Abstract
Dengue virus (DENV) causes the most prevalent arboviral infection of humans, resulting in a spectrum of outcomes, ranging from asymptomatic infection to dengue fever to severe dengue characterized by vascular leakage and shock. Previously, we determined that DENV nonstructural protein 1 (NS1) induces endothelial hyperpermeability, disrupts the endothelial glycocalyx layer (EGL) in vitro and triggers shedding of structural components, including sialic acid (Sia) and heparan sulfate. Here, using a murine model of dengue disease disease, we found high levels of Sia and NS1 circulating in mice with DENV-induced morbidity and lethal DENV infection. Further, we developed a liquid chromatography/mass spectrometry-based method for quantifying free Sia in serum and determined that the levels of free N-glycolylneuraminic acid were significantly higher in DENV-infected mice than in uninfected controls. These data provide additional evidence that DENV infection disrupts EGL components in vivo and warrant further research assessing Sia as a biomarker of severe dengue disease.
Keywords: dengue virus, NS1, vascular leakage, sialic acid, biomarker
Introduction
With an estimated 400 million infections and up to 100 million cases annually, dengue is the most prevalent human arboviral disease worldwide [1]. Dengue is caused by four dengue virus serotypes (DENV1–4) and is transmitted by Aedes aegypti and Aedes albopictus mosquitoes. DENV infection results in a spectrum of outcomes, ranging from asymptomatic infection to classic dengue fever (DF) to dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS), with the latter being characterized by vascular leakage and shock.
The immunopathogenesis of DHF/DSS constitutes a complex multifactorial relationship between viral and host factors that leads to aberrant cellular immune responses, resulting in increased vascular permeability, hypotension and shock [2]. Studies by our group and others have established that the DENV nonstructural protein 1 (NS1) also plays a key role in the pathogenesis of DENV infection by inducing endothelial hyperpermeability leading to vascular leakage [3, 4]. Using a previously established murine model of DENV infection and pathogenesis [5–7], we showed that NS1 causes morbidity and mortality when administered in combination with a sub-lethal dose of virus and that NS1 alone triggers vascular leakage in the lung, liver and small intestine [3, 4]. Further studies showed that NS1 protein from DENV and other related flavivirus disrupts the endothelial glycocalyx layer (EGL) of endothelial cells from distinct tissues in vitro by inducing the degradation and shedding of key structural components, including sialic acid (Sia) and heparan sulfate proteoglycans [8–10]. Importantly, the expression of mammalian neuraminidases, Neu1, Neu2 and Neu3, was increased in these endothelial cells, likely contributing to Sia degradation [8, 10]. Furthermore, treatment with sialidase inhibitors (e.g. zanamivir, DANA) inhibited NS1-induced endothelial hyperpermeability in vitro and dermal vascular leakage in vivo, indicating that alteration of Sia distribution on the EGL contributes to both increased permeability and vascular leakage [8, 9, 11]. While these previous studies demonstrate a role for DENV NS1 in increasing the permeability of endothelial cell monolayers [11, 12], the modulation of Sia in vivo in the context of DENV infection has not been addressed.
Sia is the common name given to a family of acidic sugars with a nine-carbon backbone that are widely expressed in animal tissues, and it is also frequently used to refer to the most common member of this group found in humans, N-acetylneuraminic acid (Neu5Ac or NANA) [13]. On glycan structures, Sia has been established as an important determinant of endothelial barrier function in in vitro studies using rat pulmonary endothelial cells [12]. In human populations, increased serum levels of EGL components such as hyaluronic acid, heparan sulfate, claudin-5 and syndecan-1 have been associated with disruption of EGL and the development of plasma leakage and severe dengue disease [14, 15], but Sia has not been evaluated.
Here, using a murine model of DENV-induced vascular leak, we assessed Sia levels in comparison to NS1 levels in mice infected with a range of DENV doses. We found that the levels of Sia and NS1 in circulation increased in tandem with viral dose and were significantly elevated in mice that succumbed to DENV infection. Further, the levels of Sia and NS1 in circulation correlated with DENV-induced morbidity and with each other. In addition, we developed a novel liquid chromatography/mass spectrometry coupled with multiple reaction monitoring (LC-MRM-MS) method for detecting free Sia in serum without the need for derivatization. Using this approach, we determined that serum levels of free N-glycolylneuraminic acid (Neu5Gc) were significantly higher in DENV-infected mice. Overall, the identification of soluble markers associated with disease severity could reveal the mechanisms of DENV pathogenesis and contribute to the development of novel therapies.
Methods
Ethics statement
All experimental procedures involving the use of animals were pre-approved and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of California, Berkeley.
Dengue virus production
DENV2 D220 was generated in our laboratory from the parental strain, DENV2 PL046 [5, 6], originally obtained from H.-Y. Lei (National Cheng Kung University, Taiwan). The virus was propagated in the Aedes albopictus C6/36 cell line [American Type Culture Collection (ATCC)].
Murine DENV infection and blood collection
In previous studies, we developed and characterized mouse-adapted DENV strains that caused lethal disease in interferon-α/β receptor-deficient C57BL/6 (Ifnar−/−) and 129Sv (AG129) mice [5, 6]. Importantly, these rodent models carry infectious virus in tissues and exhibit signs of vascular leakage as a result of DENV infection. Further, passive administration of anti-DENV antibodies enhances DENV infection and disease in this model [3, 5, 7, 16]. We have also previously demonstrated in our mouse model that the high doses of DENV2 D220 and infection under antibody-dependent enhancement (ADE) conditions yield peak viral load and mortality on day 3 post-infection [3, 5]. Ifnar−/− mice were infected with DENV by intravenous injection and were monitored twice daily for DENV-induced morbidity and mortality for 10 days post-infection. For ADE conditions, 5 µg of 4G2 (anti-envelope mAb) were administered by intraperitoneal injection 20–24 h prior to infection. Signs of morbidity were scored using a standardized scale, where 1=healthy; 2=mild signs of lethargy, some fur ruffling, no hunching; 3=fur ruffling, hunched, mild signs of lethargy; 4=ruffled, hunched and lethargic, limited morbidity; 5=moribund or dead [5]. Blood samples (0.2 ml) from DENV-infected mice were collected via submandibular bleed 3 days post-infection. Blood specimens were allowed to clot for 3 min (min) at room temperature, and serum was then separated by centrifugation and immediately stored at −80 °C.
Sia analysis
Total Sia levels (conjugated and free) in mouse sera were measured using a Mouse Sialic Acid ELISA kit (MyBioSource) as per the manufacturer’s instructions. The reported sensitivity of this assay is 0.1 ng ml−1. In addition, LC-MRM-MS was applied to measure free Sia (Neu5Ac and Neu5Gc) levels. Sia was extracted from an aliquot (30 µl) of mouse serum with LC-MRM-MS-grade acetonitrile (90 µl) containing an internal standard ([13C3]-Neu5Ac, 83.5 ng ml−1). Samples were vortexed for 2 min and incubated at −20 °C for 1 h. Samples were transferred to room temperature, briefly vortexed and centrifuged for 30 min at 13000 r.p.m. Supernatants were carefully transferred to new Eppendorf tubes and vacuum-dried. Dried metabolites were reconstituted with 30 µl of a 1 : 1 mixture of solvent A and solvent B; solvent A consisted of acetonitrile–isopropanol–water (95 : 5 : 5), whereas solvent B consisted of water–isopropanol (95 : 5). Both solvents contained 20 mM ammonium acetate, adjusted to pH 9. The metabolite extract was centrifuged and the supernatant was transferred to LC-MRM-MS vials.
An aliquot (10 µl) of each sample was subjected to LC-MRM-MS and quantification using an Agilent 1260 Infinity II LC system and an Agilent 6470 triple quadrupole mass spectrometer equipped with jet stream ionization. LC separation was achieved using a Poroshell 120 HILIC-Z LC column (2.1×150 mm, 2.7 µm) (Agilent Technologies), applying mobile phase at a flow rate of 0.30 ml min−1 at 30 °C. The LC elution of products was initiated with 100 % solvent A for 1.0 min, followed by a linear gradient to 60 % solvent A, 40 % solvent B over 8.0 min and maintenance at this solvent mixture for 2.5 min before returning to 100 % solvent A over 0.5 min. The column was re-equilibrated in 100 % solvent A for 4 min. Eluent from the column was directly introduced into the triple quadrupole mass spectrometer with electrospray ionization in the negative mode. The following MS parameters were applied: gas temperature 325 °C; drying gas flow at 8 L min−1; nebulizer pressure 40 psig; capillary voltage 3500; sheath gas flow 7 l min−1 and sheath gas temperature 325 °C. Data acquisition and analysis were performed using MassHunter software (Agilent Technologies). The compound-specific parameters, including fragmentor voltage, collision energy and ion transitions, were optimized by analysing standard compounds for multiple reaction monitoring (MRM) (Table 1). The concentration of compounds in samples was quantified by comparison with the peak area of the spiked internal standard.
Table 1.
Optimized conditions for sialic acids in multiple reaction monitoring detection
|
Compound name |
Precursor ion |
Product ion |
Fragmentor (V) |
Collision energy (V) |
Polarity |
|---|---|---|---|---|---|
|
N-acetylneuraminic acid |
308.1 |
170 |
80 |
5 |
Negative |
|
|
308.1 |
98 |
80 |
20 |
Negative |
|
|
308.1 |
87* |
80 |
5 |
Negative |
|
N-glycolylneuraminic acid |
324.1 |
186 |
80 |
10 |
Negative |
|
|
324.1 |
116 |
80 |
10 |
Negative |
|
|
324.1 |
87* |
80 |
15 |
Negative |
|
[13C3]-N-acetylneuraminic acid |
311.1 |
173 |
80 |
5 |
Negative |
|
|
311.1 |
99 |
80 |
20 |
Negative |
|
|
311.1 |
90* |
80 |
5 |
Negative |
*Quantifier ion
NS1 quantification by enzyme-linked immunosorbent assay (ELISA)
Serum levels of NS1 were assessed using a capture ELISA as previously described [3]. Briefly, Nunc MaxiSorp ELISA plates (Thermo Scientific) were coated overnight with 5 µg ml−1 of monoclonal antibody (mAb) 7E11. Plates were blocked with 1 % bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (1 % BSA-PBS), and serum samples were added (1 : 20 dilution) for 1 h at room temperature. After washing with PBS+0.05 % Tween 20 (PBS-T), biotinylated NS1-specific mAb 2B7 (50 µl/well at 2 µg ml−1) was added to each well for 1 h at room temperature. Plates were subsequently washed with PBS-T and incubated with streptavidin/horseradish peroxidase (HRP) for 40 min at room temperature. The assay was developed using 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma), according to the manufacturer’s specifications. The TMB reaction was stopped with 0.5 M H2SO4 and read at OD450 nm. The estimated limit of detection of this in-house assay using purified recombinant NS1 is 1 ng ml−1.
Statistical analysis
Data were analysed and plotted using GraphPad Prism 6 software. Differences between groups were evaluated using a two-sided Student t-test, and correlations were determined by linear regression analysis.
Results
Sia and NS1 levels in mouse serum are associated with viral dose and DENV-induced morbidity and mortality
To determine whether Sia and NS1 levels in serum were associated with DENV-induced morbidity and mortality, groups of Ifnar−/− mice were infected with increasing doses of DENV2 D220 ranging from 1×102 to 1×106 plaque-forming units (p.f.u.). In groups of mice infected with 3×105 or 1×106 p.f.u., we used ADE conditions, which have been shown to enhance DENV2 D220 infection and disease progression associated with vascular leakage [5, 6]. The tested doses and conditions allowed us to cover the spectrum of DENV infection outcomes described in the Ifnar−/− mouse model, ranging from inapparent infection to severe manifestations, including lethargy, limited mobility, fur ruffling, hunching and mortality [5]. The progression of morbidity and mortality among infected mice was dependent on the DENV dose used for infection. Animals infected with 1×102 p.f.u. did not exhibit signs of illness, and nor did they succumb to DENV infection, whereas in mice infected with 1×106 p.f.u. using ADE conditions, morbidity and mortality progressed rapidly (Fig S1, available in the online version of this article). Mortality in this model has previously been shown to be due to a vascular leak syndrome [3, 5–7].
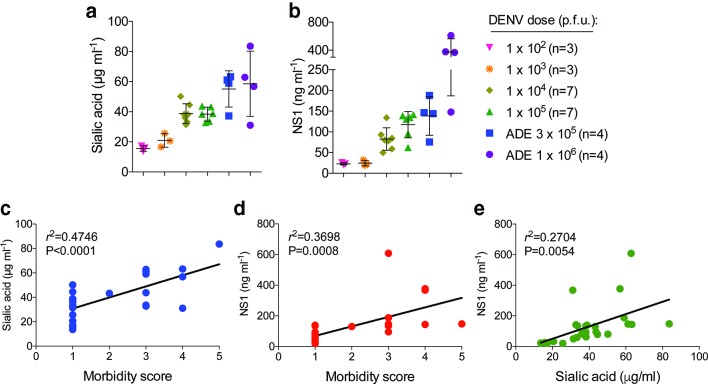
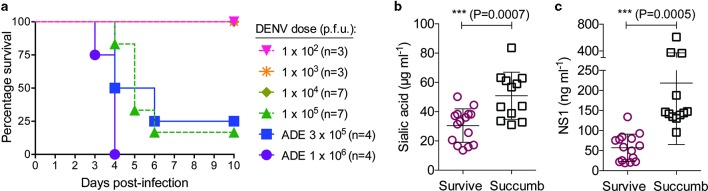
On day 3 post-infection, blood samples were collected for serum testing by ELISA, which showed that the levels of both Sia and NS1 in circulation correspondingly increased with the DENV doses used for infection (Fig. 1a–b). Accordingly, the Sia and NS1 levels in serum were the lowest among mice infected with 1×102 p.f.u. and the highest in animals infected with 1×106 p.f.u. of DENV2 D220 under ADE conditions. In addition, using a standardized morbidity scale [5], we found that DENV-induced morbidity at day 3 post-infection among infected mice was correlated with circulating amounts of Sia (r 2=0.4746, P<0.0001) and NS1 (r 2=0.3698, P=0.0008) (Fig. 1c–d). Similarly, the levels of Sia and NS1 were also moderately correlated with each other (r 2=0.2704, P=0.0054) (Fig. 1e). The mice used in these experiments were also monitored for survival (Fig. 2a), which allowed us to analyse the measured levels of Sia and NS1 in serum in relation to DENV infection outcome. This analysis showed significantly higher levels of Sia (P=0.0026) (Fig. 2b) and NS1 (P<0.0001) (Fig. 2c) among mice that succumbed to DENV infection compared to animals that survived.
Fig. 1.
Sialic acid and NS1 levels in serum of DENV-infected mice increase proportionally with infectious dose and are correlated with DENV-induced morbidity. Ifnar −/− mice were infected i.v. with increasing doses of DENV2 D220, and blood samples were collected on day 3 post-infection for serum testing by ELISA. Quantification of (a) Sia and (b) NS1 levels in serum from DENV-infected mice (mean±sd). Correlation analysis between DENV-induced (c) morbidity and Sia, (d) morbidity and NS1, and (e) Sia and NS1 levels in serum. Signs of morbidity were scored using a standardized scale, where 1=healthy; 2=mild signs of lethargy, some fur ruffling, no hunching; 3=fur ruffling, hunched, mild signs of lethargy; 4=ruffled, hunched and lethargic, limited morbidity; 5=moribund or dead. Data were combined from two experiments. ADE, antibody-dependent enhancement; p.f.u., plaque-forming units.
Fig. 2.
Circulating levels of sialic acid and NS1 are associated with DENV infection outcome. Ifnar −/− mice were infected i.v. with different doses of DENV2 D220, and survival was monitored for 10 days. These are the same mice as those tested in Fig. 1. (a) A Kaplan–Meier survival curve is shown. (b) Sia and (c) NS1 levels in serum were evaluated by ELISA on day 3 post-infection and plotted in relation to DENV infection outcome (mean±sd), with significantly higher amounts being found in animals that succumbed to the infection, with P-values as indicated (two-sided Student t-test). Data were combined from two similar experiments. ADE, antibody-dependent enhancement; p.f.u., plaque-forming units.
Quantification of free Sia by LC-MRM-MS
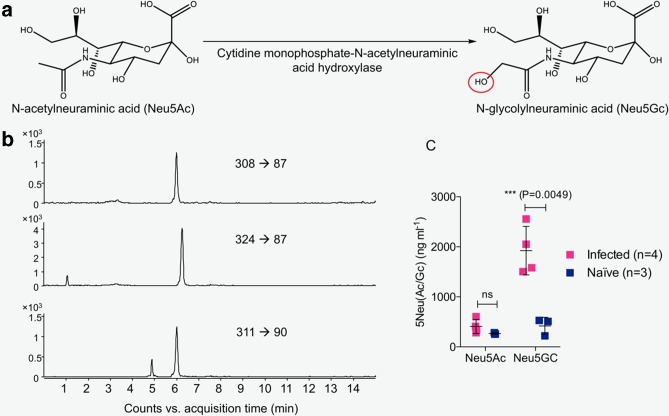
Sias are the N- or O-substituted derivatives of neuraminic acid [17]. Conversion of Neu5Ac to Neu5Gc is carried out by the enzyme cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH), which is only found in non-human mammals (Fig. 3a), as the human CMAH gene is irreversibly mutated [18, 19]. We developed a novel LC-MRM-MS assay to quantify free Neu5Ac and Neu5Gc in mouse serum (Fig. 3b). The results show a significant increase in Neu5Gc, but not Neu5Ac, in the serum of mice with severe dengue disease compared to naïve serum (Fig. 3c).
Fig. 3.
Analysis of free Sia in mouse serum using LC-MRM-MS. (a) Chemical structures of Neu5Ac and Neu5Gc. Neu5Ac is converted to Neu5Gc by the enzyme cytidine monophosphate-N-acetylneuraminic acid hydroxylase, which is only found in non-human mammals. The red circle indicates the difference between Neu5Ac and Neu5Gc. (b) LC-MRM-MS-extracted ion chromatograms of N-acetylneuraminic acid (top panel) and N-glycolylneuraminic acid (middle panel) in serum from DENV-infected mice and [13C3]-N-acetylneuraminic acid spiked into the serum (bottom panel) are shown. Three ion transitions of each compound were monitored for MRM detection; here quantifier ion transitions are shown. (c) LC-MRM-MS was used to quantify Neu5Ac and Neu5Gc in serum from mice infected with ADE 3×105 p.f.u. DENV2 D220 or uninfected (naïve) mice. Free sialic acid in the form of Neu5Gc, the predominant species in mouse serum, was increased in samples from mice with severe dengue disease. Mean±sd; P-values as indicated (two-sided Student t-test). Data were pooled from a minimum of two similar experiments. ns, not significant.
Discussion
The pathways that contribute to endothelial dysfunction and vascular leak during severe dengue disease are complex. Recently, NS1 has been shown to trigger the disruption of the EGL through various mechanisms, including the activation of endothelial sialidases and the cathepsin l-heparanase pathway [8–10]. In this study, we found that Sia and NS1 levels increased with the higher doses of DENV used for infection and correlated with DENV-induced morbidity and mortality. We tested a range of DENV doses to represent the spectrum of morbidity resulting from DENV infection in this rodent model. As determined by ELISA, the levels of Sia and NS1 in circulation at day 3 post-infection increased according to the DENV dose used for infection. Both Sia and NS1 levels correlated significantly with DENV infection severity. Taken together, these findings are consistent with previous observations indicating that the pathophysiology of DENV infection is associated with the disruption of vascular endothelium components and increased NS1 antigenaemia [14, 15, 20–22].
Sia constitutes an important component of the extracellular matrix in many different tissues [13]. Given its ample distribution, abundance and diverse structure, it is not surprising that Sia is involved in a number of cellular, physiological and immunological functions [23]. Indeed, the abundant expression of Sia on cellular and lysosomal membranes is consistent with the roles of this sugar family in the stabilization of adhesion molecules and membranes and in mediating interactions with the environment [23]. It is estimated that the typical cell displays tens of millions of Sia molecules and that concentrations on the cell surface glycocalyx are approximately 100 mM [24]. Under normal physiological conditions, extracellular Sia can be taken up by mammalian cells via macropinocytosis, arriving in the lysosomes for subsequent reutilization or degradation [17, 23, 24]. In addition, free Sia derived from cellular sources can be readily excreted in urine [17, 23]. Sia dynamics are critical for numerous biological functions. For instance, cell inflammatory responses are increased after Sia removal from cell surface ligands or receptors [25]. In particular, desialylation of TLR4 ligands is required for TLR4 signalling [26]. NS1 has been also shown to trigger secretion of proinflammatory cytokines via TLR4 activation of peripheral blood mononuclear cells (PBMCs) [4]. In this study, circulating levels of Sia and NS1 were significantly higher in mice that succumbed to DENV infection in comparison to the levels in mice that survived DENV infection. Thus, higher circulating levels of NS1 in DENV-infected mice, resulting in desialylation and concomitant increased release of Sia, could lead to the activation of TLR4 on PBMCs and an inflammatory cytokine response, which is also characteristic of severe DENV infections [27].
In previous studies, we have observed comparable survival rates in studies using ADE 3×105 p.f.u. DENV2-D220 to infect Ifnar −/− mice as seen here [9, 28]. Intriguingly, the rates of morbidity and mortality in mice infected with 1×105 p.f.u. and ADE 3×105 p.f.u. were fairly similar. We speculate that the blood collection performed at day 3 post-infection may have precipitated the rapid disease progression and increased mortality in animals infected with 1×105 p.f.u. Nonetheless, the overall data suggest that NS1-triggered disruption can lead to the release of EGL components and that this process is indeed pathogenic. In pathological conditions such as sepsis and viral haemorrhagic fevers, the shedding and degradation of Sia are often accompanied by the release of other EGL components (e.g. heparan sulfate, chondroitin sulfate and hyaluronic acid), which leads to loss of EGL barrier function and increased vascular permeability [8, 9, 29–31]. Increased levels of NS1 have been associated with the development of severe manifestations in humans as well as morbidity/mortality in mouse models of DENV infection [3, 21, 22]. Importantly, previous research in human populations showed that increased levels of hyaluronic acid, heparan sulfate, claudin-5 and syndecan-1 in serum are associated with vascular leakage, underscoring the usefulness of EGL components as markers of severe dengue disease [8, 9]. Thus, the feasibility of Sia as a marker of clinical dengue disease warrants further research.
Sia molecules are widely found in nature as components of very complex matrices, including oligosaccharide units in mucins, glycoproteins and other microbial polymers. Therefore, the existing methods for determining these compounds can be time-consuming, tedious and sometimes not specific. The quantitative methods currently used in the field to measure Sia, such as ELISA assays, typically employ unprocessed biological samples, do not differentiate between free and conjugated Sia, and cannot discriminate between closely related molecular species or isomers. High-performance liquid chromatography (HPLC) methods have the ability to discriminate closely related molecular species and isomers, but require extensive sample preparation involving derivatization with a fluorophore for detection. In contrast, LC-MRM-MS provides high mass-specific detection, yields greater sensitivity and can differentiate free from conjugated Sia, as well as molecular species and isomers, with minimal sample preparation. In this study, in addition to the widely used Sia ELISA, we also developed a targeted mass spectrometry method that accurately detected free Sia released from parental glycoconjugates in mouse serum [32]. This approach allowed us to monitor multiple ions (three ions) during analysis to achieve enhanced specificity for Sia identification and quantitation in the presence of complex biological matrices such as serum. This is a significant advancement compared to the current ELISA-based methods used in the field that suffer from selectivity and specificity problems and, as commonly used, only detect total Sia without discriminating between the conjugated versus free molecules in biological fluids. The method developed can also accurately discriminate between free Neu5Ac and Neu5Gc, the two most abundant forms of Sia, increasing our ability to discriminate between molecular species of free Sia.
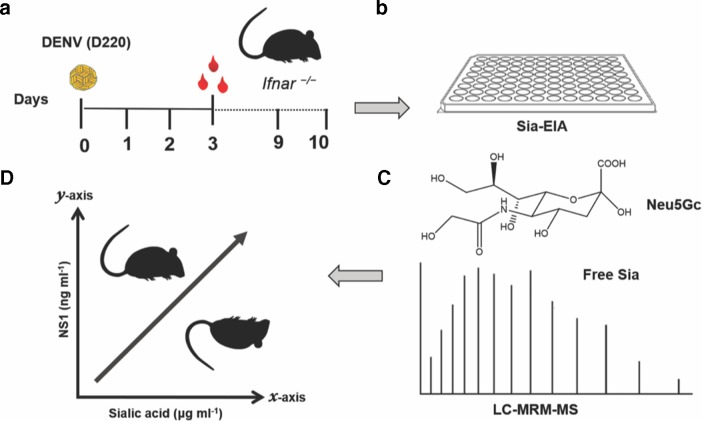
In conclusion, we demonstrated that DENV-induced morbidity in a murine model of dengue disease is correlated with increasing levels of Sia and NS1 in circulation and that the concentrations of Sia and NS1 in sera were significantly higher in mice that succumbed to DENV infection (Fig. 4). Further, the newly developed LC-MRM-MS approach for the detection of free Sia provides a useful tool for the identification of molecular signatures associated with severe dengue disease and warrants further studies assessing the utility of this method in human populations. Finally, these results extend previous research indicating that EGL disruption is associated with the hallmark characteristics of vascular leak observed in severe dengue.
Fig. 4.
DENV-induced morbidity and mortality in a murine model of dengue disease are correlated with increasing levels of Sia and NS1 in circulation. (a) Ifnar —/— mice were infected with DENV2-D220, and 3 days post-infection, circulating levels of Sia were measured by (b) ELISA and (c) a newly developed LC-MRM-MS assay. The latter approach showed that serum levels of free N-glycolylneuraminic acid (Neu5Gc), a form of Sia found in most non-human mammals, were significantly higher in DENV-infected mice. (d) High levels of NS1 and Sia were associated with increased mortality in DENV-infected mice. These findings indicate that DENV infection leads to disruption of EGL components, such as Sia, which can be measured in serum and could used as potential biomarkers for severe dengue disease in humans.
Supplementary Data
Funding information
This work was supported by grants R01 AI24493 (E. H.), U19 AI109761 (Center for Research in Diagnostics and Discovery; E. H.; program director, I. Lipkin) and R33 AI100186 (R. P. and E. H.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Author contributions
Conceptualization, D. A. E, P. R. B., H. P-G., R. P. and E.H.; methodology, D. A. E., H. P-G., M. N. I, J. T. B. and R. P.; validation and analysis, D. A. E., M. N. I, J. T. B. and R. P.; resources, R. P. and E. H.; writing – original draft preparation, D. A. E. and R. P.; writing – review and editing, P. R. B., H. P-G., M. N. I, J. T. B., R. P. and E. H.; supervision, P. R. B, R. P. and E. H.; project administration, R. P. and E. H.; funding acquisition, R. P and E. H.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
One supplementary figure is available with the online version of this article.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzman MG, Harris E, Dengue HE. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 3.Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, et al. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. 2015;7:304ra141. doi: 10.1126/scitranslmed.aaa3787. [DOI] [PubMed] [Google Scholar]
- 4.Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med. 2015;7:304ra142. doi: 10.1126/scitranslmed.aaa3863. [DOI] [PubMed] [Google Scholar]
- 5.Orozco S, Schmid MA, Parameswaran P, Lachica R, Henn MR, et al. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J Gen Virol. 2012;93:2152–2157. doi: 10.1099/vir.0.045088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, et al. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puerta-Guardo H, Glasner DR, Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016;12:e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasner DR, Ratnasiri K, Puerta-Guardo H, Espinosa DA, Beatty PR, et al. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017;13:e1006673. doi: 10.1371/journal.ppat.1006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puerta-Guardo H, Glasner DR, Espinosa DA, Biering SB, Patana M, et al. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep. 2019;26:1598–1613.:e1598. doi: 10.1016/j.celrep.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betteridge KB, Arkill KP, Neal CR, Harper SJ, Foster RR, et al. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol. 2017;595:5015–5035. doi: 10.1113/JP274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cioffi DL, Pandey S, Alvarez DF, Cioffi EA. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1067–L1077. doi: 10.1152/ajplung.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang THC, Alonso S, Ng LFP, Thein TL, Pang VJX, et al. Increased serum hyaluronic acid and heparan sulfate in dengue fever: association with plasma leakage and disease severity. Sci Rep. 2017;7:46191. doi: 10.1038/srep46191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suwarto S, Sasmono RT, Sinto R, Ibrahim E, Suryamin M. Association of endothelial glycocalyx and tight and adherens junctions with severity of plasma leakage in dengue infection. J Infect Dis. 2017;215:992–999. doi: 10.1093/infdis/jix041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7:128–139. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varki A, Schnaar RL, Schauer R, et al. Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. pp. 179–195. [Google Scholar]
- 18.Ghaderi D, Taylor RE, Padler-Karavani V, Diaz S, Varki A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. 2010;28:863–867. doi: 10.1038/nbt.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci USA. 2010;107:8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trung DT, Wills B. Systemic vascular leakage associated with dengue infections - the clinical perspective. Curr Top Microbiol Immunol. 2010;338:57–66. doi: 10.1007/978-3-642-02215-9_5. [DOI] [PubMed] [Google Scholar]
- 21.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186:1165–1168. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 22.Jayathilaka D, Gomes L, Jeewandara C, Jayarathna GSB, Herath D, et al. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat Commun. 2018;9:5242. doi: 10.1038/s41467-018-07667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varki A, Schauer R. Sialic acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 24.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci USA. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G-Y, Tang J, Zheng P, Liu Y. Cd24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amith SR, Jayanth P, Franchuk S, Siddiqui S, Seyrantepe V, et al. Dependence of pathogen molecule-induced Toll-like receptor activation and cell function on NEU1 sialidase. Glycoconj J. 2009;26:1197–1212. doi: 10.1007/s10719-009-9239-8. [DOI] [PubMed] [Google Scholar]
- 27.Srikiatkhachorn A, Mathew A, Rothman AL. Immune-Mediated cytokine storm and its role in severe dengue. Semin Immunopathol. 2017;39:563–574. doi: 10.1007/s00281-017-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espinosa DA, Beatty PR, Reiner GL, Sivick KE, Hix Glickman L, et al. Cyclic dinucleotide-adjuvanted dengue virus nonstructural protein 1 induces protective antibody and T cell responses. J Immunol. 2019;202:1153–1162. doi: 10.4049/jimmunol.1801323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly-Andersen AM, Thunberg T, Ahlm C. Endothelial activation and repair during hantavirus infection: association with disease outcome. Open Forum Infect Dis. 2014;1:ofu027. doi: 10.1093/ofid/ofu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 31.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C, Xia L, Liu L, Qu F, Li G, et al. A novel, sensitive and convenient method for determination of sialic acids in human serum utilizing ultrasonic-assisted closed in-syringe hydrolysis and derivatization prior to high performance liquid chromatography. Analytical Methods. 2016;8:554–563. doi: 10.1039/C5AY02648B. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.