Abstract
A structurally diverse family of 39 covalent triazine-based framework materials (CTFs) are synthesized by Suzuki–Miyaura polycondensation and tested as hydrogen evolution photocatalysts using a high-throughput workflow. The two best-performing CTFs are based on benzonitrile and dibenzo[b,d]thiophene sulfone linkers, respectively, with catalytic activities that are among the highest for this material class. The activities of the different CTFs are rationalized in terms of four variables: the predicted electron affinity, the predicted ionization potential, the optical gap, and the dispersibility of the CTFs particles in solution, as measured by optical transmittance. The electron affinity and dispersibility in solution are found to be the best predictors of photocatalytic hydrogen evolution activity.
Introduction
Hydrogen is essential in many industrial processes, such as the hydrogenation of unsaturated fats,1 production of stainless steel (ss)2 and ammonia,3 and crude oil purification.4 Hydrogen also has potential as a clean fuel for fuel cells that does not result in the emission of greenhouse gases at the point of use.5 Currently, hydrogen is made mostly by steam reforming of methane, which releases carbon dioxide directly and indirectly as a result of the high temperatures and pressures required for this process.6
Photocatalytic water splitting has been studied extensively as a green method for the production of hydrogen, releasing oxygen as the only side product. Light-driven water splitting was first demonstrated in 1972 for a photoelectrochemical cell with a TiO2 anode and platinum cathode.7 After this first report, many inorganic photocatalysts have been studied, also in suspension systems that do not require an applied bias.8−10 Conjugated polymer photocatalysts were studied as early as 1985,11−14 but it took the first report on carbon nitride in 200915 to trigger real interest in this field. Some limitations arise from the synthesis temperatures needed for carbon nitrides, which are typically above 300 °C, and this can result in a lack of synthetic control. A large number of studies reported improved hydrogen production rates under sacrificial conditions by varying the synthesis conditions and starting materials,16−21 by introducing doping,22,23 by controlling end groups,24−26 or by using heterojunctions27 or donor/acceptor-type systems.28 Conjugated organic polymers are typically made under much milder conditions29 than carbon nitrides and offer potential advantages in terms of synthetic control via the incorporation of different building blocks30−32 and by monomer sequence control.33 Conjugated polymers can be tailored in terms of their optical properties, as well as their driving force for proton reduction and water/sacrificial electron donor oxidation, respectively.33,34 A number of conjugated organic materials have now been studied for sacrificial hydrogen and/or oxygen production from water using light, including oligomers,35−37 linear polymers,34,38−45 covalent organic frameworks,46−54 and networks.42,55−62
Covalent triazine-based frameworks (CTFs) are a subclass of conjugated microporous polymers that have been reported to be photocatalytically active for hydrogen and oxygen evolution from water.30,63,64 The most common reactions used to prepare CTFs are the acid-catalyzed trimerization of aromatic nitriles,57,65−68 sometimes combined with sulfur and phosphorous doping,69,70 and high-temperature ionothermal synthesis.71−73 A two-step route was also reported whereby acid-catalyzed trimerizations were used to make CTFs that were then further condensed using thermal methods,74,75 or doped using ammonium halides.76 We previously used acid-catalyzed trimerization to prepare a series of CTFs with an increasing linker length between the triazine vertices (phenylene to quarterphenylene; Figure 1, CTF-1–4). In that series, the highest hydrogen evolution activity was observed for the acid-catalyzed CTF-2, which is based on a biphenylene linker.57
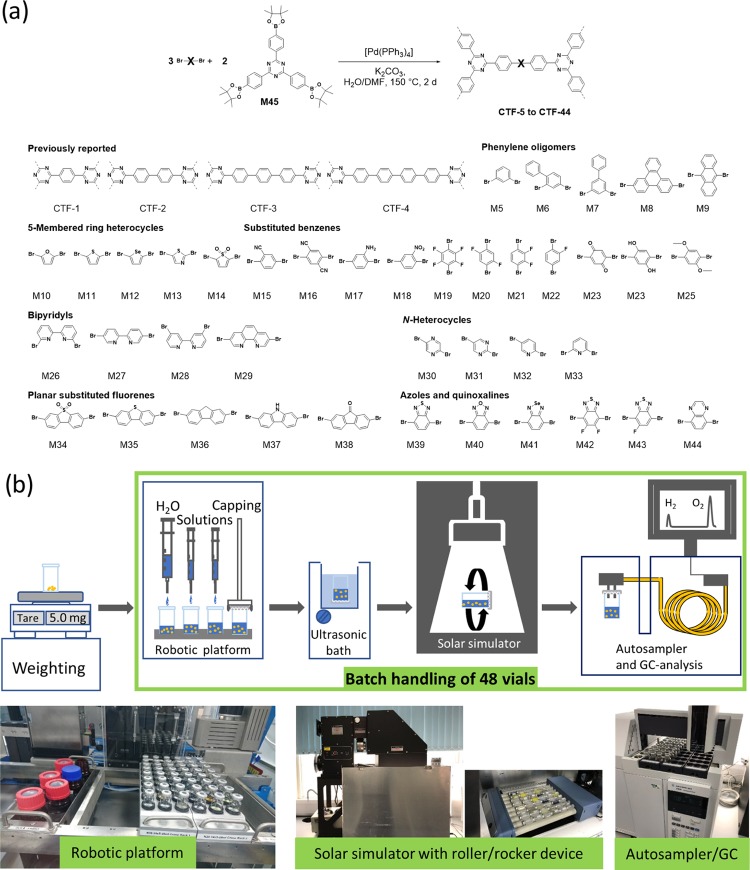
Figure 1.
(a) Synthesis of the CTF photocatalyst library, showing the linkers used. (b) High-throughput (HT) workflow for hydrogen production of suspension photocatalysts (top row) and photographs of equipment used in the workflow (bottom row).
Alkyne-linked CTFs have also recently been made using Sonogashira–Hagihara polycondensation linking tris(4-ethynylphenyl)-1,3,5-triazine with a range of nitrogen- and sulfur-containing compounds.77
To date, studies on CTFs have shown rather limited structural diversity either because of the availability of monomers,57,78 and/or the harsh synthesis conditions employed; for example, in ionothermal synthesis, partial carbonization might limit the materials’ photocatalytic activity,65,74 while acid-catalyzed cyclotrimerization is incompatible with functional groups that are acid sensitive. In this study, we present 39 new CTFs prepared by Pd(0)-catalyzed Suzuki–Miyaura polycondensation. We study these in detail for their photocatalytic hydrogen production activity (Figure 2). As such, this single study covers a significantly more diverse set of CTF materials than the 18 structurally defined photocatalytically active CTFs published so far in the combined literature.30,57,60,67,73,79−81
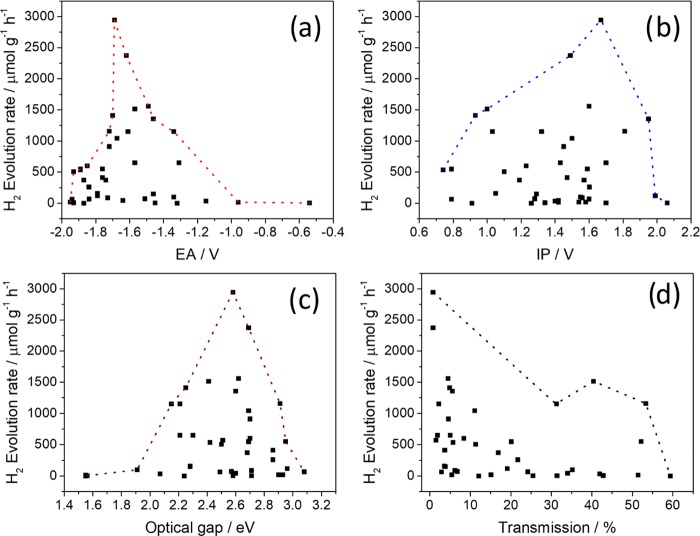
Figure 2.
(a) Hydrogen evolution rate plotted as a function of the predicted electron affinity; (b) the predicted ionization potential; (c) the measured optical gap; and (d) the measured optical transmission of the sample dispersed in the water/TEA/MeOH mixture. All hydrogen evolution rates were determined as linear fits of hydrogen produced over 5 h for 25 mg photocatalyst in water/TEA/MeOH and 3 wt % platinum co-catalyst under visible light (λ > 420 nm, 300 W Xe light source).
Experimental Section
General Procedure for the Pd(0)-Catalyzed Suzuki–Miyaura Polycondensation
A flask was charged with the dibromo monomer, 2,4,6-tris[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1,3,5-triazine, N,N-dimethylformamide, and an aqueous solution of K2CO3 (2.0 M) and degassed by bubbling with N2 for 30 min. [Pd(PPh3)4] (1.2 mol %) was added and the solution was degassed for 10 min further. The reaction mixture was then heated to 150 °C for 2 days, cooled to room temperature, and poured into water. The precipitate was collected by filtration and washed with water and methanol. Further purification was carried out by Soxhlet extraction with cyclopentyl methyl ether. The product was then recovered from the Soxhlet thimble and dried under reduced pressure at 75 °C. See the Supporting Information for results of polymer characterization.
Kinetic Hydrogen Evolution Experiments
A quartz flask was charged with the polymer powder (25 mg), water (8.3 mL), triethylamine (TEA, 8.3 mL), methanol (8.3 mL), and H2PtCl6 solution (0.02 mL, 8 wt % in H2O) and sealed with a septum. The resulting suspension was ultrasonicated for 10 min until the photocatalyst was well-dispersed before degassing by N2 bubbling for 30 min. The reaction mixture was side illuminated with a 300 W Newport Xe light source (model: 6258, ozone free) equipped with a λ > 420 nm filter for 5 h unless stated otherwise. The light source was cooled and IR light cut out by water circulating through a metal jacket with quartz windows. For the illumination under solar light, an Oriel LSH-7320 ABA LED solar simulator with an output of 1.0 sun was used (classification ICE 60904-9 2007: spectral match A, uniformity classification B, temporal stability A). Gas samples were taken with a gas-tight syringe and run on a Bruker 450-GC gas chromatograph equipped with a molecular sieve 13 × 60–80 mesh 1.5 m × 1/8 in. × 2 mm ss column at 50 °C with an argon flow of 40.0 mL min–1. Hydrogen was detected with a thermal conductivity detector referencing against standard gas with a known concentration of hydrogen. Hydrogen dissolved in the reaction mixture was not measured, and the pressure increase generated by the evolved hydrogen was neglected in the calculations. The rates were determined from a linear regression fit and the error is given as the standard deviation from the linear fit. No hydrogen evolution was observed for mixtures of water/triethylamine/H2PtCl6 under λ > 420 nm illumination or under the solar simulator illumination in the absence of a photocatalyst. No hydrogen was observed for l-ascorbic acid solution under λ > 420, >350, and >295 nm illumination (300 W Xe light source) or solar-simulated light (ABA, 400–1100 nm) in the absence of a photocatalyst. Aqueous Na2S solution showed a limited hydrogen production rate of 0.15 μmol h–1 under λ > 295 nm illumination (300 W Xe light source). For longer-term stability experiments, the photocatalysis was carried out for 5 h and the polymer was recovered through centrifugation and after decantation of the liquids, water, methanol, and triethylamine (TEA) mixtures were added for the next run. After 100 h of combined photocatalysis, the photocatalyst was isolated by decantation of the aqueous phase, washed with water and THF three times each, and dried overnight at 120 °C before analysis.
High-Throughput Hydrogen Evolution Experiments
The Agilent Technologies vials (crimp top, headspace, clear with graduation marks and write-on spot, flat bottom, 10 mL, 23 × 46 mm) were charged with 5.0 ± 0.1 mg of polymer powders and transferred to the Chemspeed Accelerator SWING for liquid transfer. Degassed jars with triethylamine, methanol, and a stock solution of H2PtCl6 were loaded into the automated liquid handling platform. The system was then closed and purged for 4 h with nitrogen. The automated liquid handling platform then dispenses the liquids as specified, which was unless stated otherwise degassed water (1.7 mL) or aqueous H2PtCl6 solution (1.7 mL, 0.24 wt % in H2O) triethylamine (1.7 mL), methanol (1.7 mL). The vials were then capped using the capper/crimper tool under inert conditions. Once capped, the samples are taken out, shaken for 5 s, and transferred to an ultrasonic bath to disperse the photocatalysts. An Oriel Solar Simulator 94123A with an output of 1.0 sun was then used to illuminate the vials on a Stuart roller bar SRT9 for the time specified (classification IEC 60904-9 2007 spectral match A, uniformity classification A, temporal stability A, 1600 W xenon light source, 12 × 12 in.2 output beam, air mass 1.5 G filter, 350–1000 nm). After photocatalysis, the gaseous products of the samples are measured on an Agilent Technologies 7890B gas chromatograph connected to an Agilent Technologies 7697A headspace sampler. No hydrogen evolution was observed for mixtures of water/triethylamine/methanol or water/triethylamine/methanol/H2PtCl6 under solar irradiation (1.0 sun) in the absence of a photocatalyst. No hydrogen production was observed in the absence of a photocatalyst.
(Time-Dependent) Density Functional Theory [(TD-)DFT] Calculations
The CTFs were modeled for the purpose of computationally screening their properties as triazine–linker–triazine (TLT) cluster models. The relevant three-dimensional structures of these TLT CTF models were generated using a multistep process starting from the SMILES codes for the linkers using the supramolecular toolkit (stk).82,83 stk is a Python library for the assembly, structure generation, and property calculation of supramolecules (using functionality from RDKit84 and Schrödinger PLC’s Macromodel software) and allowed us to automate the assembly and conformer search process. The as-assembled TLT cluster models were energy minimized using the OPLS200585,86 force field. After the relaxation step, a high-temperature OPLS2005 molecular dynamics run with regular quenches (700 K, 50 ps of simulation time, and 1000 quenches) was employed to find low-energy conformers for each cluster model. The lowest-energy conformers were finally reoptimized using density functional theory (DFT), using the B3LYP87,88 density functional and the DZP89 basis set.
The thermodynamic ability of the various CTFs, as modeled by TLT cluster models, to reduce protons and oxidize water and/or triethylamine was analyzed in the terms of the potentials associated with free electrons [electron affinity (EA)] and holes [ionization potential (IP)], as well as the exciton (IP* and EA*), relative to those of the different solution half reactions. These potentials are calculated using our standard ΔSCF approach90,91 based on DFT/time-dependent DFT (TD-DFT) calculations on polymer cluster models and the use of the COSMO92 continuum solvation model (εr 80.1, water) to approximate the dielectric environment of the polymers in the presence of water. As in our previous work33,34,38,43,57,62,90,91 and as in the CTF model generation stage, the potential calculations use the B3LYP density functional and the DZP basis set, a combination that we previously found to result in the prediction of accurate potentials for polymeric solids when compared to experimental photoelectron spectroscopy data.91 Potentials of solution half-reactions are taken from previous work.33
The absorption spectra of the CTFs are approximated by the vertical excitation spectra of the TLT cluster models, as calculated by TD-B3LYP/DZP. Similarly, the optical gap, the energy of light above (and the wavelength below) which the CTF starts absorbing light, is approximated by the energy of the lowest vertical singlet excitation.
Results and Discussion
A structurally diverse library of new CTFs was synthesized by using a trisphenyl-1,3,5-triazine building block as the node (M45) connected by various linkers (M5–44). The resulting CTFs are referred to as CTF-xx (whereby xx is the number of the dibromo monomers used in the synthesis, Mxx). The linkers cover a wide range of different aromatic units, which can be divided into the following subsets: (i) phenylene oligomers; (ii) five-membered aromatic heterocycles; (iii) substituted phenylenes; (iv) bipyridyls; (v) nitrogen heterocycles; (vi) planar heterofluorenes; and (vii) azoles and quinoxalines.
All polymers were prepared via Pd(0)-catalyzed Suzuki–Miyaura polycondensation of the corresponding dibromo monomer (M5–44) and 2,4,6-tris[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-phenyl]-1,3,5-triazine (M45, Figure 1a).38,57,62 All of the materials were synthesized under the same conditions and without further optimization for any of the monomers. As a result, the polymer yields varied. In all, 22 polymers were obtained in isolated yields exceeding 90%, and 16 polymers were obtained in isolated yields ranging from 37 to 87%. Lower yields were observed for CTF-23 (2,5-linked 1,4-benzoquinone, 21%) and CTF-24 (2,5-linked benzene-1,4-diol, 11%).
The polymers were characterized by Fourier transform infrared (FT-IR) spectroscopy (Figures S-1–S-7), thermogravimetric analysis (TGA) under air (Figures S-8–S-14), and nitrogen sorption at 77 K (Table S-1). The apparent Brunauer–Emmett–Teller (BET) surface areas (SABET) also varied across the library: 14 materials were essentially nonporous (SABET < 100 m2 g–1), 14 materials had moderate surface areas (SABET = 100–300 m2 g–1), 9 materials had higher surface areas (SABET = 300–500 m2 g–1), and 5 materials had BET surface areas greater than 500 m2 g–1. CTF-9 (9,10-linked anthracene) had the highest SABET (708 m2 g–1).
UV–vis absorption spectra were measured in the solid state (Figures S-15–S-18 and Table S-2) and the optical gaps of the polymers were calculated from the absorption onset. CTF-24 had the lowest optical gap (2,5-linked benzene-1,4-diol −1.52 eV), while CTF-26 had the highest (6,6′-linked 2,2′-bipyridine −3.08 eV). Most of the materials (30 polymers) had optical gaps in the region of 1.95–2.70 eV. As can be seen from Figure 3, there is a good correlation between the measured optical gap values and those predicted by the TD-DFT calculations. All materials are weakly emissive and fluorescence decay measurements were carried out using time-correlated single photon counting (TCSPC) in the solid state (Figures S-49–S-70 and Table S-5). The CTFs had average weighted photoluminescence (PL) lifetimes ranging from 0.1 to 4.4 ns and 7.7 ns for the reference material CTF-2. Contact angles with water were measured for pressed pellets of the polymers and found to range from 55° for 2,5-linked thiophene dioxide CTF-14 up to 106° for 1,4-linked 2,5-dimethoxybenzene CTF-25 (Figures S-22–S-24 and Table S-4); for 12 materials, a contact angle could not be measured because the water drop was absorbed by the porous polymer and swelling occurred.
Figure 3.
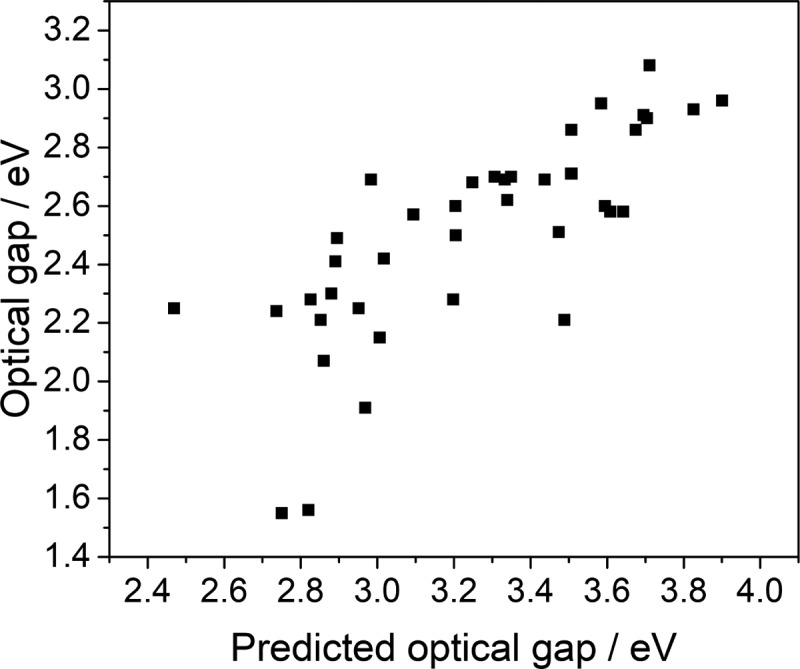
Predicted values of the optical gaps of the CTFs compared to measured values of the optical gaps.
To probe the dispersibility of the polymer particles in the reaction mixture, the transmission of light through a suspension of the photocatalysts in the water/TEA/methanol mixture was determined. The transmission values were found to range from low values of 0.8%, indicating good dispersibility (i.e., almost opaque dispersions), to high values of up to 59.4%, indicating poor dispersibility in water/TEA (triethylamine)/methanol mixtures.
The photocatalytic activity for proton reduction of the materials was tested using a high-throughput workflow: the polymers (5 ± 0.5 mg) were dispersed in mixtures of water, TEA, and methanol (1/1/1 ratio). Platinum (3 wt %) was in situ photodeposited onto the photocatalyst57 acting as a co-catalyst, which has been shown previously to improve hydrogen evolution rates (HERs).57,67 TEA acts as a hole scavenger,35 and methanol is used to ensure miscibility between water and TEA.39,93 Samples were illuminated with a solar simulator for 2 h before measuring the amount of hydrogen produced using an automated gas chromatograph.
Two materials, CTF-15 (2,5-linked benzonitrile) and CTF-34 (3,7-linked dibenzo[b,d]thiophene sulfone), were identified by this screen as the best-performing catalysts. The results of the screening measurements were compared to the traditional kinetic experiments for each photocatalyst over 5 h using a 300 W Xe light source equipped with a λ > 420 nm filter (Figures 4, S-73–S-94, and Table S-7). Again, the two most active photocatalysts were found to be CTF-15 (2946 μmol g–1 h–1) and CTF-34 (2373 μmol g–1 h–1), which had significantly higher values than that of the previously reported CTF-3 Suzuki (158 μmol g–1 h–1) when tested under the same condition. Overall, there is a good correlation between the high-throughput screening measurements and the kinetic measurements, especially when one considers the marked differences in the spectral output of the light sources for the two measurements, the different head spaces of the reaction vessels (much smaller in the high-throughput screen), and the different agitation methods (rolling/rocking in the screen versus stirring in the kinetic measurements).94
Figure 4.
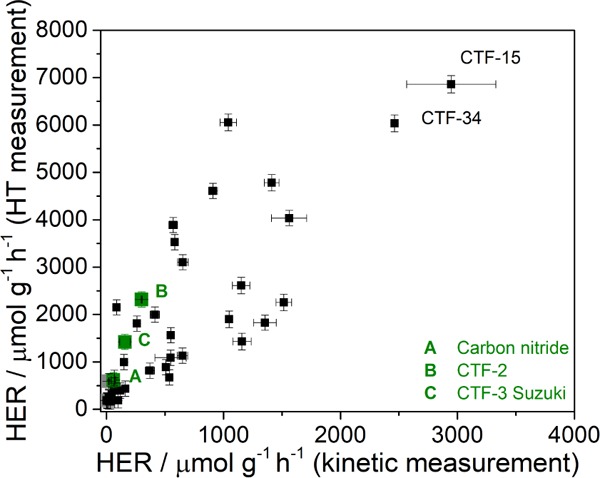
Comparison of the normalized rates of kinetic measurements (25 mg photocatalyst) compared to high-throughput measurements (5 mg photocatalyst) for each material. Conditions: water/TEA/MeOH with 3 wt % Pt co-catalyst.
Following our previous work on linear conjugated polymer photocatalysts,34 we attempted to analyze the performance of the CTFs in terms of four properties: their electron affinity (EA), ionization potential (IP), and optical gap, as well as the optical transmittance of the reaction mixture after the CTF is dispersed in it. Figure 2a–d shows the dependency of the measured hydrogen evolution rate on those four properties and dashed lines envelope the data points for all CTFs. The measured hydrogen evolution rate shows the clearest correlation with the predicted EA values (Figure 2a). EA, often approximated by (the negative of) the lowest unoccupied molecular orbital energy, governs the driving force for water reduction. None of the CTFs with EA values above −1.4 V relative to the standard hydrogen electrode have high hydrogen evolution rates (here defined to have HER >50% of the CTF with the highest activity in this study), while CTFs with even more positive EA values effectively produce no hydrogen. The observed HER is highest at around −1.7 V, but as the photocatalytic performance is not only controlled by the EA of the materials several CTFs with EA values in this range evolve only a small amount of hydrogen or show no activity (Figure 2a). Where EA governs the driving force for proton reduction, IP, often approximated by (the negative of) the highest occupied molecular orbital energy, determines the driving force for water oxidation or, as in this study, the oxidation of the hole scavenger (TEA). The measured hydrogen evolution rates lie in a broad envelope of increasing hydrogen evolution rates with increasing predicted IP. A maximum is observed at approximately +1.6 V, and then the measured hydrogen evolution rates decrease again for higher IP values (Figure 2b). In contrast to the case of EA, CTFs with high hydrogen evolution rates, as defined above, can be found across most of the IP range, and there are no IP regions in which exclusively CTFs occur that produce little or no hydrogen.
The apparent higher dependency of the measured hydrogen evolution rates of the materials on their predicted EA values (Figure 2a) when compared to the correlation of photocatalytic activity and predicted IP values of these materials (Figure 2b) can be explained when the effect of triethylamine on the pH of the photocatalysis mixtures is considered. The high pH of these mixtures of approximately 11.5 effects the potentials; hence, the proton reduction potential will lie at −0.7 V, which means that CTFs with EA values more positive than ∼−1.0 V therefore are expected to have little or no driving force for proton reduction (Figure 2a). The overall oxidation of triethylamine to give diethylamine and acetaldehyde has a predicted potential to be at −0.7 V. Therefore, it is expected that the CTFs studied here all have significant driving force for scavenger oxidation. Even the one-hole oxidation step of triethylamine (+0.7 V) to the triethylamine radical [N(CH2CH3)CHCH3], which can be an effective thermodynamic obstacle for the overall oxidation reaction, is predicted to favor most CTFs considered.
Plots of measured hydrogen evolution rates versus predicted excited-state ionization potential (IP*) and electron affinity (EA*), where the excited state (exciton) provides the electron or hole to drive the solution chemistry, look very similar to their EA and IP equivalents (Figure S-126). As a result, the correlation between the hydrogen evolution rate and the predicted IP* values is also stronger than that between the measured and predicted EA* values. Exciton dissociation, or not, thus appears to make little difference for these materials in terms of the predicted potentials.
In the case of the (computed) optical gap (Figure 2c), we find that the CTFs with the highest activity have large optical gaps. Because, as discussed above, the predicted optical gap values and experimental absorption onsets are strongly correlated, the plot of the measured HER as a function of absorption onset looks similar (Figure S-117), and we made the same observation. The fact that the most active materials have large optical gaps is counterintuitive and would have been surprising had we not observed the same behavior for linear conjugated co-polymers.34 As for those linear polymers, plotting EA/IP as a functional of optical gap in this work (Figure S-125) shows that EA and optical gap are strongly correlated; that is, the CTFs with the largest optical gaps also have the most negative EA values. Hence, for both families of materials, the linear polymers and the CTFs, the hydrogen evolution rates, at least when using TEA, are limited more by the thermodynamic driving force for proton reduction than by light absorption.
The final property that we considered was the light transmittance of dispersions of the CTF in the photocatalysis mixture, i.e., TEA/MeOH/water (Figure 2d). Values range from 100% (indicating quick settling or “creaming” of the CTF) to 0% (indicating that the CTF is well-dispersed and scattered and/or absorbed light effectively). Light transmittance is a descriptor of the dispersibility of the CTF in the photocatalysis mixture, which is affected by interaction with water and scavenger, density, and size of the CTF particles. Hydrogen evolution rates in Figure 2d are generally higher for CTFs when lowering the transmittance, and all CTFs that show high rates have a transmittance of less than 50%, therefore showing a positive correlation between the dispersion of the CTF in the reaction mixture and the performance as a photocatalyst for proton reduction.
For completeness, we also considered a number of other properties beyond those considered in our previous work but found no correlation with the hydrogen evolution rate, other than that for the fitted fluorescence lifetimes measured via TCSPC. It appears that materials with shorter lifetimes have a higher photocatalytic activity (Figure S-119). However, as in the case for the optical gap, this correlation appears counterintuitive. As the TCSPC measurements are performed in the absence of scavenger, these short lifetimes are not due to quenching but rather a sign of inherently short-lived excited states, which should be a negative effect rather than a positive one. We suspect that this is a noncausal correlation with the hydrogen evolution rate, as for the optical gap, that arises because the fluorescence lifetimes are correlated with another material property.
The above analysis demonstrates that CTFs with the highest photocataytic activities occur for specific combinations of (material) properties. The word combinations in the above sentence is important, as there are clearly also CTFs with (near-)“ideal” EA, IP, optical gap, and/or transmittance values that yet only evolve a very small amount of hydrogen or are not active at all (see also Figures 2 and S-124). In line with our previous observations for linear conjugated polymers, each of these four properties appear to be a necessary but not sufficient condition for a CTF to be an active photocatalyst.
The high-throughput system was further used to study the best-performing material, CTF-15 (2,5-linked benzonitrile), with various different scavenger systems in combination with platinum as the co-catalyst (Figure S-97 and Table S-9). The screening shows that the highest performance was obtained in this case for water/TEA/MeOH mixtures (1909 μmol g–1 h–1), followed by 5 vol % TEA in water (1830 μmol g–1 h–1). Moderate hydrogen production was observed when l-ascorbic acid and sodium sulfide were used as scavengers (116 and 139 μmol g–1 h–1, respectively) and low performance was observed for disodium ethylenediaminetetraacetate, l-cysteine, and thiophenol (101, 82, and 77 μmol g–1 h–1). FeCl2, methanol, phenol, and hydroquinone did not act as a scavenger and no hydrogen production was observed under these conditions. Furthermore, CTF-15 and CTF-34 were compared to CTF-16 and CTF-3 Suzuki in a HT screening using water/TEA/MeOH mixtures as well as sodium sulfide and l-ascorbic acid as scavengers (see Figure S-103). In all cases, CTF-15 and CTF-34 were the best-performing materials compared to CTF-16 followed by CTF-3 Suzuki, which indicates strongly that superior photocatalytsts were obtained, rather than materials that were simply better optimized for the oxidation of TEA.
The five best-performing photocatalysts, CTF-15 (2,5-linked benzonitrile), CTF-34 (3,7-linked dibenzo[b,d]thiophene sulfone), CTF-30 (2,5-linked pyrazine), CTF-12 (2,5-linked selenophene), and CTF-19 (1,4-linked tetrafluorobenzene), were characterized by scanning electron microscopy (SEM; Figures S-25 and S-26), which shows that CTF-15 and CTF-16 consist of 20–50 μm long and 1–2 μm thick rods that are bundled together, while the other polymers show oval particles in the size range 8–23 μm for CTF-34 and up to 50 μm for CTF-12. Inductively coupled plasma optical emission spectrometry (ICP-OES) analysis was carried out for a subset and palladium levels between 0.44 for CTF-34 and 1.35 wt % for CTF-5 were found.
Residual palladium has been shown to act as a co-catalyst for polymeric photocatalysts;55,95,96 in line with our previous report,57 we observe significant hydrogen production from CTF-15 and CTF-34 without added platinum (Figure S-96). However, we do not expect that this significantly affects the photocatalytic performance of these materials because platinum is added after the synthesis as the co-catalyst to saturate the performance.57
An external quantum efficiency (EQE) of 15.9% was determined with a light-emitting diode at 420 nm for CTF-15 in water/TEA/MeOH with 3 wt % Pt. This value is higher than that for the linear polymers poly(p-phenylene) with an EQE420nm = 0.4%38 and poly(dibenzo[b,d]thiophene sulfone) with an EQE420nm = 11.6%93 (both in water/TEA/MeOH), poly(benzothiadiazole-co-phenylene) with an EQE420nm = 4.0%42 from water/TEOA/3 wt % Pt, but lower than optimized nanoparticles of poly(dibenzo[b,d]thiophene sulfone) (EQE420nm = 20.4%, water/TEA/MeOH),97 co-polymer of dibenzo[b,d]thiophene–dibenzo[b,d]thiophene sulfone (EQE420nm = 20.7%, water/TEA/MeOH),34 or optimized carbon nitrides (EQE405nm = 50.7%, Pt/water/TEOA).98
The photostability of CTF-15 (2,5-linked benzonitrile CTF) was tested over 50 h under visible-light illumination (Figure 5, λ > 420 nm, Xe light source) and further 50 h on a solar simulator (Figure S-110). The CTF shows no change of activity or in post-illumination characterization, indicating good stability of this photocatalyst.
Figure 5.
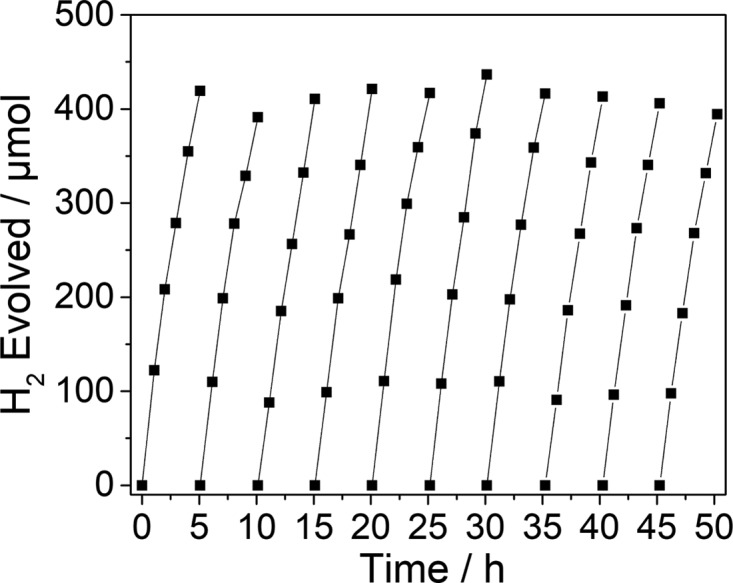
Extended photocatalysis run for CTF-15 (25 mg) in water/TEA/MeOH (1/1/1 mixture) with 3 wt % platinum as co-catalyst under visible light (λ > 420 nm, 300 W Xe light source).
Conclusions
A library of 39 new CTFs was made via Suzuki–Miyaura polycondensation, where the modularity of this approach allowed us to obtain CTFs with a much richer structural diversity than has been studied previously. All materials were characterized and studied for their photocatalytic performance using a high-throughput workflow, as well as conventional kinetic measurements. The performance of the best-performing CTFs are among the highest for this material class. We showed that the photocatalytic performance of these CTFs can be analyzed by their predicted electron affinity, predicted ionization potential, optical gap, and dispersibility in the reaction mixture. Electron affinity and dispersibility were found to be the dominant variables under the conditions used.
In this study, we measured all materials using the rapid screening method (up to 50 samples per day) and traditional kinetic methods (1–2 samples per day). This was done to establish the reliability of the screening method. The generally good correlation (Figure 4) between the screening results and the kinetic measurements suggests that this could be a powerful method in the future to discover new photocatalysts of any materials class, as well as providing valuable insights into the structure–property relationships across a diverse range of chemical functionality.
Acknowledgments
We thank the Engineering and Physical Sciences Research Council (EPSRC) for financial support under Grants EP/N004884/1 and EP/P005543/1. Dr. L. Wilbraham is acknowledged for useful discussion. K.E.J. thanks the Royal Society for a University Research Fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemmater.9b02825.
Synthetic procedures, FT-IR spectra, TGA traces, UV–vis spectra, PXRD patterns, contact angle measurements, SEM images, PL spectra and TCSPC experiments, hydrogen evolution data, ICP-OES data, post-photocatalysis characterization data, and DFT results (PDF)
Supporting structures, raw data, DFT calculations; predicted potentials and optical gap (ZIP)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Savchenko V. I.; Makaryan I. A. Palladium Catalyst for the Production of Pure Margarine: Catalyst and New Non-filtration Technology Improve Production and Quality. Platinum Met. Rev. 1999, 43, 74–82. [Google Scholar]
- Elsherif M.; Manan Z. A.; Kamsah M. Z. State-of-the-Art of Hydrogen Management in Refinery and Industrial Process Plants. J. Nat. Gas Sci. Eng. 2015, 24, 346–356. 10.1016/j.jngse.2015.03.046. [DOI] [Google Scholar]
- Häussinger P.; Lohmüller R.; Watson A. M.. Hydrogen, 6. Uses. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, 2000. [Google Scholar]
- Ramachandran R.; Menon R. K. An Overview of Industrial Uses of Hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. 10.1016/S0360-3199(97)00112-2. [DOI] [Google Scholar]
- Marbán G.; Valdés-Solís T. Towards the Hydrogen Economy?. Int. J. Hydrogen Energy 2007, 32, 1625–1637. 10.1016/j.ijhydene.2006.12.017. [DOI] [Google Scholar]
- Barelli L.; Bidini G.; Gallorini F.; Servili S. Hydrogen Production through Sorption-Enhanced Steam Methane Reforming and Membrane Technology: A Review. Energy 2008, 33, 554–570. 10.1016/j.energy.2007.10.018. [DOI] [Google Scholar]
- Fujishima A.; Honda K. Photolysis-Decomposition of Water at the Surface of an Irradiated Semiconductor. Nature 1972, 238, 37–38. 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Chen X.; Shen S.; Guo L.; Mao S. S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. 10.1021/cr1001645. [DOI] [PubMed] [Google Scholar]
- Kudo A.; Miseki Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. 10.1039/B800489G. [DOI] [PubMed] [Google Scholar]
- Yang L.; Zhou H.; Fan T.; Zhang D. Semiconductor Photocatalysts for Water Oxidation: Current Status and Challenges. Phys. Chem. Chem. Phys. 2014, 16, 6810–6826. 10.1039/c4cp00246f. [DOI] [PubMed] [Google Scholar]
- Yanagida S.; Kabumoto A.; Mizumoto K.; Pac C.; Yoshino K. Poly(p-phenylene)-Catalysed Photoreduction of Water to Hydrogen. J. Chem. Soc., Chem. Commun. 1985, 0, 474–475. 10.1039/c39850000474. [DOI] [Google Scholar]
- Shibata T.; Kabumoto A.; Shiragami T.; Ishitani O.; Pac C.; Yanagida S. Novel visible-light-driven photocatalyst. Poly(p-phenylene)-Catalyzed Photoreductions of Water, Carbonyl Compounds, and Olefins. J. Phys. Chem. A. 1990, 94, 2068–2076. 10.1021/j100368a063. [DOI] [Google Scholar]
- Matsuoka S.; Kohzuki T.; Pac C.; Ishida A.; Takamuku S.; Kusaba M.; Nakashima N.; Yanagida S. Photocatalysis of Oligo(p-Phenylenes). Photochemical Reduction of Carbon Dioxide with Triethylamine. J. Phys. Chem. A. 1992, 96, 4437–4442. 10.1021/j100190a057. [DOI] [Google Scholar]
- Matsuoka S.; Kohzuki T.; Kuwana Y.; Nakamura A.; Yanagida S. Visible-Light-Induced Photocatalysis of Poly(Pyridine-2,5-Diyl). Photoreduction of Water, Carbonyl Compounds and Alkenes with Triethylamine. J. Chem. Soc., Perkin Trans. 2 1992, 679–685. 10.1039/p29920000679. [DOI] [Google Scholar]
- Wang X.; Maeda K.; Thomas A.; Takanabe K.; Xin G.; Carlsson J. M.; Domen K.; Antonietti M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. 10.1038/nmat2317. [DOI] [PubMed] [Google Scholar]
- Schwinghammer K.; Tuffy B.; Mesch M. B.; Wirnhier E.; Martineau C.; Taulelle F.; Schnick W.; Senker J.; Lotsch B. V. Triazine-Based Carbon Nitrides for Visible-Light-Driven Hydrogen Evolution. Angew. Chem., Int. Ed. 2013, 52, 2435–2439. 10.1002/anie.201206817. [DOI] [PubMed] [Google Scholar]
- Lau V. W. H.; Mesch M. B.; Duppel V.; Blum V.; Senker J.; Lotsch B. V. Low-Molecular-Weight Carbon Nitrides for Solar Hydrogen Evolution. J. Am. Chem. Soc. 2015, 137, 1064–1072. 10.1021/ja511802c. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wang H.; Antonietti M. Graphitic Carbon Nitride “Reloaded”: Emerging Applications beyond (Photo)Catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. 10.1039/C5CS00767D. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang X.; Antonietti M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem., Int. Ed. 2012, 51, 68–89. 10.1002/anie.201101182. [DOI] [PubMed] [Google Scholar]
- Schwinghammer K.; Hug S.; Mesch M. B.; Senker J.; Lotsch B. V. Phenyl-Triazine Oligomers for Light-Driven Hydrogen Evolution. Energy Environ. Sci. 2015, 8, 3345–3353. 10.1039/C5EE02574E. [DOI] [Google Scholar]
- Chuang P.-K.; Wu K.-H.; Yeh T.-F.; Teng H. Extending the π-Conjugation of g-C3N4 by Incorporating Aromatic Carbon for Photocatalytic H2 Evolution from Aqueous Solution. ACS Sustainable Chem. Eng. 2016, 4, 5989–5997. 10.1021/acssuschemeng.6b01266. [DOI] [Google Scholar]
- Zhang J.; Sun J.; Maeda K.; Domen K.; Liu P.; Antonietti M.; Fu X.; Wang X. Sulfur-Mediated Synthesis of Carbon Nitride: Band-Gap Engineering and Improved Functions for Photocatalysis. Energy Environ. Sci. 2011, 4, 675. 10.1039/C0EE00418A. [DOI] [Google Scholar]
- Hu S.; Li F.; Fan Z.; Wang F.; Zhao Y.; Lv Z. Band Gap-Tunable Potassium Doped Graphitic Carbon Nitride with Enhanced Mineralization Ability. Dalton Trans. 2015, 44, 1084–1092. 10.1039/C4DT02658F. [DOI] [PubMed] [Google Scholar]
- Li Y.; Jin R.; Li G.; Liu X.; Yu M.; Xing Y.; Shi Z. Preparation of Phenyl Group Functionalized G-C3N4 Nanosheets with Extended Electron Delocalization for Enhanced Visible-Light Photocatalytic Activity. New J. Chem. 2018, 42, 6756–6762. 10.1039/C8NJ00298C. [DOI] [Google Scholar]
- Zhang J.; Zhang G.; Chen X.; Lin S.; Möhlmann L.; Dołęga G.; Lipner G.; Antonietti M.; Blechert S.; Wang X. Co-Monomer Control of Carbon Nitride Semiconductors to Optimize Hydrogen Evolution with Visible Light. Angew. Chem., Int. Ed. 2012, 51, 3183–3187. 10.1002/anie.201106656. [DOI] [PubMed] [Google Scholar]
- Kailasam K.; Schmidt J.; Bildirir H.; Zhang G.; Blechert S.; Wang X.; Thomas A. Room Temperature Synthesis of Heptazine-Based Microporous Polymer Networks as Photocatalysts for Hydrogen Evolution. Macromol. Rapid Commun. 2013, 34, 1008–1013. 10.1002/marc.201300227. [DOI] [PubMed] [Google Scholar]
- Fu J.; Yu J.; Jiang C.; Cheng B. G-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2017, 8, 1701503 10.1002/aenm.201701503. [DOI] [Google Scholar]
- Fan X.; Zhang L.; Cheng R.; Wang M.; Li M.; Zhou Y.; Shi J. Construction of Graphitic C3N4-Based Intramolecular Donor–Acceptor Conjugated Copolymers for Photocatalytic Hydrogen Evolution. ACS Catal. 2015, 5, 5008–5015. 10.1021/acscatal.5b01155. [DOI] [Google Scholar]
- Leclerc M., Morin J.-F., Eds. Design and Synthesis of Conjugated Polymers; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Wang K.; Yang L.-M.; Wang X.; Guo L.; Cheng G.; Zhang C.; Jin S.; Tan B.; Cooper A. Covalent Triazine Frameworks via a Low-Temperature Polycondensation Approach. Angew. Chem., Int. Ed. 2017, 56, 14149–14153. 10.1002/anie.201708548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Lan Z.-A.; Wang X. Conjugated Polymers: Catalysts for Photocatalytic Hydrogen Evolution. Angew. Chem., Int. Ed. 2016, 55, 15712–15727. 10.1002/anie.201607375. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Jin S.; Xu H.; Nagai A.; Jiang D. Conjugated Microporous Polymers: Design, Synthesis and Application. Chem. Soc. Rev. 2013, 42, 8012. 10.1039/c3cs60160a. [DOI] [PubMed] [Google Scholar]
- Sprick R. S.; Aitchison C. M.; Berardo E.; Turcani L.; Wilbraham L.; Alston B. M.; Jelfs K. E.; Zwijnenburg M. A.; Cooper A. I. Maximising the Hydrogen Evolution Activity in Organic Photocatalysts by Co-Polymerisation. J. Mater. Chem. A 2018, 6, 11994–12003. 10.1039/C8TA04186E. [DOI] [Google Scholar]
- Bai Y.; Wilbraham L.; Slater B. J.; Zwijnenburg M. A.; Sprick R. S.; Cooper A. I. Accelerated Discovery of Organic Polymer Photocatalysts for Hydrogen Evolution from Water through the Integration of Experiment and Theory. J. Am. Chem. Soc. 2019, 141, 9063–9071. 10.1021/jacs.9b03591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S.; Fujii H.; Yamada T.; Pac C.; Ishida A.; Takamuku S.; Kusaba M.; Nakashima N.; Yanagida S.; Hashimoto K.; et al. Photocatalysis of Oligo(para-phenylenes) - Photoreductive Production of Hydrogen and Ethanol in Aqueous Triethylamine. J. Phys. Chem. A. 1991, 95, 5802–5808. 10.1021/j100168a018. [DOI] [Google Scholar]
- Matsuoka S.; Fujii H.; Pac C.; Yanagida S. Photocatalysis of Oligo(p-Phenylene) Leading to Reductive Formation of Hydrogen and Ethanol from Triethylamine in Aqueous Organic Solvent. Chem. Lett. 1990, 19, 1501–1502. 10.1246/cl.1990.1501. [DOI] [Google Scholar]
- Maruo K.; Wada Y.; Yanagida S. Photocatalysis of Perfluorinated Oligo(p-Phenylene)s. Bull. Chem. Soc. Jpn. 1992, 65, 3439–3449. 10.1246/bcsj.65.3439. [DOI] [Google Scholar]
- Sprick R. S.; Bonillo B.; Clowes R.; Guiglion P.; Brownbill N. J.; Slater B. J.; Blanc F.; Zwijnenburg M. A.; Adams D. J.; Cooper A. I. Visible-Light-Driven Hydrogen Evolution Using Planarized Conjugated Polymer Photocatalysts. Angew. Chem., Int. Ed. 2016, 55, 1792–1796. 10.1002/anie.201510542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. J.; Sprick R. S.; Smith C. L.; Cowan A. J.; Cooper A. I. A Solution-Processable Polymer Photocatalyst for Hydrogen Evolution from Water. Adv. Energy Mater. 2017, 7, 1700479 10.1002/aenm.201700479. [DOI] [Google Scholar]
- Maruo K.; Yamada K.; Wada Y.; Yanagida S. Visible-Light Induced Photocatalysis of Partially Fluorinated Poly(p-Phenylene) and Related Linear Phenylene Derivatives. Bull. Chem. Soc. Jpn. 1993, 66, 1053–1064. 10.1246/bcsj.66.1053. [DOI] [Google Scholar]
- Yanagida S.; Ogata T.; Kuwana Y.; Wada Y.; Murakoshi K.; Ishida A.; Takamuku S.; Kusaba M.; Nakashima N. Synthesis of 2,′:5′,2″-Terpyridine and 2,2′:5′,2″:5″,2‴-Quaterpyridine and their Photocatalysis of the Reduction of Water. J. Chem. Soc., Perkin Trans. 2 1996, 1963–1969. 10.1039/P29960001963. [DOI] [Google Scholar]
- Yang C.; Ma B. C.; Zhang L.; Lin S.; Ghasimi S.; Landfester K.; Zhang K. A. I.; Wang X. Molecular Engineering of Conjugated Polybenzothiadiazoles for Enhanced Hydrogen Production by Photosynthesis. Angew. Chem., Int. Ed. 2016, 55, 9202–9206. 10.1002/anie.201603532. [DOI] [PubMed] [Google Scholar]
- Sprick R. S.; Wilbraham L.; Bai Y.; Guiglion P.; Monti A.; Clowes R.; Cooper A. I.; Zwijnenburg M. A. Nitrogen Containing Linear Poly(Phenylene) Derivatives for Photo-Catalytic Hydrogen Evolution from Water. Chem. Mater. 2018, 30, 5733–5742. 10.1021/acs.chemmater.8b02501. [DOI] [Google Scholar]
- Dai C.; Xu S.; Liu W.; Gong X.; Panahandeh-Fard M.; Liu Z.; Zhang D.; Xue C.; Loh K. P.; Liu B. Dibenzothiophene- S,S-Dioxide-Based Conjugated Polymers: Highly Efficient Photocatalyts for Hydrogen Production from Water under Visible Light. Small 2018, 1801839 10.1002/smll.201801839. [DOI] [PubMed] [Google Scholar]
- Lan Z.-A.; Zhang G.; Chen X.; Zhang Y.; Zhang K. A. I.; Wang X. Reducing the Exciton Binding Energy of Donor-Acceptor-Based Conjugated Polymers to Promote Charge-Induced Reactions. Angew. Chem., Int. Ed. 2019, 58, 10236–10240. 10.1002/anie.201904904. [DOI] [PubMed] [Google Scholar]
- Vyas V. S.; Haase F.; Stegbauer L.; Savasci G.; Podjaski F.; Ochsenfeld C.; Lotsch B. V. A Tunable Azine Covalent Organic Framework Platform for Visible Light-Induced Hydrogen Generation. Nat. Commun. 2015, 6, 8508 10.1038/ncomms9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegbauer L.; Schwinghammer K.; Lotsch B. V. A Hydrazone-Based Covalent Organic Framework for Photocatalytic Hydrogen Production. Chem. Sci. 2014, 5, 2789–2793. 10.1039/C4SC00016A. [DOI] [Google Scholar]
- Haase F.; Banerjee T.; Savasci G.; Ochsenfeld C.; Lotsch B. V. Structure–property–activity Relationships in a Pyridine Containing Azine-Linked Covalent Organic Framework for Photocatalytic Hydrogen Evolution†. Faraday Discuss. 2017, 201, 247–264. 10.1039/C7FD00051K. [DOI] [PubMed] [Google Scholar]
- Wang X.; Chen L.; Chong S. Y.; Little M. A.; Wu Y.; Zhu W.-H.; Clowes R.; Yan Y.; Zwijnenburg M. A.; Sprick R. S.; et al. Sulfone-Containing Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution from Water. Nat. Chem. 2018, 10, 1180–1189. 10.1038/s41557-018-0141-5. [DOI] [PubMed] [Google Scholar]
- Stegbauer L.; Zech S.; Savasci G.; Banerjee T.; Podjaski F.; Schwinghammer K.; Ochsenfeld C.; Lotsch B. V. Tailor-Made Photoconductive Pyrene-Based Covalent Organic Frameworks for Visible-Light Driven Hydrogen Generation. Adv. Energy Mater. 2018, 8, 1703278 10.1002/aenm.201703278. [DOI] [Google Scholar]
- Banerjee T.; Gottschling K.; Savasci G.; Ochsenfeld C.; Lotsch B. V. H2 Evolution with Covalent Organic Framework Photocatalysts. ACS Energy Lett. 2018, 3, 400–409. 10.1021/acsenergylett.7b01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.; Lei Y.; Ma C.; Lv W.; Li N.; Wang Y.; Xu H.; Zou Z. A (001) Dominated Conjugated Polymer with High-Performance of Hydrogen Evolution under Solar Light Irradiation. Chem. Commun. 2017, 53, 10536–10539. 10.1039/C7CC06105F. [DOI] [PubMed] [Google Scholar]
- Pachfule P.; Acharjya A.; Roeser J.; Langenhahn T.; Schwarze M.; Schomäcker R.; Thomas A.; Schmidt J. Diacetylene Functionalized Covalent Organic Framework (COF) for Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2018, 140, 1423–1427. 10.1021/jacs.7b11255. [DOI] [PubMed] [Google Scholar]
- Banerjee T.; Haase F.; Savasci G.; Gottschling K.; Ochsenfeld C.; Lotsch B. V. Single-Site Photocatalytic H2 Evolution from Covalent Organic Frameworks with Molecular Cobaloxime Co-Catalysts. J. Am. Chem. Soc. 2017, 139, 16228–16234. 10.1021/jacs.7b07489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Cai Z.; Wu Q.; Lo W. Y.; Zhang N.; Chen L. X.; Yu L. Rational Design of Porous Conjugated Polymers and Roles of Residual Palladium for Photocatalytic Hydrogen Production. J. Am. Chem. Soc. 2016, 138, 7681–7686. 10.1021/jacs.6b03472. [DOI] [PubMed] [Google Scholar]
- Li L.; Lo W. Y.; Cai Z.; Zhang N.; Yu L. Donor-Acceptor Porous Conjugated Polymers for Photocatalytic Hydrogen Production: The Importance of Acceptor Comonomer. Macromolecules 2016, 49, 6903–6909. 10.1021/acs.macromol.6b01764. [DOI] [Google Scholar]
- Meier C. B.; Sprick R. S.; Monti A.; Guiglion P.; Lee J.-S. M.; Zwijnenburg M. A.; Cooper A. I. Structure-Property Relationships for Covalent Triazine-Based Frameworks: The Effect of Spacer Length on Photocatalytic Hydrogen Evolution from Water. Polymer 2017, 126, 283–290. 10.1016/j.polymer.2017.04.017. [DOI] [Google Scholar]
- Sprick R. S.; Bonillo B.; Sachs M.; Clowes R.; Durrant J. R.; Adams D. J.; Cooper A. I. Extended Conjugated Microporous Polymers for Photocatalytic Hydrogen Evolution from Water. Chem. Commun. 2016, 52, 10008–10011. 10.1039/C6CC03536A. [DOI] [PubMed] [Google Scholar]
- Bi S.; Lan Z.; Paasch S.; Zhang W.; He Y.; Zhang C.; Liu F.; Wu D.; Zhuang X.; Brunner E.; et al. Substantial Cyano-Substituted Fully Sp2-Carbon-Linked Framework: Metal-Free Approach and Visible-Light-Driven Hydrogen Evolution. Adv. Funct. Mater. 2017, 27, 1703146 10.1002/adfm.201703146. [DOI] [Google Scholar]
- Guo L.; Niu Y.; Xu H.; Li Q.; Razzaque S.; Huang Q.; Jin S.; Tan B. Engineering Heteroatoms with Atomic Precision in Donor–acceptor Covalent Triazine Frameworks to Boost Photocatalytic Hydrogen Production. J. Mater. Chem. A 2018, 6, 19775–19781. 10.1039/C8TA07391K. [DOI] [Google Scholar]
- Zhao Y.; Ma W.; Xu Y.; Zhang C.; Wang Q.; Yang T.; Gao X.; Wang F.; Yan C.; Jiang J.-X. Effect of Linking Pattern of Dibenzothiophene-S,S-Dioxide-Containing Conjugated Microporous Polymers on the Photocatalytic Performance. Macromolecules 2018, 51, 9502–9508. 10.1021/acs.macromol.8b02023. [DOI] [Google Scholar]
- Sprick R. S.; Jiang J. X.; Bonillo B.; Ren S.; Ratvijitvech T.; Guiglion P.; Zwijnenburg M. A.; Adams D. J.; Cooper A. I. Tunable Organic Photocatalysts for Visible-Light-Driven Hydrogen Evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. 10.1021/ja511552k. [DOI] [PubMed] [Google Scholar]
- Chu S.; Wang Y.; Guo Y.; Zhou P.; Yu H.; Luo L.; Kong F.; Zou Z. Facile Green Synthesis of Crystalline Polyimide Photocatalyst for Hydrogen Generation from Water. J. Mater. Chem. 2012, 22, 15519–15521. 10.1039/c2jm32595k. [DOI] [Google Scholar]
- Zhang Z.; Long J.; Yang L.; Chen W.; Dai W.; Fu X.; Wang X. Organic Semiconductor for Artificial Photosynthesis: Water Splitting into Hydrogen by a Bioinspired C3N3S3polymer under Visible Light Irradiation. Chem. Sci. 2011, 2, 1826–1830. 10.1039/C1SC00257K. [DOI] [Google Scholar]
- Xie J.; Shevlin S. A.; Ruan Q.; Moniz S. J. A.; Liu Y.; Liu X.; Li Y.; Lau C. C.; Guo Z. X.; Tang J. Efficient Visible Light-Driven Water Oxidation and Proton Reduction by an Ordered Covalent Triazine-Based Framework. Energy Environ. Sci. 2018, 11, 1617–1624. 10.1039/C7EE02981K. [DOI] [Google Scholar]
- Lan Z.-A.; Fang Y.; Zhang Y.; Wang X. Photocatalytic Oxygen Evolution from Functional Triazine-Based Polymers with Tunable Band Structure. Angew. Chem., Int. Ed. 2018, 57, 470–474. 10.1002/anie.201711155. [DOI] [PubMed] [Google Scholar]
- Bi J.; Fang W.; Li L.; Wang J.; Liang S.; He Y.; Liu M.; Wu L. Covalent Triazine-Based Frameworks as Visible Light Photocatalysts for the Splitting of Water. Macromol. Rapid Commun. 2015, 36, 1799–1805. 10.1002/marc.201500270. [DOI] [PubMed] [Google Scholar]
- Huang W.; Wang Z. J.; Ma B. C.; Ghasimi S.; Gehrig D.; Laquai E.; Landfester K.; Zhang K. A. I. Hollow Nanoporous Covalent Triazine Frameworks via Acid Vapor-Assisted Solid Phase Synthesis for Enhanced Visible Light Photoactivity. J. Mater. Chem. A 2016, 4, 7555–7559. 10.1039/C6TA01828A. [DOI] [Google Scholar]
- Li L.; Fang W.; Zhang P.; Bi J.; He Y.; Wang J.; Su W. Sulfur-Doped Covalent Triazine-Based Frameworks for Enhanced Photocatalytic Hydrogen Evolution from Water under Visible Light. J. Mater. Chem. A 2016, 4, 12402–12406. 10.1039/C6TA04711D. [DOI] [Google Scholar]
- Cheng Z.; Fang W.; Zhao T.; Fang S.; Bi J.; Liang S.; Li L.; Yu Y.; Wu L. Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution on Phosphorus-Doped Covalent Triazine-Based Frameworks. ACS Appl. Mater. Interfaces 2018, 10, 41415–41421. 10.1021/acsami.8b16013. [DOI] [PubMed] [Google Scholar]
- Kuhn P.; Antonietti M.; Thomas A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem., Int. Ed. 2008, 47, 3450–3453. 10.1002/anie.200705710. [DOI] [PubMed] [Google Scholar]
- Bojdys M. J.; Jeromenok J.; Thomas A.; Antonietti M. Rational Extension of the Family of Layered, Covalent, Triazine-Based Frameworks with Regular Porosity. Adv. Mater. 2010, 22, 2202–2205. 10.1002/adma.200903436. [DOI] [PubMed] [Google Scholar]
- Bhunia A.; Esquivel D.; Dey S.; Fernández-Terán R. J.; Goto Y.; Inagaki S.; Van Der Voort P.; Janiak C. A Photoluminescent Covalent Triazine Framework: CO2 Adsorption, Light-Driven Hydrogen Evolution and Sensing of Nitroaromatics. J. Mater. Chem. A 2016, 4, 13450–13457. 10.1039/C6TA04623A. [DOI] [Google Scholar]
- Kuecken S.; Acharjya A.; Zhi L.; Schwarze M.; Schomacker R.; Thomas A. Fast Tuning of Covalent Triazine Frameworks for Photocatalytic Hydrogen Evolution. Chem. Commun. 2017, 53, 5854–5857. 10.1039/C7CC01827D. [DOI] [PubMed] [Google Scholar]
- Lan Z.-A.; Fang Y.; Chen X.; Wang X. Thermal Annealing-Induced Structural Reorganization in Polymeric Photocatalysts for Enhanced Hydrogen Evolution. Chem. Commun. 2019, 55, 7756–7759. 10.1039/C9CC02966D. [DOI] [PubMed] [Google Scholar]
- Cheng Z.; Zheng K.; Lin G.; Fang S.; Li L.; Bi J.; Shen J.; Wu L. Constructing a Novel Family of Halogen Doped Covalent Triazine-Based Frameworks as Efficient Metal-Free Photocatalysts for Hydrogen Production. Nanoscale Adv. 2019, 1, 2674–2680. 10.1039/C9NA00089E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz D.; Acharja A.; Ichangi A.; Lyu P.; Opanasenko M. V.; Goßler F. R.; König T. A. F.; Čejka J.; Nachtigall P.; Thomas A.; et al. Fluorescent Sulphur- and Nitrogen-Containing Porous Polymers with Tuneable Donor–Acceptor Domains for Light-Driven Hydrogen Evolution. Chem.–Eur. J. 2018, 24, 11916–11921. 10.1002/chem.201802902. [DOI] [PubMed] [Google Scholar]
- Kuhn P.; Thomas A.; Antonietti M. Toward Tailorable Porous Organic Polymer Networks: A High-Temperature Dynamic Polymerization Scheme Based on Aromatic Nitriles. Macromolecules 2009, 42, 319–326. 10.1021/ma802322j. [DOI] [Google Scholar]
- Li L.; Fang W.; Zhang P.; Bi J.; He Y.; Wang J.-Y.; Su W. Sulfur-Doped Covalent Triazine-Based Frameworks for Enhanced Photocatalytic Hydrogen Evolution from Water under Visible Light. J. Mater. Chem. A 2016, 4, 12402–12406. 10.1039/C6TA04711D. [DOI] [Google Scholar]
- Huang W.; He Q.; Hu Y.; Li Y. Molecular Heterostructures of Covalent Triazine Frameworks for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. 2019, 131, 8768–8772. 10.1002/ange.201900046. [DOI] [PubMed] [Google Scholar]
- Yu K.; Bi S.; Ming W.; Wei W.; Zhang Y.; Xu J.; Qiang P.; Qiu F.; Wu D.; Zhang F. Side-Chain-Tuned π-Extended Porous Polymers for Visible Light-Activated Hydrogen Evolution. Polym. Chem. 2019, 10, 3758–3763. 10.1039/C9PY00512A. [DOI] [Google Scholar]
- Turcani L.; Berardo E.; Jelfs K. E. STK: A Python Toolkit for Supramolecular Assembly. J. Comput. Chem. 2018, 1931–1942. 10.1002/jcc.25377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcani L. GitHub—supramolecular-toolkit/stk. https://github.com/supramolecular-toolkit/stk (accessed Sept 1, 2018).
- Landrum G. RDKit: Open-Source Cheminformatics. https://www.rdkit.org (accessed Sept 1, 2018).
- Jorgensen W. L.; Maxwell D. S.; Tirado-Rives J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. 10.1021/ja9621760. [DOI] [Google Scholar]
- Banks J. L.; Beard H. S.; Cao Y.; Cho A. E.; Damm W.; Farid R.; Felts A. K.; Halgren T. A.; Mainz D. T.; Maple J. R.; et al. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. 10.1002/jcc.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becke A. D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Stephens P. J.; Devlin F. J.; Chabalowski C. F.; Frisch M. J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. A. 1994, 98, 11623–11627. 10.1021/j100096a001. [DOI] [Google Scholar]
- Schäfer A.; Horn H.; Ahlrichs R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. 10.1063/1.463096. [DOI] [Google Scholar]
- Guiglion P.; Butchosa C.; Zwijnenburg M. A. Polymeric Watersplitting Photocatalysts; A Computational Perspective on the Water Oxidation Conundrum. J. Mater. Chem. A 2014, 2, 11996–12004. 10.1039/C4TA02044H. [DOI] [Google Scholar]
- Guiglion P.; Monti A.; Zwijnenburg M. A. Validating a Density Functional Theory Approach for Predicting the Redox Potentials Associated with Charge Carriers and Excitons in Polymeric Photocatalysts. J. Phys. Chem. C 2017, 121, 1498–1506. 10.1021/acs.jpcc.6b11133. [DOI] [Google Scholar]
- Klamt A.; Schüürmann G. COSMO: A New Approach to Dielectric Screening in Solvents with Explicit Expressions for the Screening Energy and Its Gradient. J. Chem. Soc., Perkin Trans. 2 1993, 5, 799–805. 10.1039/P29930000799. [DOI] [Google Scholar]
- Sachs M.; Sprick R. S.; Pearce D.; Hillman S. A. J.; Monti A.; Guilbert A. A. Y.; Brownbill N. J.; Dimitrov S.; Shi X.; Blanc F.; et al. Understanding Structure-Activity Relationships in Linear Polymer Photocatalysts for Hydrogen Evolution. Nat. Commun. 2018, 9, 4968 10.1038/s41467-018-07420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze M.; Stellmach D.; Schröder M.; Kailasam K.; Reske R.; Thomas A.; Schomäcker R. Quantification of Photocatalytic Hydrogen Evolution. Phys. Chem. Chem. Phys. 2013, 15, 3466. 10.1039/c3cp50168j. [DOI] [PubMed] [Google Scholar]
- Kosco J.; Sachs M.; Godin R.; Kirkus M.; Francas L.; Bidwell M.; Qureshi M.; Anjum D.; Durrant J. R.; McCulloch I. The Effect of Residual Palladium Catalyst Contamination on the Photocatalytic Hydrogen Evolution Activity of Conjugated Polymers. Adv. Energy Mater. 2018, 8, 1802181 10.1002/aenm.201802181. [DOI] [Google Scholar]
- Sprick R. S.; Bai Y.; Guilbert A. A. Y.; Zbiri M.; Aitchison C. M.; Wilbraham L.; Yan Y.; Woods D. J.; Zwijnenburg M. A.; Cooper A. I. Photocatalytic Hydrogen Evolution from Water Using Fluorene and Dibenzothiophene Sulfone-Conjugated Microporous and Linear Polymers. Chem. Mater. 2019, 31, 305–313. 10.1021/acs.chemmater.8b02833. [DOI] [Google Scholar]
- Aitchison C. M.; Sprick R. S.; Cooper A. I. Emulsion Polymerization Derived Organic Photocatalysts for Improved Light-Driven Hydrogen Evolution. J. Mater. Chem. A 2019, 7, 2490–2496. 10.1039/C8TA11383A. [DOI] [Google Scholar]
- Lin L.; Ou H.; Zhang Y.; Wang X. Tri-s-Triazine-Based Crystalline Graphitic Carbon Nitrides for Highly Efficient Hydrogen Evolution Photocatalysis. ACS Catal. 2016, 6, 3921–3931. 10.1021/acscatal.6b00922. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.