https://onlinelibrary.wiley.com/page/journal/23301619/homepage/mdc312888-sup-v001.htm
https://onlinelibrary.wiley.com/page/journal/23301619/homepage/mdc312888-sup-v002.htm
Functional movement disorders (FMDs) are common referrals to movement disorders clinics.1, 2, 3 Disability rates remain high despite appropriate diagnosis,4, 5 an unfortunate outcome given that FMDs disproportionately affect young individuals. Historically, neurologists’ role has been limited to diagnosis and referral to psychiatry. Low improvement rates show us that this system is of limited therapeutic value for patients and is dissatisfying for providers. Here we propose a new treatment model for FMD and support our viewpoint with early observations from a pilot clinic.
FMDs are defined by aberrations of motor system functioning in the absence of clear structural neuropathology. They are often syndromic, extending beyond abnormal movements to include fatigue, chronic pain, and other functional symptoms. The biopsychosocial model proposes that predisposing physical and/or psychological factors confer vulnerability to FMDs, which then become manifest in the context of precipitating events or psychological states.6 Imaging studies point to an abnormal motor–limbic interface7 and deficits in sensorimotor integration and agency.8, 9 Abnormal symptom‐related beliefs and expectations, heightened self‐directed attention,10 maladaptive conditioning,11 and autonomic and neuroendocrine changes12 are also relevant pathophysiological factors.
Functional symptoms remain underdiagnosed and relatively unknown to the general public despite being the second most common reason to see a neurologist.13 The patient experience with health care providers is frequently one of invalidation and incomplete explanation of their symptoms, which serves to further entrench maladaptive illness beliefs. These factors, coupled with the stigma associated with an explanation of physical symptoms as entirely attributable to psychological factors, create barriers for diagnosis agreement and subsequent treatment. Despite this, promising results have been shown with physiotherapy,14, 15, 16 cognitive behavioral therapy,17, 18 psychodynamic therapy,19 and integrated care models for the treatment of psychogenic nonepileptic seizures.20
Although there is general agreement that patients require a combination of psychological and physical treatment, the execution of this is challenged by healthcare delivery models that see neurologists, psychiatrists, and physiotherapists working in isolation as opposed to real‐time collaboration. On the premise that FMDs reflect maladaptive integration of “psychological” and “physical” brain functions, siloed models of care serve to perpetuate this disintegrative process. Assembling an integrated treatment team, in contrast, might serve to minimize invalidation and to hold together mind and body, as this very integration is embedded within the treatment team itself. To this end, multidisciplinary treatment models have shown good outcomes in the FMD population in both inpatient21, 22, 23 and outpatient23, 24 settings. In such models, although all therapeutic elements are there, often disciplines still practice in parallel from their individual perspectives (Fig. 1). Aybek and colleagues24 used a semiintegrated model in which 12 patients diagnosed with conversion disorder within the previous 2 weeks were seen in shared consultation by the neurologist and psychiatrist at the beginning and end of an individualized treatment plan compared to standard care. Patients receiving joint care reported good long‐term outcomes at 3 years, with significant improvement in symptom severity, and only 20% of patients had ceased working compared with 70% of patients in the standard‐care group.
Figure 1.
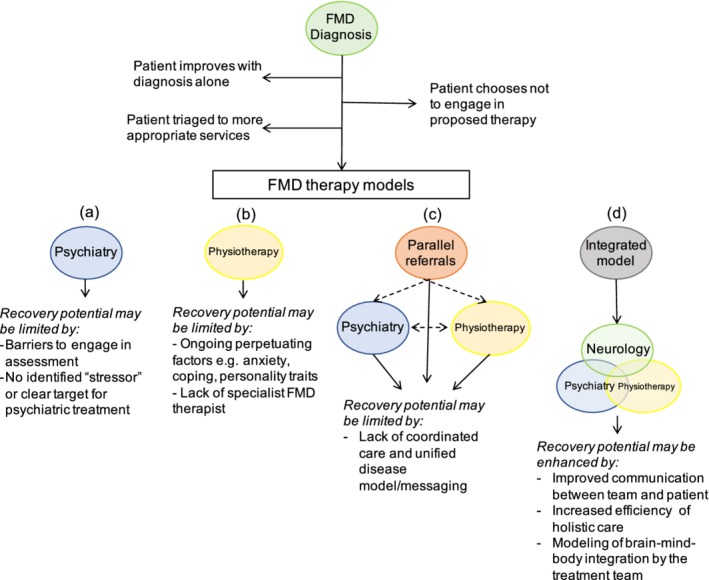
Common limitations of treatment pathways for functional movement disorders (FMDs) as a rationale for integrated care. The patient is first diagnosed by the neurologist. Some patients will improve with a diagnosis alone, others may choose not to engage in the proposed therapy and some patients may require triaging to other more appropriate services (eg, patients with a chronic pain as the dominant symptom). Remaining patients may be referred to psychiatry (1), physiotherapy (2), or both (3). Monotherapy models may miss opportunities to address relevant predisposing or perpetuating factors for FMDs (as examples, psychiatrists are rarely trained to do intensive body work that could help treat physical symptoms and a physiotherapist might struggle to treat anxiety symptoms that commonly arise during physical therapy). Multidisciplinary models offer patients a variety of treatments but may miss opportunities to demonstrate to patients that physical and psychological “systems” form parts of an integrated whole. Coordination of care in multidisciplinary models is often challenging. Integrated care is resource intensive but provides opportunities for the efficient delivery of highly individualized care that simultaneously treats the brain, body, and mind.
We propose that recovery will be optimized by individualized therapy that goes beyond multidisciplinary, where neurology, psychiatry, and physical therapy are integrated throughout the course of treatment. We created a pilot clinic to investigate the feasibility and efficacy of such a model. A total of 11 patients (8 women; mean age 35.4 ± 12.1 years, mean symptom duration 6.2 ± 7.0 years) were enrolled in a 6‐session, outpatient biweekly treatment program where therapy was simultaneously delivered by the neurologist, neuropsychiatrist, and physiotherapist in 45‐minute appointments. Therapeutic plans were individually tailored to a combination of the patients’ goals, phenomenology, and psychiatric formulation (eg, relevant personality traits, maladaptive coping). Potential treatment targets were drawn from a biopsychosocial framing of each individual FMD syndrome as occurring as a result of predisposing, precipitating, and perpetuating factors. Treatable perpetuating factors were emphasized as therapeutic targets, for example, undiagnosed anxiety. Importantly, as the formulation evolved, so did the nature of treatment. The presence of 3 providers enabled flexibility in the approach depending on how the patient's syndrome changed during the course of therapy. For example, some patients benefited from heavy application of physical therapy upfront with slower integration of psychological treatment strategies; others needed to spend more time targeting psychological factors before physical therapy could even be attempted. Creative problem‐solving around functional deficits arose organically within the sessions, resulting from a blending of the different professional perspectives. The approach is further outlined in Material S1.
The primary outcome for clinical efficacy was the Clinical Global Impression–Improvement scale, with secondary clinical and quality‐of‐life scales administered at baseline and post–program completion. At program completion, 7/11 (64%) of patients had “much” or “very much” improved, which was sustained at 3 months. Those with “mild” or no improvement (4/11) had longer disease duration and were older in age. The results are presented in Material S1. Examples of selected treatment approaches are outlined in the case vignettes.
Clinical Vignette 1
A 24‐year‐old nurse (subject 8; Video S1) presented with abrupt onset of tremor, full‐body spasms, gait disorder, and falls. Neuropsychiatric evaluation revealed longstanding symptoms of anxiety, depression, posttraumatic stress, and affect dysregulation with self‐harm. No clear trigger was identified before symptom onset. We learned that dance was an area of healthy pride for her, so we created a motor retraining program based on ballet exercises that effectively tapped into highly practiced motor programs while targeting her emotional dysregulation. Her motor symptoms improved gradually in parallel with an observed return of confidence and agency.
Clinical Vignette 2
A 31‐year‐old student (subject 9; Video S2) presented with the abrupt onset of truncal jerks associated with pain and sensory changes 2 months after a minor motor vehicle accident. His personal history was significant for exposure to civil war and violent conflict. Treatment included desensitization and uncoupling of sensory triggers from motor symptoms, cognitive behavioral therapy to target fear of symptoms/disease, and normalizing movement patterns by engaging well‐learned motor programs (eg, dribbling a soccer ball). His symptoms remitted by the fifth clinic visit, and importantly, this occurred without confronting his past history of trauma.
Clinical Vignette 3
A 49‐year‐old woman on long‐term disability (subject 2) presented with a 20‐year duration of right leg weakness and bilateral upper limb tremor. Her symptoms were preceded by a ligamentous injury to her right knee for which she underwent multiple surgeries with no improvement, leaving her with residual chronic pain/immobility. She had no formal psychiatric history. She had undergone multiple courses of physiotherapy with no benefit. Relevant factors to her formulation included a profound alexithymia, maladaptive illness beliefs, and a high degree of resistance to modifications in activity given the expectation of pain. Agreement with the diagnosis of a functional movement disorder was limited. We attempted a variety of treatments including transcranial electrical stimulation paired with motor retraining and attentional redirection exercises with demonstration of tremor improvement. Pain and fatigue were reported barriers to implementation of exercises at home. At the end of the program, the patient's functional symptoms persisted, unchanged.
This pilot clinic for FMD demonstrates the feasibility of an integrated model and has generated valuable lessons that would benefit from further exploration.
1. An Integrated Treatment Model Is an Effective Approach to Treat FMD
An integrated approach exposes the patient to treatment modalities that he or she may not have considered or been willing to explore. Neurology referral to psychiatry—particularly as the sole avenue of treatment—is experienced by patients as confusing and invalidating given the physical nature of functional symptoms.25, 26 A truly integrated model challenges the dualistic notion that mind and brain are separate and instead attempts to help patients appreciate that movement, sensory processing, cognition, and emotions are intimately connected. In our model, psychiatric comorbidities and psychosocial stressors were appropriately managed but considered perpetuating rather than causal factors. For the patient, there was meaning in having his or her neurologist witness the disclosures of anxiety and trauma and to still consider their abnormal movement as a valid disorder of the brain. Likewise, a psychiatrist who strays into physiotherapy's domain to demonstrate how stress affects body movement demonstrates important connections between emotion and body. Our cohort had chronic FMD symptoms and many had either failed prior treatment attempts or had not accepted psychiatric referral prior to participation in our program. Some had paroxysmal symptoms that would not have been traditionally seen as being amenable to physical therapy. Distilling all treatment modalities into a single session was an efficient way to deliver care, as evidenced by improved outcomes within a short (6‐session) time frame. Future controlled trials could determine if an integrated approach achieves results faster than segregated parallel therapies.
2. A Shared Treatment Environment Fosters Innovation for the Acquisition of New Clinical Skills and the Development of New Therapies
From the practitioner perspective, a joint clinic fostered the acquisition of knowledge and new therapeutic skills. The neurologist learned to screen for anxiety and dissociation in the neurological history (which also increases diagnostic yield) and to incorporate physical therapy strategies into his or her physical exam. The psychiatrist began to dovetail the demonstration of positive examination signs and the provision of psychotherapy. The physiotherapist integrated mindfulness and relaxation strategies with physical exercises. This transdisciplinary approach was both rewarding and effective. From a treatment perspective, the blurring of traditional discipline boundaries resulted in new paradigms for future study. For example, functional goal setting was the starting point for all therapeutic plans—this is an approach unfamiliar to neurologists but commonly used in physical therapy and rehabilitation medicine. Flexible treatment planning led to novel strategies such using childhood motor programs (eg, dance, fencing) to promote normal movement while targeting anxiety, confidence, and agency. In this way, symptoms previously considered the domain of psychiatry were specifically treated via integrated body‐focused work. Given their presenting deficits in motor function, patients appreciated that physical strategies were included in their treatment plans.
3. Reliable Outcome Measures for FMD Require Development
The FMD population is heterogeneous. Abolishing abnormal movements may not reflect meaningful improvement if the patient has a persistent FMD syndrome with disability, pain, and fatigue. Large, rigorous controlled studies and standardized outcome measures tied to diagnostic scales27, 28 are lacking. Quality‐of‐life questionnaires showed inconsistent results that were in many cases discrepant with the patients’ own self‐reports. Future studies should explore measurements of the presenting symptom versus measurement of the FMD syndrome and disability and the tension between subjective and objective measures in this population. Candidate outcome measures could include agreement of FMD diagnosis, measures of agency, and change in symptom threat value.
4. Some Patients Will Not Improve with Therapy
Long‐term follow‐up studies in FMD indicate that the majority of patients are the same or worse at follow‐up.4 These data likely underestimate recovery potential; although multiple studies show high percentages of disabled patients with moderate to severe quality‐of‐life impairments,29 many cohorts were largely untreated or treated with a single modality. In our experience, chronic patients with strong perpetuating factors and disagreement with the diagnosis are less likely to respond to treatment, and resources should be allocated appropriately. In chronic patients, treatment may only but still importantly accomplish prevention of further iatrogenic harm. This latter point can be very problematic because patients will often seek further investigations, and both well‐meaning and beleaguered health care providers often capitulate to these requests. Mechanisms to triage patients require further development.
This model has limitations. Given the prevalence of FMD, joint treatment as the sole clinic model is too resource intensive to be sustainable. One could envision a role for an integrated clinic as a stream of care for selected patients in addition to other streams of care such as physiotherapy alone or psychological therapy alone or as well‐placed transdisciplinary “top‐up” sessions. Development of an integrated clinic requires the participation of motivated and interested practitioners who are open to changing their practice, which can be daunting. Controlled studies of integrated multidisciplinary interventions would represent a step forward in this area, and future research should attempt to link disease mechanism and/or new phenotypes with treatment and whether this approach would generalize to other functional neurological disorders. Although our outcomes are encouraging, long‐term studies are required to see if these outcomes are sustained. We hope that sharing this preliminary experience will inspire other groups to explore deeper interprofessional collaborations and develop novel paradigms of care for this challenging and underserved patient population.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
S.C.L.: 1A, 1B, 1C, 2A, 2B, 3A
L.M.: 1A, 1B, 1C, 3A
A.E.L.: 3B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. Informed consent was obtained from patients to participate in a pilot clinic program as treatment for their symptoms. Informed consent was separately obtained for all videotapes taken as part of the routine clinical evaluation as per standard policy in the Toronto Western Hospital Movement Disorders Clinic. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
All authors declare that there is no conflict of interest. No specific funding was received for this work.
Financial Disclosures for the Previous 12 Months
S.C.L. is a recipient of the Parkinson Canada Clinical Fellowship Award. L.M. has no disclosures. A.E.L. reports the following: consultancies with Abbvie, Acorda, Biogen, Intracellular, Lundbeck, Sun Pharma, Kallyope, Retrophin, Paladin, Seelos, Theravance, Roche, and Corticobasal Degeneration Solutions; advisory boards of Jazz Pharma, PhotoPharmics, Sunovion; Honoraria from Sun Pharma, AbbVie, and Sunovion; grants from Brain Canada, Canadian Institutes of Health Research, Corticobasal Degeneration Solutions, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, and W. Garfield Weston Foundation; employment with University Health Network and University of Toronto; and royalties from Elsevier, Saunders, Wiley‐Blackwell, Johns Hopkins Press, and Cambridge University Press.
Supporting information
Material S1. Additional methods and results.
Table S1. Baseline demographic characteristics and main outcome (Clinical Global Impression–Improvement, CGI‐I) score. FMD, Functional Movement Disorder; BDI, Beck Depression Inventory; SA, State anxiety scale; TA, Trait anxiety scale; PTSD, posttraumatic stress disorder; GAD, generalized anxiety disorder; MDE, major depressive episode; NR, no response. CGI‐I scores are presented as a mean of 3 scores which were independently rendered by the 3 treating clinicians. Subject 3 was invited to return for the second cohort because of missed sessions in the first cohort.
Video S1. A 24‐year‐old nurse presented with tremor, dystonia, and abnormal gait. The baseline video demonstrates functional dystonic posturing, a variable and distractible tremor of the upper limbs, and a noneconomical gait consistent with a mixed functional movement disorder. Neuropsychiatric evaluation revealed longstanding symptoms as described in the text. Relevant factors to her formulation included chronic feelings of invalidation, deficits in self‐assertion and boundary setting and an unconscious reinforcement of physical symptoms by satisfaction of unmet emotional needs. Motor retraining was based on the patient's history of dancing that was simultaneously used to target the relevant psychological deficits in her formulation. Breathing, body awareness, and mindfulness exercises synchronized to classical music were integrated with motor retraining. The patient remitted by the final clinic and her gait normalized.
Video S2. A 31‐year‐old student presented with the abrupt onset of axial jerks following a motor vehicle accident. He developed thoracic pain that evolved into an area of sensitivity that triggered a cluster of spasms if touched. As a result, he was unable to lean back while seated on a chair or wear a backpack. Baseline video shows episodic clusters of severe truncal flexion/extension jerks while sitting and walking. He had a history of exposure to violent conflict including witnessing the murder of his father yet did not endorse symptoms of posttraumatic stress disorder. We hypothesized that his accident triggered autonomic hyperarousal that evolved to a sustained anxiety response, overlaid with catastrophic thought patterns (eg, fear of death from his symptoms.) This was overlaid with a strong conditioned response between environmental triggers (eg, the sensation of a backpack touching his back) and physical symptoms (pain and axial jerks). The treatment plan is as described in the text. The patient remitted by the fifth clinic visit, and the patient was able to dribble a soccer ball, wear a backpack, and run.
Acknowledgments
The authors acknowledge the contributions of Monique Josef and Lisa Muc, who were the physiotherapists in the clinic and also contributed to the manuscript. Dr. Connie Marras provided important guidance regarding the data analysis and manuscript preparation.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Espay AJ, Aybek S, Carson A, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018;75(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carson A, Lehn A. Epidemiology In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology. New York: Elsevier; 2016:47–60. [DOI] [PubMed] [Google Scholar]
- 3. Factor SA, Podskalny GD, Molho ES. Psychogenic movement disorders: frequency, clinical profile, and characteristics. J Neurol Neurosurg Psychiatry 1995;59(4):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry 2014;85(2):220–226. [DOI] [PubMed] [Google Scholar]
- 5. Gelauff J, Stone J. Prognosis of functional neurologic disorders In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology New York: Elsevier; 2016:523–541. [DOI] [PubMed] [Google Scholar]
- 6. Voon V, Cavanna AE, Coburn K, Sampson S, Reeve A, LaFrance WC. Functional neuroanatomy and neurophysiology of functional neurological disorders (conversion disorder). J Neuropsychiatry Clin Neurosci 2016;28(3):168–190. [DOI] [PubMed] [Google Scholar]
- 7. Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord 2011;26(13):2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol 2012;11(3):250–260. [DOI] [PubMed] [Google Scholar]
- 9. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology. New York: Elsevier; 2016:73–84. [DOI] [PubMed] [Google Scholar]
- 10. Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of “hysteria.” Brain 2012;135(11):3495–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards MJ. Neurobiologic theories of functional neurologic disorders In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology. New York: Elsevier; 2016:131–137. [DOI] [PubMed] [Google Scholar]
- 12. Bakvis P, Spinhoven P, Roelofs K. Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2009;16(3):558–560. [DOI] [PubMed] [Google Scholar]
- 13. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics? The diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010;112(9):747–751. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen G, Stone J, Edwards MJ. Physiotherapy for functional (psychogenic) motor symptoms: a systematic review. J Psychosom Res 2013;75(2):93–102. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen G, Ricciardi L, Demartini B, Hunter R, Joyce E, Edwards MJ. Outcomes of a 5‐day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol 2015;262(3):674–681. [DOI] [PubMed] [Google Scholar]
- 16. Nielsen G, Stone J, Matthews A, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry 2015;86(10):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaFrance WC, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014;71(9):997–1005. [DOI] [PubMed] [Google Scholar]
- 18. Hopp JL, Lafrance WC. Cognitive behavioral therapy for psychogenic neurological disorders. Neurologist 2012;18(6):364–372. [DOI] [PubMed] [Google Scholar]
- 19. Reuber M, Burness C, Howlett S, Brazier J, Grünewald R. Tailored psychotherapy for patients with functional neurological symptoms: a pilot study. J Psychosom Res 2007;63(6):625–632. [DOI] [PubMed] [Google Scholar]
- 20. Dworetzky BA, Baslet G, eds. Psychogenic Nonepileptic Seizures: Toward the Integration of Care. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 21. Jacob AE, Kaelin DL, Roach AR, Ziegler CH, LaFaver K. Motor retraining (MoRe) for functional movement disorders: outcomes from a 1‐week multidisciplinary rehabilitation program. PM R 2018;10(11):1164–1172. [DOI] [PubMed] [Google Scholar]
- 22. McCormack R, Moriarty J, Mellers JD, et al. Specialist inpatient treatment for severe motor conversion disorder: a retrospective comparative study. J Neurol Neurosurg Psychiatry 2014;85(8):895–900. [DOI] [PubMed] [Google Scholar]
- 23. Hubschmid M, Aybek S, Maccaferri GE, et al. Efficacy of brief interdisciplinary psychotherapeutic intervention for motor conversion disorder and nonepileptic attacks. Gen Hosp Psychiatry 2015;37(5):448–455. [DOI] [PubMed] [Google Scholar]
- 24. Aybek S, Hubschmid M, Mossinger C, Berney A, Vingerhoets F. Early intervention for conversion disorder: neurologists and psychiatrists working together. Acta Neuropsychiatr 2013;25(1):52–56. [DOI] [PubMed] [Google Scholar]
- 25. Stone J, Binzer M, Sharpe M. Illness beliefs and locus of control: A comparison of patients with pseudoseizures and epilepsy. J Psychosom Res 2004;57(6):541–547. [DOI] [PubMed] [Google Scholar]
- 26. Reid S, Crayford T, Patel A, Wessely S, Hotopf M. Frequent attenders in secondary care: a 3‐year follow‐up study of patients with medically unexplained symptoms. Psychol Med 2003;33(3):519–524. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen G, Ricciardi L, Meppelink AM, Holt K, Teodoro T, Edwards M. A simplified version of the Psychogenic Movement Disorders Rating Scale: The Simplified Functional Movement Disorders Rating Scale (S‐FMDRS). Mov Disord Clin Pract 2017;4(5):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Espay AJ, Lang AE. Phenotype‐specific diagnosis of functional (psychogenic) movement disorders. Curr Neurol Neurosci Rep 2015;15(6):32. [DOI] [PubMed] [Google Scholar]
- 29. Gelauff JM, Carson A, Ludwig L, Tijssen MAJ, Stone J. The prognosis of functional limb weakness: a 14‐year case‐control study. Brain 2019;142(7):2137–2148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Material S1. Additional methods and results.
Table S1. Baseline demographic characteristics and main outcome (Clinical Global Impression–Improvement, CGI‐I) score. FMD, Functional Movement Disorder; BDI, Beck Depression Inventory; SA, State anxiety scale; TA, Trait anxiety scale; PTSD, posttraumatic stress disorder; GAD, generalized anxiety disorder; MDE, major depressive episode; NR, no response. CGI‐I scores are presented as a mean of 3 scores which were independently rendered by the 3 treating clinicians. Subject 3 was invited to return for the second cohort because of missed sessions in the first cohort.
Video S1. A 24‐year‐old nurse presented with tremor, dystonia, and abnormal gait. The baseline video demonstrates functional dystonic posturing, a variable and distractible tremor of the upper limbs, and a noneconomical gait consistent with a mixed functional movement disorder. Neuropsychiatric evaluation revealed longstanding symptoms as described in the text. Relevant factors to her formulation included chronic feelings of invalidation, deficits in self‐assertion and boundary setting and an unconscious reinforcement of physical symptoms by satisfaction of unmet emotional needs. Motor retraining was based on the patient's history of dancing that was simultaneously used to target the relevant psychological deficits in her formulation. Breathing, body awareness, and mindfulness exercises synchronized to classical music were integrated with motor retraining. The patient remitted by the final clinic and her gait normalized.
Video S2. A 31‐year‐old student presented with the abrupt onset of axial jerks following a motor vehicle accident. He developed thoracic pain that evolved into an area of sensitivity that triggered a cluster of spasms if touched. As a result, he was unable to lean back while seated on a chair or wear a backpack. Baseline video shows episodic clusters of severe truncal flexion/extension jerks while sitting and walking. He had a history of exposure to violent conflict including witnessing the murder of his father yet did not endorse symptoms of posttraumatic stress disorder. We hypothesized that his accident triggered autonomic hyperarousal that evolved to a sustained anxiety response, overlaid with catastrophic thought patterns (eg, fear of death from his symptoms.) This was overlaid with a strong conditioned response between environmental triggers (eg, the sensation of a backpack touching his back) and physical symptoms (pain and axial jerks). The treatment plan is as described in the text. The patient remitted by the fifth clinic visit, and the patient was able to dribble a soccer ball, wear a backpack, and run.


