
receiver operating characteristic
- RSWA
REM sleep without atonia
- SM
submentalis
- STMS
The Kokmen Short Test of Mental Status
- SYN
probable synucleinopathy
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
Parkinson's Disease Foundation–American Parkinson Disease Association Summer Student fellowship, PDF-APDA-SFW-1656 and Mayo Clinic Alzheimer's Disease Research Center Grant Award from the National Institute on Aging (P50 AG016574), and the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant 1 UL1 RR024150-01. No off-label medication use.
Disclosure
S. McCarter received grant funding from a Parkinson's Disease Foundation–American Parkinson Disease Association Summer Student fellowship, PDF-APDA-SFW-1656. G. Tabatabai, H. Jong, D. Sandness, P. Timm, and K. Johnson report no disclosures relevant to the manuscript. K. Kantarci receives research support from NIH; she also serves on the Data Safety Monitoring Board for Takeda Research Inc. A. McCarter reports no disclosures relevant to the manuscript. M. Machulda receives research support from the National Institute on Aging. R. Savica receives research support from the National Center for Advancing Translational Sciences and the National Institute on Aging. P. Vemuri receives research support from the National Institute on Aging and the National Institute of Neurologic Disorders and Stroke. M. Mielke reports that she has consulted for Lysosomal Therapeutics, Inc. and Eli Lilly; she receives research support from the National Institute on Aging and unrestricted research grants from Biogen and Lundbeck. B. Boeve reports that he is an investigator in clinical trials sponsored by Axovant and GE Healthcare and a scientific advisor for the Tau Consortium; he receives research support from the National Institute on Aging, the National Institute of Neurologic Disorders and Stroke, the Mangurian Foundation, and the Little Family Foundation. M. Silber reports no disclosures relevant to the manuscript. E. St. Louis reports research support from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the National Institute on Aging. Go to Neurology.org/N for full disclosures.
References
- 1.Shea YF, Ha J, Chu LW. Comparisons of clinical symptoms in biomarker-confirmed Alzheimer's disease, dementia with Lewy bodies, and frontotemporal dementia patients in a local memory clinic. Psychogeriatrics 2015;15:235–241. [DOI] [PubMed] [Google Scholar]
- 2.Vecchierini MF. Sleep disturbances in Alzheimer's disease and other dementias. Psychol Neuropsychiatr Vieil 2010;8:15–23. [DOI] [PubMed] [Google Scholar]
- 3.McCarter SJ, St Louis EK, Boeve BF. Sleep disturbances in frontotemporal dementia. Curr Neurol Neurosci Rep 2016;16:85. [DOI] [PubMed] [Google Scholar]
- 4.Gurnani AS, Gavett BE. The differential effects of Alzheimer's disease and Lewy body pathology on cognitive performance: a meta-analysis. Neuropsychol Rev 2017;27:1–17. [DOI] [PubMed] [Google Scholar]
- 5.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017;16:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet 2015;386:1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann NY Acad Sci 2010;1184:15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003;61:40–45. [DOI] [PubMed] [Google Scholar]
- 9.Sekiguchi H, Moriwaki M, Iritani S, et al. An autopsy case of dementia with Lewy bodies clinically diagnosed to have a behavioral variant of frontotemporal dementia. Clin Neuropathol 2017;36:23–30. [DOI] [PubMed] [Google Scholar]
- 10.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 2011;77:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeve BF, Silber MH, Ferman TJ, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 2013;14:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagnon JF, Petit D, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in probable Alzheimer disease. Sleep 2006;29:1321–1325. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Wing YK, Xing J, et al. Rapid eye movement sleep behavior disorder in patients with probable Alzheimer's disease. Aging Clin Exp Res 2016;28:951–957. [DOI] [PubMed] [Google Scholar]
- 14.Lo Coco D, Cupidi C, Mattaliano A, Baiamonte V, Realmuto S, Cannizzaro E. REM sleep behavior disorder in a patient with frontotemporal dementia. Neurol Sci 2012;33:371–373. [DOI] [PubMed] [Google Scholar]
- 15.International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 16.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botha H, Duffy JR, Whitwell JL, et al. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex 2015;69:220–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 22.Ferri R, Fulda S, Cosentino FI, Pizza F, Plazzi G. A preliminary quantitative analysis of REM sleep chin EMG in Parkinson's disease with or without REM sleep behavior disorder. Sleep Med 2012;13:707–713. [DOI] [PubMed] [Google Scholar]
- 23.McCarter SJ, St Louis EK, Duwell EJ, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep 2014;37:1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarter SJ, St Louis EK, Sandness DJ, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med 2017;33:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frauscher B, Iranzo A, Gaig C, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep 2012;35:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iranzo A, Frauscher B, Santos H, et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med 2011;12:284–288. [DOI] [PubMed] [Google Scholar]
- 27.Ferri R, Manconi M, Plazzi G, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J Sleep Res 2008;17:89–100. [DOI] [PubMed] [Google Scholar]
- 28.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status: correlations with standardized psychometric testing. Arch Neurol 1991;48:725–728. [DOI] [PubMed] [Google Scholar]
- 29.Kantarci K, Lowe VJ, Boeve BF, et al. AV-1451 tau and beta-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 2017;81:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCleery J, Morgan S, Bradley KM, Noel-Storr AH, Ansorge O, Hyde C. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst Rev 2015;1:CD010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiraboschi P, Corso A, Guerra UP, et al. (123) I-2beta-carbomethoxy-3beta-(4-iodophenyl)-N-(3-fluoropropyl) nortropane single photon emission computed tomography and (123) I-metaiodobenzylguanidine myocardial scintigraphy in differentiating dementia with Lewy bodies from other dementias: a comparative study. Ann Neurol 2016;80:368–378. [DOI] [PubMed] [Google Scholar]
- 32.Kantarci K, Lesnick T, Ferman TJ, et al. Hippocampal volumes predict risk of dementia with Lewy bodies in mild cognitive impairment. Neurology 2016;87:2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manni R, Terzaghi M. Sleep-disordered breathing in dementia with Lewy bodies. Curr Neurol Neurosci Rep 2015;15:7. [DOI] [PubMed] [Google Scholar]
- 34.Aoki K, Matsuo M, Takahashi M, et al. Association of sleep-disordered breathing with decreased cognitive function among patients with dementia. J Sleep Res 2014;23:517–523. [DOI] [PubMed] [Google Scholar]
- 35.McCarter SJ, St Louis EK, Boswell CL, et al. Factors associated with injury in REM sleep behavior disorder. Sleep Med 2014;15:1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013;14:744–748. [DOI] [PubMed] [Google Scholar]
- 37.Dickson DW, Uchikado H, Fujishiro H, Tsuboi Y. Evidence in favor of Braak staging of Parkinson's disease. Mov Disord 2010;25(suppl 1):S78–S82. [DOI] [PubMed] [Google Scholar]
- 38.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 39.Sasai-Sakuma T, Frauscher B, Mitterling T, et al. Quantitative assessment of isolated rapid eye movement (REM) sleep without atonia without clinical REM sleep behavior disorder: clinical and research implications. Sleep Med 2014;15:1009–1015. [DOI] [PubMed] [Google Scholar]
- 40.McCarter SJ, St Louis EK, Sandness DJ, et al. Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep 2015;38:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]