Abstract
Protein phosphorylation is a key mediator of signal transduction, allowing for dynamic regulation of substrate activity. Whereas protein kinases obtain substrate specificity by targeting specific amino acid sequences, serine/threonine phosphatase catalytic subunits are much more promiscuous in their ability to dephosphorylate substrates. To obtain substrate specificity, serine/threonine phosphatases utilize targeting proteins to regulate phosphatase subcellular localization and catalytic activity. Spinophilin and its homologue neurabin are two of the most abundant dendritic spine-localized protein phosphatase 1 (PP1) targeting proteins. The association between spinophilin and PP1 is increased in the striatum of animal models of Parkinson’s disease (PD). However, mechanisms that regulate the association of spinophilin and neurabin with PP1 are unclear. Here, we report that the association between spinophilin and PP1α or PP1γ1 was increased by CDK5 expression and activation in a heterologous cell system. This increased association is at least partially due to phosphorylation of PP1. Conversely, CDK5 expression and activation decreased the association of PP1 with neurabin. As with dopamine depletion, methamphetamine (METH) abuse causes persistent alterations in dopamine signaling which influence striatal medium spiny neuron function and biochemistry. Moreover, both METH toxicity and dopamine depletion are associated with deficits in motor control and motor learning. Pathologically, we observed a decreased association of spinophilin with PP1 in rat striatum evaluated one month following a binge METH paradigm. Behaviorally, we found that loss of spinophilin recapitulates rotarod pathology previously observed in dopamine-depleted and METH-treated animals. Together, these data have implications in multiple disease states associated with altered dopamine signaling such as PD and psychostimulant drug abuse and delineate a novel mechanism by which PP1 interactions with spinophilin and neurabin may be differentially regulated.
Keywords: Phosphatase, methamphetamine, signaling, scaffolding proteins
Graphical Abstract
INTRODUCTION
Spinophilin is a protein phosphatase 1 (PP1) targeting protein that is enriched in dendritic spines.1,2 Spinophilin is critical in regulating normal synaptic physiology by directing PP1 to its targets such as glutamate receptors.3–8 Spinophilin interaction with PP1 is increased in both rat and mouse models of Parkinson’s disease (PD)9,10 and the expression of spinophilin is up-regulated following chronic amphetamine treatment.11,12 In addition to alterations in spinophilin interactions and expression, loss of spinophilin enhances cocaine-induced condition-place preference.13 Therefore, spinophilin expression is regulated by psychostimulants, and spinophilin modulates behavioral responses to psychostimulants. However, the mechanisms that underlie dopamine-dependent pathological changes in the spinophilin/PP1 interaction and how spinophilin contributes to striatal motor behaviors are not known. Therefore, the goals of the current study were to define novel mechanisms that are associated with dopamine modulation that regulate the spinophilin/PP1 interaction and delineate spinophilin regulation of motor behaviors and learning.
A specific binding domain on spinophilin containing residues 417–494 is necessary and sufficient for PP1 binding.14 PP1 binding occurs via four unique regions within this domain on spinophilin.15 These unique regions allow for substrate selectivity in the targeting of PP1. There are four isoforms of PP1: PP1α, PP1β, PP1γ1, and PP1γ2. While all isoforms are present, PP1α, PP1β, and PP1γ1 are highly expressed in the brain.16–19 PP1α and PP1γ1 are enriched in dendritic spines, whereas PP1β is localized to dendrites. Spinophilin associates with PP1γ1 but has less robust association with PP1β.20,21 As stated above, dopamine depletion, an animal model of Parkinson’s disease, increases the association of spinophilin with PP1;9,10 however, the mechanisms regulating this association are unclear. One putative mode by which the spinophilin PP1 interaction may be regulated is by alterations in phosphorylation of spinophilin and/or PP1. Interestingly, a recent study demonstrated that protein kinase A (PKA) activity can modulate the association of neurabin, but not spinophilin, with PP1.22 This altered association is due to differences in phosphorylation of neurabin within its PP1-binding domain. A previous study found that cyclin-dependent kinase 5 (CDK5) activity is increased in dopamine depleted animals.23 CDK5 phosphorylates spinophilin at Ser-17 within the N-terminal actin-binding domain,24 a site that has increased phosphorylation in an animal model of PD.10 In addition to spinophilin, PP1α is phosphorylated by CDK5 at Thr320.25 Moreover, PP1γ1 has a conserved Thr at residue 311 that may also be phosphorylated by the kinase.
Methamphetamine (METH) is an addictive psychostimulant drug of abuse that, when given at high doses, can lead to dopamine toxicity in rodents.26,27 METH abusers have a higher incidence of developing PD,28 and METH toxicity can lead to behavioral sensitization to dopamine agonists.29 Also, both dopamine depletion and METH toxicity are associated with motor coordination and/or motor learning deficits.30–32 Biochemically, alterations in dopamine release and levels in the synapse regulate normal synaptic protein function and signaling. Here, we report that a binge model of METH decreases the association of spinophilin with PP1 and that CDK5 phosphorylation of PP1 enhances the spinophilin/PP1 interaction. Moreover, we have observed that loss of spinophilin recapitulates motor coordination and learning deficits observed in dopamine depletion and METH toxicity. Taken together, these data give novel insight into spinophilin function in striatal-based motor behaviors as well as mechanistic changes that occur under pathological dopamine conditions that may regulate the spinophilin/PP1 interaction.
RESULTS
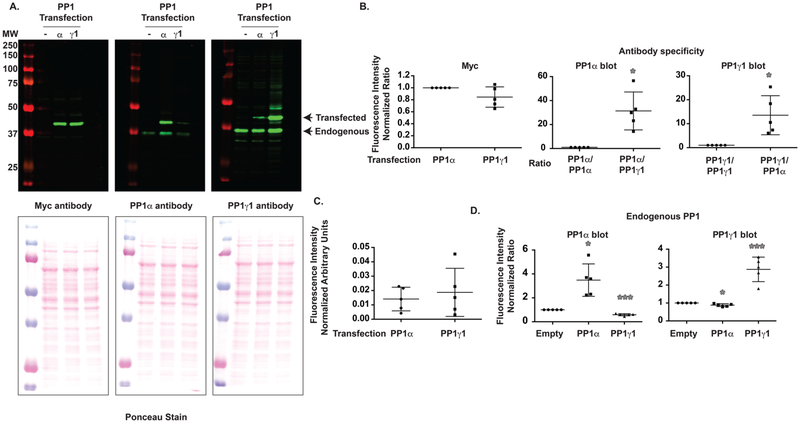
PP1 Overexpression in a Heterologous Cell System.
PP1 is a critical regulator of the phosphorylation of multiple substrates, and spinophilin is the major PP1 targeting enzyme in the PSD.33 Of the three PP1 isoforms, spinophilin does not robustly bind PP1β20 but does bind PP1α and PP1γ1. To begin to delineate mechanisms that modulate the association of spinophilin with PP1α and PP1γ1, we used a heterologous cell system (HEK293). However, we first needed to determine which PP1 isoforms are expressed in this cell system. To delineate the expression of different PP1 isoforms in a heterologous cell system, we overexpressed myc-tagged rat PP1γ1 (which differs from human PP1γ1 only by a Leu/Ile change at position 4) and myc-tagged human PP1α in HEK293 cells. We found that both PP1α and PP1γ1 were equally overexpressed using a myc-tag antibody (Figure 1A). We utilized a mouse PP1 antibody that was raised against full-length, human PP1α and a goat antibody raised against full-length, human PP1γ (see Methods). Although there were equal amounts of PP1 transfected, as detected by the myc antibody (Figure 1A left panel and Figure 1B left panel), the mouse-PP1α antibody selectively (~31.3 times) detected PP1α (Figures 1A and B middle panel), whereas the goat-PP1γ1 antibody selectively (~13.5 times) detected PP1γ1 (Figures 1A and 1B right panel). Interestingly, the mouse-PP1α antibody detected endogenous PP1 that migrated at 37 kDa, whereas the goat-PP1γ1 antibody detected a band that migrated slightly below 37 kDa (Figure 1A). This is consistent with the predicted mass of human PP1α (37 512 Da) and PP1γ1 (36 984 Da). To determine endogenous expression of PP1α and PP1γ1, we normalized for antibody differences by normalizing the detection of overexpressed PP1α or PP1γ1 to the corresponding signal detected in the myc immunoblot (Figure 1A left panel). This allows us to account for differences in detection efficiency between antibodies. It is important to note that the specificity of these antibodies may be influenced by dilution, and we use a 1:1000–1:2000 primary antibody dilution for these studies. Following additional normalization to loading (normalized to ponceau), we conclude that endogenous PP1α and PP1γ1 were detected at equal amounts (Figure 1C). This suggests that endogenous PP1α and PP1γ1 are expressed at similar levels in HEK293 cells. Interestingly, overexpression of PP1α enhanced the detection of a PP1α band migrating at the level of the endogenous PP1α, whereas overexpression of PP1γ1 enhanced the detection of a PP1γ1 band migrating at the level of endogenous PP1γ1 (Figure 1D). While we predict this band is endogenous PP1, it may be a fragment of the N-terminally tagged Myc-PP1. Conversely, overexpression of PP1α decreased endogenous PP1γ1 expression, whereas overexpression of PP1γ1 decreased endogenous PP1α expression (Figure 1D). Together, these data suggest that both PP1α and PP1γ1 are expressed in HEK293 cells at similar levels and that these two PP1 isoforms can regulate both their own expression and expression of the other isoform.
Figure 1.
PP1α and PP1γ1 are both endogenously expressed in HEK293 cells, and overexpression of PP1 regulates endogenous PP1 expression. (A) Myc-tagged PP1α or PP1γ1 was overexpressed in HEK293 cells and immunoblotted with a Myc antibody, a PP1α selective antibody, or a PP1γ1 selective antibody. (B) The PP1α selective antibody was ~31.3-fold more robust at detecting PP1α over PP1γ1 when normalized to Myc signal. Conversely, the PP1γ1 selective antibody was 13.5-fold more robust at detecting PP1γ1 over PP1α. (C) Upon normalizing to Myc intensity (to normalize for differences in antibody detections) and loading, the PP1α and PP1γ1 antibodies detected equal concentrations of PP1α and PP1γ1. (D) PP1α overexpression increased the expression of endogenous PP1α but decreased the expression of endogenous PP1γ1. Conversely, PP1γ1 overexpression increased the expression of endogenous PP1γ1 but decreased the expression of endogenous PP1α. A 1-sample t-test comparing to a theoretical value of 1 was performed. *p < 0.05, ***p < 0.001.
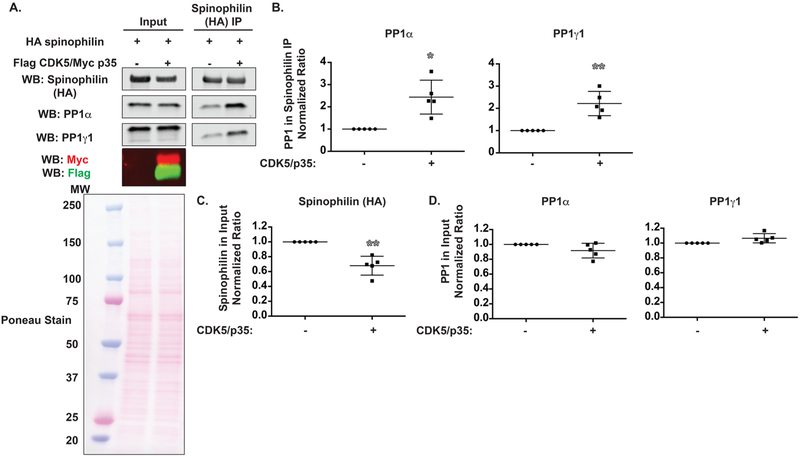
Spinophilin Interaction with Both PP1α and PP1γ1 is Enhanced by CDK5.
We previously found that spinophilin association with PP1 is increased in dopamine-depleted striatum.9,10 Dopamine activates its receptors to regulate PKA and CDK5 activity.23,34–38 HA-tagged spinophilin was overexpressed in the absence or presence of CDK5 and its activator, p35, in HEK293 cells. We evaluated the association between spinophilin and endogenous PP1α and PP1γ1 (Figure 2A). Overexpression of CDK5 and its activator p35 increased the association between spinophilin and endogenous PP1α and PP1γ1 (Figure 2B) in HEK293 cells. This increased association was not due to increased expression of spinophilin or PP1 because, in the presence of CDK5, spinophilin expression was ~34% lower (Figure 2C), whereas PP1 expression was unchanged (Figure 2D). Together, these data demonstrate that overexpression of CDK5 along with its activator p35 increases the amount of endogenous PP1 coprecipitated with overexpressed spinophilin.
Figure 2.
CDK5 increases the association between spinophilin and PP1 and decreases spinophilin expression. (A) HEK293 cells were transfected with spinophilin in the absence or presence of CDK5 and its activator, p35. Lysates and spinophilin immunoprecipitates were immunoblotted for spinophilin, PP1α, PP1γ1, Myc epitope, and Flag epitope. For lysates, 0.5% of the total was loaded for spinophilin and PP1 blots and 1.5% was loaded for Flag and Myc blots. (B) The association between spinophilin and PP1α or PP1γ1 was significantly increased by overexpression of CDK5/p35. (C) Spinophilin expression was decreased by CDK5/p35 overexpression. (D) PP1 expression was unchanged by CDK5/p35 overexpression. A 1-sample t-test comparing to a theoretical value of 1 was performed. *p < 0.05, **p < 0.01.
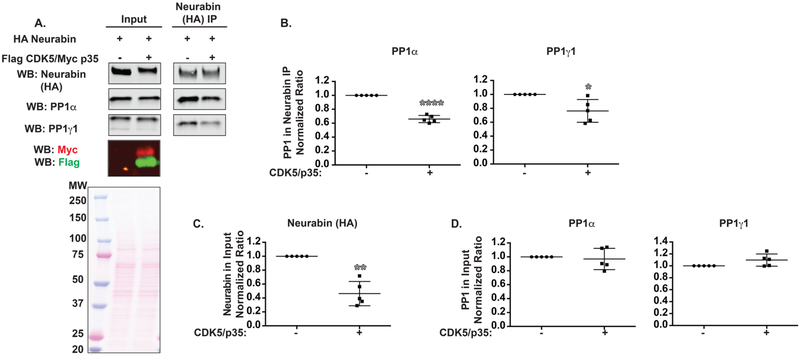
Neurabin/PP1 Interaction Is Decreased by CDK5.
The spinophilin homologue, neurabin, is also a major PP1 targeting protein in the brain.39,40 Neurabin associates with both PP1α and PP1γ1 at a similar stoichiometry as spinophilin39 but has different localization and functional roles when compared with spinophilin.1,13 Therefore, we wanted to determine if the association of neurabin with PP1α and/or PP1γ1 was also regulated by CDK5. HA-tagged neurabin was overexpressed in the absence or presence of overexpressed CDK5 and p35 (Figure 3A). In contrast to what was observed with spinophilin, PP1α and PP1γ1 association with neurabin was decreased (Figure 3B). Like spinophilin, neurabin expression was decreased in the presence of CDK5 and p35; however, this decrease was greater at ~64% (Figure 3C). Moreover, for neurabin, we observed a modest molecular weight shift in the presence of CDK5, whereas this was not observed with spinophilin (Figure S1). Furthermore, neurabin overexpression is less than that of spinophilin (Figure S1). Like spinophilin, PP1 expression was not significantly different in the presence of CDK5 and neurabin (Figure 3D). Together these data suggest that in contrast to spinophilin, CDK5 decreases the association of overexpressed neurabin with endogenous PP1.
Figure 3.
CDK5 decreases the association between neurabin and PP1 and decreases neurabin expression. (A) HEK293 cells were transfected with neurabin in the absence or presence of CDK5 and its activator, p35. Lysates and neurabin immunoprecipitates were immunoblotted for neurabin, PP1α, PP1γ1, Myc epitope, and Flag epitope. For lysates, 0.5% of the total was loaded for spinophilin and PP1 blots and 1.5% was loaded for Flag and Myc blots. (B) The association between neurabin and PP1α or PP1γ1 was significantly decreased by overexpression of CDK5/p35. (C) Neurabin expression was decreased by CDK5/p35 overexpression. (D) PP1 expression was unchanged by CDK5/p35 overexpression. A 1-sample t-test comparing to a theoretical value of 1 was performed. *p < 0.05, **p < 0.01, ****p < 0.0001.
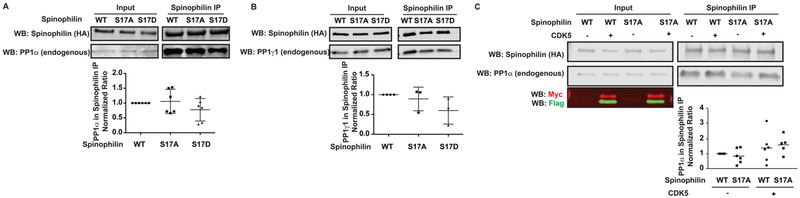
Spinophilin Phosphorylation by CDK5 at Ser17 Is Not Responsible for CDK5-Dependent Increases in the Spinophilin and PP1 Interaction.
We recently reported increased phosphorylation of spinophilin at the CDK5 site, Ser-17, and an increased association between spinophilin and PP1 in an animal model of PD.10 Phospho-mimic (S17D) or nonphosphorylateable (S17A) mutations of spinophilin at the CDK5 site did not regulate the association of spinophilin with endogenous PP1α (Figure 4A) or PP1γ1 (Figure 4B). Moreover, whereas there was a significant effect of CDK5 expression on the spinophilin interaction with PP1α (F(1,19) = 4.555; p = 0.0461) there was no effect of the mutation (S17A mutation vs WT; F(1,19) = 0.01012; p = 0.9209) (Figure 4C). This suggests that CDK5 phosphorylation of spinophilin at Ser17 is not responsible for the CDK5-dependent increase in the spinophilin/PP1 association.
Figure 4.
Spinophilin association with PP1 was not regulated by Ser17 mutations of spinophilin. HEK293 cells were transfected with WT, S17A, or S17D spinophilin and immunoprecipitated for spinophilin. Lysates and immunoprecipitates were immunoblotted for spinophilin and either (A) PP1α or (B) PP1γ1. There was no effect of the mutations on spinophilin association with PP1. (C) HEK293 cells were transfected with WT or S17A spinophilin in the absence or presence of CDK5/p35 and immunoprecipitated for spinophilin. Lysates and immunoprecipitates were immunoblotted for spinophilin and PP1α. S17A mutation had no effect on the CDK5-dependent increase in spinophilin binding to PP1. For lysates, 0.5% (A and C) or 1.33% (B) of the total was loaded for spinophilin and PP1 blots. For immunoprecipitates, 25% (A and C) or 33% (B) of the immunoprecipitate was loaded. A one-way (A and B) or a two-way ANOVA (C) was performed.
PP1 Phosphorylation Mimics Enhanced Spinophilin Binding.
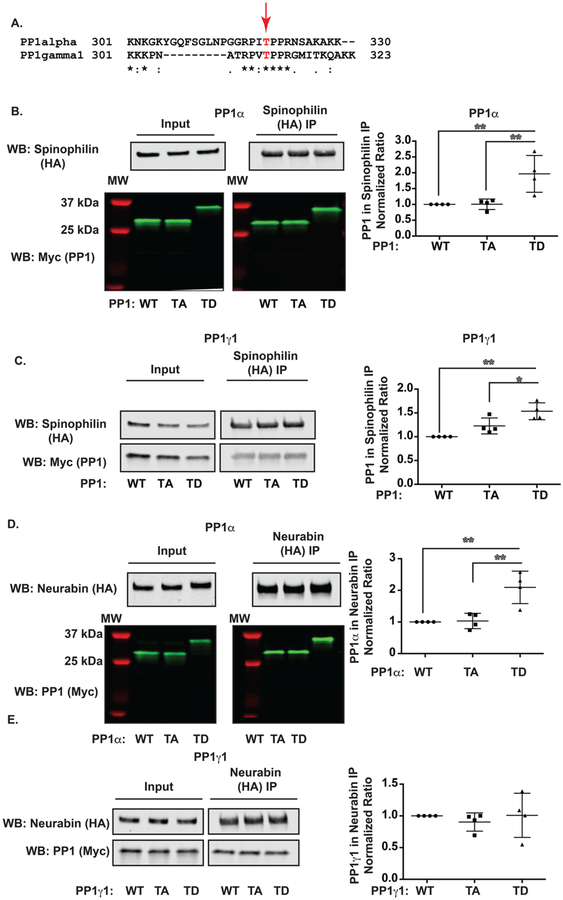
PP1α is phosphorylated by CDK5 at Thr320.25,41 Upon alignment of rat PP1γ1 and human PP1α, Thr320 of PP1α is conserved in PP1γ1 (Thr311) (Figure 5A). Moreover, CDK5/p35 overexpression increased the phosphorylation of overexpressed PP1α or PP1γ1 by 3.4 or 7-fold, respectively (Figure S2B) when the phospho signal was normalized to the Myc signal. This antibody selectively detected phosphorylated PP1 as no band was observed in either T320A or T311A mutant PP1α or PP1γ1, respectively (Figure S2B). Therefore, we wanted to determine if phosphorylation at either Thr320 (PP1α) and/or Thr311 (PP1γ1) regulates the association between spinophilin and PP1. We generated T320A and T320D (PP1α) as well as T311A and T311D mutants (PP1γ1). Interestingly, the Asp mutations led to a robust (~8 kDa) molecular weight shift in PP1α (Figure 5B) but not PP1γ1 (Figure 5C). When normalized to Myc immunoreactivity in the input, the T320/311A mutants had no effect on the association of spinophilin with PP1; however, we found that the T320/311D mutations significantly increased the association of spinophilin with PP1α (Figure 5B; ANOVA values: F(2,9) = 10.14, p = 0.0049) and PP1γ1 (Figure 5C; ANOVA values F(2,9) = 14.53, p = 0.0015). Therefore, phosphorylation of PP1 at its C-terminus by CDK5 may be partially responsible for the CDK5-dependent increase in the spinophilin/PP1 interaction.
Figure 5.
PP1 Thr320/311mutants had increased association with spinophilin. (A) Alignment of PP1 (human PP1α and rat PP1γ1). (B) HEK293 cells were transfected with spinophilin and WT, T320A, or T320D PP1α and immunoprecipitated for spinophilin. The association of spinophilin was greater with T320D compared to WT or T320A PP1α. (C) HEK293 cells were transfected with spinophilin and WT, T311A, or T311D PP1γ1 and immunoprecipitated for spinophilin. The association of T311D was greater than WT or T320A mutant. (D) HEK293 cells were transfected with neurabin and WT, T320A, or T320D PP1α and immunoprecipitated for neurabin. The association of neurabin was greater with T311D compared to WT or T311A. (E) HEK293 cells were transfected with neurabin and WT, T3110A, or T311D PP1γ1 and immunoprecipitated for neurabin. There was no change in the association of neurabin with the different PP1γ1 mutants. A one-way ANOVA followed by a Tukey posthoc test was performed. *p < 0.05, **p < 0.01.
To delineate the CDK5-dependent mechanisms that mediate the decreased PP1/neurabin association, we expressed neurabin with the PP1 CDK5 phosphorylation site mutants from above. As with spinophilin, the Thr320D mutation of PP1α also enhanced the association of neurabin with PP1α (Figure 5D; ANOVA values: F(2,9) = 14.45, p = 0.0015). This is in contrast to what was observed with CDK5 activity (Figure 3). However, neither the Thr311A nor the Thr311D mutation of PP1γ1 altered the association of neurabin with PP1γ1 (Figure 5E; ANOVA values: F(2,9) = 2.459, p = 0.2891).
CDK5 Activity and Spinophilin Association with PP1 Are Decreased by a High-Dose Regimen of METH.
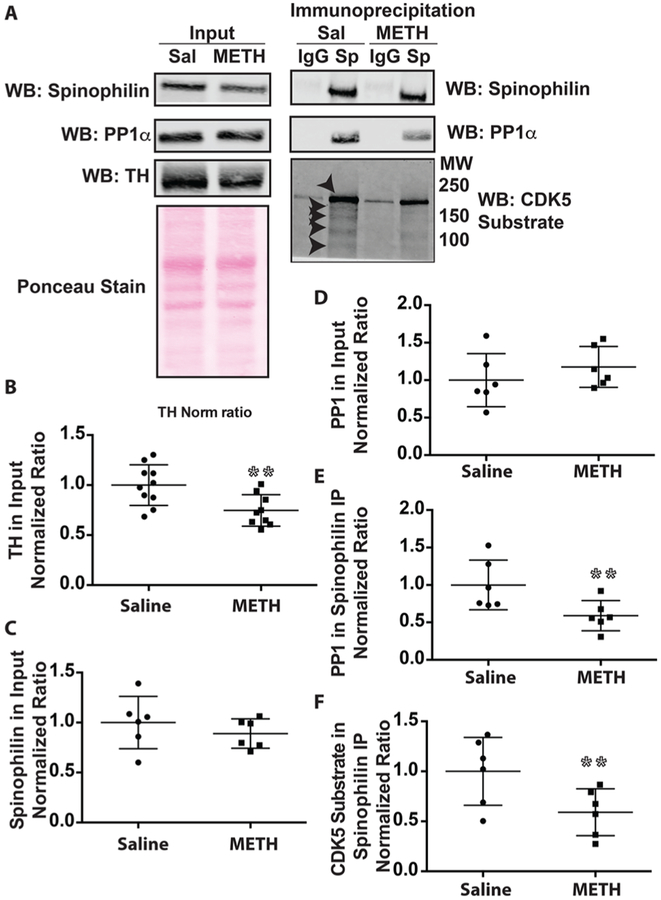
Psychostimulant drugs of abuse such as METH modulate dopamine signaling; however, their effects on the spinophilin/PP1 interaction are unknown. Twenty-eight days following a high-dose, binge regimen of METH, striata were dissected from saline or METH-treated rats. Spinophilin was immunoprecipitated from striatal lysates. Striatal lysates and/or spinophilin immunoprecipitates were immunoblotted for tyrosine hydroxylase, spinophilin, PP1α, and CDK5 substrate phosphorylation (Figure 6A). This METH regimen lead to a significant ~25% decrease in tyrosine hydroxylase immunoreactivity (Figure 6B). While METH treatment had no effect on total spinophilin or PP1α levels (Figures 6C and D), we observed a decreased association of spinophilin with PP1α (Figure 6E). To begin to identify putative mechanisms for the regulation of this association, we immunoblotted spinophilin immunoprecipitates with an antibody that detects CDK5-phosphorylated substrates (Figure S2A). Concurrent with the decreased spinophilin/PP1 interaction, there was less CDK5 substrate phosphorylation in the spinophilin immunoprecipitates in the region of the gel from 250 to 100 kDa (Figure 6F). There was one major band and four minor bands in this region (arrowheads). These data suggest that the spinophilin/PP1 interaction is decreased by a binge regimen of METH and that this decrease occurs concurrent with decreased CDK5 substrate phosphorylation of spinophilin immunoprecipitates.
Figure 6.
Spinophilin/PP1α interaction and CDK5 phosphorylation of spinophilin binding proteins is decreased following a neurotoxic regimen of METH. Striatal lysates from rats treated with 4 doses of saline or METH (10 mg/kg) were immunoprecipitated for spinophilin (A–F) or PP1 (G–H). (A) Lysates (0.75% of total homogenate) were immunoblotted for spinophilin, PP1, and TH, whereas spinophilin immunoprecipitates (9.4% of total input) were immunoblotted for spinophilin, PP1α, and phosphorylated CDK substrates. (B–D) Total levels of TH (B), spinophilin (C), and PP1α (D) were not significantly different. (E) The association between spinophilin and PP1α was decreased. (F) The amount of CDK5 substrate phosphorylation in the spinophilin immunoprecipitates was decreased. **p < 0.01.
Spinophilin KO Mice Have Deficits in Rotarod Learning.
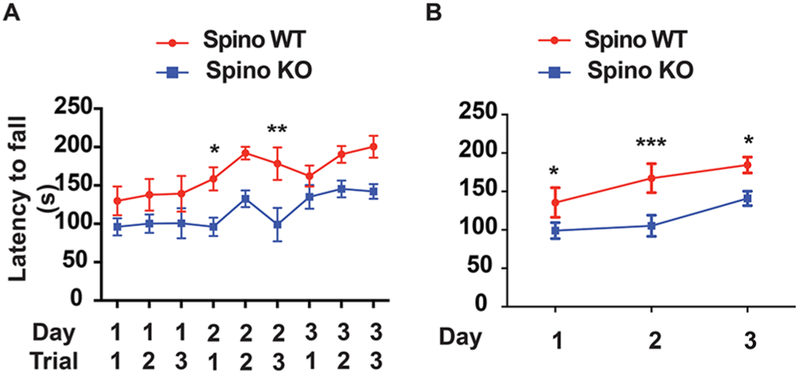
Previous studies have observed rotarod deficits in dopamine-depleted animals and following a neurotoxic regimen of METH. Moreover, loss of spinophilin is associated with enhanced sedative responses on the rotarod.42,43 However, the role of spinophilin in motor coordination and motor learning is not known. To assay rotarod behavior, control (spinophilin+/+) or whole-body spinophilin KO mice were placed on an accelerating rotarod apparatus. Three trials per day for three consecutive days were performed, and the latency to fall was recorded. All trials are shown in Figure 7A with the average of trials for each day shown in Figure 7B. A two-way ANOVA followed by a Sidak’s multiple comparison posthoc test was performed to compare across trials. Results revealed a significant trial effect (F(8,90) = 2.093; p = 0.0444) and a significant effect of genotype (F(1,90) = 54.08; p < 0.0001) with no interaction effect. A two-way ANOVA was performed on the average trial data shown in Figure 7B (day and genotype as variables). Results revealed a significant day effect (F(2,30) = 3.619; P = 0.0391) and a significant effect of genotype (F(1,30) = 30.57; p < 0.0001) with no interaction effect. When performing a Sidak’s posthoc test, all three days were significantly different between the control and KO groups. These data suggest that like both dopamine depletion and neurotoxic regimens of METH, spinophilin KO animals have impairments in rotarod behaviors and learning.
Figure 7.
Spinophilin KO mice have deficits in motor learning. Control or KO spinophilin mice were placed on an accelerating rotarod, and their latency to fall was measured. (A) Data showing all three trials from each day are shown. (B) Data showing average of the 3 trials from each day. A Holm–Sidak posthoc multiple comparison test was performed to compare Two-way ANOVA data for WT and KO latencies from each trial (A) or by day for pooled trial data (B). *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
Spinophilin Interaction with PP1α is Decreased by a Neurotoxic Regimen of METH.
The association of PP1α10 and PP1γ19 with spinophilin is increased in an animal model of PD. While acute and sensitizing regimens of METH increase CDK5 activity,44 our data suggest that a neurotoxic regimen of METH leads to a long-term decrease in CDK5 substrate phosphorylation in spinophilin immunoprecipitates. Consistent with this decrease in activity, a previous proteomics study detected increases in the tyrosine nitration protein GSTP1, a molecule that is associated with decreasing CDK5 activity,45 following METH treatment.46 While animal models of PD have a complete or near complete loss of tyrosine hydroxylase immunoreactivity in the striatum 3 weeks following lesion,9,10 we observed a 25% decrease in TH levels in METH-treated animals, suggesting less dopamine toxicity associated with neurotoxic METH treatment compared to nigral lesion. It is important to note that changes in TH levels may be due to acute alterations in dopamine that lead to compensatory changes in TH expression. However, given that these are persistent changes (observed 28 days after binge regimen of METH) and that TH immunoreactive dopamine terminals are lost following binge METH in mice,30 these changes may more likely be due to toxicity and not compensation. Moreover, these decreases in TH are in line with decreases observed in human chronic METH abusers.47 We observed a decreased association between spinophilin and PP1α in the striatum of METH-treated animals. One reason why opposite effects may occur in complete (e.g., >95% loss of dopamine neurons) vs partial lesion is that METH may increase the sensitivity to the dopamine that remains in the partial lesion. High-dose binge regimens of METH have been shown to lead to decreases in the amplitude and frequency of dopamine release but increases in the duration of release.48 Moreover, high-dose METH treatment leads to increases in D1 dopamine receptor (direct pathway) medium spiny neuron activity.49 Moreover, as is well-known, METH can cause reversal of the dopamine transporter and increases in dopamine release from vesicular pools; however, it is unlikely that these high levels of dopamine would persist for 28 days following the binge regimen. Therefore, METH treatment may lead to an increased sensitivity of striatal medium spiny neurons to the remaining dopamine as well as alterations in the mode of dopamine release.
PP1 Isoform Expression in HEK293 Cells.
While PP1α, PP1β, and PP1γ1 are highly enriched in the brain,16–19 and in particular forebrain vs cerebellum in human tissue,50 there are overlapping and unique expression patterns of the different PP1 isoforms (http://mouse.brain-map.org).15,20,39,51 Spinophilin preferentially associates with PP1α and PP1γ1 over PP1β.15,20 We observed that both PP1α and PP1γ1 isoforms were equally expressed in HEK293 cells. We were able to ascertain this as we could normalize the subunit selective antibody reactivity to total expression of each isoform by dividing the isoform antibody signal to the epitope-tag (Myc) antibody intensity of the specific overexpressed isoforms evaluated on the same samples. Overexpression of the specific PP1 isoform regulated the expression of the different endogenous PP1 isoforms with overexpression of PP1α increasing endogenous PP1α expression and decreasing endogenous PP1γ1 expression. Conversely, PP1γ1 overexpression increased endogenous PP1γ1 expression and decreased endogenous PP1α. However, the overexpression-dependent increase in PP1 expression of a band migrating at the endogenous molecular weight may be due to cleavage of the epitope tag. As an alternative explanation, it could be that PP1 levels positively modulate its own expression. Regardless, a cleavage product does not explain the PP1-mediated decrease in expression of the other PP1 isoform. PP1 is thought to be targeted to bind to DNA and regulate specific genes by PP1 interacting proteins such as NIPP1, PNUTS, and RepoMan as well as other unidentified proteins.52 Interestingly, NIPP1 was shown to have different chromatin binding capabilities depending on its ability to bind to PP1. Moreover, there was isoform specificity in PP1 targeting to different chromatin sites.52 Therefore, it is possible that a PP1α-bound form of a targeting subunit enhances the targeting subunit’s association with the PP1α promoter and causes it to be displaced from the PP1γ promoter. There is evidence for isoform specificity in PP1 targeting and regulation of PP1 activity20,21,53–56 as well as isoform specific differences in PP1 associations with different promoters,52 suggesting that unique targeting and transcriptional control may underlie PP1 autoregulation of its expression.
Spinophilin and Neurabin Association with PP1 is Differentially Regulated by CDK5.
CDK5 overexpression in a heterologous cell line increased the interaction between spinophilin and both PP1 isoforms. In contrast, CDK5 decreased the interaction of neurabin with PP1α and PP1γ1. CDK5 decreased the expression of both spinophilin (30%) and neurabin (64%). This is most likely not due to a loss of cells as we normalize to ponceau stain, and we did not see a significant decrease in endogenous PP1 expression under the different conditions. Neurabin, but not spinophilin, had a modest molecular weight shift in the presence of overexpressed CDK5 (Figure S1). This difference may be due to a different number of CDK5 phosphorylation sites on each protein or a different phosphorylation site. Spinophilin is known to be phosphorylated by CDK5 at Ser-17.24 While Neurabin has a conserved Ser-17, it is not phosphorylated by CDK5 at that site; rather, it is phosphorylated at Ser-95.57 While phosphorylation of Ser-17 on spinophilin does not have an effect on F-actin binding, Ser-95 phosphorylation on neurabin does.24,57 CDK5 overexpression regulating spinophilin and neurabin stability is a plausible function of CDK5 as it has previously been shown that CDK5 overexpression decreased the stability of the protein CLOCK.58 In regard to CDK5-dependent regulation of protein stability, it may be that alterations in spinophilin and/or neurabin phosphorylation may directly regulate protein stability and/or may modulate ubiquitination of the protein. Whereas we did not see any laddering in the presence of CDK5, it may be that the molecular weight shift is too great to detect on this gel. Future studies will need to determine if CDK5 activity regulates spinophilin and neurabin protein stability in vivo and the mechanisms of this regulation.
Mechanisms Underlying CDK5-Dependent Increase in the Spinophilin Interaction with PP1.
Phosphomimic mutation (S17D) of spinophilin on its known CDK5-site, Ser17, had no effect on its association with PP1α or PP1γ1. In fact, if anything, the phosphomimic had a trend for a decreased association with both PP1 isoforms, suggesting that phosphorylation at this site is not responsible for the CDK5-dependent increase in association. PP1α is also phosphorylated by CDK5 at Thr320, and phosphorylation at this site is important in cellular differentiation.25 Specifically, Hou et al. found that overexpression of CDK5 along with p35 in neuronal cultures increased PP1 phosphorylation at Thr320.41 Activation of synaptic NMDARs decrease CDK5 activity and consequently decrease PP1 phosphorylation at Thr320, leading to increased PP1 activity.41 Xia and colleagues also determined that NMDAR signaling increased the association between PP1 and inhibitor-2 and that these changes were due to alterations in inhibitor-2 phosphorylation.41 However, the role of PP1 phosphorylation on modulating its interaction with spinophilin is unknown. While the overall structure of the PP1 isoforms is highly similar, the C-terminal regions of the different isoforms diverge.59 However, both isoforms have a conserved Thr residue at Thr320 RPITPPR (PP1α) or Thr311 RPVTPPR (PP1γ1).
Mutation of PP1 to an Asp at Thr320 (PP1α) caused a dramatic molecular weight shift in the migration of PP1α, but mutation at Thr311 did not affect the mobility of PP1γ1. These differences in migration may be due to variances in the sequence surrounding the phosphorylateable threonine. Specifically, in contrast to PP1γ1, PP1α has an additional stretch of 9 amino acid residues upstream of the phosphorylated threonine. Interestingly, one of these residues, a proline, is at the end of this stretch. This proline may allow for differential flexibility than that observed in PP1γ1. Interestingly, Asp substitutions at these sites increased the binding of spinophilin to PP1α and PP1γ1 when normalized to spinophilin levels in the IP and expression of the different PP1 mutants in the input. This normalization was performed as the Asp mutants had qualitatively less immunoreactivity than the WT or Ala mutants. This is consistent with qualitatively less immunoreactivity of overexpressed PP1α or PP1γ1 in the presence of overexpressed CDK5/p35. It is unclear why this is, but one possible explanation is that PP1 phosphorylation or TD mutation may modulate the stability of the protein. Spinophilin binds to multiple sites on PP1 in addition to the RVxF binding pocket.15 The Asp mutants on PP1 only increased spinophilin binding by approximately twofold, whereas CDK5 overexpression increased the association by approximately threefold. This could be due to the Asp mutants not fully recapitulating a phosphorylated threonine or additional CDK5-dependent changes occurring. CDK5 regulates and/or is regulated by different kinases.60–62 Therefore, additional sites on spinophilin or PP1 that are regulated downstream of CDK5 overexpression (e.g., other kinases) may also contribute to the alterations in the spinophilin PP1 interaction. Moreover, given that T320/Thr311 phosphorylation of PP1α or PP1γ1 decreases PP1 activity and PP1 can autodephosphorylate,25,41,63–65 we cannot rule out that the overexpression of differentially active PP1 may play a role in altering the spinophilin or neurabin association with PP1.
In contrast to spinophilin, CDK5 activity decreased the neurabin/PP1 interaction, an effect that was not due to PP1 phosphorylation as T311D on PP1γ1 had no effect on the association of PP1γ1 with neurabin and the T320D of PP1α actually enhanced PP1 binding to neurabin. This may be of interest as both spinophilin and neurabin bind to PP1 via their RVxF motifs but may have additional sites that can modulate this interaction. For instance, neurabin has a PKA-phosphorylateable residue near the PP1 binding domain that is lacking in spinophilin. This residue has been recently shown to modulate the association between neurabin and PP1.22 Moreover, CDK5-dependent differences in spinophilin and neurabin binding to different PP1 isoforms may modify competition in the association of PP1 with neurabin or spinophilin. This may be of interest as spinophilin and neurabin have both overlapping and differential expression patterns.1 Moreover, spinophilin and neurabin may heterodimerize,21 and therefore, altering binding to neurabin may regulate PP1 targeting to spinophilin. Therefore, there appear to be differential roles of mutation at the Thr311/320 site in the two PP1 isoforms on modulating the association with these two, major neuronal PP1-targeting proteins. Moreover, given this complexity, we cannot rule out that additional mechanisms are contributing to dopamine depletion or METH toxicity-dependent modulation of the spinophilin/PP1 interaction.
We observed decreases in CDK5 substrate phosphorylation in spinophilin immunoprecipitates in METH-treated samples. Whereas this antibody specifically detected CDK5-dependent phosphorylation as evidenced in HEK293 cells overexpressing CDK5 and p35 (Figure S2A), we cannot rule out that other CDKs or kinases that have similar phosphorylation motifs are also detected by this antibody. Future studies will need to directly test if Thr320 phosphorylation of PP1α and/or PP1γ1 is responsible for the decreased association between spinophilin and PP1 in METH-treated rodents.
Spinophilin Function in Modulating Behavioral Pathologies Associated with Dopamine Depletion and METH Toxicity.
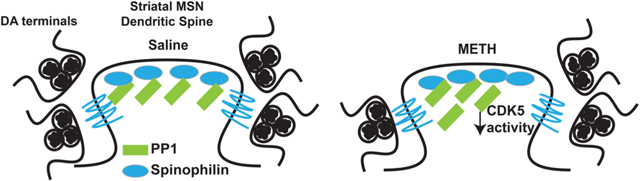
The current biochemical data suggest CDK5-dependent regulation of the spinophilin PP1 interaction. Moreover, these studies suggest differences in CDK5 substrate phosphorylation in spinophilin immunoprecipitates isolated from METH-treated rats as well as decreases in the spinophilin–PP1 interaction under these conditions. Behaviorally, both METH toxicity30,31 and dopamine depletion32 lead to deficits in rotarod balance and learning. Interestingly, we observed deficits in both basal rotarod performance and delays in motor learning in whole-body spinophilin KO mice. These data further demonstrate a role of spinophilin in mediating striatal behaviors such as motor learning. Given that spinophilin is the most abundant PP1 targeting protein in the PSD,33 alterations in spinophilin-dependent PP1 targeting may underlie these pathologies. Furthermore, given that the spinophilin/PP1 interaction is enhanced in animal models of PD9,10 and decreased in METH toxicity (present study), we posit that biphasic regulation of spinophilin’s association with PP1 has deleterious effects. A decreased interaction between spinophilin and PP1 may attenuate targeting of PP1 and enhance phosphorylation of its substrates.8 Conversely, increased association of spinophilin with PP1 may lead to decreases in PP1 activity as spinophilin is known to prevent PP1 activity toward specific substrates.15
Taken together, our data demonstrate mechanisms by which the spinophilin/PP1 interaction are regulated and suggest an influential role of spinophilin on modulating striatal behaviors.
METHODS
Animals.
Male Sprague–Dawley rats (180–275 g, Harlan, Indianapolis, IN) were used for METH treatment studies. For rotarod studies, 3–5.5-month old male spinophilin KO (B6N(Cg)-Ppp1r9btm1.1(KOMP)Vlcg/J N = 6) or male (N = 4) or female (N = 2) control, WT littermate mice were used. All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals as disseminated by the U.S. National Institutes of Health and were approved by Indiana University School of Medicine and/or Indiana University–Purdue University School of Science Animal Care and Use Committees.
METH Treatment.
Rats were i.p. injected with 4 doses of METH (10 mg/kg, Sigma, St. Louis, MO, Cat. M8750) or saline (1 mL/kg), once every 2 h for 4 total injections, as previously described.66
DNA Plasmids and Mutagenesis.
Mammalian expression constructs were amplified by PCR and inserted into pDonr221 (ThermoFisher Scientific, Waltham, MA) using Gateway cloning with BP recombinase (ThermoFisher Scientific). Following insertion of the cDNA encoding different proteins (see below) into the donor vector, the cDNAs in the donor vectors were recombined into the mammalian expression vector, pcDNA3.1 nDEST, containing different N-terminal epitope tags (V5, Myc, HA, or Flag) using LR recombinase. The following cDNAs were used as templates to generate the mammalian expression vectors: human spinophilin10 (Addgene no. 87122), neurabin (isoform-4; Transomic Technologies, Huntsville, AL BC130449-TCH1003), rat PP1γ1,20 human PP1α (PBC004482, Transomic Technologies), human CDK5 (Addgene no. 23699), and human p35 (Addgene no. 23779). CDK5 and p35 were gifts from William Hahn and David Root67 and obtained from Addgene. Mutagenesis to generate PP1 mutants was performed as previously described.10 DNA quantification was performed on a Cytation3 imager (BioTek, Winooski, VT). DNA inserts and mutations were sequence verified (Genewiz, South Plainfield, NJ).
Transfections.
Human embryonic kidney cells (HEK293FT; ThermoFisher) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) that contained 10% fetal bovine serum, 584 mg/L L-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. Culture flasks were incubated at a constant 37 °C and 5% CO2 (Panasonic Healthcare; Secaucus, NJ). Cells were transfected with appropriate DNAs and PolyJet reagent (SignaGen Laboratories, Gaithersburg, MD) as per the manufacturers’ instructions.
Cell Lysis.
For spinophilin immunoprecipitates from METH-treated rats, one rat striatum was homogenized and sonicated in 2 mL of a modified radio-immunoprecipitation assay (RIPA) buffer containing 20 mM Tris, 150 mM NaCl, 2 mM EDTA, protease inhibitor cocktail (ThermoFisher Scientific or Bimake, Houston, TX), phosphatase inhibitors (20 mM sodium fluoride, 20 mM sodium orthovanadate, 20 mM β-glycerophosphate, and 10 mM sodium pyrophosphate; Sigma-Aldrich or ThermoFisher Scientific), 1% NP-40 (v/v; ThermoFisher Scientific), 1% sodium deoxycholate (w/v; ThermoFisher Scientific). Following overnight transfection, HEK293 cells were sonicated in 1.6 mL KCl lysis buffer (150 mM KCl, 1 mM DTT, 2 mM EDTA, 50 mM Tris-HCl, pH 7.5, 1% (v/v) Triton X-100, 20 mM NaF, 20 mM β-glycerophosphate, 20 mM NaVO3, 10 mM Na pyrophosphate, 1× protease inhibitor cocktail) or low-ionic strength Tris buffer (10 mM DTT, 5 mM EDTA, 2 mM Tris-HCl pH 7.5, 1% Triton X-100 with protease and phosphatase inhibitors as above).
Immunoprecipitations.
Spinophilin or HA-tagged spinophilin or neurabin were immunoprecipitated from rat brain or HEK293 cell lysates, respectively, as previously described.10 Endogenous spinophilin was immunoprecipitated from 37.5% of the total rat lysate using 3 μg of a goat spinophilin polyclonal antibody (Santa Cruz Biotechnology, Dallas TX; SC-14774). HA-tagged spinophilin or neurabin was immunoprecipitated from 50% of the total HEK293 cell lysate using 1–2 μg of a goat HA polyclonal antibody (Bethyl Laboratories, Montgomery, TX; A190–238A).
Immunoblotting.
For the METH experiments evaluating spinophilin immunoprecipitates, 1% of the input and 25% of the spinophilin immunoprecipitates were immunoblotted for spinophilin and PP1. For HEK293 studies, 0.5–1.5% of the input and 25–33% of the immunoprecipitates were immunoblotted with the appropriate primary antibody (see below). These studies were performed similar to previously described.10 For protein detection, the following primary antibodies were used: spinophilin: rabbit polyclonal HA (Santa Cruz Biotechnology, SC-805), goat polyclonal HA, goat spinophilin, or rabbit monoclonal spinophilin (Cell Signaling Technology, Danvers, MA; 14136S); rabbit monoclonal CDK5 substrate motif antibody (Cell Signaling Technology, 9477); rabbit Thr320 phsopho-PP1 antibody (Cell Signaling Technology, 2581); mouse monoclonal Myc epitope tag antibody (Santa Cruz Biotechnology, sc-40) or goat Myc (Bethyl A190–104A), goat polyclonal PP1γ antibody (Santa Cruz Biotechnology, SC-6108), mouse monoclonal PP1α antibody (Santa Cruz Biotechnology, SC-7482), rabbit tyrosine hydroxylase (TH; Santa Cruz Biotechnology, SC-14007), rabbit DDDDK (Flag) antibody (Bethyl A190–102A), mouse Flag antibody (Sigma-Aldrich F3165), Thr320 PP1α antibody (Cell Signaling Technology, 2581; previously validated25,41). Antibody dilutions were used at 1:500–1:2000 for immunoblotting. Following overnight incubation at 4 °C, the following secondary antibodies were used for fluorescence detection: Donkey anti goat IgG (H+L) Alexa Fluor 790 (Life Technologies A11370; 1:10 000 dilution), donkey anti mouse IgG (H +L) Alexa Fluor 790 (Jackson Immunoresearch, West Grove, PA, no. 715-655-151, 1:50 000 dilution), Donkey anti rabbit IgG (H+L) Alexa Fluor 790 (Jackson Immunoresearch no. 711-655-152, 1:50 000 dilution). Donkey anti goat Alexa Fluor 680 (Life Technologies A21084; 1:10 000 dilution). Imaging was performed on an Odyssey CLx system (LI-COR Biosicences, Lincoln, NE). Images were acquired under automatic mode to ensure a linear range. No saturated pixels were detected. Moreover, ponceau staining was shown to be linear between 0.63 μg and 78 μg of total protein lysate (striatal lysates) and fluorescence immunoblotting was shown to be linear for spinophilin, PP1α, and PP1γ1 (0.13–78.3 μg total protein lysate) (Figure S3).
Rotarod.
For the accelerating rotarod (3 cm width); male whole-body spinophilin KO (spinophilin − / −; B6N (Cg) - Ppp1r9btm1.1(KOMP)Vlcg/J) or male or female control wild-type mice were placed on the Rotamex-5 (Columbus Instruments) apparatus. Mice were subjected to an accelerating rotarod (4–40 rpm in 300 s) for 3 successive trials on 3 consecutive days. A series of photocell beams located above the rotating rod with a temporal resolution of 0.1 rpm (0.1 cm/sec) detected when the mouse was no longer on the rod. To circumvent any false fall recordings, any mouse that was able to grip the bar and rotate with the bar for 2 rotations or greater was considered to have failed the trial and the recording was not used. Latency to fall was documented for each mouse, and a 120–180 s rest was given to all mice before the next trial.
Data Analysis and Statistics.
For HEK293 cell experiments, each experiment was run on a separate gel, and each data point is a separate transfection, performed in a separate set of 25 cm2 flasks isolated on a separate day or on the same day from a separate 75 cm2 parent flask. To calculate total protein expression, the fluorescence value derived from a specific antibody (e.g., HA) was divided by the value generated from the same ponceau stained membrane. To quantify the corresponding ponceau-stained lane, a portion of the lane was measured using Image J. For quantitation of lysates, ~10 μg of total protein was loaded. Similar total protein concentrations as we were loading have been shown to generate a linear signal by ponceau staining (Figure S3 and ref 68). The ponceau stain value was used to normalize loading for endogenous proteins. To compare across multiple gels, the value of the experimental group was normalized to the value of the control group on each immunoblot. Therefore, no error was obtained for the control conditions. For METH spinophilin immunoprecipitation experiments, an N of 3 animals was immunoblotted on one gel and a separate N of 3 animals was evaluated on a separate gel. A ratio was generated from each gel to obtain error bars for both saline and METH treated samples. To statistically analyze each experimental group, a 1-sample t-test was used to compare each experimental group to a theoretical mean of 1. For comparing the effect of CDK5 expression on WT and S17A mutant spinophilin, a two-way ANOVA was performed. For comparing across the three different PP1 mutations (WT, TA, and TD), a one-way ANOVA followed by a Tukey’s multiple comparison posthoc test was performed. For the rotarod studies, a two-way ANOVA followed by a Holm–Sidak multiple comparison posthoc test was performed to compare control and spinophilin KO across the different trials or days. Statistical analyses and graphing were performed using Prism 6.0 (GraphPad, LaJolla, CA). Graphs and immunblotting images were assembled using Photoshop and Illustrator (Adobe Systems Incorporated, San Jose, CA).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Andy Hudmon (Indiana University School of Medicine) and Roger Colbran (Vanderbilt University) for critical discussion of the manuscript. We thank Mr. Victor Olafusi for help in generation of the PP1 mutants.
Funding
Support for these studies was from NIH (Grants K01NS073700 and R21DA041876 to A.J.B.), Department of Biology Start-up funds (to A.J.B.), and a Summer Undergraduate Research Program Fellowship (C.W.M.).
ABBREVIATIONS
- PP1
protein phosphatase 1
- METH
methamphetamine
- PD
Parkinson’s disease
- CDK5
cyclin-dependent kinase 5
- HEK293
human embryonic kidney 293FT cells
- PSD
postsynaptic density
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneuro.8b00144.
Spinophilin and neurabin immunoblots with and without CDK5/p35 overexpression; CDK5 substrate antibody and Thr320 PP1 antibody with and without CDK5/p35 overexpression; linearity of Ponceau S staining; and PP1α, PP1γ1, and spinophilin immunoblots (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Muly EC, Allen P, Mazloom M, Aranbayeva Z, Greenfield AT, and Greengard P (2004) Subcellular distribution of neurabin immunolabeling in primate prefrontal cortex: comparison with spinophilin. Cereb Cortex 14, 1398–1407. [DOI] [PubMed] [Google Scholar]
- (2).Muly EC, Smith Y, Allen P, and Greengard P (2004) Subcellular distribution of spinophilin immunolabeling in primate prefrontal cortex: localization to and within dendritic spines. J. Comp. Neurol 469, 185–197. [DOI] [PubMed] [Google Scholar]
- (3).Baucum AJ 2nd, Jalan-Sakrikar N, Jiao Y, Gustin RM, Carmody LC, Tabb DL, Ham AJ, and Colbran RJ (2010) Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach. Mol. Cell. Proteomics 9, 1243–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Evans JC, Robinson CM, Shi M, and Webb DJ (2015) The Guanine Nucleotide Exchange Factor (GEF) Asef2 Promotes Dendritic Spine Formation via Rac Activation and Spinophilin-dependent Targeting. J. Biol. Chem 290, 10295–10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Grossman SD, Futter M, Snyder GL, Allen PB, Nairn AC, Greengard P, and Hsieh-Wilson LC (2004) Spinophilin is phosphorylated by Ca2+/calmodulin-dependent protein kinase II resulting in regulation of its binding to F-actin. J. Neurochem 90, 317–324. [DOI] [PubMed] [Google Scholar]
- (6).Hsieh-Wilson LC, Benfenati F, Snyder GL, Allen PB, Nairn AC, and Greengard P (2003) Phosphorylation of spinophilin modulates its interaction with actin filaments. J. Biol. Chem 278, 1186–1194. [DOI] [PubMed] [Google Scholar]
- (7).Hu XD, Liu YN, Zhang ZY, Ma ZA, Suo ZW, and Yang X (2015) Spinophilin-Targeted Protein Phosphatase-1 Alleviated Inflammatory Pain by Negative Control of MEK/ERK Signaling in Spinal Cord Dorsal Horn of Rats. J. Neurosci 35, 13989–14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, and Greengard P (1999) Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat. Neurosci 2, 13–17. [DOI] [PubMed] [Google Scholar]
- (9).Brown AM, Baucum AJ, Bass MA, and Colbran RJ (2008) Association of protein phosphatase 1 gamma 1 with spinophilin suppresses phosphatase activity in a Parkinson disease model. J. Biol. Chem 283, 14286–14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hiday AC, Edler MC, Salek AB, Morris CW, Thang M, Rentz TJ, Rose KL, Jones LM, and Baucum AJ 2nd. (2017) Mechanisms and Consequences of Dopamine Depletion-Induced Attenuation of the Spinophilin/Neurofilament Medium Interaction. Neural Plast. 2017, 4153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Boikess SR, and Marshall JF (2008) A sensitizing d-amphetamine regimen induces long-lasting spinophilin protein upregulation in the rat striatum and limbic forebrain. European journal of neuroscience 28, 2099–2107. [DOI] [PubMed] [Google Scholar]
- (12).Boikess SR, O’Dell SJ, and Marshall JF (2010) A sensitizing D-amphetamine dose regimen induces long-lasting spinophilin and VGLUT1 protein upregulation in the rat diencephalon. Neurosci. Lett 469, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Allen PB, Zachariou V, Svenningsson P, Lepore AC, Centonze D, Costa C, Rossi S, Bender G, Chen G, Feng J, Snyder GL, Bernardi G, Nestler EJ, Yan Z, Calabresi P, and Greengard P (2006) Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience 140, 897–911. [DOI] [PubMed] [Google Scholar]
- (14).Hsieh-Wilson LC, Allen PB, Watanabe T, Nairn AC, and Greengard P (1999) Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1. Biochemistry 38, 4365–4373. [DOI] [PubMed] [Google Scholar]
- (15).Ragusa MJ, Dancheck B, Critton DA, Nairn AC, Page R, and Peti W (2010) Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat. Struct. Mol. Biol 17, 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).da Cruz e Silva EF, Fox CA, Ouimet CC, Gustafson E, Watson SJ, and Greengard P (1995) Differential expression of protein phosphatase 1 isoforms in mammalian brain. J. Neurosci 15, 3375–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Smith GD, Wolf DP, Trautman KC, da Cruz e Silva EF, Greengard P, and Vijayaraghavan S (1996) Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol. Reprod 54, 719–727. [DOI] [PubMed] [Google Scholar]
- (18).Strack S, Kini S, Ebner FF, Wadzinski BE, and Colbran RJ (1999) Differential cellular and subcellular localization of protein phosphatase 1 isoforms in brain. J. Comp. Neurol 413, 373–384. [PubMed] [Google Scholar]
- (19).Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, and Greengard P (1996) Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol. Reprod 54, 709–718. [DOI] [PubMed] [Google Scholar]
- (20).Carmody LC, Baucum AJ 2nd, Bass MA, and Colbran RJ (2008) Selective targeting of the gamma1 isoform of protein phosphatase 1 to F-actin in intact cells requires multiple domains in spinophilin and neurabin. FASEB J. 22, 1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).MacMillan LB, Bass MA, Cheng N, Howard EF, Tamura M, Strack S, Wadzinski BE, and Colbran RJ (1999) Brain actin-associated protein phosphatase 1 holoenzymes containing spinophilin, neurabin, and selected catalytic subunit isoforms. J. Biol. Chem 274, 35845–35854. [DOI] [PubMed] [Google Scholar]
- (22).Gao J, Hu XD, Yang H, and Xia H (2018) Distinct Roles of Protein Phosphatase 1 Bound on Neurabin and Spinophilin and Its Regulation in AMPA Receptor Trafficking and LTD Induction. Mol. Neurobiol, DOI: 10.1007/s12035-018-0886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Yamamura Y, Morigaki R, Kasahara J, Yokoyama H, Tanabe A, Okita S, Koizumi H, Nagahiro S, Kaji R, and Goto S (2013) Dopamine signaling negatively regulates striatal phosphorylation of Cdk5 at tyrosine 15 in mice. Front. Cell. Neurosci 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Futter M, Uematsu K, Bullock SA, Kim Y, Hemmings HC Jr., Nishi A, Greengard P, and Nairn AC (2005) Phosphorylation of spinophilin by ERK and cyclin-dependent PK 5 (Cdk5). Proc. Natl. Acad. Sci. U. S. A 102, 3489–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Li T, Chalifour LE, and Paudel HK (2007) Phosphorylation of protein phosphatase 1 by cyclin-dependent protein kinase 5 during nerve growth factor-induced PC12 cell differentiation. J. Biol. Chem 282, 6619–6628. [DOI] [PubMed] [Google Scholar]
- (26).Cadet JL, Sheng P, All S, Rothman R, Carlson E, and Epstein C (1994) Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J. Neurochem 62, 380–383. [DOI] [PubMed] [Google Scholar]
- (27).Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, and Hall ED (1996) Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res. 738, 172–175. [DOI] [PubMed] [Google Scholar]
- (28).Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, and Hanson GR (2015) Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment. Drug Alcohol Depend. 146, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wallace TL, Gudelsky GA, and Vorhees CV (1999) Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J. Neurosci 19, 9141–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ares-Santos S, Granado N, Espadas I, Martinez-Murillo R, and Moratalla R (2014) Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining. Neuropsychopharmacology 39, 1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Tulloch IK, Afanador L, Baker L, Ordonez D, Payne H, Mexhitaj I, Olivares E, Chowdhury A, and Angulo JA (2014) Methamphetamine induces low levels of neurogenesis in striatal neuron subpopulations and differential motor performance. Neurotoxic. Res 26, 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Iancu R, Mohapel P, Brundin P, and Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res 162, 1–10. [DOI] [PubMed] [Google Scholar]
- (33).Colbran RJ, Bass MA, McNeill RB, Bollen M, Zhao S, Wadzinski BE, and Strack S (1997) Association of brain protein phosphatase 1 with cytoskeletal targeting/regulatory subunits. J. Neurochem 69, 920–929. [DOI] [PubMed] [Google Scholar]
- (34).Herve D, Le Moine C, Corvol JC, Belluscio L, Ledent C, Fienberg AA, Jaber M, Studler JM, and Girault JA (2001) Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J. Neurosci 21, 4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Shiflett MW, and Balleine BW (2011) Molecular substrates of action control in cortico-striatal circuits. Prog. Neurobiol 95, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sibley DR, and Monsma FJ Jr. (1992) Molecular biology of dopamine receptors. Trends Pharmacol. Sci 13, 61–69. [DOI] [PubMed] [Google Scholar]
- (37).Surmeier DJ, Ding J, Day M, Wang Z, and Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 30, 228–235. [DOI] [PubMed] [Google Scholar]
- (38).Tritsch NX, and Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Terry-Lorenzo RT, Carmody LC, Voltz JW, Connor JH, Li S, Smith FD, Milgram SL, Colbran RJ, and Shenolikar S (2002) The neuronal actin-binding proteins, neurabin I and neurabin II, recruit specific isoforms of protein phosphatase-1 catalytic subunits. J. Biol. Chem 277, 27716–27724. [DOI] [PubMed] [Google Scholar]
- (40).Hu XD, Huang Q, Roadcap DW, Shenolikar SS, and Xia H (2006) Actin-associated neurabin-protein phosphatase-1 complex regulates hippocampal plasticity. J. Neurochem 98, 1841–1851. [DOI] [PubMed] [Google Scholar]
- (41).Hou H, Sun L, Siddoway BA, Petralia RS, Yang H, Gu H, Nairn AC, and Xia H (2013) Synaptic NMDA receptor stimulation activates PP1 by inhibiting its phosphorylation by Cdk5. J. Cell Biol 203, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lu R, Chen Y, Cottingham C, Peng N, Jiao K, Limbird LE, Wyss JM, and Wang Q (2010) Enhanced hypotensive, bradycardic, and hypnotic responses to alpha2-adrenergic agonists in spinophilin-null mice are accompanied by increased G protein coupling to the alpha2A-adrenergic receptor. Mol. Pharmacol 78, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, and Limbird LE (2004) Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science (Washington, DC, U. S.) 304, 1940–1944. [DOI] [PubMed] [Google Scholar]
- (44).Chen PC, and Chen JC (2005) Enhanced Cdk5 activity and p35 translocation in the ventral striatum of acute and chronic methamphetamine-treated rats. Neuropsychopharmacology 30, 538–549. [DOI] [PubMed] [Google Scholar]
- (45).Sun KH, Chang KH, Clawson S, Ghosh S, Mirzaei H, Regnier F, and Shah K (2011) Glutathione-S-transferase P1 is a critical regulator of Cdk5 kinase activity. J. Neurochem 118, 902–914. [DOI] [PubMed] [Google Scholar]
- (46).Zhang F, Chen L, Liu C, Qiu P, Wang A, Li L, and Wang H (2013) Up-regulation of protein tyrosine nitration in methamphetamine-induced neurotoxicity through DDAH/ADMA/NOS pathway. Neurochem. Int 62, 1055–1064. [DOI] [PubMed] [Google Scholar]
- (47).Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, and Kish SJ (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med 2, 699–703. [DOI] [PubMed] [Google Scholar]
- (48).Howard CD, Daberkow DP, Ramsson ES, Keefe KA, and Garris PA (2013) Methamphetamine-induced neurotoxicity disrupts naturally occurring phasic dopamine signaling. European journal of neuroscience 38, 2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Mark KA, Soghomonian JJ, and Yamamoto BK (2004) High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J. Neurosci 24, 11449–11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Carlyle BC, Kitchen RR, Kanyo JE, Voss EZ, Pletikos M, Sousa AMM, Lam TT, Gerstein MB, Sestan N, and Nairn AC (2017) A multiregional proteomic survey of the postnatal human brain. Nat. Neurosci 20, 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, and Jones AR (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. [DOI] [PubMed] [Google Scholar]
- (52).Verheyen T, Gornemann J, Verbinnen I, Boens S, Beullens M, Van Eynde A, and Bollen M (2015) Genome-wide promoter binding profiling of protein phosphatase-1 and its major nuclear targeting subunits. Nucleic Acids Res. 43, 5771–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Terrak M, Kerff F, Langsetmo K, Tao T, and Dominguez R (2004) Structural basis of protein phosphatase 1 regulation. Nature 429, 780–784. [DOI] [PubMed] [Google Scholar]
- (54).Carmody LC, Bauman PA, Bass MA, Mavila N, DePaoli-Roach AA, and Colbran RJ (2004) A protein phosphatase1gamma1 isoform selectivity determinant in dendritic spine-associated neurabin. J. Biol. Chem 279, 21714–21723. [DOI] [PubMed] [Google Scholar]
- (55).Colbran RJ, Carmody LC, Bauman PA, Wadzinski BE, and Bass MA (2003) Analysis of specific interactions of native protein phosphatase 1 isoforms with targeting subunits. Methods Enzymol. 366, 156–175. [DOI] [PubMed] [Google Scholar]
- (56).Scotto-Lavino E, Garcia-Diaz M, Du G, and Frohman MA (2010) Basis for the isoform-specific interaction of myosin phosphatase subunits protein phosphatase 1c beta and myosin phosphatase targeting subunit 1. J. Biol. Chem 285, 6419–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Causeret F, Jacobs T, Terao M, Heath O, Hoshino M, and Nikolic M (2007) Neurabin-I is phosphorylated by Cdk5: implications for neuronal morphogenesis and cortical migration. Mol. Biol. Cell 18, 4327–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kwak Y, Jeong J, Lee S, Park YU, Lee SA, Han DH, Kim JH, Ohshima T, Mikoshiba K, Suh YH, Cho S, and Park SK (2013) Cyclin-dependent kinase 5 (Cdk5) regulates the function of CLOCK protein by direct phosphorylation. J. Biol. Chem 288, 36878–36889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Peti W, Nairn AC, and Page R (2013) Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280, 596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Lim AC, Hou Z, Goh CP, and Qi RZ (2004) Protein kinase CK2 is an inhibitor of the neuronal Cdk5 kinase. J. Biol. Chem 279, 46668–46673. [DOI] [PubMed] [Google Scholar]
- (61).Banks AS, McAllister FE, Camporez JP, Zushin PJ, Jurczak MJ, Laznik-Bogoslavski D, Shulman GI, Gygi SP, and Spiegelman BM (2015) An ERK/Cdk5 axis controls the diabetogenic actions of PPARgamma. Nature 517, 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Sharma P, Veeranna, Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, and Pant HC (2002) Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J. Biol. Chem 277, 528–534. [DOI] [PubMed] [Google Scholar]
- (63).Goldberg J, Huang HB, Kwon YG, Greengard P, Nairn AC, and Kuriyan J (1995) Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 376, 745–753. [DOI] [PubMed] [Google Scholar]
- (64).Shimada M, Haruta M, Niida H, Sawamoto K, and Nakanishi M (2010) Protein phosphatase 1gamma is responsible for dephosphorylation of histone H3 at Thr 11 after DNA damage. EMBO Rep. 11, 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Wang X, Swain JE, Bollen M, Liu XT, Ohl DA, and Smith GD (2004) Endogenous regulators of protein phosphatase-1 during mouse oocyte development and meiosis. Reproduction 128, 493–502. [DOI] [PubMed] [Google Scholar]
- (66).Northrop NA, Halpin LE, and Yamamoto BK (2016) Peripheral ammonia and blood brain barrier structure and function after methamphetamine. Neuropharmacology 107, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, and Garraway LA (2010) COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468, 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Gustin RM, Bichell TJ, Bubser M, Daily J, Filonova I, Mrelashvili D, Deutch AY, Colbran RJ, Weeber EJ, and Haas KF (2010) Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiol. Dis 39, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.