Abstract
Herpesviruses, including herpes simplex virus-1, encode and express a DNA polymerase that is required for replication of their dsDNA genomes. The catalytic subunit of this enzyme contains a 3′-5′ exonuclease that is involved in proofreading during replication. Although certain mutations that severely impair exonuclease activity are not lethal to the virus, it was reported that virus containing the substitution of alanine for aspartate 368 (D368A), which ablates exonuclease activity, could not be recovered, raising the possibility that this activity is essential for viral replication. To investigate this issue, we produced virus containing this mutation (D368A Pol) using a complementing cell line. D368A Pol virus was unable to form plaques on non-complementing cells. Viral DNA synthesis and polymerase activity were severely inhibited in D368A-infected cells, as was expression of the enzyme, suggesting that effects on polymerase expression rather than on exonuclease activity per se largely explain the lethal phenotype of this mutation.
Keywords: Herpes smplex virus-1, DNA polymerase, 3’-5’ exonuclease, DNA replication
Herpesviruses encode DNA replication machinery that is crucial for copying their dsDNA genomes and for lytic replication within the host. For herpes simplex virus-1 (HSV-1) this machinery includes the catalytic subunit of the DNA polymerase (Pol), which is a family B polymerase that contains four domains: the pre-NH2-terminal domain, the NH2-terminal domain (NTD), the 3′-5′ exonuclease domain (Exo), and the 5′-3′ polymerase domain [1–3]. Residues that are important for HSV-1 Pol 5′-3′ DNA polymerase activity include multiple conserved regions [2, 4–7] within the palm, finger and thumb subdomains that form the canonical catalytic centre for 5′-3′ polymerization activity [1, 3, 8].
HSV-1 Pol also contains an intrinsic 3′-5′ exonuclease activity that is important for proofreading during DNA replication [2, 7, 9, 10]. Mutations affecting the Exo domain of HSV-1 Pol have been studied extensively for the mapping of catalytic residues within the active site [9–12], their effects on fidelity (reviewed in [13]), antiviral resistance (reviewed in [14]), and strand displacement synthesis [15, 16]. Residues required for this 3′-5′ exonuclease activity have been mapped within three conserved regions (Exo I, II and III) [1, 2, 7, 17]. Specific mutations within each of these regions of HSV Pol (Exo I mutant aspartate 368 substituted with alanine (D368A); Exo II mutant E460D and G464V; Exo III mutants Y577H and YD12) severely inhibit 3′-5′ exonuclease activity in vitro and have been recombined into the virus, for characterization in cell culture [9–11]. The Exo II mutants studied so far (E460D and G464V) have been reported to severely affect polymerase activity in vitro and had major effects on viral replication in cell culture [10, 12]. The Y577H and YD12 Exo III mutants, on the other hand, only exhibited a ~three-fold defect on virus replication, while maintaining at least 80 % of polymerase activity and reducing exonuclease activity to 2 % or less, providing evidence that this loss of exonuclease activity and the mutator phenotype associated with it are not lethal to viral replication [9].
The Exo I mutant D368A, which exhibits severely impaired exonuclease activity, likely through the disruption of metal ion coordination within the 3′-5′ exonuclease active site [11, 12, 18], retains nearly wild-type (WT) levels of polymerase activity following expression using recombinant baculovirus in insect cells and purification [11, 12, 16, 18]. Multiple attempts to recombine this mutation into HSV-1 using marker rescue of a temperature-sensitive (ts) pol mutant were unsuccessful, despite successful rescue of the ts mutation and successful introduction of a different mutation using the same protocol, leading the authors to raise the possibilities that the D368A mutation is lethal and that the exonuclease activity is essential for viral replication [11]. These attempts to generate the D368A Pol virus did not utilize a complementing cell line [11], however. Thus, the failure to derive the mutant was a negative result and prevented a quantitative assessment of the effects of the mutation on viral replication, DNA synthesis, polymerase activity, and expression. Because the aforementioned Exo III mutants with severely impaired exonuclease activity have been reported to have relatively minor defects in virus production [9], we set out to reinvestigate the phenotype of the D368A mutation.
In this study, we generated D368A Pol virus in a complementing cell line. The D368A mutation was introduced into an HSV-1 KOS bacterial artificial chromosome (BAC) encoding FLAG-tagged Pol [19] using Red two-step recombination [20] and the PCR primers GGGGCATGAGCGACCTACCGGCATACAAGCTCATGTGCTTCGCTAT CGAATGCAAGGCTAGGGATAACAGGGTAATCGATT T (forward) and GGAAAGGCCAGCTCGTCCTCCCCCC CCGCCTTGCATTCGATAGCGAAGCACATGAGCTTGTATGGCCAGTGTTACAACCAATTACC (reverse). The resulting BAC was transfected into PolB3 cells (generously provided by C. Hwang), which inducibly express WT Pol upon infection [9]. Additionally, to test whether any off-target mutations are responsible for the resulting phenotype, a rescued derivative of the D368A Pol virus (D368A Rescue) was produced by recombining the WT sequence into the D368A Pol BAC using the primers GGGGCATGAGCGACCTACCGGCATACAAGCTCATGTGCTTCGATATC GAATGCAAGGCGGGGGTAGGGATAACAGGGTAATC GATTT (forward) and GAAAGGCCAGCTCGTCCTCC CCCCCCGCCTTGCATTCGATATCGAAGCACATGAGCTTGTATGGCCAGTGTTACAACCAATTAACC (reverse) and transfecting this BAC into PolB3 cells. Additionally, the D368A Pol and D368A Rescue viral genomes were collected, and the pol gene was sequenced to confirm that no other mutations were present.
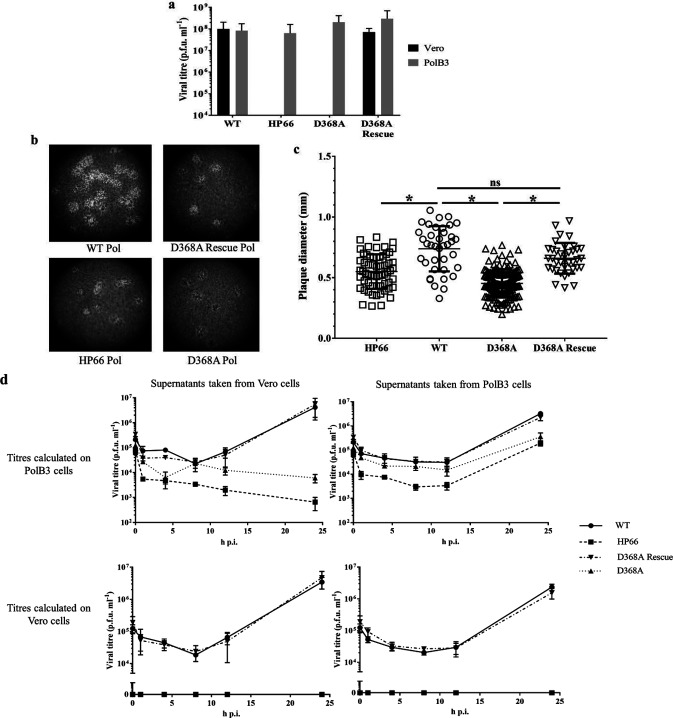
First, we assessed any viral replication defects of the D368A Pol virus (Fig. 1). Viruses generated from PolB3 cells were collected 5 days post-transfection, and the supernatants were titrated on either Vero or PolB3 cells alongside WT virus and, as a negative control, a virus (HP66) with an insertion of the Escherichia coli β-galactosidase gene replacing much of the pol gene [21]. The cells were fixed and stained using crystal violet at 72 h post-infection (p.i.). Whereas the WT virus could form plaques efficiently upon infection of either Vero or PolB3 cells, the negative control, HP66, was unable to form any plaques on Vero cells, but could form plaques on complementing PolB3 cells (Fig. 1a). Like the negative control, the D368A Pol virus was unable to form any plaques on Vero cells even at the lowest dilution tested but could do so on PolB3 cells. As expected, D368A Rescue could form plaques efficiently on Vero cells, indicating that the inability of the D368A mutant to form plaques on Vero cells is due to the designed mutation. These data show that the D368A virus is severely inhibited for plaque formation in the absence of complementing Pol protein which is consistent with the suggestion in the previous report that the D368A mutation is lethal [11].
Fig. 1.
D368A Pol virus replication is severely inhibited in non-complementing cells, and is also affected in complementing cells. (a) Assay for plaque formation of the denoted viruses titrated side-by-side on Vero and complementing PolB3 cells. The lower limit of the vertical axis denotes the limit of detection for plaque formation in this assay. (b) Twenty times magnification images of wells from plaque assays fixed at 72 h p.i. and stained with crystal violet. (c) Plaque diameters for each denoted virus infection on PolB3 cells were analysed using one-way analysis of variance (ANOVA) with Šídák correction for multiple comparisons. *, P-value <0.0001, ns, not significant. (d) Single-cycle replication kinetics for the denoted viruses collected from either Vero or PolB3 cells, titrated on both Vero and PolB3 cells. Vero cells (left column) or PolB3 cells (right column) were infected with the indicated viruses [multiplicity of infection (m.o.i.) of 10], the cells were washed at 1 h p.i. and supernatants were collected at time points ranging from 4 to 24 h p.i. The supernatants were then titrated on either PolB3 cells (top row) or Vero cells (bottom row), and the resulting titres of the supernatants are indicated on the y-axis of each graph.
Despite the high titres observed, the plaques formed on PolB3 cells by HP66 and D368A Pol were smaller in size than those of the WT and D368A Rescue viruses. To quantify this difference, the crystal violet-stained plates were imaged at 20× magnification (Fig. 1b), and the plaque diameters were measured using ImageJ software, compiled and analysed by one-way analysis of variance (ANOVA) using GraphPad Prism software (Fig. 1c). Both the HP66 and the D368A plaques were significantly smaller than the WT and D368A Rescue plaques, suggesting that while these viruses form plaques readily on PolB3 cells, they are either producing less infectious virus per cell or are otherwise less efficient at viral spread to surrounding cells.
To investigate the replication of HP66 and D368A Pol in Vero and PolB3 cells, single-cycle replication kinetics were assessed. Viruses were plaque-purified, and high-titre stocks were obtained using PolB3 cells. Vero cells (Fig. 1d, left column) and PolB3 cells (Fig. 1d, right column) were infected with WT, HP66, D368A, or D368A Rescue viruses (m.o.i. 10), incubated at 37 °C for 1 h and washed three times with PBS before the media were replaced, and the supernatants were collected at 1, 4, 8, 16 and 24 h p.i. The resulting supernatants were titrated on both PolB3 cells (Fig. 1d, top row) and Vero cells (Fig. 1d, bottom row). Additionally, the titres at the zero time point were calculated from the back titres of the inocula. The positive controls, WT Pol and D368A Rescue, were able to replicate similarly in either Vero or PolB3 cells (Fig. 1d). The negative control, HP66, did not produce any plaque-forming units above what had been seen at 1 h p.i. when infecting the Vero cells; indeed, the titres dropped after that time point (Fig. 1d, top left). When HP66 virus was grown on PolB3 cells, a 10- to 20-fold defect compared to WT was detected at 24 h p.i. (Fig. 1d, top right). The likely cause for this defect in the PolB3 cells will be discussed below. As with HP66, following the infection of Vero cells, the titres of D368A decreased over the time course (Fig. 1d, top left). In the PolB3 cells, we detected a nine-fold defect in viral replication associated with the D368A mutation compared to the WT (Fig. 1d, top right). We also titrated the supernatants on Vero cells to see whether there was any evidence for reversion to a WT phenotype, and none was detected (Fig. 1d, bottom left and right). Combined, these results suggest that the D368A mutation is lethal.
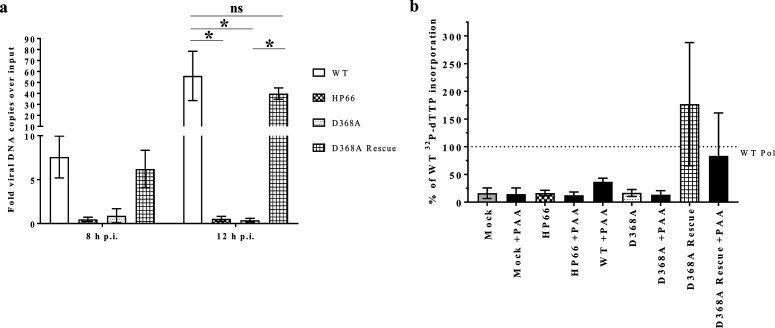
We next assessed whether the D368A Pol virus was capable of viral DNA synthesis in non-complementing cells. Vero cells were infected at an m.o.i. of 10 and cell lysates were collected at 1, 8, and 12 h p.i. The lysates were diluted 20-fold, and DNA was purified alongside lysates of mock-infected cells containing serial dilutions of spiked viral genome standards. The samples were amplified using primers specific to the viral thymidine kinase (tk) gene or the cellular 1,3-alpha-galactosyltransferase gene, the tk values were compared to the standard curve of spiked KOS viral genomes, and the amounts of cellular DNA detected in each sample were used to normalize the viral DNA values as reported previously [19]. The viral copy numbers are reported as the fold viral genomes replicated over the input viral genomes (1 h p.i. time point) (Fig. 2a). The positive controls, WT and D368A Rescue viruses, were able to replicate viral DNA above the input number of genomes by at least 40-fold. The negative control, HP66, on the other hand, did not detectably produce any viral DNA copies over those present at 1 h p.i. D368A Pol resembled HP66; viral DNA copy number never increased over the input levels. The infection of PolB3 cells with the D368A and HP66 viruses resulted in viral DNA synthesis, but with a 10- to 20-defect similar to that seen for virus production relative to the WT and D368 Rescue viruses (data not shown). Thus, the D368A Pol virus is highly impaired for viral DNA synthesis during infection.
Fig. 2.
D368A Pol virus is deficient in viral DNA synthesis and for polymerase activity. (a) qPCR analysis of viral genome copy numbers at 8 and 12 h p.i. compared to those present following adsorption [1 h p.i.; analysed using one-way analysis of variance (ANOVA) with Šídák correction for multiple comparisons]. *, P-value <0.002, ns, not significant. (b) 32P dTTP incorporated into activated salmon sperm DNA in the presence of high ammonium sulfate concentrations compared to the WT incorporation after 20 min in the presence or absence of HSV-1 Pol inhibitor PAA. The data from three assays were combined and plotted as a percentage of incorporated radioactivity compared to WT Pol, as marked with the dotted line, with the standard deviations denoted by the error bars.
We and others have purified soluble D368A Pol from recombinant baculovirus-infected Sf9 insect cells and showed that it exhibits near WT levels of 5′-3′ polymerase activity [11, 12, 18], so we wanted to examine whether there was any active HSV Pol in the infected cells. To this end, we utilized an assay [10] that can discern viral polymerase activity in cell lysates by taking advantage of HSV Pol’s resistance to or even activation by high ammonium sulfate concentrations that are inhibitory to cellular polymerases [22]. In this assay, DNA polymerase activity from infected 293 T cell lysates was quantified using scintillation counting of filter-bound 32P-dTTP incorporated into activated salmon sperm DNA. Incorporation over that of the mock-infected and HP66-infected lysates was detected from lysates of WT- and D368A Rescue-infected cells in the absence of an HSV Pol inhibitor, phosphonoacetic acid (PAA), but was inhibited upon PAA addition to the reaction (Fig. 2b). In contrast, there was no ammonium sulfate-resistant polymerase activity above that of the mock-infected samples associated with the D368A Pol virus-infected cell lysates.
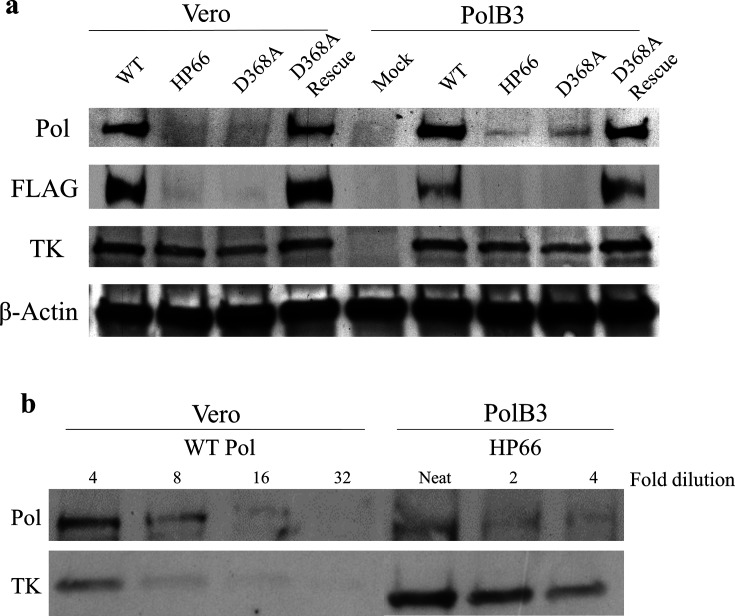
Since we were unable to identify any viral DNA synthesis or viral polymerase activity in D368A Pol virus-infected cells, we next examined Pol expression during infection using Western blotting (Fig. 3a). Vero and PolB3 cells were infected at an m.o.i. of 10, and the cell lysates were collected in Laemmli buffer at 8 h p.i. The samples were boiled at 95 °C for 5 min and run on a 4–20 % polyacrylamide SDS-PAGE gel (Bio-Rad), transferred onto polyvinylidene difluoride (PVDF) membrane, blocked, and probed using the following antibodies: α-Pol (mouse 1051c, generously provided by C. Knopf), α-FLAG (mouse M2, Sigma-Aldrich; to detect FLAG-tagged Pol expressed from the virus, but not from the pol gene resident in PolB3 cells), α-TK (goat polyclonal, generously provided by W. Summers) and α-β-Actin (8226, Abcam). Following washing, the blots were incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) (Southern Biotech), and washed again. Finally, chemiluminescence solution (Pierce) was added to detect signal from the membranes using film.
Fig. 3.
D368A Pol expression is substantially decreased in infected cells compared to WT. (a) Western blots of cell lysates collected at 8 h p.i. and detected using antibodies specific for the proteins listed on the left. Whereas the antibody for Pol detects protein expressed both by the virus and the PolB3 cells, the FLAG antibody only detects Pol expression from the virus and does not detect expression of the WT pol gene in PolB3 cells. (b) Lysates of WT-infected Vero cells or HP66-infected PolB3 cells were serially diluted as indicated at the top, and then probed with the antibodies against the proteins indicated on the left.
Vero and PolB3 cells infected with WT and the D368A Rescue viruses produced readily detectable bands indicative of Pol expression at 8 h p.i. In contrast, Vero cells infected with the D368A Pol virus did not exhibit detectable Pol expression above the background levels seen following infection with the null mutant, HP66 (Fig. 3a). Interestingly, in PolB3 cells infected with either HP66 or D368A, Pol expression was only detected with the mouse anti-Pol antibody, and not with the anti-FLAG antibody (Fig. 3a, right side), suggesting that only the complementing WT Pol encoded by the PolB3 cells was detectably expressed. To estimate how much Pol was expressed in PolB3 cells infected with HP66 relative to WT-infected Vero cells, we performed a Western blot on dilutions of lysates (Fig. 3b). Probing with anti-TK antibody revealed similar levels of TK in four-fold dilutions of lysates of WT-infected Vero and HP66-infected PolB3 cells. (Fig. 3b) Probing with the mouse anti-Pol antibody indicated that the levels of Pol were 8- to 16-fold lower in HP66-infected PolB3 cells than in WT-infected Vero cells (Fig. 3b), which was confirmed by densitometry. Thus, this relatively low level of Pol is sufficient for efficient formation of plaques by HP66 and D368A in PolB3 cells, yet the fold reduction in Pol correlates well with and explains the 10- to 20-fold reduction in the yield of HP66 relative to WT in PolB3 cells (Fig. 1b-d). The dilution series also revealed that the limit of detection of Pol in Vero cells was 1/32 to 1/64 the level of WT Pol (Fig. 3b and data not shown), indicating that D368A Pol expression was >32-fold less than that of WT. Based on the just-mentioned correlation of Pol expression and yield, we expect at least a 32-fold effect on virus yield. If this is combined with the ~three-fold replication defect of viable Exo III mutants with unimpaired polymerase activity [9], we expect titres that are similar to those in the eclipse phase for D368A Pol in Vero cells (Fig. 1d, top left).
Combined, these data are consistent with the view that the D368A Pol mutant is unable to express enough Pol protein to facilitate DNA synthesis or virus production within non-complementing cells. It is possible that the mutation affects polymerase activity, too, but the defect in Pol expression largely explains the replication defect. The results corroborate previous failed attempts to produce the D368A Pol virus in the absence of a complementing cell line [11] and suggest that the D368A mutation inhibits pol mRNA accumulation or Pol protein synthesis, or leads to premature degradation of Pol within infected cells. In contrast, Exo III mutants, which have a ~three-fold defect in replication, must be reasonably well expressed. However, to the best of our knowledge, the expression levels of these mutant enzymes have not been tested.
The only available HSV-1 Pol structure does not reveal metal ion occupancy within the exonuclease active site [1], but in the structure of the similar family B polymerase, RB69 Pol, residues within the Exo III domain play a less critical role in metal ion coordination compared to conserved residues in the Exo I and II domains, which are held together by the metal ion [23]. We speculate, then, that the substitution of D368 with A results in loss of metal ion coordination and thus local unfolding of the Exo domain, making Pol more susceptible to protease digestion in Vero cells. Further work is required to determine the step at which expression is inhibited.
Funding information
The NIH provided funding to support this project (R01AI019838).
Acknowledgements
We thank the members of the Coen and Hogle labs for their support and input, especially Jean Pesola for her input on qPCR methods and analysis and Han Chen and Adrian Wilkie for their help designing and troubleshooting assays. Additionally, we would like to thank Charles Hwang, Charles Knopf, and William Summers for their generous donation of reagents.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
No experimental work with humans or animals is described in this article.
Footnotes
Abbreviations: ANOVA, analysis of variance; Exo, exonuclease; HSV, herpes simplex virus; m.o.i., multiplicity of infection; NTD, NH2-terminal domain; PAA, phosphonacetic acid; Pol, catalytic subunit of DNA polymerase; PVDF, polyvinylidene difluoride; tk, thymidine kinase; WT, wild-type.
References
- 1.Liu S, Knafels JD, Chang JS, Waszak GA, Baldwin ET, et al. Crystal structure of the herpes simplex virus 1 DNA polymerase. J Biol Chem. 2006;281:18193–18200. doi: 10.1074/jbc.M602414200. [DOI] [PubMed] [Google Scholar]
- 2.Knopf CW, Weisshart K. The herpes simplex virus DNA polymerase: analysis of the functional domains. Biochim Biophys Acta. 1988;951:298–314. doi: 10.1016/0167-4781(88)90100-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang TS, Wong SW, Korn D. Human DNA polymerase alpha: predicted functional domains and relationships with viral DNA polymerases. Faseb J. 1989;3:14–21. doi: 10.1096/fasebj.3.1.2642867. [DOI] [PubMed] [Google Scholar]
- 4.Boehmer PE, Lehman IR. Herpes simplex virus DNA replication. Annu Rev Biochem. 1997;66:347–384. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- 5.Dorsky DI, Crumpacker CS. Expression of herpes simplex virus type 1 DNA polymerase gene by in vitro translation and effects of gene deletions on activity. J Virol. 1988;62:3224–3232. doi: 10.1128/jvi.62.9.3224-3232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haffey ML, Novotny J, Bruccoleri RE, Carroll RD, Stevens JT, et al. Structure-function studies of the herpes simplex virus type 1 DNA polymerase. J Virol. 1990;64:5008–5018. doi: 10.1128/jvi.64.10.5008-5018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisshart K, Kuo AA, Hwang CB, Kumura K, Coen DM. Structural and functional organization of herpes simplex virus DNA polymerase investigated by limited proteolysis. J Biol Chem. 1994;269:22788–22796. [PubMed] [Google Scholar]
- 8.Hubscher U, Maga G, Spadari S. Eukaryotic DNA polymerases. Annu Rev Biochem. 2002;71:133–163. doi: 10.1146/annurev.biochem.71.090501.150041. [DOI] [PubMed] [Google Scholar]
- 9.Hwang YT, Liu BY, Coen DM, Hwang CB. Effects of mutations in the Exo III motif of the herpes simplex virus DNA polymerase gene on enzyme activities, viral replication, and replication fidelity. J Virol. 1997;71:7791–7798. doi: 10.1128/jvi.71.10.7791-7798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbs JS, Weisshart K, Digard P, Debruynkops A, Knipe DM, et al. Polymerization activity of an alpha-like DNA polymerase requires a conserved 3'-5' exonuclease active site. Mol Cell Biol. 1991;11:4786–4795. doi: 10.1128/MCB.11.9.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall JD, Orth KL, Sander KL, Swihart BM, Senese RA. Mutations within conserved motifs in the 3'-5' exonuclease domain of herpes simplex virus DNA polymerase. J Gen Virol. 1995;76:2999–3008. doi: 10.1099/0022-1317-76-12-2999. [DOI] [PubMed] [Google Scholar]
- 12.Kühn FJ, Knopf CW. Herpes simplex virus type 1 DNA polymerase. Mutational analysis of the 3'-5'-exonuclease domain. J Biol Chem. 1996;271:29245–29254. doi: 10.1074/jbc.271.46.29245. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Stroud J, Song L, Parris DS. Kinetic approaches to understanding the mechanisms of fidelity of the herpes simplex virus type 1 DNA polymerase. J Nucleic Acids. 2010;2010:1–15. doi: 10.4061/2010/631595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Trego KS, Song L, Parris DS. 3' to 5' exonuclease activity of herpes simplex virus type 1 DNA polymerase modulates its strand displacement activity. J Virol. 2003;77:10147–10153. doi: 10.1128/JVI.77.18.10147-10153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Wu Z, Cardoso MC, Parris DS. Processing of lagging-strand intermediates in vitro by herpes simplex virus type 1 DNA polymerase. J Virol. 2010;84:7459–7472. doi: 10.1128/JVI.01875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernad A, Blanco L, Lázaro JM, Martín G, Salas M. A conserved 3'–5' exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989;59:219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- 18.Lawler JL, Mukherjee P, Coen DM. HSV-1 DNA polymerase RNase H activity acts in a 3'-5' direction and is dependent on the 3'-5' exonuclease active site. J Virol. 2017;92:e01813. doi: 10.1128/JVI.01813-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrell SL, Coen DM. The pre-NH2-terminal domain of the herpes simplex virus 1 DNA polymerase catalytic subunit is required for efficient viral replication. J Virol. 2012;86:11057–11065. doi: 10.1128/JVI.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tischer BK, von Einem J, Kaufer B, Osterrieder N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques. 2006;40:191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 21.Marcy AI, Yager DR, Coen DM. Isolation and characterization of herpes simplex virus mutants containing engineered mutations at the DNA polymerase locus. J Virol. 1990;64:2208–2216. doi: 10.1128/jvi.64.5.2208-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissbach A, Hong SC, Aucker J, Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973;248:6270–6277. [PubMed] [Google Scholar]
- 23.Shamoo Y, Steitz TA. Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell. 1999;99:155–166. doi: 10.1016/s0092-8674(00)81647-5. [DOI] [PubMed] [Google Scholar]