ABSTRACT
Background
We describe the clinical and neuropathological features of a patient with T‐cell‐mediated paraneoplastic limbic encephalitis, parkinsonism, hypothermia, and narcolepsy‐like presentation associated with endometrial carcinoma.
Objectives
This patient with prominent parkinsonism and narcolepsy broadens the phenotype of known paraneoplastic syndromes and demonstrates the importance of investigation for occult malignancy even in the absence of paraneoplastic antibodies.
Methods
This is a case report with diagnosis confirmed at postmortem.
Results
Paraneoplastic antibodies were not detected. The initial improvement with immunosuppression was short lived, and postmortem neuropathological examination demonstrated encephalitis with predominant T‐cell infiltration affecting the hypothalamus and extending to the brainstem, suggestive of a paraneoplastic syndrome.
Conclusions
Although the possibility of a novel antibody cannot be ruled out, consideration must also be given to recent demonstration of purely T‐cell‐mediated neuronal destruction in the context of paraneoplastic syndromes.
Keywords: Paraneoplastic, limbic encephalitis, parkinsonism, endometrial carcinoma
Paraneoplastic neurologic syndromes (PNS) are rare complications of underlying cancer. Central nervous system involvement is typically associated with limbic encephalitis, encephalomyelitis, subacute cerebellar degeneration, or opsoclonus myoclonus. The main research focus has been on identifying an increasing number of onconeural antibodies to intracellular antigens associated with a clinical paraneoplastic syndrome. As a result, most clinicians will use ever‐expanding panels of autoimmune or paraneoplastic antibodies when screening a patient for possible autoimmune or PNS. It remains likely that most of these paraneoplastic antibodies are markers for an underlying malignancy and are not pathogenic. Furthermore, there is also evidence that onconeural antigen specific T‐cells can attack neuronal tissue with shared antigenicity to the tumor tissue causing PNS.1 Thus the unsuspecting clinician may miss the diagnosis of PNS if he or she relies solely on an antibody screen and also may miss an opportunity to guide treatment toward cell therapy rather than antibodies alone.
Small‐cell lung cancer and breast and gynecological cancer are the most frequently associated malignancies.2 PNS often present subacutely and are usually associated with markers of inflammation in the cerebrospinal fluid (CSF) such as elevated protein, elevated IgG, and an oligoclonal band pattern suggestive of intrathecal synthesis.
For definitive diagnosis of PNS, Graus and colleagues3 recommend that the syndrome meet 1 of 4 criteria (Table 1). PNS often precede detection of the malignancy itself and can lead to delayed diagnosis and increased morbidity. Unfortunately, to date the treatments of PNS are limited in their efficacy compared to the autoimmune neurological disorders probably because the antigenic stimulus is intracellular. Still, early detection and diagnosis of PNS is crucial, as appropriate management can sometimes improve both quality of life and survival.
Table 1.
Criteria for paraneoplastic syndrome diagnosis, adapted from Graus and colleagues3
|
Involvement of the hypothalamus can cause narcolepsy and has been described with anti‐Ma1 and Ma2 antibodies,4, 5 and also with ANNA2 (anti‐Ri).6 Mandel‐Brehm and colleagues7 very recently described brainstem encephalitis with hearing loss associated with Kelch‐11 antibodies and seminomas. Ma1 and 2 are intracellular proteins largely expressed in the brainstem, midbrain, basal ganglia, hypothalamus, and limbic structures.8 Paraneoplastic parkinsonism is extremely rare. It has been described in association with anti‐Ma29 and anti‐CRMP510 and recently in association with LGI1 and new uncharacterized antibodies.11
Here, we report a case of autopsy‐confirmed paraneoplastic, T‐cell‐mediated limbic encephalitis with parkinsonism, hypothermia, and narcolepsy‐like presentation without an identifiable antibody in serum (CSF not tested) associated with endometrial carcinoma.
Case Report
A 65‐year‐old woman presented to the neurology service with subacute parkinsonism (reduced blink rate, masked facies, bradykinesia, rigidity, jerky saccades, and bradyphrenia). The patient also reported uncontrollable daytime sleepiness and sudden episodes of muscle weakness triggered by laughter, consistent with narcolepsy and cataplexy. Her history included hypertension and estrogen‐receptor positive breast carcinoma, diagnosed 13 years previously and treated with wide local excision, 6 cycles of chemotherapy, and 5 years of hormonal therapy. Five weeks after symptom onset, she deteriorated and was admitted to her local hospital with increasing drowsiness, confusion, hypothermia (body temperature of 27°C), and bradycardia (38 beats per minute). The patient became comatose (Glasgow Coma Scale of 6) and required intubation. After 5 days, she was transferred to our hospital. Computed tomography of the brain was normal, and the patient was started on empiric cephalosporin and acyclovir but showed no improvement. CSF albumin (0.536 g/L, normal 0.140–0.200 g/L), IgG (0.125g/l, normal 0.020–0.040g/L), and white cells (6 cells/μL, normal 0–5 cells/μL) were raised, and unmatched oligoclonal bands were present in the CSF. HIV, syphilis, and other viral screening were negative. Because of the suspicion of a paraneoplastic syndrome (subacute encephalopathy and autonomic dysfunction), the patient received intravenous methylprednisolone (1 g/day) for 5 days followed by a tapering dose of oral prednisolone. The patient demonstrated an initial response to steroid treatment with reduced levels of confusion and increased mobility.
Magnetic resonance imaging of the brain showed fluid‐attenuated inversion recovery (FLAIR) hyperintensities in the anterior and medial temporal lobes, mammillary bodies, and dorsal midbrain (Fig. 1A–C). Electroencephalogram showed nonspecific background slowing. A Western blot analysis of serum was negative for anti‐Hu, Ri, Yo, Ma1, Ma2, anti‐CV2/CRMP5 and antiamphiphysin. At the time of testing, other newer antibodies associated with autoimmune parkinsonism, sleep disorders, and encephalopathy (IgLON5) or PNS (Kelch‐11) were not available for testing.
Figure 1.
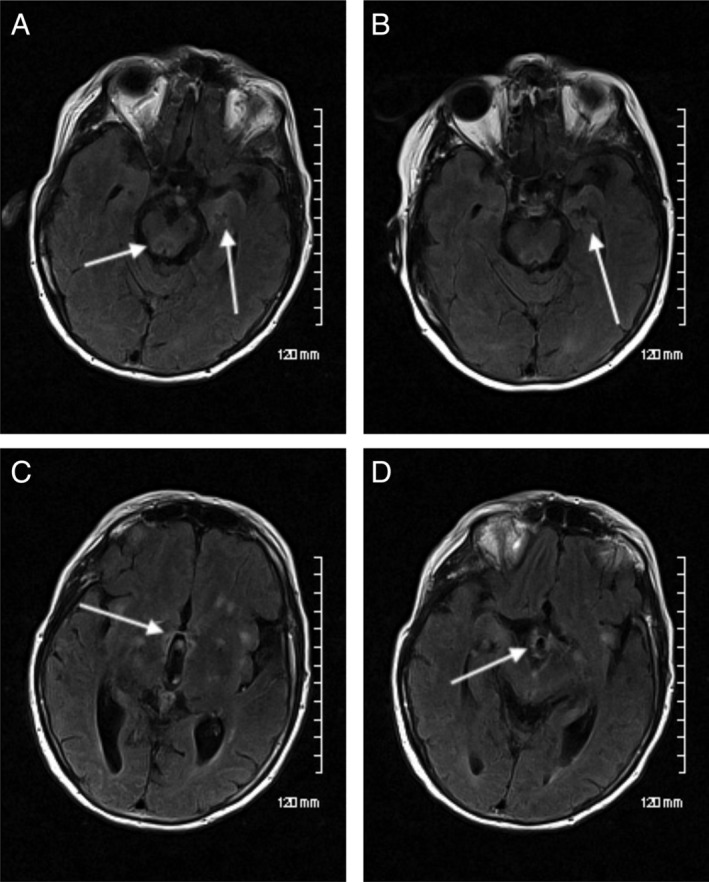
Initial axial FLAIR sequences demonstrating high signal in the left medial temporal lobe and dorsal midbrain (A), mammillary bodies (B), hypothalamic region (C), and patchy high signal in the subcortical insular regions (C). Axial FLAIR sequence after steroid treatment demonstrating interval improvement of the high signal in the left medial temporal lobe (indicated by arrow; D). FLAIR, fluid‐attenuated inversion recovery.
CSF hypocretin levels were low at 190 pg/ml (normal >200 pg/ml). Hypocretin levels between 100 and 200 are not uncommon in patients with neurological disorders associated with sleep disturbance, such as focal hypothalamic lesions and head trauma.12 Levels <110 pg/ml are highly suggestive of narcolepsy.13
F‐18 glucose body positron emission tomography/computed tomography demonstrated increased uterine endometrial metabolic activity to a maximum standardized uptake value of 31. Endometrial curettings showed a high‐grade, poorly differentiated adenocarcinoma. Hysterectomy and lymph node dissection were performed, and pathology showed a high‐grade adenocarcinoma stage pT1a, N0; FIGO stage 1A.
Carboplatin and paclitaxel chemotherapy were commenced. The patient initially responded to treatment with an improvement of hypothermia from 35.7°C to 36.8°C 4 days post the first cycle. Follow‐up magnetic resonance imaging showed a slight decrease in the size and intensity of the FLAIR hyperintensities (Fig. 1D). Electroencephalogram also showed improvement of background slowing. Despite treatment, the patient subsequently deteriorated suddenly and died of autonomic crisis within 1 year of the onset of symptoms.
Pathological Findings
Postmortem histopathological examination showed encephalitis with neuronophagia (Fig. 2A), gliosis (Fig. 2B), microglial nodules (Fig. 2C), and perivascular and parenchymal inflammation (Fig. 2D) predominantly in the hypothalamic area and extending to involve the medial temporal lobes and especially the amygdala. Extension into the brainstem with involvement of the substantia nigra without depigmentation was also a feature. The cortex, including the hippocampi, was relatively unaffected. The cerebellum showed normal molecular, purkinje, and granular cell layers, and the dentate nucleus was unaffected. The spinal cord was also unaffected.
Figure 2.
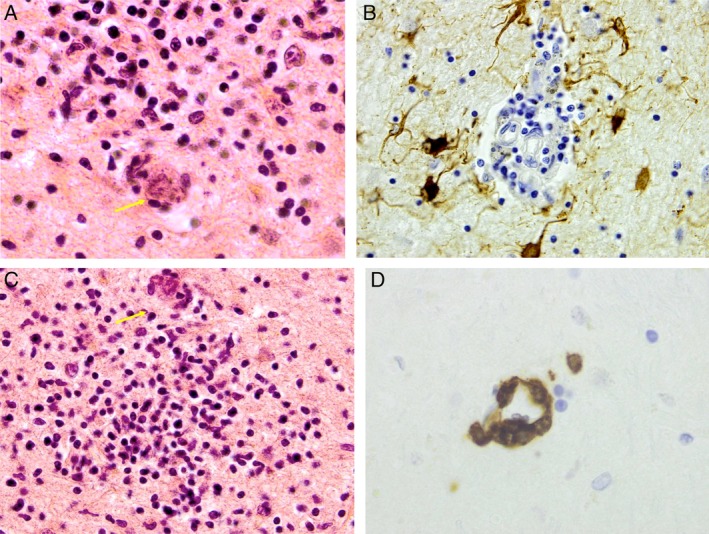
Neuropathological images from the hypothalamus showing (A) neuronophagia, hematoxylin and eosin × 60 (note arrow highlighting neurone); (B) gliosis–GFAP × 40; (C) microglial nodule, hematoxylin and eosin × 40 (note arrow highlighting neurone); and (D) perivascular lymphocytes CD3 × 60. GFAP, Glial fibrillary acidic protein.
Immunocytochemistry showed lymphocytic infiltration of B‐cells and T‐cells. The majority of lymphocytes were CD8 suppressor T‐cells. Alpha‐synuclein staining was negative for Lewy bodies, and there was also no evidence of tau or amyloid deposits. The brain section immunostaining for viral antigens was negative. Subsequent histological examination of the endometrial tumor with hematoxylin and eosin staining did not reveal any significant inflammatory infiltrate.
Discussion
We report a patient with antibody‐negative, T‐cell‐predominant paraneoplastic limbic encephalitis with hypothalamic and brainstem involvement. Our patient's clinical syndrome is similar to that described with anti‐Ma2 antibody positive paraneoplastic encephalitis with limbic and brainstem encephalitis, hypothalamic involvement manifesting with excessive daytime sleepiness and narcolepsy, low CSF hypocretin levels, and atypical parkinsonism.14 Similar to our patient, anti‐Ma2 paraneoplastic syndromes do not respond well to immunosuppression or other treatments probably because it is an intracellular protein. In our patient, the pathology was primarily T‐cell‐mediated parenchymal and perivascular inflammation associated with uterine adenocarcinoma. The clinical and radiological features were consistent with an inflammatory process primarily in, but not limited to, the hypothalamus. This was confirmed at autopsy. Pure T‐cell‐mediated neuronal destruction is rare but has been described with Rasmussen's encephalitis, and we have described previously antibody‐negative, T‐cell‐mediated paraneoplastic dentate ganglionopathy.15 Paraneoplastic antibody negativity with autopsy‐proven, T‐cell‐mediated neuronal destruction is rarely described. Table S1 lists 4 such examples available in the literature.
It is possible that our patient had an unknown or novel paraneoplastic antibody or autoimmune antibody (eg, IgLON5). However, we did not detect the most likely antibodies (anti‐Ma1, Ma2 or ANNA‐2 [anti‐Ri]) for the clinical syndrome, nor did we detect any known paraneoplastic antibody in an extended paraneoplastic panel. Furthermore, the autopsy showed a T‐cell‐predominant process rather than a B‐cell‐mediated antibody process. Moreover, the paraneoplastic antibodies are thought to be markers for an associated cancer and not pathogenic. Pittock and colleagues6 reported paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti‐Ri), and at autopsy 1 patient had diffuse infiltration by CD8+ T‐lymphocytes with axonal loss and gliosis in the brainstem and spinal cord tracts. We did not test the patient's CSF for paraneoplastic antibodies, and it is known that combined CSF and serum testing is more sensitive than testing serum alone.16
IgLON5 autoimmunity is a recently described rare disorder associated with the insidious onset of sleep and brainstem disorders with parkinsonism and a median disease onset of 62 years and is associated with an accumulation of phosphorylated tau.17 Although we were unable to test for this particular antibody in our patient, at autopsy there was no evidence of tau deposits, making IgLON5 autoimmunity highly unlikely.
Paraneoplastic parkinsonism is incredibly rare. In our patient it was most likely the result of the basal ganglia and substantia nigra inflammation and dysfunction. The hypothermia and narcolepsy‐like features were most likely the result of neuronal destruction in the hypothalamus. Although a low hypocretin level of 190 pg/mg did not meet the definitive diagnostic criteria for narcolepsy, it is consistent with a neurological sleep disorder.13
Autoimmune and paraneoplastic conditions with broader phenotypes are increasingly recognized as our awareness and understanding develops. The treatments vary, but more options must be explored, particularly those targeted toward intracellular antigens associated with worse outcomes, and perhaps cell‐mediated neuronal injury is more likely to be involved in the pathogenesis of these disorders. Remarkably, there is also a significant overlap between autoimmune (IgLON5) or paraneoplastic pathology with that of neurodegenerative disease. In summary, the clinician must keep a high index of suspicion and pursue the diagnosis of PNS even in patients who are “antibody negative” as not all PNS have been discovered yet, the antibody titer may be low in the body fluid (serum, CSF) tested, or the patient has a T‐cell‐mediated disease. For this reason, a strong index of suspicion is required to avoid missing a critical and possibly treatable diagnosis.
Author Roles
(1) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique; (2) A. Neuropathology.
D.B.: 1A
O.C.M.: 1B
C.F.: 1B
F.B.: 1B, 2A
B.M.: 1B
T.L.: 1B
Disclosures
Ethical Compliance Statement: The authors confirm that ethical approval from an institutional review board was not required. Informed patient consent was obtained for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: No funding was provided in the production of the manuscript. The authors have no conflicts of interest to declare.
Financial Disclosures for the Previous 12 Months: Dr. TIm Lynch received grant support from the Health Research Board in Ireland.
Supporting information
Supplementary Table S1 Other antibody negative with autopsy proven T‐cell‐mediated neuronal destruction.
Acknowledgments
We acknowledge the contributions and feedback of Dr. Valerie Reid, Department of Neurophysiology, Mater Misericordiae University Hospital, Dublin, Ireland; Professor Eoin Kavanagh, Department of Radiology, Mater Misericordiae Hospital, Dublin, Ireland; and Professor Conor O'Keane, Department of Pathology, Mater Misericordiae University Hospital, Dublin, Ireland.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Pilli D, Zou A, Brilot F, et al. Expanding role of T cells in human autoimmune diseases of the central nervous system. Front Immunol 2017;8:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pelosof L, Gerber, D . Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 2010;85(9):838–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graus F, Delattre J, Antoine J, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalmau J, Rosenfeld M. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008;7(4):327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams C, McKeon A, Silber M, Kumar R. Narcolepsy, REM sleep behaviour disorder and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Arch Neurol 2011;68(4):521–524. [DOI] [PubMed] [Google Scholar]
- 6. Pittock S, Parisi J, Lennon V, et al. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti‐Ri). Arch Neurol 2010;67(9):1109–1115. [DOI] [PubMed] [Google Scholar]
- 7. Mandel‐Brehm C, Dubey D, Pittock S, et al. Kelch‐like protein 11 antibodies in seminoa‐assoicated paraneoplastic encephalitis. N Engl J Med 2019;381(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lein E, Hawrylycz M, Ao N, et al. Genome‐wide atlas of gene expression in the adult mouse brain. Nature 2007;445(7124):168–176. [DOI] [PubMed] [Google Scholar]
- 9. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti‐Ma2‐associated encephalitis. Brain 2004;127(8):1831–1844. [DOI] [PubMed] [Google Scholar]
- 10. Yap SM, Lynch T, MacMahon P, Murray B. Paraneoplastic atypical parkinsonism with anti‐CRMP5 antibodies and severe caudate and putaminal hypometabolism on 18‐fluorodeoxyglucose positron emission tomography of the brain. Mov Disord Clin Pract 2017;4:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kannoth S, Anandakkuttan A, Mathai A, Sasikumar AN, Nambiar V. Autoimmune atypical parkinsonism—a group of treatable parkinsonism. J Neurol Sci 2016;362:40–46. [DOI] [PubMed] [Google Scholar]
- 12. Ripley B, Overeem S, Fujiki N, et al. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology 2001;57(12):2253–2258. [DOI] [PubMed] [Google Scholar]
- 13. Mignot E, Lammers G, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 2002;59(10):1553–1562. [DOI] [PubMed] [Google Scholar]
- 14. Dalmau J, Graus F, Villarejo A. Clinical analysis of anti‐Ma2‐associated encephalitis. Brain 2004;127(8):1831–1844. [DOI] [PubMed] [Google Scholar]
- 15. O'Toole O, Murphy O, Farrell M, et al. Paraneoplastic dentate ganglionopathy: clinical and neuroimmunologic studies. Clin Neuropathol 2015;34(1):34–39. [DOI] [PubMed] [Google Scholar]
- 16. McKeon A, Pittock S, Lennon A. CSF complements serum for evaluating paraneoplastic antibodies and NMO‐IgG. Neurology 2011;76(12):1108–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Honorat J, Pittock S, McKeon A. IgLON 5antibody. Neurological accompaniments and outcomes in 20 patients. Neurology 2017;4(5):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drlicek M, Bodenteich A, Grisold W, et al. T cell‐mediated paraneoplastic ganglionitis—an autopsy case. Acta Neuropathol 2000;99(5):599–602. [DOI] [PubMed] [Google Scholar]
- 19. Graus F, Escudero D, Dalmau J, et al. Syndrome and outcome of antibody‐negative limbic encephalitis. Eur J Neurol 2018; 25(8):1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Other antibody negative with autopsy proven T‐cell‐mediated neuronal destruction.


