Abstract
Scarring of the vocal fold lamina propria (LP) can cause considerable voice disorders due to reduced pliability in scar tissue, attributed in part to abnormal extracellular matrix (ECM) deposition produced by the fibrotic vocal fold fibroblast (fVFF). Cytokines with anti-fibrotic potential have been investigated to limit abnormal LP ECM, but are limited by the need for repeat injections. Moreover, the potentially significant role played by activated macrophages (AMOs) is usually not considered even though the interaction between AMO and fibrotic fibroblasts is known to regulate scar formation across different tissues. AMO are also regulated by cytokines that are used for LP scar removal, but little is known about AMO behaviors in response to these cytokines within the context of LP scar. In the present study, we evaluated anti-fibrotic effects of hepatocyte growth factor (HGF), interleukin-10 (IL-10) and interleukin- 6 (IL-6) in a 3D, in vitro fVFF-AMO co-culture system using poly(ethylene glycol) diacrylate (PEGDA) hydrogels. Data from all cytokines was synthesized into a heat-map that enabled assessment of specific associations between AMO and fVFF phenotypes. Cumulatively, our results indicated that both HGF and IL-10 are potentially anti-fibrotic (reduction in fibrotic markers and enhancement in normal, anti-fibrotic VFF markers), while IL-6 displays more complex, marker specific effects. Possible associations between AMO and fVFF phenotypes were found and may highlight a potential desirable macrophage phenotype. These data support the therapeutic potential of HGF and IL-10 for LP scar treatment, and shed light on future strategies aimed at targeting specific AMO phenotypes.
Keywords: vocal fold scar, cytokine treatment, 3D cell culture, macrophages, fibroblasts
INTRODUCTION
Scarring in vocal fold lamina propria (LP) is a debilitating condition that can lead to voice disorders from hoarseness, fatigue or even total loss of voice based on severity of scarring.1–3 Upon scarring, healthy LP tissue is replaced by stiff, fibrotic tissue with a disorganized extracellular matrix (ECM). The major source of abnormal ECM in LP scar is the resident vocal fold fibroblasts (VFFs) which are activated toward a fibrotic, myofibroblastic phenotype (fVFF). Histological characterization of vocal fold scar over the past several years has revealed a number of alterations in ECM including: (1) formation of thick and disorganized collagen bundles,4 (2) increased presence of alpha-smooth muscle actin (α-SMA), fibronectin and biglycan, and (3) decreased presence of hyaluronic acid and decorin.2,5–7 Traditional treatments for vocal fold scarring, including surgery and augmentation using collagen or fat, have proved insufficient due, in part, to their inability to reverse the abnormal ECM production and elicit healthy tissue regeneration.3,8
Researchers have been investigating bioactive treatments for vocal fold scar such as anti-fibrotic growth factors and cytokines including basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF). Both cytokines have demonstrated the capacity to improve LP biomechanical properties in vivo, reverse undesirable ECM production such as collagen and α-SMA, and promote hyaluronic acid expression both in vitro and in vivo.9–13 While simple and partially effective, cytokine injections are limited by rapid in vivo absorption and potential need for multiple dosing, potentially causing secondary injuries and further exacerbating scar formation.14 To prolong cytokine retention, different biomaterial-based loading systems have been developed, successfully extending release up to 1 month in vivo.11,15,16 However, these loading systems are still incapable of completely restoring vocal fold function, partly attributed to the following: (1) unknown desired cytokine dose and unclear underlying biological mechanism(s) and (2) necessity for repeated surgeries in most cases.9,11,17 These factors are important for several reasons. First, determining/understanding the underlying biological mechanisms is essential for the development of future strategies aimed at completely restoring vocal fold function. Second, injury to the vocal fold through cutting (as would be performed during surgery) has been shown to induce a significant activated macrophage (AMO)-based inflammatory response,18 and macrophages have been shown to directly influence fVFF behavior.19
Macrophages are highly plastic, professional phagocytes that reside in nearly every tissue. Their phenotype is often identified by the specific protein marker profiles that enable them to interact with their surroundings, including other cell types in the tissue. Interactions between macrophages and fibroblasts are critical in wound healing and tissue repair in contexts such as skin, lung and liver injury. Generally, macrophages take on a variety of phenotypes through the processes of wound healing and fibrosis. While many macrophage phenotypes (defined by the molecules they secrete/produce) often have both pro- and anti-fibrotic properties,20 the anti-inflammatory/pro-resolving AMO which appears during the final stage of tissue repair has shown capacity of fibrosis resolution through secretion of interleukin-10 (IL-10) and other molecules.21–23 Aside from this knowledge, the specific correlations between a given macrophage phenotype and the resulting fibroblast phenotype has yielded contradictory and inconsistent results, owing to a variety of factors including: (1) non-uniform definitions of a macrophage phenotype,24,25 (2) lack of studies which examine multiple fibroblast and macrophage phenotypic markers at a time, and (3) context-dependent nature of a given cytokine/growth factor on fibrosis. For example, pro-inflammatory AMO products such as TNF-α often exert both pro-fibrotic (stimulate fibroblast migration and activation in skin, kidney and lung19) and anti-fibrotic properties (accelerate pulmonary fibrosis resolution, promote vocal fold ECM degradation26,27). Similarly, anti-inflammatory AMO markers such as arginase-1 (Arg-1) and VEGF all exhibit both pro- and anti-fibrotic effects under specific environments.20,28,29 Because of these reasons, at present, the beneficial or detrimental contributions of macrophages in fibrosis, including the scarred vocal fold, are unclear. Generally, the anti-inflammatory/pro-resolving macrophage phenotype has emerged as a candidate for anti-fibrotic outcomes.
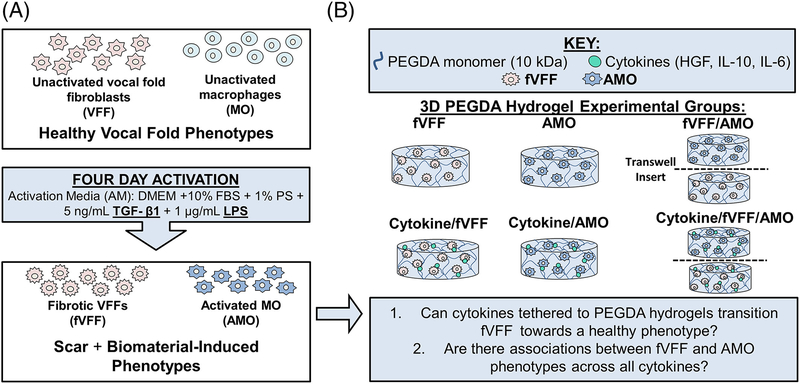
It is likely that any successful biomaterial-based strategy for VF scar treatment must address the underlying cell and tissue biology (interactions between AMO and fVFF) driving inflammation, wound healing, and fibrosis. Ideally, the approach would promote favorable phenotypes in both fVFF and AMO. Unfortunately, the desired AMO phenotype is still largely unclear, rendering it difficult to design a targeted strategy for the purpose of fibrosis resolution. With these design criteria in mind, the goals of the present study were: (1) to evaluate anti-fibrotic potential of several additional bioactive molecules with important roles in inflammation and fibrosis and (2) to utilize fVFF and AMO phenotypic assessments across all cytokine/growth factor treatments to gain a deeper understanding of associations between fVFF and AMO. Toward these goals, we have tested the anti- fibrotic benefit of three promising cytokines, including HGF, IL-10 and interleukin-6 (IL-6), using a 3D co-culture design with fVFF-AMO encapsulated in poly(ethylene glycol) diacrylate (PEGDA) hydrogels (Figure 1).
FIGURE 1.
Overall experimental design for the study. (A) Scar and biomaterial-induced vocal fold phenotypes (fVFF and AMO) were experimentally induced with activation media (AM) containing TGF-β1 and LPS for 4 days. (B) Effects of various cytokines on fVFF and AMO were tested utilizing 3D PEGDA hydrogels. The transition of fVFF to a more normal phenotype was examined in mono- and co-cultures containing select, tethered cytokines (HGF, IL-10, and IL-6). Data from all treatments (HGF, IL-10, and IL-6) are examined to find patterns of associations between fVFF and AMO phenotypes.
MATERIALS AND METHODS
Cell expansion and activation
Vocal fold fibroblasts (VFFs) were isolated from primary explants of the mid-membranous LP of 6–12-month-old pigs, an accepted animal model for the human vocal fold LP.2,30 The cryopreserved VFF were thawed and expanded at 37°C/5% CO2 in cell culture media, which consists of Dulbecco’s Modified Eagle’s Medium (DMEM, Cellgro) supplemented with 10% fetal bovine serum (FBS, Hyclone), 2 ng mL−1 bFGF, 100 U mL−1 penicillin and 100 pg mL−1 streptomycin (Gibco). A cryopreserved mouse macrophage cell line, Raw 264.7 (ATCC), was thawed at 37°C and expanded in a monolayer culture. Macrophages were maintained at 37°C/5% CO2 in cell culture media containing DMEM supplemented with 10% FBS (Hyclone), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin.
Vocal fold fibroblast and macrophages were seeded and activated for 4 days before encapsulation with activation media (AM, cell culture media supplemented with 1 μg mL−1 lipopolysaccharide [LPS] from Salmonella enterica serotype enteritidis [Sigma Aldrich] and 5 ng mL−1 transforming growth factor-β1 [TGF-β1, recombinant human, Millipore GF111]) [Figure 1(A)]. LPS and TGF-β1 were chosen to mimic the complex microenvironment during chronic scar and acute inflammation, and we have previously shown this treatment to result in a fVFF phenotype and an AMO phenotype similar to that seen in biomaterial-treated dermal wounds.13
Hydrogel fabrication and maintenance
Poly(ethylene glycol) diacrylate was synthesized from PEGdiol (10 kDa; Sigma-Aldrich) at ~92% acrylation as reported previously.31 Immobilized cytokines and cell adhesion peptide were prepared through PEGylation.32,33 Recombinant human hepatocyte growth factor (rhHGF; Millipore GF414), recombinant human interleukin-10 (rhIL-10; Millipore GF419) and recombinant murine interleukin-6 (rmIL-6; R&D Systems 406-ML/CF) and the cell adhesion peptide NH2-Arg-Gly-Asp-Ser-COOH (RGDS; American Peptide) were reacted with acryloyl-PEG-succinimidyl valeric acid (ACRL- PEG-SVA) (3.4 kDa; Laysan Bio) at the molar ratio of 1:6 (for HGF), 1:3 (for IL-10 and IL-6) and 1:1 (for RGDS), respectively. The resulting ACRL-PEG-cytokine was used immediately after preparation (to avoid bioactivity loss with freeze-thaw cycles) and ACRL-PEG-RGDS was purified by dialysis, then lyophilized and stored at −80°C until use.
Hydrogels were fabricated by photo-crosslinking of a precursor solution containing 100 mg mL−1 10 kDa PEGDA (92% acrylate), 1 mM ACRL-PEG-RGDS alone or with addition of ACRL-PEG-cytokine in phosphate-buffered saline (PBS; Gibco) and 1 vol/vol % of the photoinitiator lrgacure (262 mg mL−1 in 70% ethanol; Sigma Aldrich). The final concentration of ACRL-PEG-cytokine was adjusted to 200 ng mL−1 for HGF and 50 ng mL−1 for IL-10 and IL-6, based on previous studies showing their potential anti- fibrotic and/or anti-inflammatory benefits.34–36 The precursor solution underwent sterilization and endotoxin removal using 0.2 μm Acrodisc® Units with Mustang® E Membrane filter (Pall Life Science). PEGDA molecular weight and concentration were selected to yield hydrogels with average dynamic elastic moduli appropriate to vocal fold applications,37 and RGDS concentration was selected based on previous work.13
After being activated for 4 days, fVFF (passage 6) and AMO were harvested and re-suspended in the corresponding precursor solution at a cell density of ~5 × 106 cells mL−1 for each cell type. Hydrogel discs were cast in a 48-well plate (BD Falcon) with 200 μL of cell suspension per well and 6 min exposure to long wavelength UV light for polymerization (Spectroline; ~6 mWcm−2, 365 nm). Hydrogel formulations are shown in Figure 1(B). Polymerized hydrogels were transferred to their respective mono-culture and co-culture groups in 12-well plates (BD Falcon) with cell culture inserts (BD Falcon; 12 mm diameter, 0.8 μm pores). For co-culture groups, AMO-containing hydrogels were placed on top of the insert and fVFF-containing hydrogels below [Figure 1(B)]. Hydrogel constructs were immersed in activation media and maintained at 37°C/5% CO2 for 72 h. Media was changed daily. Hydrogels were harvested at 72 h, flash frozen in liquid nitrogen and stored at −80°C.
Endpoint analyses
Fibrotic vocal fold fibroblast and activated macrophage constructs were homogenized and lysed for protein extraction, as previously reported.13,38 Briefly, hydrogels were placed in contact with lysis binding buffer (Invitrogen) (300 μL for AMO-containing gels and 350 μL for fVFF-containing gels), homogenized using a plastic RNase free pestle (Kimble Chase) and incubated at room temperature for 15 min. Following incubation, the samples were centrifuged for 5 min at 10,000 rpm and protein-containing supernatant was collected. Total DNA was measured by Quant-iT™ PicoGreen dsDNA Assay (Invitrogen) following the manufacturer’s protocol. DNA levels were utilized for normalization of protein levels on a per cell basis.
Western blot
Western blots were used to semiquantitatively compare levels of proteins expressed by fVFF to evaluate phenotypic changes that occurred with individual cytokine treatment, as described previously.13,39 Targeted proteins of each sample were quantified via integrated band densitometry, normalized to DNA amount loaded, and further normalized to the fVFF mono-culture control experimental group. Primary antibodies associated with profibrotic and anti-fibrotic properties were selected to assess fVFF phenotype in response to cytokine treatment (Table S1). An in depth justification for selecting these proteins to analyze fVFF phenotype is provided in detail in the “Justification of Experimental Parameters” section of this manuscript.
MAGPIX immunoassay multiplexing
Protein production of select cytokines and growth factors from AMO were quantitatively assessed with MAGPIX immunoassay multiplexing (Luminex). AMO protein lysates were reacted with a MILLI- PEX® MAP kit of mouse cytokine/chemokine containing tumor necrosis factor alpha (TNF-α), IL-6, IL-10, interleukin 12 p40 (IL-12), vascular endothelial growth factor A (VEGF), and arginase-1 (Arg-1) following the manufacture’s protocol (Millipore). Concentrations of protein of interest were obtained from a median fluorescence intensity relative to a standard curve. Results were first normalized to sample DNA concentration and then subsequently normalized to the AMO mono-culture control experimental group. An in depth justification for selecting these proteins to analyze macrophage phenotype is provided in detail in the “Justification of experimental parameters” section of this manuscript.
Justification of experimental parameters
In vitro evaluation of anti-fibrotic potential with PEGDA hydrogels
Many reasons for utilizing PEGDA hydrogels for vocal fold scar restoration have been justified previously.13 In brief, PEGDA hydrogels can be used as a platform for prolonging cytokine release (3–6 months due to hydrolysis mediated degradation of tethered proteins)40 to greater extents than other VFF material systems (i.e. gelatin).41 Prolonged retention may circumvent problems associated with multiple dosing (i.e. cost and secondary injuries14). Since no cytokine in our study (HGF, IL-10, and IL-6) requires internalization to change cell behaviors,42–45 covalently tethering of these molecules is believed to prolong their effective dosage. In addition, previous studies have demonstrated enhanced growth factor bioactivity with PEG-tethering relative to treatment with the growth factor in solution.46 Lastly, PEGDA-based hydrogels with the formulation utilized herein have mechanical properties which are able to preserve the vocal fold mucosal wave with low phonation threshold pressure47 and can be injected and cross-linked in vivo through trans-epithelial light exposure.48 Relative to in vivo models, the 3D in vitro models with PEGDA provide a more controlled platform to gain deeper understanding of the underlying cellular mechanisms behind specific therapeutic effects of cytokines.
Culture duration and pre-activation with LPS/TGF-β1
Biomaterial insertion can elicit a foreign body response (FBR), leading to non-specific protein adsorption, inducing inflammation and promoting fibrosis.49 In our study, hydrogel discs were cultured for 72 h after encapsulation in order to assess their short-term anti-fibrotic effects and immediate immunomodulatory potential of cytokines in the acute phase of biomaterial implantation-associated FBR to prevent such potential undesirable effects from happening in vivo.
In order to establish cell phenotypes consistent with scar and short-term biomaterial-insertion, activation media containing LPS and TGF-β1was used.18,50 We have previously demonstrated that this activation induces normal VFF to take on characteristics of a fibrotic phenotype, and AMO to take on phenotype similar to that seen in chronic scar as well as many biomaterial-treated dermal wounds51,52 (loosely, a phenotype between the traditional pro- and antiinflammatory classifications13).
Tethered anti-fibrotic cytokine and growth factor selection
Generally, all selected bioactive molecules (HGF, IL-10, and IL-6) were chosen based on their known or potential anti-fibrotic properties on vocal fold and/or other tissues. Moreover, these molecules potentially induce an antiinflammatory AMO phenotype similar to macrophages found to have potential anti-fibrotic effects.53–55 Specifically, HGF was previously reported to improve LP scar tissue biomechanical properties and reverse fibrotic ECM production in vivo and in vitro11,56,57 and was reported to attenuate inflammation associated with many diseases, including during vocal fold reconstruction.15
Interleukin-10 has been shown to regulate dermal and lung fibroblasts, in part, through reducing expression of collagen and α-SMA expression35,58–60, but its effect on vocal fold scar has not been studied yet. On the other hand, IL-10 has been consistently used an anti-inflammatory molecule as well as a marker for anti-inflammatory/pro-resolving AMO.22,55,61
Interleukin-6 is a pleiotropic cytokine with respect to fibrosis regulation, exhibiting both pro- or anti-fibrotic prop- erties.62,63 Research about IL-6 as a potential anti-fibrotic mediator in LP scar is limited, but one study indicated that IL-6 exhibited anti-fibrotic benefit on VFF activated by TGF- β1.36 IL-6 effects on inflammation and AMO phenotype is also pleiotropic with evidence supporting both pro- and antiinflammatory roles.64,65 Limited studies have focused on direct evaluation of IL-6 as a treatment to AMO, but IL-6 knockout mice displayed dysregulation in the balance between pro- and anti-inflammatory AMO in skin, indicating the immunomodulatory role of IL-6 on AMO phenotype.65 Cumulatively, HGF, IL-10, and IL-6 all possess potential for reducing scar and inducing a pro-resolving AMO phenotype. Therefore, their potential effects in the unique and complex LP scar condition are worth further assessment.
Markers assessed for VFF and AMO phenotype evaluation
Histological characterization of scarred vocal fold tissue over the past several years has revealed a number of alterations in ECM including: (1) formation of thick and disorganized collagen bundles, (2) increased presence of α-SMA, fibronectin and biglycan, and (3) decreased presence of hyaluronic acid and decorin. In the present study, we looked into changes directly in protein expression of all above fibrotic proteins (α-SMA, Col-1, and biglycan) and anti-fibrotic proteins (decorin and HAS-2) in fVFF. This panel of markers is generally more extensive than previous studies, most of which have only investigated 2–3 markers.10,11,34,36,57,58,66–68
A panel of markers were selected to evaluate AMO behavior based on the following commonly-studied roles in either inflammation (TNF-α IL-6 and the ratio between IL- 10 and IL-12 (IL-10/IL-12) or wound healing (Arg-1 and VEGF). Moreover macrophages contribute to fibrosis through the production of many of these proteins 20 although the specific contribution of each is difficult to discern for reasons denoted in the “Introduction”. Previous studies related to characterizing macrophage phenotype (in response to cytokines53,65, 69–76 or in vocal fold injuries18,77) only examined two to three AMO markers, usually limited to a specific role (i.e. inflammation). Our panel goes beyond this by examining several additional inflammation markers as well as wound healing markers, each with a distinct role in the wound healing process. Generally, TNF-α has been identified as a pro-inflammatory cytokine with an important role in multiple inflammation associated diseases,78 and has also been demonstrated to exert both pro- and anti-fibrotic properties.79–81 IL-6 is a highly pleo- tropic, context-dependent cytokine with respect to inflammation (both pro- and anti-inflammatory functions64) and fibrosis (pro- and anti-fibrotic properties36,62,63), meaning its specific role depends on a variety of factors, including tissue type, signaling pathways, dosage, and timing.42,62,63,82–85 Rather than analyzing IL-10 and IL-12 separately, the ratio of IL-10 to IL-12 has been identified as a more meaningful phenotypic readout for characterizing macrophage phenotype,29 with an increase in this ratio representing antiinflammatory processes61,86,87 and/or the resolution of inflammation.72 Arg-1 and VEGF have been demonstrated to be important for wound healing with respect to collagen synthesis and angiogenesis, respectively.88,89
Statistical analyses
All data is reported as the mean ± standard error of the mean. Means were compared using a two-way ANOVA (n = 3–9 samples per group). This statistical test is appropriate when the experimental design seeks to test the effects of two independent variables (the presence or absence of cytokine and the presence or absence of co-culture) on a dependent variable (production of a specific protein). A two-way ANOVA was utilized to determine significance resulting from main effects of either independent variable (i.e. the cytokine or the co-culture) or significance resulting from the interaction between these two variables. For all tests, a p <0.05 was considered significant and SPSS software was utilized. For the purposes of constructing the heat-map, a p <0.10 was utilized to determine proteins that were weakly affected (non-significantly) by the cytokine in question. This value was selected somewhat arbitrarily but reflects an acceptable level of significance in other fields. Moreover, there were a number of proteins that fell within the 0.05–0.10 range when the next closest p-value was 0.206.
RESULTS
Fibrotic VFF and activated macrophages phenotype change in response to cytokines
After culturing in activated media for 72 h, fVFF and AMO containing hydrogels were collected and fVFF and AMO phenotypes were characterized via protein level assessments (western blot and MAGPIX). For the purpose of presenting the data in a simplistic and logical manner and since one goal of this study was to determine anti-fibrotic/antiinflammatory potential of select cytokines, the following sections will be divided into effects from individual cytokines on fVFF and AMO.
HGF effects on fVFF and AMO
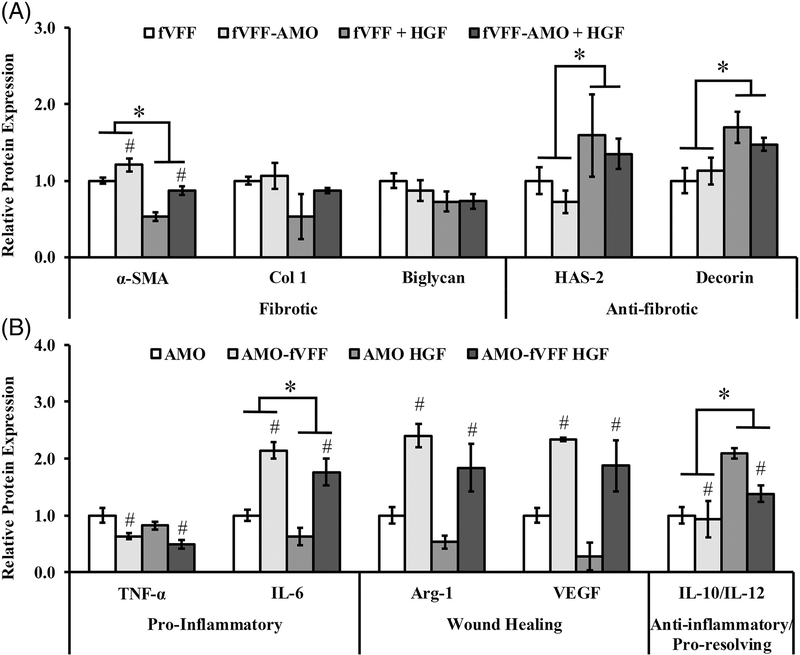
Relative to fVFF encapsulated in PEG-only hydrogels, the incorporation of HGF resulted in a significant reduction in the fibrotic marker α-SMA [p < 0.001, Figure 2(A)]. Other fibrotic markers such as Col-1 and biglycan were statistically unaffected by HGF incorporation, although they showed a decreasing trend [p = 0.089 and 0.100; Figure 2(A)]. In contrast to fibrotic markers, HGF-tethered hydrogels significantly increased the production of anti-fibrotic markers HAS-2 and decorin relative to PEG-only controls [p = 0.012 and 0.004; Figure 2(A)]. These data suggest that HGF can limit the fibrotic phenotype and simultaneously enhance anti-fibrotic phenotype of fVFF in the context of 3D mono- and co-culture conditions.
FIGURE 2.
Relative protein expression of a panel of markers indicating fVFF and AMO phenotype following 72 h culture in various 3D PEGDA hydrogel experimental groups with or without addition of HGF. (A) Fibrotic and anti-fibrotic/healthy markers were utilized to assess fVFF phenotype. (B) Pro-inflammatory, wound healing, or anti-inflammatory/pro-resolving markers were utilized to assess AMO phenotype. * denotes a significant main effect resulting from HGF treatment relative to no treatment. # denotes a significant main effect of the co-culture relative to mono-culture.
Relative to AMO encapsulated in PEG-only hydrogels, the incorporation of HGF resulted in a significant decrease in IL- 6 expression, often considered as a pro-inflammatory marker [p = 0.039; Figure 2(B)]. In contrast, HGF-tethered hydrogels significantly increased the ratio of IL-10/IL-12, an antiinflammatory marker [p < 0.001, Figure 2(B)]. No statistical differences were noted between PEG-only and PEG-HGF hydrogels with respect to TNF-α (p = 0.103), Arg-1 (p = 0.059) or VEGF (p = 0.057); (Figure 2(B)). These data suggest that HGF partly suppresses the pro-inflammatory while promoting an anti-inflammatory, pro-resolving phenotype in AMO, with minimal effect on wound healing markers.
IL-10 effects on fVFF and AMO
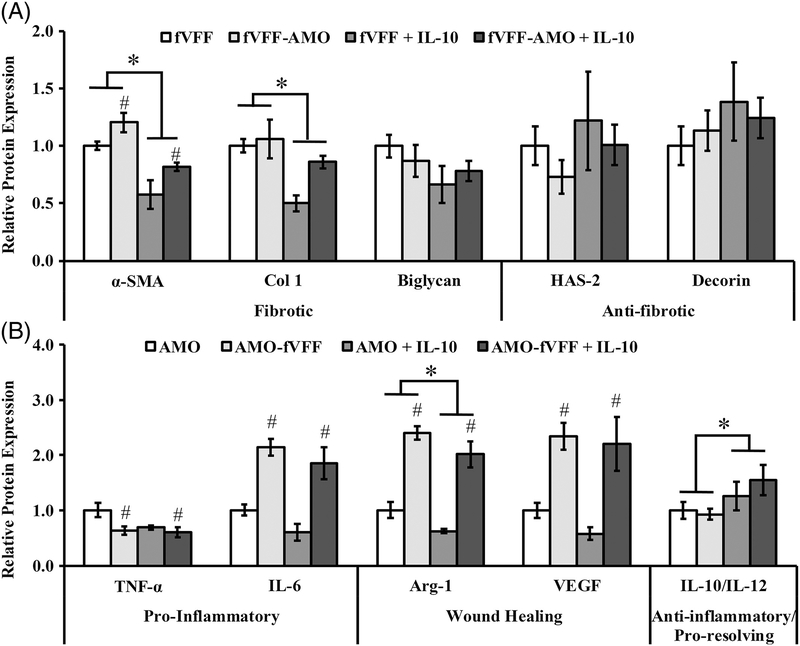
Relative to fVFF encapsulated in PEG-only hydrogels, the incorporation of IL-10 resulted in a significant reduction in the fibrotic markers α-SMA and Col-1 [p < 0.001 and 0.003, Figure 3(A)]. Biglycan was statistically unaffected by HGF incorporation, although it showed a decreasing trend [p = 0.073; Figure 3(A)]. In contrast to fibrotic markers, IL-10 did not influence the production of anti-fibrotic markers HAS-2 or decorin, relative to PEG-only controls [Figure 3(A)]. These data suggest that IL- 10 can limit the fibrotic phenotype without altering the anti- fibrotic phenotype of fVFF in the context of 3D mono- and co-culture conditions.
FIGURE 3.
Relative protein expression of a panel of markers indicating fVFF and AMO phenotype following 72 h culture in various 3D PEGDA hydrogel experimental groups with or without addition of IL-10. (A) Fibrotic and anti-fibrotic/healthy markers were utilized to assess fVFF phenotype. (B) Pro-inflammatory, wound healing, or anti-inflammatory/pro-resolving markers were utilized to assess AMO phenotype. * denotes a significant main effect resulting from IL-10 treatment relative to no treatment. # denotes a significant main effect of the co-culture relative to mono-culture.
Relative to AMO encapsulated in PEG-only hydrogels, the incorporation of IL-10 resulted in a significant increase in the ratio of IL-10/IL-12, a resolving marker [p = 0.033, Figure 3(B)]. In contrast, the wound healing marker Arg-1 was significantly decreased in AMO encapsulated in IL-10 containing hydrogels relative to PEG-controls [p = 0.034; Figure 3(B)]. No differences were noted between PEG-only and PEG-IL-10 hydrogels with respect to IL-6, TNF-α, or VEGF [Figure 3(B)]. These data suggest that IL-10 promotes an anti-inflammatory phenotype with a concurrent suppression in select wound-healing markers (i.e. Arg-1) in AMO.
IL-6 effects on fVFF and AMO
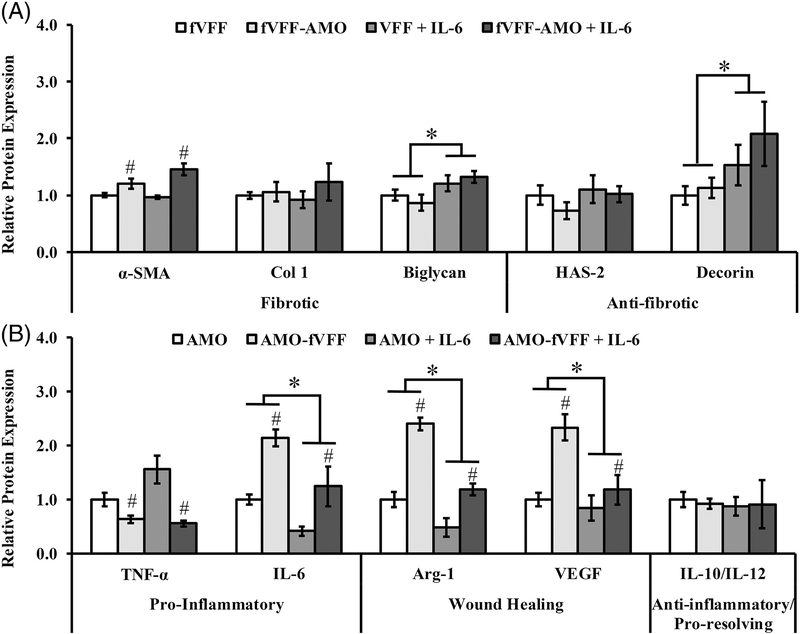
Relative to fVFF encapsulated in PEG-only hydrogels, the incorporation of IL-6 resulted in a significant increase in the fibrotic marker biglycan [p = 0.015, Figure 4(A)]. Other fibrotic markers such as α-SMA and Col-1 were unaffected by IL-6 incorporation [Figure 4(A)]. Similar to the fibrotic markers, IL-6 exhibited a marker specific influence on anti-fibrotic markers. Specifically, decorin was significantly increased while HAS-2 remained unchanged in PEG-IL-6 hydrogels relative to PEG- only controls (p = 0.008 and 0.347; Figure 4). These data suggest that IL-6 contributes to a complex, marker- dependent fVFF phenotype with both fibrotic and antifibrotic features in the context of 3D mono- and co-culture conditions.
FIGURE 4.
Relative protein expression of a panel of markers indicating fVFF and AMO phenotype following 72 h culture in various 3D PEGDA hydrogel experimental groups with or without addition of IL-6. (A) Fibrotic and anti-fibrotic/healthy markers were utilized to assess fVFF phenotype. (B) Pro-inflammatory, wound healing, or anti-inflammatory/pro-resolving markers were utilized to assess AMO phenotype. * denotes a significant main effect resulting from IL-6 treatment relative to no treatment. # denotes a significant main effect of the co-culture relative to mono-culture.
Relative to AMO encapsulated in PEG-only hydrogels, the incorporation of IL-6 resulted in a significant decrease in the wound healing markers Arg-1 and VEGF [p < 0.001 and p = 0.007, Figure 4(B)], and significant decrease in endogenous expression of IL-6 (p < 0.001, Figure 4B). No differences were noted between PEG-only and PEG-IL-6 hydrogels with respect to TNF-α or IL-10/IL-12 [Figure 4 (B)]. These data suggest that IL-6 partly suppresses the proinflammatory phenotype and more fully suppresses the wound healing phenotype in AMO.
Summary of cytokine effects
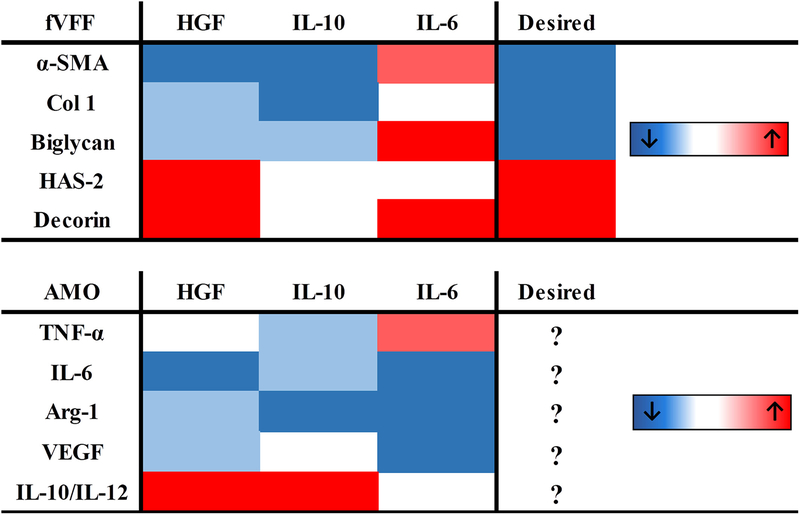
To summarize the above data and enable comparisons of the cytokine in question on the individual phenotypes of fVFF and AMO, a heat map was generated (Figure 5). Results from all markers were color-coded with directionality and degree of change (dark blue: significantly decreased, p < 0.05; light blue: decreased, p < 0.10; dark red: significantly increased, p < 0.05; light red: increased, p < 0.10). The rightmost column indicates the most desirable VFF phenotype based on previous findings on healthy VFF characteristics: decrease in pro-fibrotic ECM proteins (α-SMA, Col-1, biglycan) and increase in anti-fibrotic/healthy ECM proteins (HAS-2 and decorin). There is no rightmost column for the AMO phenotype because this phenotype is largely unknown.
FIGURE 5.
Heat-map summarizing cytokine effects on fVFF (top) and AMO (bottom) phenotypic markers. Color code was determined based off significance values as denoted in “Materials and Methods” section. Red and blue colors represent an increase or decrease of the protein in response to cytokine treatment, respectively.
Cumulatively, data from both fVFF and AMO indicated two major points: (1) HGF and IL-10 displayed the most anti-fibrotic/anti-inflammatory, pro-resolving potential (i.e. more boxes in same colors as desired VFF phenotype; blue boxes in the inflammatory AMO markers and red boxes in anti-inflammatory/pro-resolving marker of AMO) (2) IL-6 exerted pleiotropic effects on fibrotic markers and reduced the wound healing AMO markers (boxes with both consistent and opposite color as desired fVFF markers; blue boxes in AMO wound healing markers).
DISCUSSION
Vocal fold scar treatment remains problematic owing, at least in part, to limited understanding of the interactions between fVFF and AMO. Certain cytokines (HGF, IL-10, and IL-6) are emerging as potential anti-fibrotic, bioactive molecules but have not been investigated in depth for vocal fold repair. Moreover, the potentially desirable effects of any above cytokine treatment on AMO phenotype is unclear. To address these issues, the goals of the present study were: (1) to determine potential anti-fibrotic benefit of HGF, IL-10, and IL-6 in a 3D in vitro model of VF scar and (2) to gain a deeper understanding of associations between fVFF and AMO in the context of VF scar. Toward our first goal, we first compare results from each individual cytokine to those previously reported in literature. Afterwards (and toward our second goal), we discuss a heat-map integrating effects from each cytokine to determine a potential AMO phenotype associated with an anti-fibrotic fVFF phenotype.
Protein level analyses of fVFF revealed an overall beneficial effect of HGF, transitioning fVFF toward a more normal, healthy phenotype (↓α-SMA, ↑HAS-2, and ↑decorin). Changes found in α-SMA and HAS-2 are consistent with previously reported in vitro studies.36,66 To our knowledge, the decrease in decorin in response to HGF is a novel finding, as this has not yet been measured on a cellular level in response to HGF treatment. With respect to AMO phenotype, HGF suppressed IL-6 protein expression and increased the IL-10/IL-12 ratio in the present study. These findings are in agreement with the overall anti-inflammatory effects of HGF on macrophage phenotype. Specifically, pre-treatment of HGF was able to suppress IL-6 and increase IL-10 expression from LPS-activated bone marrow derived macrophages and RAW 264.7 cell line,53,73 remarkably similar to our results.
Similar to HGF, protein level analyses of fVFF revealed an overall beneficial effect of IL-10, transitioning fVFF toward more normal, healthy phenotype (↓α-SMA, ↓Col-1). Although IL-10 has not yet been investigated in the context of vocal fold scarring, these beneficial effects are generally consistent with those reported in dermal scar treatment.35,58,59,90 For example, Shi and colleagues58 have found that IL-10 reduces Col-1, Col-3, and α-SMA protein and gene expression. However, our decorin results are different than those of Yamamoto et al.35 which demonstrated that IL-10 increased decorin in fibroblasts stimulated with TGF-β1. This inconsistency may be explained by a variety of factors (i.e. skin fibroblast vs. VFF, gene expression vs. protein expression, and different treatment times). With respect to AMO phenotype, IL-10 treatment increased the IL-10/IL-12 ratio, a marker for anti-inflammatory, pro-resolving macrophages, in agreement with the general anti-inflammatory and pro-resolving role of this cytokine in many contexts.91–94
In contrast to the strictly anti-fibrotic effects of HGF and IL-10, IL-6 treatment demonstrated a limited, marker-dependent, pleotropic effect (↓biglycan and ↑decorin). These results may reflect the overall pleiotropic effects of IL-6 in scar formation, which depend on a variety of factors including tissue, dosage and timing.62,63,85 While assessments in vocal fold contexts have been limited, our α-SMA data is different from Vyas et al.,36 where IL-6 treatment was found to reduce α-SMA expression. Differences between the current study and these findings can be attributed to any combination of the following: pre-activation formulation (with or without LPS), treatment duration (72 h vs. 24 h), and/or culture dimension (3D vs. 2D). For the AMO response, IL-6 treatment significantly suppressed endogenous IL-6, Arg-1, and VEGF expression. These effects are inconsistent with existing studies, but none were conducted within the context of VF scar.75,95,96 Cumulatively, IL-6 exhibited complex, marker-dependent effects on both fVFF and AMO.
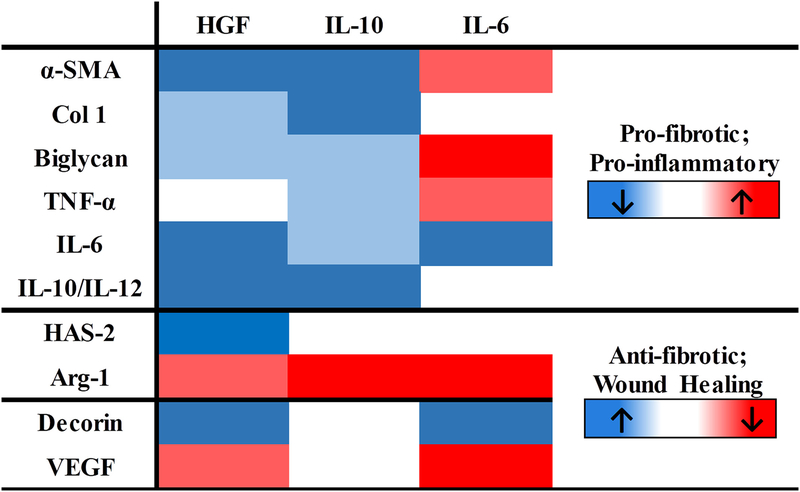
Our second aim was to gain deeper insight into associations between AMO and fVFF phenotypes in order to better target a desired AMO phenotype potentially associated with an anti-fibrotic outcome. To better interpret potential phenotypic associations between AMO and fVFF, we integrated data across all cytokines to reorganize the heat map with specific clusters of proteins from fVFF and AMO cells (Figure 6). Cumulatively two major associations were found: (1) a decrease in pro-fibrotic markers of fVFF was associated with a decrease in markers favoring inflammation (i.e. ↓TNF- a, ↓IL-6, and ↑IL-10/IL-12 ratio) from AMO (blue boxes from top panel appear together) and (2) specific wound healing macrophage markers were associated with specific anti-fibrotic fVFF markers (increased HAS-2 only occurred when Arg-1 was weakly reduced; lower VEGF was associated with increased level of decorin).
FIGURE 6.
Reorganized heat-map to illustrate potential associations between fVFF and AMO phenotypes. Color code was determined based off significance values as denoted in “Materials and Methods” section. In this heat-map, red and blue colors indicate a change in the direction of the markers associated with a given phenotype (i.e. pro-fibrotic markers) in response to cytokine treatment.
Our data suggests that a decrease in pro-inflammatory markers (TNF-α, IL-6) and an increase in anti-inflammatory, pro-resolving markers (i.e. IL-10/IL-12) may favor antifibrotic outcomes through the reduction in fibrotic proteins. This conclusion is supported through several studies demonstrating pro-fibrotic effects of excess inflammation across scarring contexts (vocal fold, kidney and intestine18,80,97,98) as well as the emerging importance for the resolution of inflammation in fibrosis. While IL-6 is a pleiotropic cytokine in inflammation,64,65 this cytokine clustered well as a proinflammatory marker in the current study, potentially indicating a pro-fibrotic role in VF contexts.
In contrast to the relationship between inflammatory cytokines and pro-fibrotic proteins, associations between the wound healing markers and anti-fibrotic proteins were found. Our inverse relationship between decorin and VEGF is supported through previous studies demonstrating decorin’s capacity to inhibit VEGF expression and angiogenesis.99,100 While opposing effects of VEGF and decorin could be confirmed with literature support, direct literature support for the potentially positive relationship between Arg-1 and HAS-2 is lacking. At present, we have no explanation for this finding.
Several limitations to the present study merit comment. First, while we were able to generate general phenotypic associations between markers, any potential cause-effect relationships between these phenotypes remains to be determined. Second, although we may have identified desirable AMO phenotypic characteristics, designing a therapy which elicits these general responses from AMO could be challenging. Third, while the current study extensively profiled fVFF and AMO phenotypes with several phenotypic readouts, the selected proteins are by no means exhaustive. Including additional markers may help uncover novel and more specific associations. To address these issues, future work will need to assess the temporal evolution of the phenotype for each cell type with additional phenotypic markers and design a high throughput screening platform to identify compounds which elicit the desired AMO phenotype. Lastly, while tethering growth factors has been shown to preserve bioactivity for other molecules like bone-morphogenetic protein-2,46 any potential loss in bioactivity in the tethered molecules utilized herein (HGF, IL-6, and IL-10) relative to their soluble form will need to be determined in future work.
CONCLUSIONS
Utilizing a PEGDA hydrogel based fVFF-AMO co-culture model, the present study revealed the potential anti-fibrotic benefit of HGF and IL-10. These cytokines transitioned fVFF toward a more normal, healthy phenotype - assessed through a reduction in fibrosis associated markers and increase in normal tissue associated markers. IL-6 exhibited both pro- and anti-fibrotic features in our culture environment. Specific associations between AMO and VFF phenotype have been investigated, and an AMO phenotype with relatively lower expression of TNF-α, IL-6, IL-12, and VEGF, relatively higher expression of IL-10, and a minor reduction of Arg-1 was identified as a potential AMO phenotype with anti-fibrotic properties.
Supplementary Material
Acknowledgments
Contract grant sponsor: NIH; contract grant number: R01-DC013508
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Benninger MS, Alessi D, Archer S, Bastian R, Ford C, Koufman J, Sataloff RT, Spiegel JR, Woo P. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg 1996;115(5): 474–482. [DOI] [PubMed] [Google Scholar]
- 2.Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice 2006;20(1):110–120. [DOI] [PubMed] [Google Scholar]
- 3.Graupp M, Bachna-Rotter S, Gerstenberger C, Friedrich G, Fröhlich-Sorger E, Kiesler K, Gugatschka M. The unsolved chapter of vocal fold scars and how tissue engineering could help us solve the problem. Eur Arch Oto-Rhino-Laryngol 2016;273(9):2279–2284. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau B, Hirano S, Chan RW, Welham NV, Thibeault SL, Ford CN, Bless DM. Characterization of chronic vocal fold scarring in a rabbit model. J Voice 2004;18(1):116–124. [DOI] [PubMed] [Google Scholar]
- 5.Jetté ME, Hayer SD, Thibeault SL. Characterization of human vocal fold fibroblasts derived from chronic scar. The Laryngoscope 2013;123(3):738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousseau B, Hirano S, Scheidt TD, Welham NV, Thibeault SL, Chan RW, Bless DM. Characterization of vocal fold scarring in a canine model. The Laryngoscope 2003;113(4):620–627. [DOI] [PubMed] [Google Scholar]
- 7.Tateya T, Sohn JH, Tateya I, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol 2005;114(3): 183–191. [DOI] [PubMed] [Google Scholar]
- 8.Rosen CA. Phonosurgical vocal fold injection: procedures and materials. Otolaryngol Clin North Am 2000;33(5):1087–1096. [DOI] [PubMed] [Google Scholar]
- 9.Bless DM, Welham NV, Hirano S, Nagai H, Montequin DW, Rousseau B, Ford CN. Growth factor therapy for vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol 2004;113(10): 777–785. [DOI] [PubMed] [Google Scholar]
- 10.Hirano S, Bless DM, Rousseau B, Welham N, Montequin D, Chan RW, Ford CN. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. The Laryngoscope 2004;114(3):548–556. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto Y, Hirano S, Kitani Y, Suehiro A, Umeda H, Tateya I, Kanemaru S, Tabata Y, Ito J. Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel. The Laryngoscope 2010; 120(1):108–113. [DOI] [PubMed] [Google Scholar]
- 12.Suehiro A, Hirano S, Kishimoto Y, Rousseau B, Nakamura T, Ito J. Treatment of acute vocal fold scar with local injection of basic fibroblast growth factor: a canine study. Acta Otolaryngol 2010; 130(7):844–850. [DOI] [PubMed] [Google Scholar]
- 13.Erndt-Marino JD, Jimenez-Vergara AC, Diaz-Rodriguez P, Kulwatno J, Diaz-Quiroz JF, Thibeault S, Hahn MS. In vitro evaluation of a basic fibroblast growth factor-containing hydrogel toward vocal fold lamina propria scar treatment. J Biomed Mater Res Part B: Appl Biomater 2018;106:1258–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ban MJ, Park JH, Kim JW, Park KN, Lee JY, Kim HK, Lee SW. The efficacy of fibroblast growth factor for the treatment of chronic vocal fold scarring: From animal model to clinical application. Clin Exp Otorhinolaryngol 2017;10(4):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction. J Biomed Mater Res Part A 2010;93(4):1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiwatashi N, Hirano S, Mizuta M, Tateya I, Kanemaru S-i, Nakamura T, Ito J, Kawai K, Suzuki S. Biocompatibility and efficacy of collagen/gelatin sponge scaffold with sustained release of basic fibroblast growth factor on vocal fold fibroblasts in 3-dimensional culture. Ann Otol Rhinol Laryngol 2015;124(2):116–125. [DOI] [PubMed] [Google Scholar]
- 17.Ohno T, Hirano S, Kanemaru S-i, Yamashita M, Umeda H, Suehiro A, Tamura Y, Nakamura T, Ito J, Tabata Y. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol 2007; 116(10):762–769. [DOI] [PubMed] [Google Scholar]
- 18.King SN, Guille J, Thibeault SL. Characterization of the leukocyte response in acutevocal fold injury. PLoS One 2015;10(10):e0139260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 2014;102(2): 258–269. [DOI] [PubMed] [Google Scholar]
- 20.Adhyatmika A, Putri KS, Beljaars L, Melgert BN. The elusive antifi- brotic macrophage. Front Med 2015;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluth D Pro-resolution properties of macrophages in renal injury. Kid Int 2007;72(3):234–236. [DOI] [PubMed] [Google Scholar]
- 22.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44(3):450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution- phase macrophages possess a unique inflammatory phenotype that is controlled bycAMP. Blood 2008;112(10):4117–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev 2017;122:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redente EF, Keith RC, Janssen W, Henson PM, Ortiz LA, Downey GP, Bratton DL, Riches DW. Tumor necrosis factor-a accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am J Respirat Cell Mol Biol 2014;50(4):825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Thibeault SL. Role of tumor necrosis factor-α in wound repair in human vocal fold fibroblasts. The Laryngoscope 2010; 120(9):1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wynn TA, Barron L. Macrophages: Master Regulators of Inflammation and Fibrosis. Semin Liver Dis; 2010;30(3):pp. 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn MS, Teply BA, Stevens MM, Zeitels SM, Langer R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials 2006;27(7):1104–1109. [DOI] [PubMed] [Google Scholar]
- 31.Erndt-Marino JD, Hahn MS. Probing the response of human osteoblasts following exposure to sympathetic neuron-like PC-12 cells in a 3D coculture model. J Biomed Mater Res Part A 2017;105(4): 984–990. [DOI] [PubMed] [Google Scholar]
- 32.Leslie-Barbick JE, Moon JJ, West JL. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulo- genesis in poly (ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed 2009;20(12):1763–1779. [DOI] [PubMed] [Google Scholar]
- 33.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 2005;26(16):3227–3234. [DOI] [PubMed] [Google Scholar]
- 34.Krishna P, Regner M, Palko J, Liu F, Abramowitch S, Jiang J, Wells A. The effects of decorin and HGF-primed vocal fold fibroblasts in vitro and ex vivo in a porcine model of vocal fold scarring. The Laryngoscope 2010;120(11):2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto T, Eckes B, Krieg T. Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: differential regulation by transforming growth factor-β and monocyte chemoattractant protein-1. Bioche Biophys Res Commun 2001;281(1):200–205. [DOI] [PubMed] [Google Scholar]
- 36.Vyas B, Ishikawa K, Duflo S, Chen X, Thibeault SL. Inhibitory effects of hepatocyte growth factor and interleukin-6 on transforming growth factor-β1 mediated vocal fold fibroblast- myofibroblast differentiation. Ann Otol Rhinol Laryngol 2010; 119(5):350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao H, Munoz-Pinto D, Qu X, Hou Y, Grunlan MA, Hahn MS. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater 2008;4(5):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erndt-Marino J, Trinkle E, Hahn MS. Hyperosmolar potassium (K+) treatment suppresses osteoarthritic chondrocyte catabolic and inflammatory protein production in a 3-dimensional in vitro model. Cartilage 2017; 1947603517734028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erndt-Marino JD, Munoz-Pinto DJ, Samavedi S, Jimenez- Vergara AC, Diaz-Rodriguez P, Woodard L, Zhang D, Grunlan MA, Hahn MS. Evaluation of the osteoinductive capacity of polydopamine-coated poly (ε-caprolactone) diacrylate shape memory foams. ACS Biomater Sci Eng 2015;1(12):1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Browning M, Cereceres S, Luong P, Cosgriff-Hernandez E. Determination of the in vivo degradation mechanism of PEGDA hydrogels. J Biomed Mater Res Part A 2014;102(12):4244–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiwatashi N, Hirano S, Mizuta M, Kobayashi T, Kawai Y, Si K, Nakamura T, Ito J, Kawai K, Suzuki S, et al. The efficacy of a novel collagen-gelatin scaffold with basic fibroblast growth factor for the treatment of vocal fold scar. J Tissue Eng Regenerat Med 2017;11(5):1598–1609. [DOI] [PubMed] [Google Scholar]
- 42.Rose-John S IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 2012;8(9): 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parikh RA, Wang P, Beumer JH, Chu E, Appleman LJ. The potential roles of hepatocyte growth factor (HGF)-MET pathway inhibitors in cancer treatment. OncoTargets Therapy 2014;7:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter MR. The molecular basis of IL-10 function: From receptor structure to the onset of signaling In: Fillatreau Simon, and O’Garra Anne, editors. Interleukin-10 in health and disease. Berlin Heidelberg: Springer; 2014. pp. 191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, Muise AM, Snapper SB. Interleukin 10 receptor signaling: Master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol 2014;122:177–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H-W, Chen C-H, Tsai C-L, Lin I-H, Hsiue G-H. Heterobifunctional poly (ethylene glycol)-tethered bone morphogenetic protein-2- stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Eng 2007;13(5):1113–1124. [DOI] [PubMed] [Google Scholar]
- 47.Karajanagi SS, Lopez-Guerra G, Park H, Kobler JB, Galindo M, Aanestad J, Mehta DD, Kumai Y, Giordano N, d’Almeida A. Assessment of canine vocal fold function after injection of a new biomaterial designed to treat phonatory mucosal scarring. Ann Otol Rhinol Laryngol 2011;120(3):175–184. [DOI] [PubMed] [Google Scholar]
- 48.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci USA 1999;96(6):3104–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones KS. Effects of biomaterial-induced inflammation on fibrosis and rejection. 2008;20:130–136. Elsevier. [DOI] [PubMed] [Google Scholar]
- 50.Branco A, Bartley SM, King SN, Jetté ME, Thibeault SL. Vocal fold myofibroblast profile of scarring. The Laryngoscope 2016;126(3): E110–E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witherel CE, Graney PL, Freytes DO, Weingarten MS, Spiller KL. Response of human macrophages to wound matrices in vitro. Wound Repair Regen 2016;24(3):514–524. [DOI] [PubMed] [Google Scholar]
- 52.Lynn AD, Blakney AK, Kyriakides TR, Bryant SJ. Temporal progression of the host response to implanted poly (ethylene glycol)- based hydrogels. J Biomed Mater Res Part A 2011;96(4):621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamimoto M, Mizuno S, Nakamura T. Reciprocal regulation of IL-6 and IL-10 balance by HGF via recruitment of heme oxygenase-1 in macrophages for attenuation of liver injury in a mouse model of endotoxemia. Int J Mol Med 2009;24(2):161–170. [DOI] [PubMed] [Google Scholar]
- 54.Kusunoki H, Taniyama Y, Otsu R, Rakugi H, Morishita R. Antiinflammatory effects of hepatocyte growth factor on the vicious cycle of macrophages and adipocytes. Hypertens Res 2014;37(6): 500–506. [DOI] [PubMed] [Google Scholar]
- 55.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol 2002;169(5):2253–2263. [DOI] [PubMed] [Google Scholar]
- 56.Kishimoto Y, Hirano S, Suehiro A, Tateya I, Kanemaru S-i, Nakamura T, Ito J. Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts. Ann Otol Rhinol Laryngol 2009;118(8):606–611. [DOI] [PubMed] [Google Scholar]
- 57.Luo Y, Kobler JB, Zeitels SM, Langer R. Effects of growth factors on extracellular matrix production by vocal fold fibroblasts in 3-dimensional culture. Tissue Eng 2006;12(12):3365–3374. [DOI] [PubMed] [Google Scholar]
- 58.Shi J-H, Guan H, Shi S, Cai W-X, Bai X-Z, Hu X-L, Fang X-B, Liu J-Q, Tao K, Zhu X-X. Protection against TGF-β1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res 2013;305(4): 341–352. [DOI] [PubMed] [Google Scholar]
- 59.Reitamo S, Remitz A, Tamai K, Uitto J. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Investigat 1994;94(6): 2489–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagome K, Dohi M, Okunishi K, Tanaka R, Miyazaki J-i, Yamamoto K In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-β in the lung. Thorax 2006;61(10):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park-Min K-H, Antoniv TT, Ivashkiv LB. Regulation of macrophage phenotype by long-term exposure to IL-10. Immunobiology 2005; 210(2–4):77–86. [DOI] [PubMed] [Google Scholar]
- 62.Gallucci RM, Lee EG, Tomasek JJ. IL-6 modulates alpha-smooth muscle actin expression in dermal fibroblasts from IL-6-deficient mice. J Investigat Dermatol 2006;126(3):561–568. [DOI] [PubMed] [Google Scholar]
- 63.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine 2000;12(6):671–676. [DOI] [PubMed] [Google Scholar]
- 64.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta (BBA) Mol Cell Res 2011;1813(5):878–888. [DOI] [PubMed] [Google Scholar]
- 65.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X-F, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Investigat 1998;101(2):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirano S, Bless D, Heisey D, Ford C. Roles of hepatocyte growth factor and transforming growth factor β1 in production of extracellular matrix by canine vocal fold fibroblasts. The Laryngoscope 2003;113(1):144–148. [DOI] [PubMed] [Google Scholar]
- 67.Kumai Y, Kobler JB, Park H, Galindo M, Herrera VL, Zeitels SM. Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. The Laryngoscope 2010;120(2):330–337. [DOI] [PubMed] [Google Scholar]
- 68.Jiang D, Jiang Z, Han F, Zhang Y, Li Z. HGF suppresses the production of collagen type III and α-SMA induced by TGF-β1 in healing fibroblasts. Eur J Appl Physiol 2008;103(5):489–493. [DOI] [PubMed] [Google Scholar]
- 69.Mosser DM. The many faces of macrophage activation. J Leukocyte Biol 2003;73(2):209–212. [DOI] [PubMed] [Google Scholar]
- 70.MacMicking J, Xie Q-w, Nathan C. Nitric oxide and macrophage function. Ann Rev Immunol 1997;15(1):323–350. [DOI] [PubMed] [Google Scholar]
- 71.Trinchieri G Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003;3(2):133–146. [DOI] [PubMed] [Google Scholar]
- 72.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J Immunol 2001;166(11): 6861–6868. [DOI] [PubMed] [Google Scholar]
- 73.Coudriet GM, He J, Trucco M, Mars WM, Piganelli JD. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: implications for inflammatory mediated diseases. PLoS One 2010;5(11):e15384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gazzinelli RT, Oswald I, James S, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol 1992;148(6):1792–1796. [PubMed] [Google Scholar]
- 75.Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One 2014;9(4): e94188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL- 1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood 1994;83(1):113–118. [PubMed] [Google Scholar]
- 77.King SN, Chen F, Jetté ME, Thibeault SL. Vocal fold fibroblasts immunoregulate activated macrophage phenotype. Cytokine 2013; 61(1):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Popa C, Netea MG, Van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 2007;48(4): 751–762. [DOI] [PubMed] [Google Scholar]
- 79.Piguet P-F, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respirat J 1994;7(3):515–518. [DOI] [PubMed] [Google Scholar]
- 80.Meldrum KK, Misseri R, Metcalfe P, Dinarello CA, Hile KL, Meldrum DR. TNF-α neutralization ameliorates obstruction- induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol 2007;292(4):R1456–R1464. [DOI] [PubMed] [Google Scholar]
- 81.Morimoto Y, Gai Z, Tanishima H, Kawakatsu M, Itoh S, Hatamura I, Muragaki Y. TNF-a deficiency accelerates renal tubular interstitial fibrosis in the late stage of ureteral obstruction. Exp Mol Pathol 2008;85(3):207–213. [DOI] [PubMed] [Google Scholar]
- 82.Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol 2011;2011:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banerjee I, Fuseler JW, Intwala AR, Baudino TA. IL-6 loss causes ventricular dysfunction, fibrosis, reduced capillary density, and dramatically alters the cell populations of the developing and adult heart. Am J Physiol Heart Circulat Physiol 2009;296(5): H1694–H1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M. IL-6 induces an antiinflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 2003;4(6):551–556. [DOI] [PubMed] [Google Scholar]
- 85.McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, Kozma SC, Drew AF. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol 2010;184(12):7219–7228. [DOI] [PubMed] [Google Scholar]
- 86.Ip WE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Antiinflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017;356(6337):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol 2005;16(12):3651–3660. [DOI] [PubMed] [Google Scholar]
- 88.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Investigat Dermatol 2013;133(10):2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153(2):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, Liechty KW. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Investigat Dermatol 2008;128(7): 1852–1860. [DOI] [PubMed] [Google Scholar]
- 91.Makita N, Hizukuri Y, Yamashiro K, Murakawa M, Hayashi Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int Immunol 2014;27(3):131–141. [DOI] [PubMed] [Google Scholar]
- 92.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology 2004;25(12):677–686. [DOI] [PubMed] [Google Scholar]
- 93.Koscsó B, Csóka B, Kókai E, Németh ZH, Pacher P, Virág L, Leibovich SJ, Haskó G. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukocyte Biol 2013;94(6): 1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008;13:453–461. [DOI] [PubMed] [Google Scholar]
- 95.Lagathu C, Bastard J-P, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun 2003; 311(2):372–379. [DOI] [PubMed] [Google Scholar]
- 96.Huang S-P, Wu M-S, Shun C-T, Wang H-P, Lin M-T, Kuo M-L, Lin J-T. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci 2004;11(4): 517–527. [DOI] [PubMed] [Google Scholar]
- 97.Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem 2005;280(43): 36099–36109. [DOI] [PubMed] [Google Scholar]
- 98.Verjee LS, Verhoekx JS, Chan JK, Krausgruber T, Nicolaidou V, Izadi D, Davidson D, Feldmann M, Midwood KS, Nanchahal J. Unraveling the signaling pathways promoting fibrosis in Dupuyt- ren’s disease reveals TNF as a therapeutic target. Proc Natl Acad Sci USA 2013;110(10):E928–E937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol 2015;43:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Du S, Wang S, Wu Q, Hu J, Li T. Decorin inhibits angiogenic potential of choroid-retinal endothelial cells by downregulating hypoxia-induced Met, Rac1, HIF-1α and VEGF expression in cocultured retinal pigment epithelial cells. Exp Eye Res 2013;116: 151–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.