Abstract
Noroviruses are genetically diverse RNA viruses associated with acute gastroenteritis in mammalian hosts. Phylogenetically, they can be segregated into different genogroups as well as P (polymerase)-groups and further into genotypes and P-types based on amino acid diversity of the complete VP1 gene and nucleotide diversity of the RNA-dependent RNA polymerase (RdRp) region of ORF1, respectively. In recent years, several new noroviruses have been reported that warrant an update of the existing classification scheme. Using previously described 2× standard deviation (sd) criteria to group sequences into separate clusters, we expanded the number of genogroups to 10 (GI-GX) and the number of genotypes to 49 (9 GI, 27 GII, 3 GIII, 2 GIV, 2 GV, 2 GVI and 1 genotype each for GVII, GVIII, GIX [formerly GII.15] and GX). Viruses for which currently only one sequence is available in public databases were classified into tentative new genogroups (GNA1 and GNA2) and genotypes (GII.NA1, GII.NA2 and GIV.NA1) with their definitive assignment awaiting additional related sequences. Based on nucleotide diversity in the RdRp region, noroviruses can be divided into 60 P-types (14 GI, 37 GII, 2 GIII, 1 GIV, 2 GV, 2 GVI, 1 GVII and 1 GX), 2 tentative P-groups and 14 tentative P-types. Future classification and nomenclature updates will be based on complete genome sequences and will be coordinated and disseminated by the international norovirus classification-working group.
Keywords: Norovirus, acute gastroenteritis, classification, 2xSD criteria, genogroup, genotype, P-group, P-type
Introduction
The genus Norovirus in the family Caliciviridae consists of a genetically diverse group of viruses infecting a wide range of mammalian host species that include humans, dogs, cats, pigs, mice, sheep and cattle [1–8]. To add to the diversity, several new unclassified noroviruses have recently been identified in bats, sea lions and harbour porpoise [9–12] and in non-human primates (unpublished GenBank submissions). Noroviruses found in humans are genetically and antigenically divergent and the leading cause of acute gastroenteritis in people of all ages worldwide associated with an estimated 70 000–200 000 deaths annually [13, 14].
Noroviruses are non-enveloped viruses with a single-stranded RNA genome approximately 7.5 kb in length. At the 5′-end, the RNA is covalently linked to a viral protein (VPg) and the 3′-end of the genome is polyadenylated. The genome of most noroviruses is organized into three ORFs, except for murine noroviruses, which contain a fourth ORF. ORF1 encodes a polyprotein that generates six non-structural proteins (NS1/2 to NS7) after post-translational cleavage by the viral protease [2, 15]. ORF2 encodes the major structural protein (VP1) that has shell (S) and protruding (P) domains. The S domain surrounds the viral RNA and the P domain, which consists of the P2 domain, is linked to the S domain through a flexible hinge [2, 14]. The P2 sub-domain is highly variable, harbours major neutralization epitopes and interacts with histo-blood group antigens (HBGAs) [16]. ORF3 encodes a minor structural protein (VP2), which is located inside the virus particle and has been proposed to be involved in capsid assembly and genome encapsidation [17] and, as described recently for feline calicivirus, entry of the virion into the host cell [18]. Complete VP1 amino acid sequences and the ORF1 NS7 region (which encodes the RNA-dependent RNA polymerase [RdRp]) nucleotide sequences are the basis of the current genetic classification of noroviruses [19].
The need to organize norovirus strains into different genetic groups or clusters was recognized in the mid-1990s when noroviruses were primarily divided into genogroups and genotypes based on partial RdRp sequences [20–23]. When more sequences became available, classification shifted to designate genogroups and genotypes based on the complete VP1 amino acid sequence with 20 % sequence difference used as a cut-off threshold for new genotypes, which was later adjusted to 15 % [24, 25]. Due to the lack of internationally accepted standards for norovirus classification several research groups used a small nucleotide region of the 5′-end of VP1 (termed region C) to classify genotypes, which although appropriate for typing noroviruses, led to inconsistent classification of some strains [25, 26].
To allow efficient communication of epidemiologically important norovirus lineages, in 2013 researchers from the Norovirus Classification Working Group (NCWG) proposed a universal standardized nomenclature and typing system for genotyping of GI and GII noroviruses using phylogenetic clustering of the complete VP1 amino acid sequences [19]. Based on an analysis of the then available sequence data, noroviruses were classified into six genogroups (GI to GVI) [19, 27], with a proposed seventh genogroup (GVII) [27]. These genogroups were further divided into more than 40 genotypes [28]. Viruses of GI, GII and GIV infect humans however GII also includes three genotypes (GII.11, GII.18 and GII.19) detected in faecal specimens from swine and GIV viruses include a genotype (GIV.2) that has only been detected in carnivore species (cats and dogs) [2, 27, 29]. Since the nomenclature criteria could not be met for distinguishing GII.4 variants, it was decided that the subtyping of GII.4 strains into variants will be based on phylogenetic clustering and that new GII.4 variants will only be recognized after they become epidemic in at least two geographically diverse locations [19]. Over the past two decades, eight GII.4 variants have been circulating each replacing a previous dominant variant and since 2012 GII.4 Sydney is the most contemporary GII.4 variant [19].
In addition, as norovirus diversity may be further increased through recombination, and circulating recombinant strains appear as distinct epidemiological entities, dual typing was proposed to include diversity at the level of the partial RdRp sequences in strain designations [19]. Although several recombination breakpoints have been identified across the norovirus genome, they are most frequently found in the ORF1-ORF2 junction region [29–36]. Dual typing (ORF1-RdRp=P type, ORF2=genotype) is now used routinely in many laboratories worldwide and at least 14 GI P-types and 27 GII P-types as well as 9 GI capsid genotypes and 22 GII capsid genotypes have been described [2, 19, 27]. Inclusion of norovirus diagnostic testing by real-time quantitative reverse transcription PCR into clinical and public health routine in recent years [27, 37–39] has confirmed the importance of GI and GII noroviruses globally [13, 31, 33, 35, 37, 40–44]. The increasing use of pathogen genome sequencing to unravel modes of transmission, sources of outbreaks, or to study the burden of infections has led to identification of several new candidate norovirus genogroups and genotypes since the last norovirus classification update in 2013 [9, 12, 33, 40, 41, 43, 45–49]. In this paper, we update the classification scheme for noroviruses by proposing new genogroups and genotypes based on the complete capsid amino acid sequences using the previously agreed criteria [19]. To provide a uniform basis to norovirus classification in the face of recombination and potential ‘orphan’ sequences, and to eliminate orphan ORF1 naming system (e.g. GI.Pa, GI.Pb, GII.Pa, GII.Pe, etc.), we grouped nucleotide sequences from the partial RdRp region into polymerase (P)-groups and P-types independently from the classification of their corresponding capsid genogroups and genotypes, respectively.
Results
Nomenclature criteria for new genogroups and genotypes
VPI designations
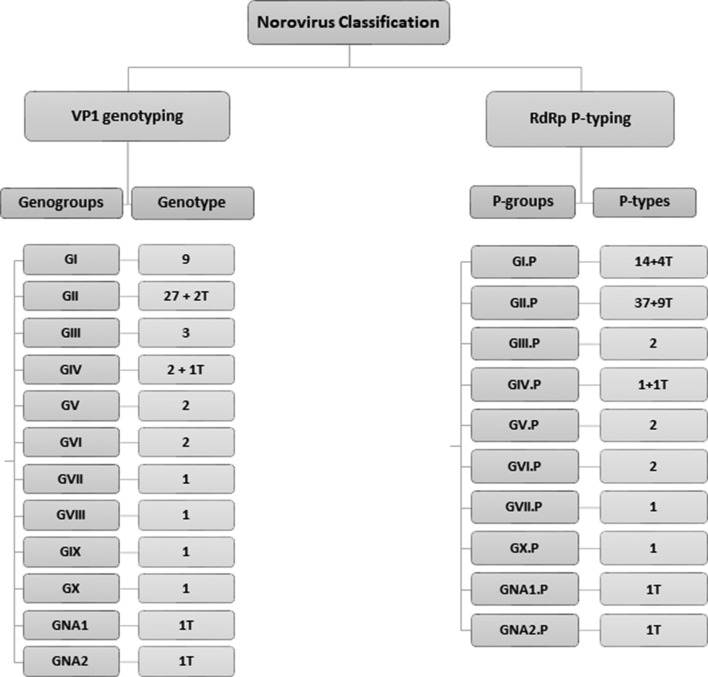
We followed the genotype designation and numbering as reported previously [19]. Sequences from strains that could not be typed, and for which at least two non-identical complete VP1 sequences from geographically diverse locations were available, were identified as candidate novel genogroups or genotypes (Fig. 1). After confirmation that they formed separate phylogenetic clusters using the 2×sd criteria [19], defined as the average distance between all sequences within a new genogroup or genotype and its nearest cluster(s), should not overlap within two standard deviations (2×sd) from each other, the next available number for new genogroups (e.g. GVIII) and genotypes (e.g. GII.23) was assigned. Genogroups or genotypes for which only a single complete VP1 sequence or only sequences from a single geographic location were available were referred to as not assigned (NA). For example, GNA1 and GNA2 for not yet assigned new genogroups and GII.NA1 and GII.NA2 for not yet assigned new genotypes [19] (Fig. 1). New GII.4 variants were named only when they became epidemic in at least two geographically diverse locations and were given the name of the city of the first full-length capsid sequence available in the public domain, e.g. GII.4 Sydney [19].
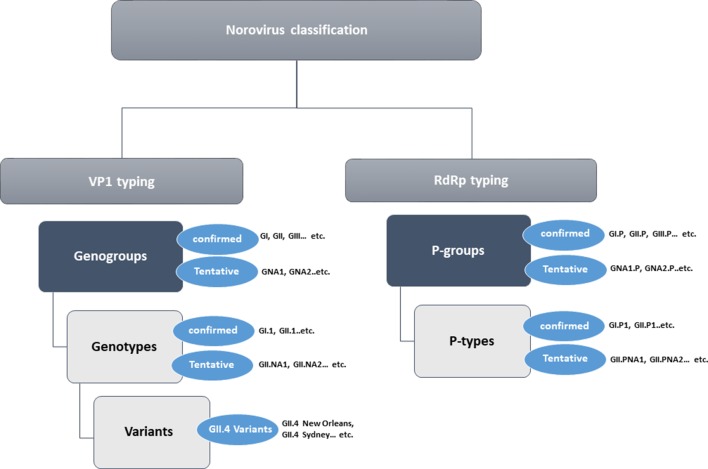
Fig. 1.
Classification of noroviruses into genogroups, genotypes, variants, P-groups and P-types. Tentative genogroups, genotypes, P-groups and P-types are currently represented only by a single sequence or multiple non-identical sequences from a single geographic location and are therefore named as non-assigned (NA).
RdRp designations
At the genogroup level, RdRp clusters were referred to as P-groups and at the genotype level as P-types. To distinguish VP1 genogroups or genotypes from P-groups or P-types, a capital P was introduced after the Roman genogroup (e.g. GI.P, GII.P, GIII.P) or genotype (GI.P1, GII.P3) designation. To eliminate ‘orphan’ P-types from the norovirus classification scheme, all RdRp nucleotide sequences for which at least two sequences from two geographic locations were available were assigned a P-type number independent of their corresponding VP1 genotype (Table 1b). When only one RdRp sequence was available, we assigned ‘NA’ to those P-types with a number after NA (e.g. GI.PNA1, GII.NA2) (Fig. 1).
Table 1.
Classification of norovirus prototype strains in current and new genotype assignment as determined by (a) complete VP1 amino acid and (b) partial (762 nt) RdRp relatedness
|
Strain designation |
GenBank accession no. |
Current norovirus genotype |
New norovirus genotype |
|---|---|---|---|
|
(a) VP1 | |||
|
GI/Hu/US/1968/GI.1/Norwalk |
GI.1 |
GI.1 |
|
|
GI/Hu/GB/1991/GI.2/Southampton |
GI.2 |
GI.2 |
|
|
GI/Hu/SA/1990/GI.3/DesertShield395 |
GI.3 |
GI.3 |
|
|
GI/Hu/JP/1987/GI.4/Chiba407 |
GI.4 |
GI.4 |
|
|
GI/Hu/GB/1989/GI.5/Musgrove |
GI.5 |
GI.5 |
|
|
GI/Hu/DE/1997/GI.6/BS5(Hesse) |
GI.6 |
GI.6 |
|
|
GI/Hu/GB/1994/GI.7/Winchester |
GI.7 |
GI.7 |
|
|
GI/Hu/US/2001/GI.8/Boxer |
GI.8 |
GI.8 |
|
|
GI/Hu/CA/2004/GI.9/Vancouver730 |
GI.9 |
GI.9 |
|
|
GII/Hu/US/1971/GII.1/Hawaii |
GII.1 |
GII.1 |
|
|
GII/Hu/GB/1994/GII.2/Melksham |
GII.2 |
GII.2 |
|
|
GII/Hu/CA/1991/GII.3/TV24 |
GII.3 |
GII.3 |
|
|
GII/Hu/GB/1993/GII.4/Bristol |
GII.4 |
GII.4 |
|
|
GII/Hu/GB/1990/GII.5/Hillingdon |
GII.5 |
GII.5 |
|
|
GII/Hu/GB/1990/GII.6/Seacroft |
GII.6 |
GII.6 |
|
|
GII/Hu/GB/1990/GII.7/Leeds |
GII.7 |
GII.7 |
|
|
GII/Hu/NL/1998/GII.8/Amsterdam |
GII.8 |
GII.8 |
|
|
GII/Hu/US/1997/GII.9/VA97207 |
GII.9 |
GII.9 |
|
|
GII/Hu/DE/2000/GII.10/Erfurt546 |
GII.10 |
GII.10 |
|
|
GII/Po/JP/1997/GII.11/Sw918 |
GII.11 |
GII.11 |
|
|
GII/Hu/GB/1990/GII.12/Wortley |
GII.12 |
GII.12 |
|
|
GII/Hu/US/1998/GII.13/Fayetteville |
GII.13 |
GII.13 |
|
|
GII/Hu/US/1999/GII.14/M7 |
GII.14 |
GII.14 |
|
|
GII/Hu/US/1999/GII.16/Tiffin |
GII.16 |
GII.16 |
|
|
GII/Hu/US/2002/GII.17/CS-E1 |
GII.17 |
GII.17 |
|
|
GII/Po/US/2003/GII.18/OH-QW101 |
GII.18 |
GII.18 |
|
|
GII/Po/US/2003/GII.19/OH-QW170 |
GII.19 |
GII.19 |
|
|
GII/Hu/DE/2002/GII.20/Luckenwalde591 |
GII.20 |
GII.20 |
|
|
GII/Hu/IQ/2002/GII.21/IF1998 |
GII.21 |
GII.21 |
|
|
GII/Hu/JP/2003/GII.22/Yuri |
GII.22 |
GII.22 |
|
|
GII/Hu/PE/2010/GII.23/Loreto1847 |
– |
GII.23 |
|
|
GII/Hu/PE/2013/GII.24/Loreto1972 |
– |
GII.24 |
|
|
GII/Hu/CN/2007/GII.25/Beijing53931 |
GII.22 |
GII.25 |
|
|
GII/Hu/NI/2005/GII.26/Leon4509 |
– |
GII.26 |
|
|
GII/Hu/PE/2012/GII.27/Loreto0959 |
– |
GII.27 |
|
|
GII/Hu/PE/2013/GII.NA1/Loreto1257 |
– |
GII.NA1 |
|
|
GII/Hu/PE/2008/GII.NA2/PNV06929 |
– |
GII.NA2 |
|
|
GIII/Bo/DE/1980/GIII.1/Jena |
GIII.1 |
GIII.1 |
|
|
GIII/Bo/GB/1976/GIII.2/Newbury2 |
GIII.2 |
GIII.2 |
|
|
GIII/Ov/NZ/2007/GIII.3/Norsewood30 |
GIII.3 |
GIII.3 |
|
|
GIV/Hu/NL/1998/GIV.1/Alphatron |
GIV.1 |
GIV.1 |
|
|
GIV/Ca/IT/2006/GIV.2/Pistoia-387 |
GIV.2 |
GIV.2 |
|
|
GIV/Hu/US/2016/GIV.NA1/WI7002 |
– |
GIV.NA1 |
|
|
GV/Mu/US/2002/GV.1/MNV-1 |
GV.1 |
GV.1 |
|
|
GV/Rn/HK/2011/GV.2/HKU-CT2 |
GV.2 |
GV.2 |
|
|
GVI/Ca/IT/2007/GVI.1/Bari91 |
GVI.1 |
GVI.1 |
|
|
GVI/Ca/PT/2007/GVI.2/C33-Viseu |
GVI.2 |
GVI.2 |
|
|
GVII/Hu/HK/2007/GVII.1/Ca026F |
GVII.1 |
GVII.1 |
|
|
GVIII/Hu/JP/2004/GVIII.1/Chiba-040502 |
– |
GVIII.1 |
|
|
GIX/Hu/US/1999/GIX.1/J23 |
GII.15 |
GIX.1 |
|
|
GX/Rs/CN/2010/GX.1/YN2010 |
– |
GX.1 |
|
|
GNA1/Pp/NL/2012/GNA1.1/120906 |
– |
GNA1.1 |
|
|
GNA2/Zc/HK/2008/GNA2.1/PF080916-2 |
– |
GNA2.1 |
|
|
Strain designation |
GenBank accession no. |
Current norovirus P-type |
New norovirus P-type |
|---|---|---|---|
|
(b) RdRp | |||
|
GI/Hu/US/1968/GI.P1/Norwalk |
GI.P1 |
GI.P1 |
|
|
GI/Hu/GB/1991/GI.P2/Southampton |
GI.P2 |
GI.P2 |
|
|
GI/Hu/US/1998/GI.P3/VA98115 |
GI.P3 |
GI.P3 |
|
|
GI/Hu/JP/1987/GI.P4/Chiba 407 |
GI.P4 |
GI.P4 |
|
|
GI/Hu//Hu/2013/GI.P5/Siklos-HUN5407 |
GI.P5 |
GI.P5 |
|
|
GI/Hu/DE/1997/GI.P6/BS5(Hesse) |
GI.P6 |
GI.P6 |
|
|
GI/Hu/SE/2008/GI.P7/LillaEdet| |
GI.P7 |
GI.P7 |
|
|
GI/Hu/JP/2007/GI.P8/Nagoya |
GI.P8 |
GI.P8 |
|
|
GI/Hu/CH/2012/GI.P9/CAIQ12110628 |
GI.P9 |
GI.P9 |
|
|
GI/Hu/SA/1990/GI.P10/DesertShield395 |
GI.Pa |
GI.P10 |
|
|
GI/Hu/CH/2007 GI.P11/Beijing53997 |
GI.Pb |
GI.P11 |
|
|
GI/Hu/JP/2000/GI.P12/SzUG1 |
GI.Pc |
GI.P12 |
|
|
GI/Hu/FR/2003/GI.P13/Vesoul576 |
GI.Pd |
GI.P13 |
|
|
GI/Hu/JP/1979/GI.P14/Otofuke |
GI.Pf |
GI.P14 |
|
|
GI/Hu/JP/2002/GI.PNA1/WUG1 |
GI.Pb |
GI.PNA1 |
|
|
GI/Hu/BD/2011/GI.PNA2/Dhaka1882 |
– |
GI.PNA2 |
|
|
GI/Hu/IN/2007/GI.PNA3/V1707 |
– |
GI.PNA3 |
|
|
GI/Hu/JP/1998/GI.PNA4/No20-Saitama-98–17 |
– |
GI.PNA4 |
|
|
GII/Hu/US/1971/GII.P1/Hawaii |
GII.P1 |
GII.P1 |
|
|
GII/Hu/GB/1994/GII.P2/Melksham |
GII.P2 |
GII.P2 |
|
|
GII/Hu/CA/1991/GII.P3/TV24 |
GII.P3 |
GII.P3 |
|
|
GII/Hu/GB/1993/GII.P4/Bristol |
GII.P4 |
GII.P4 |
|
|
GII/Hu/HU/1999/GII.P5/MOH |
GII.P5 |
GII.P5 |
|
|
GII/Hu/JP/2002/GII.P6/SaitamaU16 |
GII.P6 |
GII.P6 |
|
|
GII/Hu/JP/2002/GII.P7/SaitamaU4 |
GII.P7 |
GII.P7 |
|
|
GII/Hu/JP/2002/GII.P8/SaitamaU25 |
GII.P8 |
GII.P8 |
|
|
GII/Po/JP/2003/GII.P11/swine43 |
GII.P11 |
GII.P11 |
|
|
GII/Hu/JP/2005/GII.P12/Sakai-04–179 |
GII.P12 |
GII.P12 |
|
|
GII/Hu/FR/2004/GII.P13/Briancon870 |
GII.P13 |
GII.P13 |
|
|
GII/Hu/US/1999/GII.P15/Sapporo/HK299 |
GII.P15 |
GII.P15 |
|
|
GII/Hu/DE/2000/GII.P16/Neustrelitz260 |
GII.P16 |
GII.P16 |
|
|
GII/Hu/JP/2014/GII.P17/Kawasaki323 |
GII.P17 |
GII.P17 |
|
|
GII/Po/US/2003/GII.P18/OH-QW101 |
GII.P18 |
GII.P18 |
|
|
GII/Hu/GE/2005/GII.P20/Leverkusen267 |
GII.P20 |
GII.P20 |
|
|
GII/Hu/FR/2004/GII.P21/Pont de Roide673 |
GII.P21 |
GII.P21 |
|
|
GII/Hu/JP/2003/GII.22/YURI |
GII.P22 |
GII.P22 |
|
|
GII/Hu/PE/2010/GII.P23/Loreto1847 |
– |
GII.P23 |
|
|
GII/Hu/PE/2013/GII.P24/Loreto1972 |
– |
GII.P24 |
|
|
GII/Hu/BD/2012/GII.P25/Dhaka1928 |
– |
GII.P25 |
|
|
GII/Hu/NI/2005/GII.P26/Leon4509 |
– |
GII.P26 |
|
|
GII/Hu/PE/2012/GII.P27/Loreto0959 |
– |
GII.P27 |
|
|
GVIII/Hu/JP/2011/GII.P28/Gira2HS |
– |
GII.P28 |
|
|
GII/Hu/JP/2004/GII.P29/SN2000JA |
GII.Pa |
GII.P29 |
|
|
GII/Hu/US/1976/GII.P30/SnowMountain |
GII.Pc |
GII.P30 |
|
|
GII/Hu/JP/2007/GII.P31/OC07138 |
GII.Pe |
GII.P31 |
|
|
GII/Hu/FR/1999/GII.P32/S63 |
GII.Pf |
GII.P32 |
|
|
GII/Hu/AU/2008/GII.P33/NSW199U |
GII.Pg |
GII.P33 |
|
|
GII/Hu/JP/1997/GII.P34/OC97007 |
GII.Ph |
GII.P34 |
|
|
GII/Hu/GR/1997/GII.P35/E3 |
GII.Pj |
GII.P35 |
|
|
GII/Hu/JP/1996/GII.P36/OC96065 |
GII.Pk |
GII.P36 |
|
|
GII/Hu/IN/2006/GII.P37/PunePC24 |
GII.Pm |
GII.P37 |
|
|
GII/Hu/CN/2007/GII.P38/Beijing53931 |
GII.Pn |
GII.P38 |
|
|
GII/Hu/US/1974/GII.P39/CHDC5191 |
GII.P1 |
GII.P39 |
|
|
GII/Hu/JP/2004/GII.P40/OsakaNI |
GII.P22 |
GII.P40 |
|
|
GII/Hu/AUS/1983/GII.P41/GoulburnValleyG5175B |
GII.Pg |
GII.P41 |
|
|
GII/Hu/PE/2013/GII.PNA1/Loreto1257 |
– |
GII.PNA1 |
|
|
GII/Hu/PE/2008/GII.PNA2/PNV06929 |
– |
GII.PNA2 |
|
|
GII/Hu/US/1974/GII.PNA3/CHDC2094 |
GII.P1 |
GII.PNA3 |
|
|
GII/Po/JP/1997/GII.PNA4/Sw918 |
GII.P11 |
GII.PNA4 |
|
|
GII/Hu/BD/2012/GII.PNA5/Dhaka1940 |
GII.P22 |
GII.PNA5 |
|
|
GII/Hu/AR/2015/GII.PNA6/Arg13099 |
– |
GII.PNA6 |
|
|
GII/Hu/JP/1999/GII.PNA7/No35-Saitama-99–1 |
– |
GII.PNA7 |
|
|
GII/Hu/ZA/2013/GII.PNA8/Empangeni_10403 |
– |
GII.PNA8 |
|
|
GII/Hu/AR/2019/GII.PNA9/Arg15813 |
– |
GII.PNA9 |
|
|
GIII/Bo/DE/1980/GIII.P1/Jena |
GIII.P1 |
GIII.P1 |
|
|
GIII/Bo/UK/1976/GIII.P2/Newbury2 |
GIII.P2 |
GIII.P2 |
|
|
GIV/Hu/US/1998/GIV.P1/FtLauderdale-560 |
GIV.P1 |
GIV.P1 |
|
|
GIV/Hu/US/2016/GIV.PNA1/WI7002 |
– |
GIV.PNA1 |
|
|
GV/Mu/USA/2002/GV.P1/MNV1 |
GV.P1 |
GV.P1 |
|
|
GV/Rn/HK/2011/GV.P2/HKU-CT2 |
GV.P2 |
GV.P2 |
|
|
GVI/Ca/IT/2007/GVI.P1/Bari-91 |
GVI.P1 |
GVI.P1 |
|
|
GVI/Ca/PT/2007/GVI.P2/C33-Viseu |
GVI.P2 |
GVI.P2 |
|
|
GVII/Ca/HK/2007/GVII.P1/HKU_Ca026F |
GVII.P1 |
GVII.P1 |
|
|
GX/Rs/CN/2010/GX.P1/YN2010 |
– |
GX.P1 |
|
|
GNA1/Pp/NL/2012/GNA1.P1/120906 |
– |
GNA1.P1 |
|
|
GNA2/Zc/HK/2008/GNA2.P1/PF080916-2 |
– |
GNA2.P1 |
|
For dual-typing nomenclature, we propose the following designations of norovirus strains: GI.genotype[P-type] or GII.genotype[P-type], first listing the capsid genotype followed by the P-type between brackets (Table 2). For those strains where VP1 and RdRp sequences segregate into different genogroups the proposed designations are Genogroup.genotype[P-group.P-type] (Table 2).
Table 2.
Examples of new proposed dual-typing designations of norovirus strains
|
Current designation |
New designation |
|---|---|
|
GI.P6-GI.6 |
GI.6[P6] |
|
GI.Pd-GI.3 |
GI.3[P13] |
|
GII.P1-GII.1 |
GII.1[P1] |
|
GII.P12-GII.3 |
GII.3[P12] |
|
GII.Pe-GII.4 Sydney |
GII.4 Sydney[P31] |
|
GII.P16-GII.4 Sydney |
GII.4 Sydney[P16] |
|
GII.P4 New Orleans-GII.4 Sydney |
GII.4 Sydney[P4 New Orleans] |
|
GII.Pe-GII.17 |
GII.17[P31] |
|
GII.P15-GII.15 |
GIX.1[GII.P15] |
|
GVI.P1-GIV.2 |
GIV.2[GVI.P1] |
Strain designation for GenBank submission
With the species name (norovirus GI, norovirus GII) in the organism field, we propose a minor update of the current cryptogram [19] when submitting sequences to GenBank as follows: Organism/host/country code (two letter)/year of sample collection/genotype[P-type]/strain name. For example: Norovirus GII/Hu/US/2014/GII.4 Sydney[P4 New Orleans]/Pierce0249 or Norovirus GII/Hu/PE/2013/GII.24[P24]/Loreto1972.
Norovirus prototype strains
Prototype strains for each new genogroup or genotype include those strains for which the first full-length VP1 amino acid sequence and at least 762 nucleotides at the 3′-end of the RdRp region were available in public-domain databases (GenBank, EMBL, DDBJ). Prototype strains of novel genogroup/types are the first sequences of that lineage deposited into public-domain databases. The prototype strains for all genotypes and P-types are listed in Table 1.
Classification of VP1 sequences
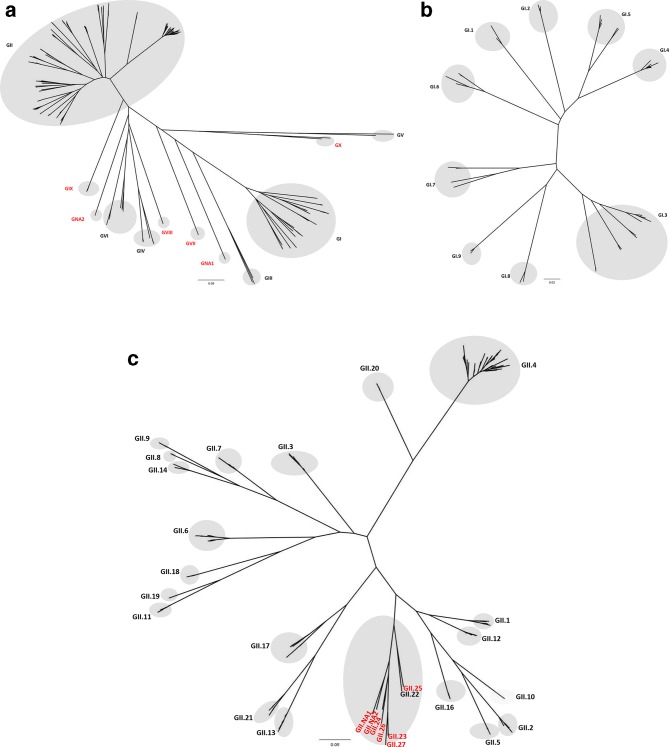
We applied 2×sd criteria to classify 305 complete VP1 amino acid sequences (Table S1, available in the online version of this article) into known and tentative new norovirus genogroups and genotypes. We identified and confirmed four new genogroups including GVII (dog), GVIII (human), GIX (human) (re-classified from GII.15) and GX (bat) and two tentative new genogroups: GNA1 (harbour porpoise) and GNA2 (sea lion) (Fig. 2a, Table S2). The 2×sd criteria could not be applied to GNA1 and GNA2 viruses (each representing a tentative new genogroup) as at least two sequences per cluster are required to calculate the standard deviation for within cluster distances. We confirmed five new genotypes: GII.23, GII.24, GII.25, GII.26 and GII.27 (Fig. 2b and c and Table S2) as well as two tentative new GII genotypes: GII.NA1 and GII.NA2 (Table S2 and Fig. 2b and c), along with one tentative new GIV genotype (GIV.NA1).
Fig. 2.
Phylogenetic classification of noroviruses based on VP1 amino acid sequences into ten norovirus genogroups and two non-assigned (NA) genogroups (a), GI genotypes (b) and GII genotypes (c). Phylogenetic analysis was performed using maximum likelihood (PhyML). Resulting trees were plotted and edited in FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Newly identified genogroups and genotypes are labelled in red.
Classification of RdRp sequences
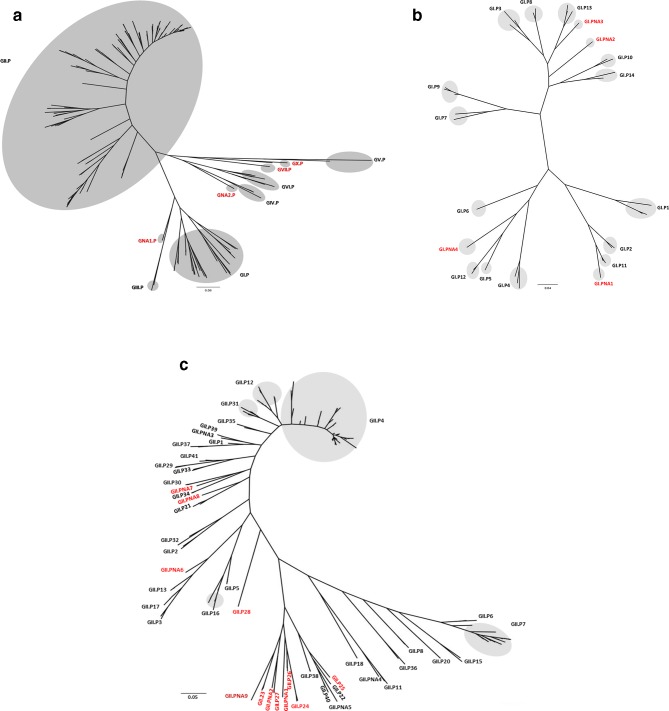
Phylogenetic analysis of 232 partial RdRp nucleotide sequences (Table S3) from all norovirus genogroups confirmed eight (GI.P, GII.P, GIII.P, GIV.P, GV.P, GVI.P, GVII.P and GX.P) P-groups and two tentative (GNA1.P and GNA2.P) P-groups (Fig. 3a, Table S4). The RdRp sequences of GIV viruses that infect humans clustered together and were classified as GIV.P groups whereas GIV and GVI viruses from cats, lions and dogs clustered together and are classified as GVI.P groups (Fig. S1).
Fig. 3.
Phylogenetic trees of partial (762 nucleotides at 3′-end of ORF1) RdRp sequences of norovirus genogroups (a), GI P-types (b) and GII P-types (c). Tentative P-groups and P-types with only a single sequence or multiple non-identical sequences from a single geographic location are referred to as non-assigned (NA). Phylogenetic analysis was carried out using maximum likelihood (ML) with PhyML. The resulting trees were plotted and edited in FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Newly identified genogroups and genotypes are labelled in red. In Fig 3c, only GII P-types with highly divergent clusters are shown with a gray circle for clarity.
The RdRp sequences of GI viruses could be divided into 14 P-types and 4 tentative P-types (GI.PNA1, GI.PNA2, GI.PNA3 and GI.PNA4) with one available sequence or multiple strains from the same geographic location (Tables 1b, S4, and Fig. 3b). Similarly, RdRp sequences of GII viruses could be separated into 37 P-types (GII.P1- GII.P8, GII.P11-GII.P13, GII.P15-GII.P18, GII.P20-GII.P41) and 9 tentative P-types (GII.PNA1-GII.PNA9) (Table S4, Fig. 3c). GIII viruses could be divided into two P-types (GIII.P1-P2); GIV into one confirmed (GIV.P1) and one tentative (GIV.PNA1); GV into two (GV.P1 and GV.P2); GVI into two (GVI.P1-P2); GVII into one (GVII.P1) and GX into one (GX.P1) P-type(s) (Table S4). GNA1 and GNA2 P-groups both include a single strain and therefore are designated as GNA1.P1 and GNA2.P1. The RdRp sequences of GVIII and GIX viruses, which cluster with GII viruses, were assigned P-types GII.P28 and GII.P15, respectively (Table S4).
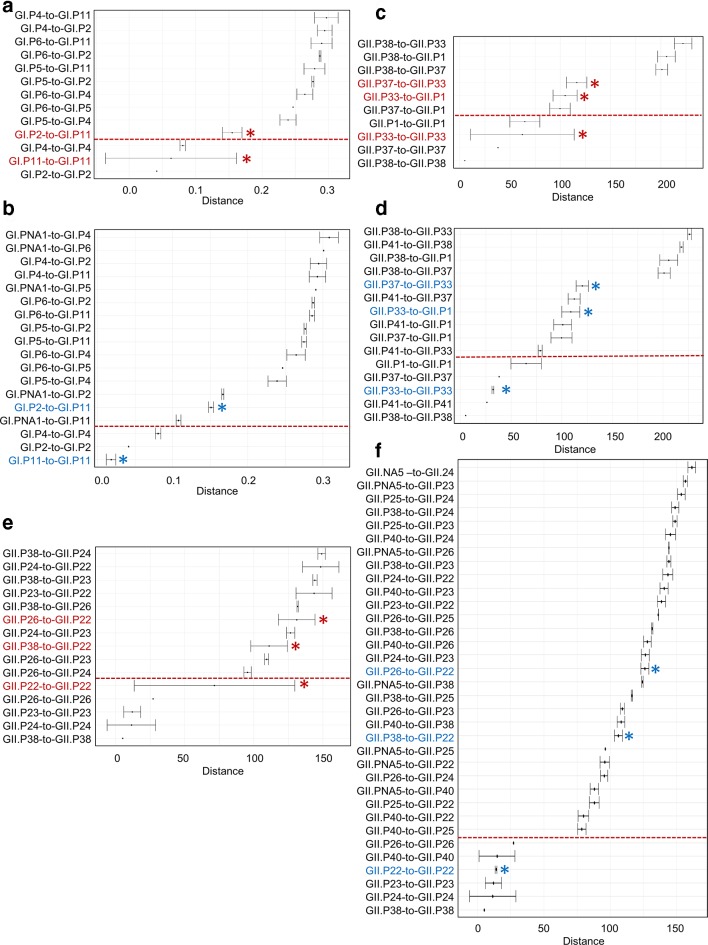
Viruses previously typed as GI.Pb, GII.P1, GII.P11, GII.P22 and GII.Pg did not meet the 2×sd criteria, either due to overlapping standard deviations (GI.Pb, GII.P22 and GII.Pg) (Fig. 4) or due to high intra-genotype diversity among the sequences (GII.P1 and GII.P11). To group these strains into P-types that meet the 2×sd criteria, viruses from clusters GI.Pb, GII.P1, GII.P11 and GII.Pg were re-classified into eight P-types and four non-assigned P-types including (GI.P11 [previous GI.Pb], GII.P1, GII.P11, GII.P22, GII.P33 [previous P-type GII.Pg], GII.P39, GII.P40, GII.P41, GI.PNA1, GII.PNA3, GII.PNA4 and GII.PNA5) (Fig. 4, Table 1b and Table S4). Since segregation of these P-types was based on partial RdRp sequences, we also analysed available complete ORF1 sequences, which confirmed the need of re-classification into additional P-types (Figs S2 and S3). However, since no complete ORF1 sequences were available, we were unable to complete analyses for GII.P11 viruses.
Fig. 4.
Patristic and P-distance comparison of GI.P11 (previously GI.Pb) (a and b), GII.P33 viruses (c and d) and GII.P22 viruses (e and f) with their phylogenetically closest P-types. Error bars represent 2xsd for each P-type comparison. The red * and font indicate the overlap of 2×sd error bars for viruses within a cluster and its closest P-types (a, b and e). After re-assigning GI.P11 viruses into two clusters (GI.P11 and GI.PNA1) (c), GII.P33 into GII.P33 and GII.P41 (d) and GII.P22 into three clusters (GII.P22, GII.P40 and GII.PNA5) (f), no overlap of 2×sd error bars within or in-between P-types was present (blue * and font). Y-axis represents comparison of distances within and in-between P-types. Below the dotted line, distances within P-type are indicated and above the dotted line, distances between P-types are indicated. (2×sd criteria: phylogenetic distances of sequences within a P-type should not overlap with distances between different P-types.)
Discussion
We updated the number and classification of norovirus genogroups and genotypes statistically supported by 2×sd criteria (Fig. 5). Until recently, noroviruses were officially grouped into six genogroups (GI–GVI) [2]. Our updated analysis demonstrates that, based on VP1 amino acid sequence diversity, the number of genogroups in the genus Norovirus can be expanded to ten (GI–GX), as well as two tentative genogroups, which will need to be confirmed when additional full VP1 or partial RdRp sequence becomes available. Viruses in these ten genogroups can be further divided into 49 confirmed capsid genotypes based on amino acids of the complete VP1 and 60 confirmed P-types based on partial nucleotide sequences of RdRp regions.
Fig. 5.
Updated norovirus classification scheme with the number of genogroups and genotypes based on complete VP1 amino acids and the number of P-groups and P-types based on a partial region (762 nucleotides) of the RNA-dependent RNA polymerase (RdRp) at the 5′-end of ORF1. P=P-group, T=tentative genotype/P-type (when only a single sequence or multiple non-identical sequences from a single geographic location as available, they are non-assigned (NA).
Over the last decade, several new norovirus sequences have been detected from new hosts. These included canine norovirus sequences that were identified in faecal swab specimens of dogs in Hong Kong, China in 2007 [27, 49] as well as in sewage samples from Uruguay in 2012 [47]. Our analyses confirmed the classification of these viruses into a new genogroup, GVII. During routine surveillance of stool samples from children with acute gastroenteritis in Chiba, Japan in 2004, a tentative new GII genotype was reported [50] and a genetically similar strain from Yuzawa, Japan in 2011 was submitted to GenBank. We confirmed that the VP1 sequences of these strains represent a novel genogroup, GVIII.
Among GII viruses, GII.15 viruses have phylogenetically always been an outlier. With five VP1 sequences including one complete genome available in GenBank, we confirmed that based on 2×sd criteria, GII.15 viruses should be re-classified into a separate genogroup, designated as GIX. A multi-year virome analysis of pharyngeal and anal swab samples from a large collection of major bat species in China showed several novel norovirus sequences that could be classified into a separate genogroup (GX) [12]. Tentative new genogroups were also detected in harbour porpoises [9] (GNA1) and sea lions (GNA2). Applying the 2×sd criteria to the classification of phylogenetic clusters of GIV and GVI suggests that the three GIV genotypes and two GVI genotypes may each represent separate genogroups, however, we decided not to re-classify them but wait until additional GIV and GVI sequences become available.
In recent years, several new GII and GIV genotypes from different geographic locations around the world have been reported [41, 43, 45, 46, 48]. With the addition of five novel genotypes GII.23, GII.24, GII.25, GII.26 and GII.27, we now recognize 26 GII genotypes (GII.1–GII.27) (excluding GII.15, which now represents a novel genogroup) along with two tentative new GII genotypes (GII.NA1 and GII.NA2). The new GII genotypes were identified in samples collected from humans in Peru, Guatemala, Ecuador, Bangladesh, Germany, Argentina and the USA [43, 45, 48]. A tentative new GIV genotype was recently identified in wastewater (Brazil, Japan) and in stool samples from an acute gastroenteritis outbreak in a restaurant in the USA ([41, 46] J Vinjé, personal communication). However, since only one complete GIV genome sequence is currently available, classification into a new genotype is pending additional sequences and therefore this genotype is temporarily designated as not assigned (NA) (GIV.NA1[PNA1]).
Until now, classifying noroviruses into P-types was based on a naming system that used P-type designation linked to the corresponding VP1 genotype of the strain and when no VP1 sequence was known, orphan P-types and/or P types with alphabet letters were assigned [19]. Since an increasing number of recombinant strains have been recognized in recent years [33, 37, 40, 51], we propose an update for the norovirus classification system in which P-types and VP1 genotypes are assigned independently. We also propose designating dual types with the capsid genotype listed first followed by P-type (e.g. GI.6[P10], GII.4 Sydney[P16], GIX.1[GII.P15], GIV.2[GVI.P1]).
Five P-types, some of which include strains dating back to the 1970–1980s, were grouped into 12 P-types to meet 2×sd criteria. Of note, P-types (GII.P6 and GII.P7) have overlapping standard deviations and several GII.P4 types also did not satisfy the 2×sd criteria because of overlapping standard deviations with GII.P12, GII.P31 (former GII.Pe) and GII.P35 (former GII.Pj) viruses (Figs S4 and S5). Since all three P-types are historic types, especially GII.P4 strains, which are of epidemiological importance worldwide and the current P-typing classification is based on relatively short nucleotide sequence fragments (762 nucleotides), we decided that we will re-visit this issue in our next classification update, which is expected to be based on complete ORF1 sequences.
RdRp sequences of GIV, GVI, GVIII, GIX viruses segregate independently of their corresponding capsid genogroups. For example, RdRp sequences of GVIII, GIX viruses cluster with GII viruses, and were designated as GII.P28 and GII.P15, respectively. Similarly, RdRp sequences of GIV and GVI viruses clustered into two distinct groups with sequences from GIV viruses that infect humans clustering together with other GIV strains, whereas sequences from GIV and GVI viruses that infect cats, lions and dogs clustered together as GVI strains. Thus, inter-genogroup recombination, which is considered a rare event among noroviruses [52, 53], may have occurred among viruses infecting humans (GVIII.1[GII.P28]; GIX.1[GII.P15]) and among viruses infecting canines (GIV.2[GVI.P1]). In addition, several recently reported RdRp sequences without an associated VP1 sequence were included in our updated classification scheme [33, 40].
One major limitation of the present classification is that current P-typing is based on a relatively short nucleotide sequence fragment (762 nucleotides) at the 3′-end of ORF1. Reassuringly, phylogenetic clustering of these shorter RdRp sequences was identical with segregation of P-types based on the complete RdRp sequences (~1500 nt), which were available for 85 % of the sequences used in our study. Additional complete RdRp sequences or ideally complete genome sequences for all reference strains will help to improve the robustness of the present classification system for which several protocols have been described recently [54–56].
With consensus from the NCWG, we propose to base future nomenclature and classification updates on complete ORF1 and VP1 sequences of new genetic lineages of viruses in the genus Norovirus. The updated nomenclature, including new genogroup, genotypes and new P-type names are available via publicly accessible typing tools https://www.rivm.nl/mpf/norovirus/typingtool and https://norovirus.ng.philab.cdc.gov both of which use the same set of GenBank-available norovirus reference sequences. For high-quality sequences that cannot be typed using these typing tools, researchers have the option to contact members of the NCWG for additional analyses. Database administrators of both typing tools will coordinate among each other to periodically monitor public databases for novel genotypes and update the reference sequences accordingly.
Methods
GenBank sequences used for analysis
A total of 305 complete ORF2 sequences (ranging from 530 to 580 amino acids in length), representing the genetic diversity of all norovirus genogroups and genotypes including reference strains used by CaliciNet [37] and NoroNet [44] databases were downloaded from GenBank (last download date: March 2019) (Table S1). In addition, 232 partial nucleotide sequences (762 nucleotides) of the RdRp region at the 3′-end of ORF1 were also downloaded (Table S3).
Phylogenetic analyses
ORF2 nucleotide sequences of all 305 norovirus strains were translated into amino acids and aligned using ClustalW [57]. In addition, separate alignments were carried out for VP1 amino acid sequences of GI (n=50) and GII (n=218) noroviruses. Sequences of the remaining 37 strains belonged to GIII (n=3), GIV (n=11), GV (n=2), GVI (n=8), GVII (n=2) and unassigned genogroups (n=11). For the RdRp region, an alignment of all 232 norovirus sequences was generated, as well as separate alignments for GI (n=44) and GII (n=163) sequences. Phylogenetic analyses were performed as described previously [19]. Briefly, phylogenetic trees were computed with PhyML [58, 59] through the web server at the ATGC Montpellier Bioinformatics platform (http://www.atgc-montpellier.fr/phyml/) and the resulting trees were plotted and edited in FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Known genogroups and genotypes were identified using the prototype sequences [2, 19, 27]. Phylogenetic distance matrices were computed using Patristic [60]. Mean distances and standard deviations (sd) values within and between phylogenetic clusters were computed in R [61]. According to 2×sd criteria, the average distance between all sequences within a new genogroup or genotype and its nearest cluster should not overlap within two standard deviations (2×sd) from each other [19]. New genogroups or genotypes were assigned by comparing the average distance between all sequences within and between phylogenetic cluster(s) (genogroup or genotype) (Fig. 1). Segregation of sequences into genogroups was based on 2×sd values followed by each individual genogroup, subdividing them further into genotypes.
Supplementary Data
Funding information
This study was supported by the EU H2020 grant COMPARE (grant agreement number 643476) and ZonMw TOP grant under grant number 91213058, CDCs intramural food safety funds and the NIAID Intramural Research Program. We thank A. Kroneman and S. Nooij for excellent technical assistance and R. Tatusov and L. Barclay for critically reviewing the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: Bo, bovine; Ca, canine; DDBJ, DNA Data Bank of Japan; EMBL, European Molecular Biology Laboratory; G, genogroup; HBGA, histo-blood group antigen; Hu, human; Mu, murine; NA, not assigned; NCWG, Norovirus Classification Working Group; ORF, open reading frame; Ov, ovine; PCR, polymease chain reaction; P-groups, polymerase groups; Po, porcine; Pp, Phocoena phocoena; P-types, polymerase types; RdRp, RNA dependent RNA polymerase; Rn, Rattus norvegicus; RNA, ribonucleic acid; Rs, Rhinolophus sinicus; SD, standard deviation; VP, viral protein; Zc, Zalophus californianus.
Four supplementary tables and five supplementary figures are available with the online version of this article.
References
- 1.Di Martino B, Di Profio F, Melegari I, Sarchese V, Cafiero MA, et al. A novel feline norovirus in diarrheic cats. Infect Genet Evol. 2016;38:132–137. doi: 10.1016/j.meegid.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green K. Caliciviridae: The noroviruses. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 586–608. [Google Scholar]
- 3.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. Stat1-Dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 4.Liu BL, Lambden PR, Günther H, Otto P, Elschner M, et al. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J Virol. 1999;73:819–825. doi: 10.1128/jvi.73.1.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesquita JR, Barclay L, Nascimento MSJ, Vinjé J. Novel norovirus in dogs with diarrhea. Emerg Infect Dis. 2010;16:980–982. doi: 10.3201/eid1606.091861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Q, Zhang W, Yang S, Cui L, Hua X. Complete genome sequence of a new-genotype porcine norovirus isolated from piglets with diarrhea. J Virol. 2012;86:7015–7016. doi: 10.1128/JVI.00757-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q-H, Han MG, Cheetham S, Souza M, Funk JA, et al. Porcine noroviruses related to human noroviruses. Emerg Infect Dis. 2005;11:1874–1881. doi: 10.3201/eid1112.050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf S, Williamson W, Hewitt J, Lin S, Rivera-Aban M, et al. Molecular detection of norovirus in sheep and pigs in New Zealand farms. Vet Microbiol. 2009;133:184–189. doi: 10.1016/j.vetmic.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 9.de Graaf M, Bodewes R, van Elk CE, van de Bildt M, Getu S, et al. Norovirus infection in harbor porpoises. Emerg Infect Dis. 2017;23:87–91. doi: 10.3201/eid2301.161081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi M, Lin X-D, Chen X, Tian J-H, Chen L-J, et al. The evolutionary history of vertebrate RNA viruses. Nature. 2018;556:197–202. doi: 10.1038/s41586-018-0012-7. [DOI] [PubMed] [Google Scholar]
- 11.Teng JLL, Martelli P, Chan W-M, Lee HH, Hui S-W, et al. Two novel noroviruses and a novel norovirus genogroup in California sea lions. J Gen Virol. 2018;99:777–782. doi: 10.1099/jgv.0.001071. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Yang L, Ren X, He G, Zhang J, et al. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. Isme J. 2016;10:609–620. doi: 10.1038/ismej.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bányai K, Estes MK, Martella V, Parashar UD. Viral gastroenteritis. The Lancet. 2018;392:175–186. doi: 10.1016/S0140-6736(18)31128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Liu H, Wilen CB, Sychev ZE, Desai C, et al. A secreted viral nonstructural protein determines intestinal norovirus pathogenesis. Cell Host Microbe. 2019;25:845–857.:e845. doi: 10.1016/j.chom.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutson AM, Atmar RL, Marcus DM, Estes MK. Norwalk virus-like particle hemagglutination by binding to H histo-blood group antigens. J Virol. 2003;77:405–415. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vongpunsawad S, Venkataram Prasad BV, Estes MK. Norwalk virus minor capsid protein VP2 associates within the VP1 shell domain. J Virol. 2013;87:4818–4825. doi: 10.1128/JVI.03508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conley MJ, McElwee M, Azmi L, Gabrielsen M, Byron O, et al. Calicivirus VP2 forms a portal-like assembly following receptor engagement. Nature. 2019;565:377–381. doi: 10.1038/s41586-018-0852-1. [DOI] [PubMed] [Google Scholar]
- 19.Kroneman A, Vega E, Vennema H, Vinjé J, White PA, et al. Proposal for a unified norovirus Nomenclature and genotyping. Arch Virol. 2013;158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ando T, Monroe SS, Gentsch JR, Jin Q, Lewis DC, et al. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and southern hybridization. J Clin Microbiol. 1995;33:64–71. doi: 10.1128/jcm.33.1.64-71.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green SM, Lambden PR, Caul EO, Ashley CR, Clarke IN. Capsid diversity in small round-structured viruses: molecular characterization of an antigenically distinct human enteric calicivirus. Virus Res. 1995;37:271–283. doi: 10.1016/0168-1702(95)00041-N. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama M, Ueda Y, Kawamoto H, Han-jun Y, Saito K, et al. Detection and sequencing of Norwalk-like viruses from stool samples in Japan using reverse transcription-polymerase chain reaction amplification. Microbiol Immunol. 1996;40:317–320. doi: 10.1111/j.1348-0421.1996.tb03343.x. [DOI] [PubMed] [Google Scholar]
- 23.Vinjé J, Koopmans MP. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J Infect Dis. 1996;174:610–615. doi: 10.1093/infdis/174.3.610. [DOI] [PubMed] [Google Scholar]
- 24.Vinjé J, Green J, Lewis DC, Gallimore CI, Brown DW, et al. Genetic polymorphism across regions of the three open reading frames of "Norwalk-like viruses". Arch Virol. 2000;145:223–241. doi: 10.1007/s007050050020. [DOI] [PubMed] [Google Scholar]
- 25.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, et al. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama T, Shinohara M, Uchida K, Fukushi S, Hoshino FB, et al. Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to norovirus in Japan. J Clin Microbiol. 2004;42:2988–2995. doi: 10.1128/JCM.42.7.2988-2995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinjé J. Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol. 2015;53:373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Graaf M, van Beek J, Koopmans MPG. Human norovirus transmission and evolution in a changing world. Nat Rev Microbiol. 2016;14:421–433. doi: 10.1038/nrmicro.2016.48. [DOI] [PubMed] [Google Scholar]
- 29.Ford-Siltz L, Mullis L, Sanad Y, Tohma K, Lepore C, et al. Genomics analyses of GIV and GVI noroviruses reveal the distinct clustering of human and animal viruses. Viruses. 2019;11:204. doi: 10.3390/v11030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bull RA, White PA. Mechanisms of GII.4 norovirus evolution. Trends Microbiol. 2011;19:233–240. doi: 10.1016/j.tim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Lim KL, Hewitt J, Sitabkhan A, Eden JS, Lun J, et al. A multi-site study of norovirus molecular epidemiology in Australia and New Zealand, 2013-2014. PLoS One. 2016;11:e0145254. doi: 10.1371/journal.pone.0145254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahar JE, Bok K, Green KY, Kirkwood CD. The importance of intergenic recombination in norovirus GII.3 evolution. J Virol. 2013;87:3687–3698. doi: 10.1128/JVI.03056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mans J, Murray TY, Nadan S, Netshikweta R, Page NA, et al. Norovirus diversity in children with gastroenteritis in South Africa from 2009 to 2013: GII.4 variants and recombinant strains predominate. Epidemiol Infect. 2016;144:907–916. doi: 10.1017/S0950268815002150. [DOI] [PubMed] [Google Scholar]
- 34.Mathijs E, Muylkens B, Mauroy A, Ziant D, Delwiche T, et al. Experimental evidence of recombination in murine noroviruses. J Gen Virol. 2010;91:2723–2733. doi: 10.1099/vir.0.024109-0. [DOI] [PubMed] [Google Scholar]
- 35.Medici MC, Tummolo F, Martella V, Giammanco GM, De Grazia S, et al. Novel recombinant GII.P16_GII.13 and GII.P16_GII.3 norovirus strains in Italy. Virus Res. 2014;188:142–145. doi: 10.1016/j.virusres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Cockrell SK, Kolawole AO, Rotem A, Serohijos AWR, et al. Isolation and analysis of rare norovirus recombinants from coinfected mice using Drop-Based Microfluidics. J Virol. 2015;89:7722–7734. doi: 10.1128/JVI.01137-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannon JL, Barclay L, Collins NR, Wikswo ME, Castro CJ, et al. Genetic and epidemiologic trends of norovirus outbreaks in the United States from 2013 to 2016 demonstrated emergence of novel GII.4 recombinant viruses. J Clin Microbiol. 2017;55:2208–2221. doi: 10.1128/JCM.00455-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jothikumar N, Lowther JA, Henshilwood K, Lees DN, Hill VR, et al. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Appl Environ Microbiol. 2005;71:1870–1875. doi: 10.1128/AEM.71.4.1870-1875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, et al. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41:1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degiuseppe JI, Gomes KA, Hadad MF, Parra GI, Stupka JA. Detection of novel GII.17 norovirus in Argentina, 2015. Infect Gen Evol. 2017;47:121–124. doi: 10.1016/j.meegid.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Fioretti JM, Fumian TM, Rocha MS, Dos Santos IdeAL, Carvalho-Costa FA, et al. Surveillance of noroviruses in Rio de Janeiro, Brazil: occurrence of new GIV genotype in clinical and wastewater samples. Food Environ Virol. 2018;10:1–6. doi: 10.1007/s12560-017-9308-2. [DOI] [PubMed] [Google Scholar]
- 42.Kuroda M, Masuda T, Ito M, Naoi Y, Doan YH, et al. Genetic diversity and intergenogroup recombination events of sapoviruses detected from feces of pigs in Japan. Infect Gen Evol. 2017;55:209–217. doi: 10.1016/j.meegid.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Pietsch C, Ennuschat N, Härtel S, Liebert UG. Within-Host evolution of virus variants during chronic infection with novel GII.P26-GII.26 norovirus. J Clin Virol. 2018;108:96–102. doi: 10.1016/j.jcv.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 44.van Beek J, de Graaf M, Al-Hello H, Allen DJ, Ambert-Balay K, et al. Molecular surveillance of norovirus, 2005-16: an epidemiological analysis of data collected from the NoroNet network. Lancet Infect Dis. 2018;18:545–553. doi: 10.1016/S1473-3099(18)30059-8. [DOI] [PubMed] [Google Scholar]
- 45.Chhabra P, Aswath K, Collins N, Ahmed T, Olórtegui MP, et al. Near-Complete genome sequences of several new norovirus genogroup II genotypes. Genome Announc. 2018;6:e00007-18. doi: 10.1128/genomeA.00007-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitajima M, Rachmadi AT, Iker BC, Haramoto E, Gerba CP. Genetically distinct genogroup IV norovirus strains identified in wastewater. Arch Virol. 2016;161:3521–3525. doi: 10.1007/s00705-016-3036-z. [DOI] [PubMed] [Google Scholar]
- 47.Lizasoain A, Tort LFL, García M, Gómez MM, Leite JPG, et al. Sewage surveillance reveals the presence of canine GVII norovirus and canine astrovirus in Uruguay. Arch Virol. 2015;160:2839–2843. doi: 10.1007/s00705-015-2571-3. [DOI] [PubMed] [Google Scholar]
- 48.Tohma K, Saito M, Mayta H, Zimic M, Lepore CJ, et al. Complete genome sequence of a nontypeable GII norovirus detected in Peru. Genome Announc. 2018;6:e00095-18. doi: 10.1128/genomeA.00095-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tse H, Lau SKP, Chan WM, Choi GKY, Woo PCY, et al. Complete genome sequences of novel canine noroviruses in Hong Kong. J Virol. 2012;86:9531–9532. doi: 10.1128/JVI.01312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada M, Ogawa T, Kaiho I, Shinozaki K. Genetic analysis of noroviruses in Chiba Prefecture, Japan, between 1999 and 2004. J Clin Microbiol. 2005;43:4391–4401. doi: 10.1128/JCM.43.9.4391-4401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsushima Y, Shimizu T, Ishikawa M, Komane A, Okabe N, et al. Complete genome sequence of a recombinant GII.P16-GII.4 norovirus detected in Kawasaki City, Japan, in 2016. Genome Announc. 2016;4:e01099-16. doi: 10.1128/genomeA.01099-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bull RA, Tanaka MM, White PA. Norovirus recombination. J Gen Virol. 2007;88:3347–3359. doi: 10.1099/vir.0.83321-0. [DOI] [PubMed] [Google Scholar]
- 53.Ludwig-Begall LF, Mauroy A, Thiry E. Norovirus recombinants: recurrent in the field, recalcitrant in the lab - a scoping review of recombination and recombinant types of noroviruses. J Gen Virol. 2018;99:970–988. doi: 10.1099/jgv.0.001103. [DOI] [PubMed] [Google Scholar]
- 54.Brown JR, Roy S, Ruis C, Yara Romero E, Shah D, et al. Norovirus whole-genome sequencing by SureSelect target enrichment: a robust and sensitive method. J Clin Microbiol. 2016;54:2530–2537. doi: 10.1128/JCM.01052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fonager J, Stegger M, Rasmussen LD, Poulsen MW, Rønn J, et al. A universal primer-independent next-generation sequencing approach for investigations of norovirus outbreaks and novel variants. Sci Rep. 2017;7:813. doi: 10.1038/s41598-017-00926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parra GI, Squires RB, Karangwa CK, Johnson JA, Lepore CJ, et al. Static and evolving norovirus genotypes: implications for epidemiology and immunity. PLoS Pathog. 2017;13:e1006136. doi: 10.1371/journal.ppat.1006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 59.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 60.Fourment M, Gibbs MJ. PATRISTIC: a program for calculating patristic distances and graphically comparing the components of genetic change. BMC Evol Biol. 2006;6:1. doi: 10.1186/1471-2148-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. ISBN. 2014:3-900051-07-0 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.